Abstract
The stress-induced host cell factors initiating the expression of the herpes simplex virus lytic cycle from the latent viral genome are not known. Previous studies have focused on the effect of specific viral proteins on reactivation, i.e., the production of detectable infectious virus. However, identification of the viral protein(s) through which host cell factors transduce entry into the lytic cycle and analysis of the promoter(s) of this (these) first protein(s) will provide clues to the identity of the stress-induced host cell factors important for reactivation. In this report, we present the first strategy developed for this type of analysis and use this strategy to test the established hypothesis that the herpes simplex virus ICP0 protein initiates reactivation from the latent state. To this end, ICP0 null and promoter mutants were analyzed for the abilities (i) to exit latency and produce lytic-phase viral proteins (initiate reactivation) and (ii) to produce infectious viral progeny (reactivate) in explant and in vivo. Infection conditions were manipulated so that approximately equal numbers of latent infections were established by the parental strains, the mutants, and their genomically restored counterparts, eliminating disparate latent pool sizes as a complicating factor. Following hyperthermic stress (HS), which induces reactivation in vivo, equivalent numbers of neurons exited latency (as evidenced by the expression of lytic-phase viral proteins) in ganglia latently infected with either the ICP0 null mutant dl1403 or the parental strain. In contrast, infectious virus was detected in the ganglia of mice latently infected with the parental strain but not with ICP0 null mutant dl1403 or FXE. These data demonstrate that the role of ICP0 in the process of reactivation is not as a component of the switch from latency to lytic-phase gene expression; rather, ICP0 is required after entry into the lytic cycle has occurred. Similar analyses were carried out with the ΔTfi mutant, which contains a 350-bp deletion in the ICP0 promoter, and the genomically restored isolate, ΔTfiR. The numbers of latently infected neurons exiting latency were not different for ΔTfi and ΔTfiR. However, ΔTfi did not reactivate in vivo, whereas ΔTfiR reactivated in ∼38% of the mice. In addition, ICP0 was detected in ΔTfiR-infected neurons exiting latency but was not detected in those neurons exiting latency infected with ΔTfi. We conclude that while ICP0 is important and perhaps essential for infectious virus production during reactivation in vivo, this protein is not required and appears to play no major role in the initiation of reactivation in vivo.
It is estimated that over five billon people are presently infected with herpes simplex virus (HSV) type 1 (HSV-1) worldwide. The perpetual HSV pandemic is due in large part to the ability of the virus to establish latent infections that persist for the life of the host. These latent infections periodically reactivate, which can lead to contagious surface lesions, stromal keratitis (an important cause of blindness), and rare but fatal encephalitis (59).
During acute infection of sensory ganglia, some neurons are productively infected and die, while concomitantly in other neurons a latent infection is established (23, 44). After the acute stage of infection ends, viral proteins are very rarely detected during latency, and these rare positive neurons are thought to be undergoing reactivation (12, 39, 42, 48). Viral genetics, inoculation titer, and route of infection are important parameters that influence the number of neurons in which latency is established (41). In mice, as many as 25% of the neurons in a trigeminal ganglion (TG) can become latently infected, and the individual neurons in this reservoir contain variable numbers of viral genomes ranging from 1 to >1,000 (36, 38-41, 46, 53-55). These values are in general agreement with estimates of latency in human TG (7, 27, 58). The latent virus can reactivate following a variety of stressful stimuli (59), and the percentage of neurons in which latency is established as well as the average number of viral genomes present have been shown to correlate with the frequency of reactivation in the mouse model (38-41, 53-55). The molecular mechanisms that regulate reactivation are not known.
A central question is how reactivation from latency is initiated in neurons in the absence of the virion proteins that are critical for initiating lytic-phase transcription from the viral genome, such as VP16. Various stressful stimuli can lead to reactivation from latency (Fig. 1). These systemic stresses induce changes in the physiologic state of sensory neurons that contain the latent viral genome (42). If stress-induced changes result in detectable viral proteins in a neuron, the viral genome has exited the latent state. Thus, the process of reactivation has been initiated in these neurons. The initiation of reactivation is highly correlated with progression to infectious virus production (reactivation) with wild-type strains of HSV-1 (39).
FIG. 1.
Schematic of HSV reactivation from the latent state following stress. Stressful stimuli induce changes within sensory neurons that induce virus reactivation from the latent state. Many studies have shown that infectious virus is produced in latently infected ganglia when explanted in vitro (49). Recently, more-refined analysis at the single-cell level has demonstrated that many thousands of neurons harbor the latent viral genome but that the latent virus in most neurons does not exit the latent state following explant in vitro or hyperthermic stress in vivo. In a very few neurons (<0.1%) viral lytic-phase proteins can be detected, indicating virus entry into the lytic cycle (36, 38, 39, 41-45, 53-55), which we term the initiation of reactivation. Once the latent state has been exited, the lytic-phase proteome is expressed and infectious virus is produced, peaking at 22 h poststress in vivo (44). It has been hypothesized that stress-induced signals operate on the ICP0 promoter and that ICP0 initiates the exit from the latent state by modifying the host cell environment as well as transactivating other viral genes (11, 15, 16).
A favored hypothesis is that the promoter for the viral ICP0 gene responds to stress-induced signals and the ICP0 protein serves to initiate reactivation from latency (11, 15, 16, 20, 28). ICP0 is a multifunctional protein that can regulate the levels of both viral and host proteins at the transcriptional, translational, and posttranslational levels (11, 14). Another potential mechanism for ICP0 altering host and perhaps viral gene expression is the interaction of ICP0 with host cell histone deacetylases (13, 21, 32, 34, 61). Indeed, several studies have demonstrated that ICP0 null mutants reactivate less efficiently (as measured by the detection of infectious virus) than the wild type when assayed in vitro (1, 3, 6, 16, 20).
Importantly, excising and explanting the ganglia result in rapid, widespread changes in the physiology of the ganglion cells, including neurons. These changes do not occur during reactivation in vivo (42), raising the possibility that in the explant setting, the role of viral proteins such as ICP0 could be obviated or obscured. Since it is critical to unambiguously determine the viral gene(s) and its (their) promoter(s) that initiate reactivation, we examined the potential role of ICP0 for the initiation of reactivation in vivo. The long-term goal for these studies is to identify the responsive viral promoter elements to gain insight into the cellular factors and, ultimately, the signaling cascade that mediate reactivation following subjection to stressful stimuli.
Distinguishing between a requirement for ICP0 in the initiation of reactivation and one for the efficient production and/or detection (i.e., plaquing efficiency) of infectious virions has not been attempted. We have recently developed approaches that can distinguish between these possibilities. For example, while viral thymidine kinase (TK)-negative mutants do not produce infectious virus (reactivate), they do express lytic-phase viral proteins (initiate reactivation) in sensory neurons following hyperthermic stress in vivo (42, 45). Therefore, the number of neurons in which HSV exits latency can be quantified in the absence of infectious virus production.
Here we report the results of experiments designed to directly investigate the role of ICP0 in the initiation of reactivation from latency in vivo. The strain 17syn+ ICP0 null mutants dl1403 (50) and FXE (10) did not reactivate in vivo (as assayed by infectious virus production), and the KOS-based ICP0 promoter mutant, ΔTfi (9), was severely impaired. Thus, ICP0 appears to be required for the production of detectable infectious virus during reactivation in vivo. However, the ICP0 null mutants did enter the lytic cycle as efficiently as the parental strain, as evidenced by the number of neurons expressing viral lytic-phase proteins. Further, ΔTfi promoter mutant initiated reactivation as efficiently as ΔTfiR and the parental wild-type virus. We conclude that the initiation of reactivation occurs by a mechanism that does not require ICP0 function.
MATERIALS AND METHODS
Viral strains and stock production.
Virus stocks of laboratory strains 17syn+ and KOS/M and of the various mutants employed in this study were generated by routine propagation on rabbit skin cell (RSC) monolayers. Infected cells were harvested and sonicated, and the titer of each stock was determined by serial-dilution plaque assay on RSC monolayers. The wild-type HSV-1 strain 17syn+ was originally obtained from John H. Subak-Sharpe at the Medical Research Council Virology Unit in Glasgow, Scotland. The ICP0 null mutants dl1403 and FXE were kind gifts of Nigel Stow and Roger Everett at the Medical Research Council Virology Unit in Glasgow, Scotland, and their construction and characterization have been described previously (10, 50). ΔTfi and its genomically restored companion virus, ΔTfiR, were a kind gift of David Leib at Washington University, and their construction and characterization have been reported previously (8, 9).
Construction of viral mutants.
All viral DNA sequences were derived from the isolate of strain 17syn+ described above. The methods for inserting promoter/reporter gene cassettes into the gC locus were previously detailed (52-54, 57). Briefly, the promoter/reporter cassette was inserted into the gC gene at the XbaI site at bp 97669 on the viral genome in the orientation opposite to that of gC to limit promoter occlusion or interference effects. All restriction enzyme sites and base pair numberings are referred to as the corresponding positions in the published HSV-1 sequence of strain 17syn+ (24, 30) as present in GenBank (NID g1944536). To generate the ICP0 promoter/beta galactosidase (β-Gal) reporter mutant employed here, a 563-bp fragment containing the ICP0 promoter and the entire 5′ untranslated region through the methionine translation start codon of ICP0 was fused in frame with the β-Gal reading frame, flanked by XbaI sites, and inserted into the gC locus at bp 96318 in a manner exactly analogous to a previously described insertion of a reporter cassette into the gJ locus (56).
Inoculation of mice.
All procedures involving animals were approved by the Children's Hospital or the University of Cincinnati Institutional Animal Care and Use Committee and were in compliance with NIH guidelines. Animals were housed in American Association for Laboratory Animal Care-approved quarters. Male outbred Swiss Webster mice (22 to 25 g in weight; Harlan Laboratories) were used throughout these studies. Prior to inoculation, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg of body weight). A 10-μl drop containing 1 × 105 PFU was placed onto each scarified corneal surface. As indicated in the text, in some experiments the inoculum titer was altered to achieve uniform levels of latent infections as previously detailed (38, 55).
Replication in vivo.
Groups of mice were infected as described above, and at the indicated times postinfection three mice from each inoculation group were individually assayed for virus. Eyes and TG were removed, homogenized in 1 ml of culture medium, clarified by centrifugation at 800 × g for 3 min, and assayed for infectious virus titer on RSC monolayers.
Quantification of latent infections by contextual analysis of latency.
Mice were infected as described above and maintained for at least 40 days postinoculation (p.i.) Enriched neuron populations were obtained, and individual neurons were assayed for the presence of the latent viral genome as described previously (36, 38-41, 46, 53-55). To assess the rare neurons that contain very high viral genome copy numbers, samples containing 10 neurons were employed as described previously (36, 38, 39, 53, 55). Products were electrophoresed, blotted, probed with an internal 32P-labeled oligonucleotide, and quantified on a PhosphorImager (Molecular Dynamics) using ImageQuant software.
In vivo reactivation.
Latent HSV was induced to reactivate in the ganglia of mice in vivo by use of hyperthermic stress (44). At 22 h postinduction, TG pairs were removed and homogenized in cell culture medium, and the entire homogenate was adsorbed to an RSC monolayer in a single well of a six-well dish (Falcon). After 1 h, the wells were rinsed, maintained for 3 days, and examined for the presence of infectious virus. The ICP0 null and promoter mutant ganglion extracts were plated on the ICP0-expressing L7 cell line (35) (a kind gift of Neal DeLuca, University of Pittsburgh).
Explant reactivation.
Latently infected ganglia were aseptically removed, placed into minimal essential medium supplemented with 5% newborn calf serum, and incubated at 37°C in a 5% CO2 incubator as described previously (42). At the indicated times postexplant, ganglia were homogenized and plated exactly as described previously for reactivation in vivo.
Antibodies and immunohistochemistry.
HSV proteins were detected in whole ganglia processed as described previously (22, 39). Ganglia were incubated overnight in phosphate-buffered saline containing 2% bovine serum albumin, 5% dimethyl sulfoxide, 5% normal horse serum, and 0.5 μl/ml of the rabbit anti-HSV antibody (AXL237; Accurate) or rabbit anti-ICP0 antibody (Zymed) as described previously (45). After being rinsed for 5 h with multiple changes of phosphate-buffered saline, ganglia were incubated overnight in horseradish peroxidase conjugate, rinsed for 5 h, and developed in 0.1 M Tris (pH 8.2) containing 250 μg diaminobenzidine and 0.004% H2O2 as described previously (39, 42, 56).
Analysis of RNA expression.
Fifty male Swiss Webster mice were inoculated via corneal abrasion with 105 PFU of HSV-1 strain 17syn+. The efficiency of acute replication was monitored by replication kinetic analysis of eyes and trigeminal ganglia pooled from three mice/group on days 2, 4, and 6 p.i. At >40 days p.i., 27 mice were subjected to HS, which induces reactivation of latent virus in vivo. Ganglia from 15 of the mice were harvested at 20 h post-HS and analyzed for infectious virus as described previously. The remaining mice were sacrificed at 1, 3, 8, and 20 h post-HS, and ganglia (six at each time point) were snap-frozen in liquid nitrogen. Ganglia from three untreated latently infected mice were harvested and snap-frozen in a manner identical to that used for the HS group. RNA was extracted from the tissue by utilizing Ultraspec (Biotecx) according to the manufacturer's directions. The quality and quantity of RNA were assessed on ethidium-stained agarose gels. The annealing temperatures were optimized for each primer pair and ranged from 60 to 65°C. A dilution series of template DNA was used to compare the relative efficiencies of the primer pairs utilized. Probed products were exposed to phosphorimaging screens and quantified using Imagequant software.
Forward (F), reverse (R), and probe (P) oligonucleotides employed in these studies are as follows: ICP0 upstream of mRNA cap site, CCATTGGGGGAATCGTCACTG (F), TTCTGTGGTGATGCGGAGAGG (R), and AGTGCCGAGGTGCAAATGC (P); ICP0 unspliced RNA, AGCAGGGGGGCAGGACTTTG (F), TTCTGTGGTGATGCGGAGAGG (R), and CGTCAATCAGCACCCACGAG (P); spliced ICP0 mRNA, GCATTTGCACCTCGGCACTC (F), GGTCTCGGTCGCAGGGAAAC (R), and CCTCTCCGCATCACCACAGAAG (P); ICP4 upstream of mRNA cap site, CCTCCGCTGCTCCTCCTTC (F), GCGAGCGTCTGACGGTCTG (R), and CGACACGGATCCACGACCC (P); ICP4, CGACACGGATCCACGACCC (F), GATCCCCCTCCCGCGCTTCGTCCG (R), and ACCGCCAGAGACAGACCGTCAGA (P); ICP22, TCCGACGACAGAAACCCACC (F), GACCACAGTGGCTTCCCCC (R), and CGAGCAGGAAGCGGTCCAC (P); ICP27, AAGATGTGCATCCACCACAACC (F), GCAATGTCCTTAATGTCCGCC (R), and CCTTTCTCCAGTGCTACCTGAAGG (P); ICP47, TGGAAATGGCGGACACCTTC (F), ATTACGGGGACTGTCGGTCAC (R), and CCACACCCAAGGATGCGTTG (P); and SKD3, AGAACGCACCGAGCTCCCATAC (F), CATTAGAGCTGCATCCTTGTTGG (R), and TCCTAGCCCCGAAGAAACACTC (P). Other oligonucleotide sets for various HSV-1 regions mentioned in the text but not shown in Fig. 2 are available on request.
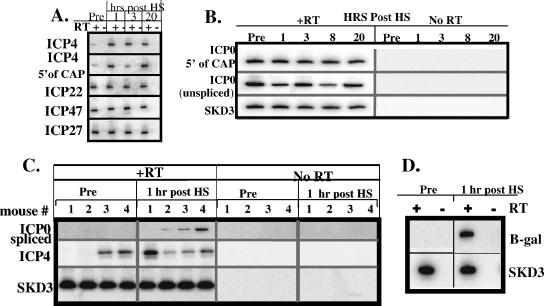
FIG. 2.
Viral gene transcription during latency and following hyperthermic stress-induced reactivation. (A) RNA related to ICP4, ICP22, ICP47, and ICP27 detected by RT-PCR before and at times indicated after hyperthermic stress. + and − indicate the presence and absence, respectively, of reverse transcriptase in the reaction. RNAs were detected prior to hyperthermic stress within the normally transcribed regions of these genes, as well as upstream of the TATA boxes in the promoters (labeled 5′ of CAP for ICP4; others are not shown). (B) Detection of unspliced ICP0 transcripts and transcripts originating 5′ of the normal ICP0 start site both before and at the indicated times after hyperthermic stress. +RT and No RT indicate the presence and absence, respectively, of reverse transcriptase in the reaction. SKD3 is a mouse ribosomal protein mRNA utilized as a control. Most if not all of the RNA detected is transcribed from somewhere upstream of the ICP0 promoter. (C and D) Total RNA derived from the TG of individual animals (indicated by numbers above the lanes) was assayed for the presence of spliced ICP0 and ICP4 (C) and β-Gal (D) transcripts both before and 1 hour after hyperthermic stress. The results suggest that the ICP0 promoter is upregulated within 1 h poststress.
Reverse transcription-PCR (RT-PCR).
One half of the total RNA from six ganglia was treated with DNase (RNase free) for 45 min in the presence of RNase inhibitor. The DNase was heat inactivated (70°C/15 min), and the RNA was divided into six 8-μl aliquots, each of which was incubated with a downstream primer for 10 min at 70°C. The reverse transcriptase (RT) reaction mix was added to each RNA/primer sample, which was then divided into two aliquots. The temperature was reduced to 50°C, and Superscript II (Invitrogen) was added to one of each primer sample pair. cDNA synthesis was halted after 1 h by heating samples to 70°C for 15 min. One half of each reaction mix with and without RT (5 μl) was added to 44.75 μl of PCR mix containing the appropriate amplification primers. Following a “hot start,” 0.25 μl of Taq was added, and amplification was carried out for 35 rounds. Ten microliters of the PCR product was electrophoresed through acrylamide gels, transferred to nylon membrane, probed with a 32P-labeled internal oligonucleotide, and analyzed as described above.
RESULTS
Analysis of viral transcriptional changes in trigeminal ganglia from latently infected mice after hyperthermic stress.
If ICP0 initiates reactivation from latency, it follows that ICP0 mRNA would be the first viral transcript produced following a reactivation stimulus. Arguing against this possibility is the previous report that viral transcripts of the early class were detected prior to immediate early (IE) mRNA in explanted latently infected ganglia (51). We examined the kinetics of RNA expression during reactivation in vivo. Latently infected animals were subjected to hyperthermic stress, a procedure that rapidly induces reactivation (44), and RT-PCR was utilized to determine the viral transcripts detected at early times after hyperthermic stress.
Mice were infected on scarified corneas with 1 × 105 PFU of strain 17syn+ and maintained for at least 40 days p.i. Total RNA was isolated from pooled ganglia, and cDNAs were generated from specific primers and amplified by PCR as described in Materials and Methods. Initially, assays were performed to detect RNAs corresponding to the five immediate early genes of HSV-1 as well as to the beta/early gene encoding thymidine kinase, and RNAs corresponding to all of these regions were detected prior to hyperthermic stress (Fig. 2, top panels, and data not shown). Using this semiquantitative approach, no consistent increases in the levels of any of these RNAs were detected at 1, 3, or 20 h after hyperthermic stress. RNAs were also detected 5′ of the known transcription start sites of ICP0, ICP22/47, ICP27, and ICP4 (Fig. 2A and B and data not shown). Since these RNAs were detected within the promoter sequences, these RNAs were not transcribed from the IE gene promoters. Kramer and Coen first reported viral transcription from the TK and ICP4 loci as well as 5′ of the ICP4 transcription start site during latency (19), and the presence of virus-related RNAs other than LAT during latency has been detected in other studies as well (4, 5, 19, 31, 33). The findings presented here confirm and extend these results by demonstrating that by use of RT-PCR analyses, RNAs corresponding to many regions of the viral genome are detectable during latency.
To determine if spontaneous reactivation events could account for the presence of these transcripts, individual latently infected ganglia were assayed. Viral RNAs related to ICP4, ICP0, ICP22, ICP47, ICP27 (all encoded by IE genes), TK, VP5 (encoded by early genes), VP16 (encoded by a leaky late gene), and UL15 (encoded by a late gene) were detected in about 80% of the ganglia examined (Fig. 2C and data not shown). However, viral protein-positive neurons (evidence of initiation of reactivation) were found in fewer than 1 in 15 ganglia (reference 39 and data below), supporting the consistent but low frequency of spontaneous reactivation in mice that has been observed by others (12, 48). This strongly suggests that the RNA detected was not the result of spontaneous reactivation. The biological significance of this transcriptional activity during latency is not known, but the continuous presence of these RNAs does obfuscate the interpretation of RT-PCR analyses during reactivation.
Of interest is the fact that spliced forms of ICP0 and UL15 mRNAs were not detectable in latently infected ganglia prior to hyperthermic stress but were detected after hyperthermic stress (Fig. 2C). Significantly, properly spliced ICP0 RNA was detected within 1 hour after hyperthermic stress, whereas spliced UL15 transcripts were not detected until later. Thus, the presence of spliced ICP0 mRNA transcripts might serve as an early marker for reactivation.
Upregulation of transcription from the ICP0 promoter is detectable within 1 hour after hyperthermic stress.
We next determined whether the detection of spliced ICP0 mRNA correlated temporally with the upregulation of the ICP0 promoter, as it was possible that hyperthermic stress induced the processing of preexisting ICP0-related transcripts. Mice were latently infected as described above with an ICP0 promoter/reporter virus in which the Escherichia coli β-Gal gene was expressed from a 562-bp ICP0 promoter in the gC locus. RNA was isolated from TG both prior to and 1 hour after hyperthermic stress.
β-Gal-related RNA was not detected in untreated animals but was found at 1 hour after hyperthermic stress in treated animals (Fig. 2D). These results demonstrate that the ICP0 promoter is upregulated within 1 hour poststress. Further, since the reporter construct resides within the unique long segment, this upregulation was position independent and was not the result of a generalized upregulation of transcription from the terminal or internal repeat sequences. Histochemical evidence of β-Gal enzymatic activity was detectable in a few neurons by 6 h posttreatment (not shown), suggesting that neurons in which reactivation occurs could be the source of the RNA detected.
ICP0 is required for reactivation from latency in vivo.
To determine the requirement of ICP0 for reactivation in vivo, three ICP0 mutant viruses were employed (Fig. 3). In mutant dl1403, a large deletion removes much of the carboxy terminus of the ICP0 protein (50), and in mutant FXE, a small deletion removes the zinc ring finger binding domain of ICP0 (10). Both mutations eliminate the transactivating function of ICP0 (10). The ΔTfi mutant contains a 350-bp deletion in the ICP0 promoter that results in a reduction in ICP0 protein production at early times p.i. in Vero cells. Importantly, the ICP0 protein produced is fully functional (8, 9).
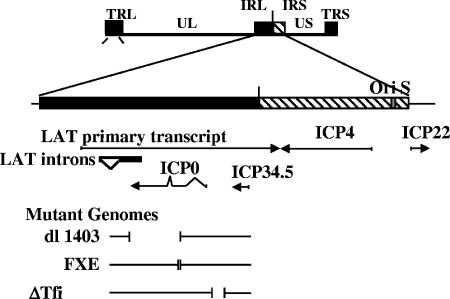
FIG. 3.
A schematic representation of the viral mutants used in this study. Top: the HSV viral genome with the unique long (UL) and unique short (US) regions as well as the terminal and internal repeat sequences (TR and IR) indicated. The internal repeat region is shown enlarged below, with the mRNAs transcribed within this region indicated by arrows. Short vertical bars indicate the extents of the deletions present in the mutants employed in this study.
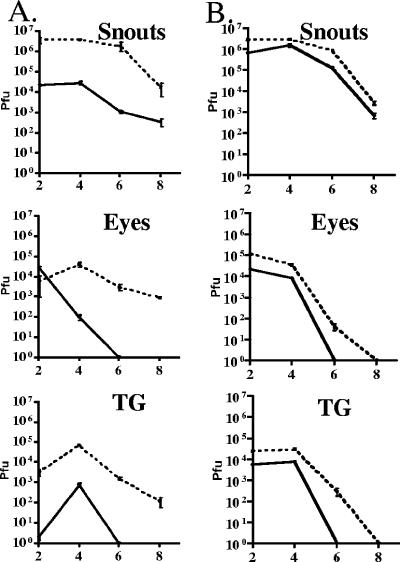
Mice were infected on scarified corneas and shaved and abraded whisker pads with virus as indicated below (see Fig. 5 and 7). Lytic-phase replication kinetics in inoculated surface tissues and in the trigeminal ganglia were determined as described in Materials and Methods. These data are shown graphically in Fig. 4. As reported previously, there was a marked reduction in the titer of infectious virus detected in mice infected with mutants dl1403 and FXE (not shown) at the corneal surface, snout, and in the nervous system (Fig. 4A), whereas the replication of ΔTfi was reduced compared to that of the rescue ΔTfiR at these sites, but less so (Fig. 4B) (9). The ratio of the area under the curve (AUC) for dl1403/17syn+ in the ganglia was 0.012 compared to 0.25 for ΔTfi/ΔTfiR. In the eyes, snouts, and TG, the AUC for strain 17syn+ was significantly greater than that of dl1403 (P < 0.0001). All of the AUC for ΔTfi were significantly lower than those for ΔTfiR as well (P = 0.0023 [snout]; P < 0.0001 [eyes and TG]).
FIG. 5.
ICP0 null mutants do not reactivate in vivo. Mice were infected on the cornea (strain 17syn+) or the cornea plus the snout (dl1403 and FXE) with the indicated inoculation titers. The percentages of neurons in which latency was established were determined by PCR analysis of individual neurons as described in Materials and Methods. The numbers within the bars are the numbers of neurons positive/tested. Additional animals were subjected to hyperthermic stress, and ganglia were analyzed for infectious virus at 22 h poststress. The percentages of mice positive for infectious virus and the numbers of mice positive/tested are indicated.
FIG. 7.
ΔTfi establishes latent infections efficiently but does not reactivate in vivo. Groups of mice were infected with the indicated titers of ΔTfi and ΔTfiR on both scarified corneas and snouts as described in Materials and Methods. The mice were maintained for at least 40 days postinfection. Top: Purified neurons derived from six trigeminal ganglia were analyzed by CXA for the presence of viral genomes as described in Materials and Methods. The gray bars indicate the percentages of neurons that were latently infected with ΔTfiR, and the black bars indicate the percentages latently infected with ΔTfi. The numbers of neurons positive for the viral genome/number of neurons tested are shown for the bars. Bottom: groups of animals were subjected to hyperthermic stress to induce reactivation as described in Materials and Methods. At 22 h postinduction, TG were removed, homogenized, and assayed for the presence of infectious virus. The gray bars indicate the percentages of ΔTfiR-infected animals positive for virus. The black bars indicate the percentages of ΔTfi-infected mice positive for virus. The numbers of animals positive/tested are shown for the bars.
FIG. 4.
In vivo replication of dl1403 and parental strain 17syn+ (A) and the ΔTfi and ΔTfiR mutants (B). Mice were inoculated on scarified corneas and abraded snouts. At the indicated times, tissues from three mice per inoculation group were examined for infectious virus as described in Materials and Methods. Solid lines represent ICP0 mutants and dashed lines wild type.
Additional animals were maintained for 40 days p.i. and subjected to either axotomy/explant or hyperthermic stress. Ganglia homogenates were plated on the ICP0-complementing cell line L7 (35). All ganglia from mice latently infected with the ICP0 null and promoter mutants reactivated within 9 days when placed into explant cultures, a finding similar to those from previous studies (1, 3, 6, 9, 16, 20). In vivo, however, while latent virus reactivated in 70% (7/10) animals infected with wild-type 17syn+ 22 h after hyperthermic stress, the reactivation of latent virus was not detected in animals infected with the ICP0 null mutants dl1403 and FXE at this time (0/20 and 0/20, respectively) (Fig. 5). In order to rule out the possibility that in the absence of ICP0 in vivo reactivation was merely delayed, additional mice latently infected with dl1403 were examined at 48 h after hyperthermic stress. As was observed at 22 h after hyperthermic stress, virus was not detected in any of the mice examined (0/18).
Establishment of latency.
ICP0 null mutants have been shown by quantitative PCR on total ganglion DNA to establish latent infections less efficiently than wild-type virus (1, 3, 16, 20). We have shown previously that the number of neurons in the latent pool is directly correlated to reactivation frequency (38, 41, 55). Therefore, a reduction in the number of latently infected neurons in ICP0 null mutant-infected mice could account for the apparent inability of these mutants to reactivate in vivo. Since one goal of these experiments was to determine the specific role of ICP0 in reactivation in vivo, it was important to determine precisely how the lack of ICP0 affected the establishment of latent infections. Contextual analysis (CXA), in which individual neurons are examined by PCR for the presence of the viral genome (36, 38, 41, 54), was employed to determine the percentage of neurons latently infected.
Ganglia from additional mice from the groups described above were analyzed. Mutants dl1403 and FXE established latent infections in 6.9% or ∼1,400 and 5.8% or ∼1,200 neurons per ganglion, respectively. 17syn+ established latency in 25% or ∼6,000 neurons per ganglion (Fig. 5). Clearly, the reduction in the number of latent infections established by the mutants would have a negative impact on the frequency of reactivation, as we have previously shown (36, 38, 40-42, 46, 53-56). Therefore, it was important to employ strategies to equalize the number of latent infections in mutant- and wild type-infected mice.
It was not possible to substantially increase the number of latent infections established by the mutants by altering the inoculation titer. In addition, the strategy of immunosuppression, previously reported to enhance the establishment of KOS-based ICP0 mutants (16), resulted in unacceptable levels of mortality with 17syn+-based mutants. Therefore, the inoculation titer of strain 17syn+ was reduced to yield roughly equivalent levels of establishment of latency (Fig. 5). When the inoculation titer was reduced to 1,000 PFU of strain 17syn+, latency was established in about 6.5% or ∼1,300 neurons per ganglion. Viral reactivation was detected in 20% of these mice (4/20) within 22 h after hyperthermic stress, while none was detected in the animals infected with dl1403 (0/20) or FXE (0/20). In a second experiment, virus reactivated in 6/12 mice latently infected with 17syn+, whereas no reactivation was detected in mice latently infected with dl1403 (0/56; P < 0.0001) (see Fig. 9A).
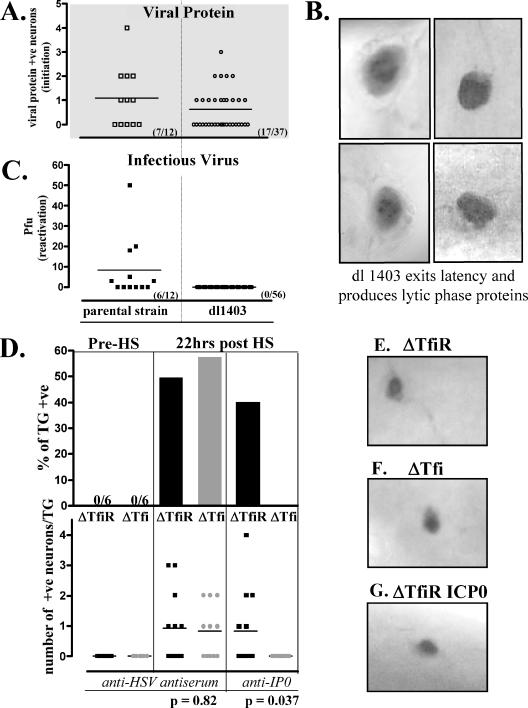
FIG. 9.
Quantification of the initiation of reactivation from latency at the single-neuron level. Additional mice latently infected with dl1403, ΔTfi, or ΔTfiR were subjected to hyperthermic stress to induce virus reactivation. At 22 h posttreatment, trigeminal ganglia were removed and processed for the immunohistochemical detection of viral proteins as detailed in Materials and Methods. (A) Scattergram showing the numbers of positive neurons detected in individual ganglia from animals latently infected with dl1403 or with the parental strain. Seventeen of 37 ganglia tested from dl1403-infected mice contained one or more positive (+ve) neurons. (B) Photomicrographs of four representative neurons expressing viral proteins in the ganglia of mice latently infected with dl1403 at 22 h after hyperthermic stress (brown precipitate). (C) None of 56 ganglia tested from dl1403-infected mice were positive for virus. (D) Percentages of ganglia containing viral protein-positive neurons from mice latently infected with ΔTfi and ΔTfiR before and 22 h after hyperthermic stress (top), and numbers of positive neurons in each ganglion (bottom). The numbers of viral protein-positive neurons detected in the ganglia of mice infected with ΔTfi and with ΔTfiR were similar poststress (P = 0.82). However, ICP0 was not detected in ganglia from ΔTfi group (P = 0.037). (E, F, and G) Photomicrographs of representative neurons in latently infected ganglia immunohistochemically stained for lytic viral proteins (E and F) or for ICP0 (G) at 22 h after hyperthermic stress are shown.
In vivo reactivation of ΔTfi.
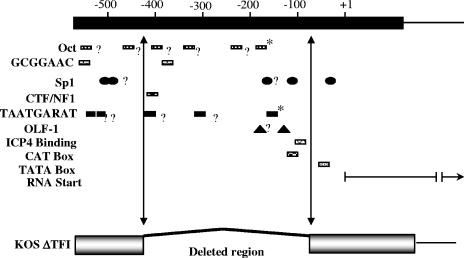
If the expression of ICP0 is the precipitating event that initiates the reactivation of HSV from the latent state, the ICP0 gene promoter must contain elements that respond to signals induced by stress in latently infected neurons. Previously, Davido and Leib showed that ΔTfi reactivated from latency in explant cultures in vitro with the same frequency and kinetics as the wild-type parental and genomically restored virus (9). This finding was unexpected because the deletion in the ICP0 promoter in ΔTfi removes all of the signals known to regulate IE gene expression (Fig. 6). The authors suggested that promoter elements that regulate ICP0 expression during acute infection might be distinct from those involved in regulation during reactivation (9). However, it remained possible that the promoter elements deleted from ΔTfi could play an important role in the regulation of reactivation in vivo.
FIG. 6.
Schematic representation of the ICP0 gene promoter region and the deletion present in ΔTfi. Top: Base pair numbers relative to the start site of the ICP0 mRNA (horizontal arrow at + 1). Shown are known or potential regulatory elements of the ICP0 gene. Within the 180 bp upstream of the RNA start site is the known Octa/TAATGARAT sequence that confers IE expression kinetics to ICP0 (marked with asterisks), sp1 binding sites, a CAT box, an OLF-1 site, an ICP4 binding site, and a TATA box. Other sites with homology to known factor binding sites, including four additional potential TAATGARAT sites (labeled with question marks), are also indicated. Vertical arrows indicate the limits of the deletion in the ICP0 promoter in the ΔTfi mutant.
Confirming prior results (9), ΔTfi reactivated in explant with timing and frequency the same as those for wild-type KOS and ΔTfiR. At 22 h postexplant, infectious virus was detected in 44% (7/16) of TG pairs latently infected with ΔTfi and in 47% (7/15) of those latently infected with ΔTfiR. However, when examined in vivo, infectious virus could not be recovered from ΔTfi mutant-infected TG pairs after hyperthermic stress (0/20), even though infectious virus was recovered from 6/16 TG pairs latently infected with ΔTfiR (P = 0.0041; Fisher's exact test) (Fig. 7). Importantly, the ganglia induced to reactivate in vitro and in vivo were analyzed identically. We have shown for ganglia latently infected with the wild-type strain KOS and 17syn+ that the number of neurons positive for viral proteins and the amount of virus detected in the reactivating ganglia are not different at 22 h postinduction following either explant in vitro or hyperthermic stress in vivo (42). Since this assay successfully detected infectious virus in the ΔTfi mutant-explanted ganglia, trivial reasons for the negative results in vivo, such as the less efficient reactivation of strain KOS (41) or inefficient plaquing of the ICP0 mutants, could be ruled out.
As detailed above, it was necessary to quantify the number of latently infected neurons in TG infected with ΔTfi and ΔTfiR to determine if a reduction in latent infections could account for the absence of reactivation of ΔTfi in vivo. Of the 244 PCRs performed on individual latently infected neurons from ΔTfi-infected mice, 44 (18%) were positive for the viral genome. In ΔTfiR-infected TG, 25.9% of neurons (54/208) contained the latent viral genome (Fig. 7).
To determine the reactivation phenotypes at equal levels of latency, the number of neurons latently infected was either decreased (ΔTfiR) or increased (ΔTfi) by decreasing or increasing inoculation titer, respectively. In the first experiment, an inoculation titer of 4 × 105 PFU of ΔTfiR resulted in ganglia containing ∼17% of neurons latently infected (17/100), very close to the 18% (44/244) observed for ΔTfi-infected TG. As anticipated, mice latently infected with ΔTfiR did show reactivation. In this case, 37.5% (6/16) mice tested positive for infectious virus after hyperthermic stress. In the second experiment, in mice inoculated with 2 × 107 PFU of ΔTfi, latency was established in 28% (23/82) of neurons. In this group of mice, reactivation of the ΔTfi mutant was detected, but in only 1 of 20 mice (5%). This was significantly less than the 47% (9/19) reactivation observed in ΔTfiR-infected mice, in which 26% (54/208) of the neurons were latently infected (P < 0.0033; Fisher's exact test) (Fig. 7).
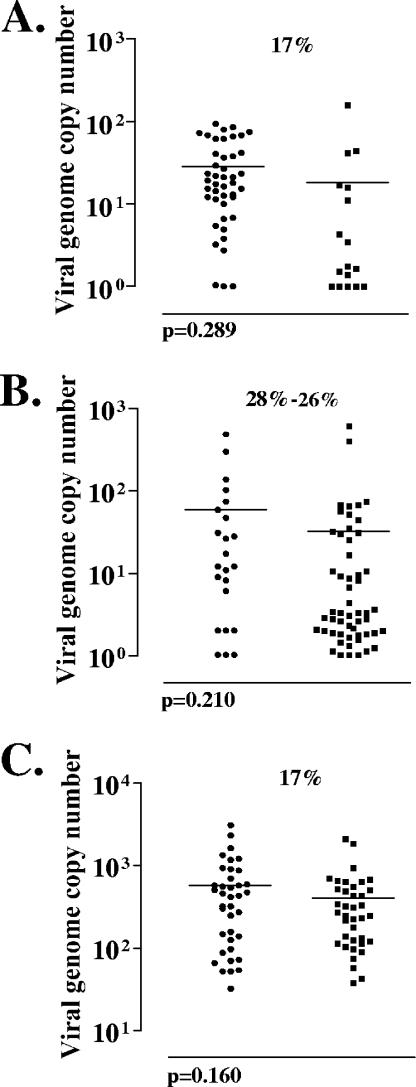
Although the numbers of latently infected neurons in the trigeminal ganglia were nearly identical, it was still possible that the latent infections established by ΔTfi differed qualitatively from those established by ΔTfiR. It has been shown previously that the number of viral genome copies in individual neurons can differ among latent pools and that a reduced average genome copy correlates with a reduction in reactivation frequency (55). Quantitative PCR analysis of individual neurons (CXA) was employed to determine the number of latent HSV genomes in individual latently infected neurons (Fig. 8). In those animals in which 17% of the neurons were latently infected, there was no significant difference in the average genome copy number between ΔTfi and ΔTfiR (29 and 18 latent genomes per neuron, respectively; P = 0.210; Student's t test) (Fig. 8A). Likewise, no significant difference in genome copy number was seen in those animals described above in which latency was established in approximately 28% of the TG neurons (means were 60 and 32 in ΔTfi- and ΔTfiR-infected ganglionic neurons, respectively; P = 0.289; Student's t test) (Fig. 8B).
FIG. 8.
The numbers of viral genome copies present in individual latently infected neurons. The numbers of copies of the viral genome present in each of the positive neurons from Fig. 6 are shown. Each point represents a single neuron. Shown are viral genome copies in neurons from ganglia latently infected with ΔTfi and ΔTfiR when 17% of neurons were latently infected (A), when 28% and 26% of the neurons were latently infected (B), and when 17% of neurons were latently infected but analyzed in groups of 10 neurons instead of individually (C). In this last case, each point represents 10 neurons.
Rare latently infected neurons contain very high numbers of latent genomes (36, 38, 40, 41, 53-56). In order to detect these very-high-copy-number neurons and compare this characteristic of ΔTfi and ΔTfiR latent pools, samples containing 10 neurons each were analyzed, and again there was no significant difference seen between TG latently infected with ΔTfi and TG infected with ΔTfiR (mean copy number per 10 neurons, 567 and 396, respectively; P = 0.160; Student's t test) (Fig. 8C).
Thus, by adjusting inoculum titers of ΔTfi and ΔTfiR, the number of latent infections established and the number of latent viral genomes present in individual neurons were made roughly equal, although the ΔTfi-infected neurons consistently contained a higher average viral genome copy number. Under these conditions, ΔTfiR reactivated in vivo following hyperthermic stress, whereas ΔTfi was severely impaired. These results demonstrate that in contrast to what is seen for explant reactivation in vitro (9), the region of the ICP0 promoter deleted from the ΔTfi mutant is required for efficient reactivation in vivo.
ICP0 is not required for the initial stages of reactivation in vivo.
In the experiments described above, the end point was the detection of infectious virus. While this assay fulfills the requirement for the functional definition of reactivation, it does not discriminate between the failure to enter the lytic cycle from the latent state (initiation of reactivation) and the inability to progress to infectious virus production after lytic-phase gene expression has begun. We have recently demonstrated using whole-ganglion immunohistochemistry that neurons latently infected with a TK null mutant do express lytic-phase viral proteins after induction in vivo; by definition, however, these neurons do not reactivate, since infectious virus is not produced (42, 45). It is therefore possible to use this approach to discriminate between a requirement for ICP0 in the earliest stages of reactivation from its known functions for efficient viral replication. We employed whole-ganglion immunohistochemistry to examine whether viral proteins were expressed in neurons latently infected with dl1403, FXE, or ΔTfi following hyperthermic stress in vivo. The failure to detect viral proteins would indicate that ICP0 was required for the earliest steps in reactivation. Conversely, the detection of viral proteins would demonstrate that ICP0 was not required for the initiation of reactivation in vivo.
Counter to the hypothesis that ICP0 initiates reactivation from latency, neurons expressing lytic viral proteins were detected at 22 h after hyperthermic stress in the ganglia of mice latently infected with dl1403, FXE, or ΔTfi. About 46% (17/37) of the trigeminal ganglia from mice latently infected with the ICP0 deletion mutant dl1403 contained one or more neurons in which reactivation had been initiated (as evidenced by lytic-phase viral protein expression) by 22 h after hyperthermic stress in vivo (Fig. 9A). Clearly, these viral protein-positive neurons had exited the latent state (Fig. 9B), although virus could not be detected (0/56) (Fig. 9A). The frequency of ganglia containing one or more positive neurons was not different from that seen in mice infected with the wild type (7/12 [58%]; P = 0.52; Fisher's exact test). In these mice, 6/12 ganglia tested were positive for virus, compared to 0/56 for the dl1403-infected mice (P < 0.0001) (Fig. 9C). As expected from prior results (12, 37, 39), no viral protein-positive neurons were detected in latently infected ganglia prior to hyperthermic stress (0/6 ganglia in each group) (Fig. 9D).
In ΔTfi- and ΔTfiR-infected mice, the percentages of ganglia containing one or more neurons expressing lytic-phase viral proteins at 22 h after hyperthermic stress were not different (7/12 and 6/12, respectively) (Fig. 9D). Also, the number of neurons in which viral protein synthesis was initiated following hyperthermic stress in ganglia latently infected with the ΔTfi promoter mutant (10 neurons in 12 ganglia) was not different from that seen in ganglia latently infected with ΔTfiR (11 neurons in 12 ganglia) (Fig. 9D). Representative viral protein-expressing neurons are shown in Fig. 9E, F, and G. Additional ganglia were examined for ICP0 protein expression poststress. Five of 12 ganglia from mice infected with ΔTfiR were positive, whereas 0/12 ganglia from mice infected with ΔTfi were positive (P = 0.037; Fisher's exact test) (Fig. 9D). Taken together, these results demonstrate that ICP0 does not play an important role in the initiation of reactivation from latency and that the proper regulation of ICP0 is required for efficient progression to virus production once latency has been exited.
DISCUSSION
Several converging lines of evidence have led to the hypothesis that the herpes simplex virus ICP0 gene is responsible for initiating reactivation from the latent state (reviewed in reference 11). In models of HSV quiescence in cultured cells, ICP0 can induce viral gene expression when provided in trans, and ICP0 either is or is not required for reentry into lytic-phase transcription depending on the model system (11, 15, 18, 25, 26, 60). With animal models of HSV latency, it has proven difficult to directly test this hypothesis. In particular, the importance of ICP0 for general virus replication and for the efficient establishment of latent infections makes it critical to distinguish between the roles of this gene product during the acute stage of infection when latency is established and those during reactivation from latency per se. Additionally, the importance of ICP0 for virus replication is dependent the cell's physiological state (2). Axotomy and explant of latently infected ganglia rapidly induces changes in neuronal physiology that do not occur during reactivation in vivo, and such changes could obscure the roles of viral proteins in this process (42).
In this study, we employed methods that quantify latency and reactivation at the single-cell level in vivo (i) to evaluate the role of ICP0 in reactivation in the intact host and (ii) to test the hypothesis that ICP0 is required for the initiation of reactivation from latency. Our results demonstrate (i) that ICP0 function is required for efficient reactivation in vivo (ICP0 null mutants did not reactivate); (ii) that the promoter elements contained within the 350-bp deletion from the ΔTfi mutant that are fully dispensable during reactivation in vitro are critical for reactivation in vivo; and (iii) that ICP0 is not required for the efficient initiation of the lytic cycle from the latent state, but properly regulated ICP0 is required for the progression to infectious virus production once the lytic cycle has been initiated.
ICP0 function is required for efficient reactivation (infectious virus production) in vivo.
Two well-characterized ICP0 null mutants, dl1403 (50) and FXE (10), were employed to determine the requirement for ICP0 for reactivation in vivo following hyperthermic stress (44). As others have reported (1, 6, 15, 16, 20, 50), ICP0 null mutants in strains KOS and 17syn+ reactivate in vitro. Halford and Schaffer demonstrated that ICP0 is important for efficient reactivation in vitro by use of quantitative kinetic assays (16). In vivo, we show that functional ICP0 is critical for reactivation. No reactivation of dl1403 was detected in the 76 mice examined following hyperthermic stress. The levels of latency of the ICP0 null mutants were low; however, the parental strain, 17syn+, did reactivate at these same low levels of latency. Further, neurons latently infected with dl1403 did express lytic-phase viral proteins with kinetics the same as those of neurons infected with the wild type following hyperthermic stress in vivo. However, it is formally possible that dl1403 would reactivate in vivo to some extent if high levels of latency could be established.
At these low levels of latency, mutant dl1403 does reactivate in 100% of the ganglia when ganglia are excised and placed into culture (50). We emphasize that dl1403 exits the latent state in vivo following stress as efficiently as the wild type when the levels of latency are the same. However, a detectable level of infectious virus is not produced in the ganglia. The reason why ICP0 is required for the progression to virus production in vivo but not in the in vitro setting is not known. However, it seems likely that some change in the physiologic state of neurons that occurs in explanted ganglia is responsible for the suppression of the need for ICP0. We have shown that cell cycle-associated proteins such as geminin, cdk2, and cdk4 are rapidly upregulated in neurons within hours after explant. These physiologic changes are accompanied by gross morphological changes and neuronal apoptosis (42). Our as-yet-unpublished studies utilizing chip array technology suggest that many additional host genes not altered following hyperthermic stress are up- or downregulated to a significant degree in explanted ganglia, and it is reasonable to anticipate that other parameters, such as the phosphorylation state of proteins, might also be altered in the in vitro setting. Any of these changes could be linked to the different reactivation phenotypes observed for ICP0 null mutants in vitro and in vivo.
ICP0 promoter elements dispensable during reactivation in vitro are essential for efficient reactivation in vivo.
It is reasonable to assume that the proper regulation of the ICP0 gene in neurons would be important during reactivation. We have shown that promoter elements that map upstream of −145 in the ICP0 promoter are essential for expression in neurons (56). However, Davido and Leib found that ΔTfi, which contains a 350-bp deletion between −70 and −420 in the ICP0 promoter, reactivated with wild-type efficiency and kinetics in vitro (9). It was therefore of interest to determine how this mutant reactivated in vivo.
It was possible to adjust inoculum titers of ΔTfi and ΔTfiR (a genomically restored isolate) so that all measurable parameters of latency, including the number of latently infected neurons and the number of viral genomes present in individual neurons, were similar. Following hyperthermic stress, only one animal latently infected with ΔTfi showed reactivation (2.5%), whereas 43% of the mice infected with ΔTfiR showed reactivation. In contrast, the frequency of in vitro reactivation of ΔTfi 22 h postexplant was indistinguishable from that of the ΔTfiR. Importantly, we have shown that the numbers of positive ganglia and the amounts of virus produced at 22 h postexplant and after hyperthermic stress are not different (42). Since all ganglia (22 h postexplant and 22 h after HS) were processed and analyzed identically on an ICP0-complementary cell line, virus would have been detected if it had been produced in vivo as it was in vitro. Our results in vitro are in agreement with previous studies that showed that ΔTfi reactivated as efficiently and with the same kinetics as its genomically restored variant, ΔTfiR, in explant (9). The disparity between the findings in these systems emphasizes the importance of investigating putatively reactivation-associated processes in an in vivo setting as well as in vitro.
ICP0 does not initiate reactivation following hyperthermic stress in vivo.
ICP0-related transcripts were detected in all latently infected ganglia assayed prior to stress. The production of virally related RNA during latency has previously been reported, but its biological significance is not yet known (4, 5, 19, 31, 33). In contrast the findings of a previous report (5), properly spliced ICP0 transcripts were not detected prior to stress in this study; this discrepancy may reflect differences in the virus strains or in the sensitivities of the assays employed. Spliced transcripts were detected at 1 h poststress, and this occurred concomitantly with the rapid upregulation of the ICP0 promoter. Clearly, the upregulation of the ICP0 promoter and the production of spliced ICP0 transcripts were early events, but it was not clear if the expression of ICP0 initiated reactivation. Indeed, we have shown that there is a posttranscriptional constraint on the production of ICP0 protein during reactivation in vivo that is mediated by LAT (56), and so the detection of RNA does not necessarily correlate with the production of protein.
Whole-ganglion immunohistochemistry can be employed to detect viral proteins in neurons in vivo. Using this approach to examine hundreds of latently infected ganglia, we have determined that prior to stress about 1 in 15 ganglia latently infected with strain 17syn+ contain a single positive neuron (∼6.7%) (39, 42, 45), consistent with the observations of others (12, 17, 29, 47). At 22 h poststress, ∼70 to 80% of the ganglia contain one or more positive neurons (39, 42, 45). Importantly, using this assay we have demonstrated that thymidine kinase mutants which do not reactivate (produce infectious virus) do enter into the lytic cycle at the same frequency as the rescued and parental strains at the same level of latency (42, 45). In this report, we utilized this approach to investigate for the first time whether mutations in the ICP0 gene result in reduced ability to exit the latent state or a reduced ability to produce infectious virus once latency has been exited.
The fact that viral proteins were detected in ganglia latently infected with dl1403 at 22 h after hyperthermic stress demonstrates that ICP0 is not required for the earliest stages of reactivation (initiation of reactivation). Importantly, the ICP0 null mutant exited latency (initiated reactivation) as efficiently as the parental strain when levels of latency were the same. These initiated reactivation events did not progress to infectious virus production, but reactivation was begun, as defined by a switch from latency to lytic-phase viral protein expression.
Interpreting the findings with ΔTfi is complicated, because this mutant is capable of producing fully functional ICP0. Abundant ICP0 protein was detected in ganglia acutely infected with ΔTfi (not shown), consistent with the detection of significant levels of ICP0 mRNA in ganglia (9). However, ICP0 protein, readily detected in ganglia latently infected with ΔTfiR poststress, was not detected in ganglia latently infected with ΔTfi poststress (Fig. 9). In these same ganglia, the numbers of neurons expressing other lytic-phase viral proteins were not different between the two groups (Fig. 9). Thus, the absence of detectable ICP0 did not reduce the capacity of ΔTfi to enter the lytic phase from the latent state. Taken together with the findings presented above for the ICP0 null mutant, we conclude that ICP0 does not appear to play any important role in the initiation of reactivation from latency. Rather, the proper expression of functional ICP0 is important for progression to virus production once reactivation has been initiated by some as-yet-unknown mechanism.
Acknowledgments
We thank Nigel Stow for mutants dl1403 and dl1403R, Roger Everett for mutant FXE, David Leib for ΔTfi and ΔTfiR mutants, and Neal Deluca for theL7 cell line.
This work was funded by Public Health Service grants 5RO1 AI 32121 and 5RO1 EY 13168 and also by support to N.M.S. from the Astellas USA Foundation.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai, W. Z., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, S. H., M. F. Kramer, P. A. Schaffer, and D. M. Coen. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, S. H., L. Y. Lee, D. A. Garber, P. A. Schaffer, D. M. Knipe, and D. M. Coen. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 76:4764-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements, G. B., and N. D. Stow. 1989. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J. Gen. Virol. 70:2501-2506. [DOI] [PubMed] [Google Scholar]
- 7.Cohrs, R. J., J. J. Laguardia, and D. Gilden. 2005. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal ganglia. Virus Genes 31:223-227. [DOI] [PubMed] [Google Scholar]
- 8.Davido, D. J., and D. A. Leib. 1998. Analysis of the basal and inducible activities of the ICPO promoter of herpes simplex virus type 1. J. Gen. Virol. 79:2093-2098. [DOI] [PubMed] [Google Scholar]
- 9.Davido, D. J., and D. A. Leib. 1996. Role of cis-acting sequences of the ICPO promoter of herpes simplex virus type 1 in viral pathogenesis, latency and reactivation. J. Gen. Virol. 77:1853-1863. [DOI] [PubMed] [Google Scholar]
- 10.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 12.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu, H., Y. Liang, G. Mandel, and B. Roizman. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. USA 102:7571-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbour, D. A., T. J. Hill, and W. A. Blyth. 1983. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J. Gen. Virol. 64:1491-1498. [DOI] [PubMed] [Google Scholar]
- 18.Harris, R. A., R. D. Everett, X. X. Zhu, S. Silverstein, and C. M. Preston. 1989. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 63:3513-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomonte, P., J. Thomas, P. Texier, C. Caron, S. Khochbin, and A. L. Epstein. 2004. Functional interaction between class II histone deacetylases and ICP0 of herpes simplex virus type 1. J. Virol. 78:6744-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luque, J. M., W. B. Adams, and J. G. Nicholls. 1998. Procedures for whole-mount immunohistochemistry and in situ hybridization of immature mammalian CNS. Brain Res. Brain Res. Protoc. 2:165-173. [DOI] [PubMed] [Google Scholar]
- 23.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150-160. [DOI] [PubMed] [Google Scholar]
- 24.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 25.Miller, C. S., R. J. Danaher, and R. J. Jacob. 2006. ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J. Virol. 80:3360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minaker, R. L., K. L. Mossman, and J. R. Smiley. 2005. Functional inaccessibility of quiescent herpes simplex virus genomes. Virol. J. 2:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell, B. M., D. C. Bloom, R. J. Cohrs, D. H. Gilden, and P. G. Kennedy. 2003. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J. Neurovirol. 9:194-204. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Openshaw, H., J. I. McNeill, X. H. Lin, J. Niland, and E. M. Cantin. 1995. Herpes simplex virus DNA in normal corneas: persistence without viral shedding from ganglia. J. Med. Virol. 46:75-80. [DOI] [PubMed] [Google Scholar]
- 30.Perry, L. J., and D. J. McGeoch. 1988. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:2831-2846. [DOI] [PubMed] [Google Scholar]
- 31.Pesola, J. M., J. Zhu, D. M. Knipe, and D. M. Coen. 2005. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J. Virol. 79:14516-14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon, A. P., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezuchova, I., M. Kudelova, V. Durmanova, A. Vojvodova, J. Kosovsky, and J. Rajcani. 2003. Transcription at early stages of herpes simplex virus 1 infection and during reactivation. Intervirology 46:25-34. [DOI] [PubMed] [Google Scholar]
- 34.Roizman, B., H. Gu, and G. Mandel. 2005. The first 30 minutes in the life of a virus: unREST in the nucleus. Cell Cycle 4:1019-1021. [DOI] [PubMed] [Google Scholar]
- 35.Samaniego, L. A., N. Wu, and N. A. DeLuca. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J. Virol. 71:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawtell, N. M. 2005. Detection and quantification of the rare latently infected cell undergoing herpes simplex virus transcriptional activation in the nervous system in vivo. Methods Mol. Biol. 292:57-72. [DOI] [PubMed] [Google Scholar]
- 38.Sawtell, N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawtell, N. M. 2003. Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J. Virol. 77:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawtell, N. M., D. I. Bernstein, and L. R. Stanberry. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J. Infect. Dis. 180:821-823. [DOI] [PubMed] [Google Scholar]
- 41.Sawtell, N. M., D. K. Poon, C. S. Tansky, and R. L. Thompson. 1998. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 72:5343-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawtell, N. M., and R. L. Thompson. 2004. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J. Virol. 78:7784-7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J. Virol. 66:2157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawtell, N. M., R. L. Thompson, and R. L. Haas. 2006. Herpes simplex virus DNA synthesis is not a decisive regulatory event in the initiation of lytic viral protein expression in neurons in vivo during primary infection or reactivation from latency. J. Virol. 80:38-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawtell, N. M., R. L. Thompson, L. R. Stanberry, and D. I. Bernstein. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J. Infect. Dis. 184:964-971. [DOI] [PubMed] [Google Scholar]
- 47.Shimeld, C., T. J. Hill, W. A. Blyth, and D. L. Easty. 1990. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J. Gen. Virol. 71:397-404. [DOI] [PubMed] [Google Scholar]
- 48.Shimeld, C., J. L. Whiteland, N. A. Williams, D. L. Easty, and T. J. Hill. 1996. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J. Gen. Virol. 77:2583-2590. [DOI] [PubMed] [Google Scholar]
- 49.Stevens, J. G., and M. L. Cook. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843-845. [DOI] [PubMed] [Google Scholar]
- 50.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 51.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and N. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, R. L., S. K. Rogers, and M. A. Zerhusen. 1989. Herpes simplex virus neurovirulence and productive infection of neural cells is associated with a function which maps between 0.82 and 0.832 map units on the HSV genome. Virology 172:435-450. [DOI] [PubMed] [Google Scholar]
- 53.Thompson, R. L., and N. M. Sawtell. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson, R. L., and N. M. Sawtell. 2000. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J. Virol. 74:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson, R. L., M. T. Shieh, and N. M. Sawtell. 2003. Analysis of herpes simplex virus ICP0 promoter function in sensory neurons during acute infection, establishment of latency, and reactivation in vivo. J. Virol. 77:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson, R. L., E. K. Wagner, and J. G. Stevens. 1983. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology 131:180-192. [DOI] [PubMed] [Google Scholar]
- 58.Wang, K., T. Y. Lau, M. Morales, E. K. Mont, and S. E. Straus. 2005. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J. Virol. 79:14079-14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitley, R. J. 2001. Herpes simplex viruses, p. 2461-2510. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 60.Wilcox, C. L., R. L. Smith, R. D. Everett, and D. Mysofski. 1997. The herpes simplex virus type 1 immediate-early protein ICP0 is necessary for the efficient establishment of latent infection. J. Virol. 71:6777-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Y., and C. Jones. 2001. The bovine herpesvirus 1 immediate-early protein (bICP0) associates with histone deacetylase 1 to activate transcription. J. Virol. 75:9571-9578. [DOI] [PMC free article] [PubMed] [Google Scholar]