Abstract
Understanding the characteristics of the virus-specific T-lymphocyte response that will confer optimal protection against the clinical progression of AIDS will inform the development of an effective cellular immunity-based human immunodeficiency virus vaccine. We have recently shown that survival in plasmid DNA-primed/recombinant adenovirus-boosted rhesus monkeys that are challenged with the simian immunodeficiency virus SIVmac251 is associated with the preservation postchallenge of central memory CD4+ T lymphocytes and robust gamma interferon (IFN-γ)-producing SIV-specific CD8+ and CD4+ T-lymphocyte responses. The present studies were initiated to extend these observations to determine which virus-specific T-lymphocyte subpopulations play a primary role in controlling disease progression and to characterize the functional repertoire of these cells. We show that the preservation of the SIV-specific central memory CD8+ T-lymphocyte population and a linked SIV-specific CD4+ T-lymphocyte response are associated with prolonged survival in vaccinated monkeys following challenge. Furthermore, we demonstrate that SIV-specific IFN-γ-, tumor necrosis factor alpha-, and interleukin-2-producing T lymphocytes are all comparably associated with protection against disease progression. These findings underscore the contribution of virus-specific central memory T lymphocytes to controlling clinical progression in vaccinated individuals following a primate immunodeficiency virus infection.
Accruing data suggest a central role for cellular immune responses in controlling human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication. CD8+ cytotoxic T lymphocytes (CTL) mediate viral containment, and CD4+ T lymphocytes provide the helper function needed for the generation and maintenance of these effector cells (5, 10-12, 23, 26, 27). In fact, HIV vaccine strategies developed for the elicitation of these cellular immune responses are currently being evaluated in nonhuman primate and human clinical trials (3, 8, 14, 15, 17-19, 22, 24). However, we still know very little about the functional capabilities of the HIV/SIV-specific T lymphocytes that mediate optimal antiviral activity.
As CD8+ T lymphocytes develop following exposure to viral antigen, they evolve into distinct subsets of cells with distinguishable functional profiles (9, 13, 21). Central memory CD8+ T lymphocytes home to lymphoid organs, produce predominantly interleukin-2 (IL-2), and have a high capacity to proliferate. In contrast, effector memory CD8+ T lymphocytes migrate to peripheral tissues, display a cytolytic function, produce primarily gamma interferon (IFN-γ), and lack proliferative capacity. While evidence from murine studies suggests that central memory CD8+ T lymphocytes are particularly effective at controlling viral replication and disease progression following infection by several viruses (29), we know very little about the relative importance of effector and memory CD8+ T-lymphocyte subpopulations in controlling HIV/SIV replication.
The functional repertoire of T-lymphocyte populations can also be assessed on the basis of their production of effector molecules. T lymphocytes can make cytotoxic granules, including granzyme and perforin, that allow the cells to mediate killing. They can also produce a variety of soluble factors, including chemokines such as macrophage inflammatory protein 1β and cytokines such as IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α). It is well documented that chronic infection with HIV/SIV results in profound abnormalities in a lymphocyte's production of a variety of these effector molecules (1, 2, 6, 7, 25). However, we have little data to allow us to predict the profile of lymphocyte effector molecules that will confer the best protection against HIV/SIV replication.
We have recently shown that vaccination with a plasmid DNA prime/recombinant adenovirus (rAd) boost regimen conferred a survival advantage to rhesus monkeys following SIVmac251 challenge (16). This survival advantage was associated with the preservation postchallenge of central memory CD4+ T lymphocytes. Moreover, the monkeys that survived longest postchallenge maintained the most robust IFN-γ-producing SIV-specific CD8+ and CD4+ T-lymphocyte responses. However, we did not evaluate which virus-specific T-lymphocyte subpopulations played a primary role in controlling disease progression, and we did not characterize the functional repertoire of the virus-specific T lymphocytes. An in depth understanding of the character and activity of SIV-specific T cells will be of central importance in clarifying the immune mechanisms and defining the correlates of protection conferred by HIV vaccination. The present studies were undertaken to define the virus-specific T-lymphocyte responses associated with protection against the progression of clinical AIDS in this cohort of monkeys.
MATERIALS AND METHODS
Animals and viruses.
Thirty Mamu-A*01− rhesus monkeys (Macaca mulatta) were used in this study. All animals were maintained in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. The challenge virus used in this study was uncloned pathogenic SIVmac251.
Plasma viral RNA levels.
Plasma viral RNA levels were measured by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies per ml (Bayer Diagnostics, Berkeley, CA).
Vaccination.
Monkeys (six per group) were established in five groups receiving the following regimens: (i) DNA-SIVmac239 gag/pol/nef prime followed by rAd-SIVmac239 gag/pol boost, (ii) DNA-SIVmac239 env prime followed by rAd-SIVmac239 env boost, (iii) DNA-SIVmac239 gag/pol/nef and DNA-SIVmac239 env prime followed by rAd-SIVmac239 gag/pol and rAd-SIVmac239 env boost, (iv) rAd-SIVmac239 gag/pol and rAd-SIVmac239 env only, and (v) sham DNA and empty Ad. These vaccine constructs were described previously (16). The animals received the DNA on a schedule of 0, 4, and 8 weeks at a dose of 4 mg/plasmid construct/inoculation and the rAd at week 40 at a dose of 1011 particles/construct/inoculation.
Antibodies.
The antibodies used in this study were purchased from BD Biosciences. All reagents were validated and titrated using rhesus monkey peripheral blood mononuclear cells (PBMCs). The antibodies used in this study were anti-TNF-α-fluorescein isothiocyanate (MAb11), anti-CD95-phycoerythrin (DX2), anti-IFN-γ-phycoerythrin-Cy7 (B27), anti-CD28-PerCP-Cy5.5 (L293), anti-IL-2-allophycocyanin (MQ1-17H12), anti-CD4-AmCyan (L200), anti-CD3-Pacific blue (SP34-2), and anti-CD8α-Alexa fluor 700 (RPA-T8).
PBMC stimulation and intracellular cytokine staining.
Purified PBMCs were isolated from EDTA-anticoagulated blood and frozen in the vapor phase of liquid nitrogen. Cells were later thawed and allowed to rest for 6 h at 37°C in a 5% CO2 environment. The viability of these cells was >90%. PBMCs were then incubated at 37°C in a 5% CO2 environment for 6 h in the presence of RPMI 1640-10% fetal calf serum alone (unstimulated), a pool of 15-mer Gag peptides (5 μg/ml [each peptide]), or staphylococcal enterotoxin B (5 μg/ml; Sigma-Aldrich) as a positive control. All cultures contained monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences). The cultured cells were stained with monoclonal antibodies specific for cell surface molecules (CD3, CD4, CD8, CD28, and CD95) and with an amine dye (Invitrogen) to discriminate live from dead cells. After being fixed with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with antibodies specific for IFN-γ, TNF-α, and IL-2. Labeled cells were fixed in 1.5% formaldehyde-phosphate-buffered saline. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star). Approximately 200,000 to 1,000,000 events were collected per sample. The background level of cytokine staining varied within different samples and different cytokine patterns but was typically <0.01% of the CD4+ T cells (median, 0%) and <0.05% of the CD8+ T cells (median, 0.01%). All data are reported after background correction. The only samples considered positive were those in which the percentage of cytokine-staining cells was at least twice that of the background or in which there was a distinct population of brightly cytokine-positive cells, and the cutoffs for a positive response ranged from 10 events (double or triple cytokines) to as many as a few hundred events (single cytokines).
Statistical analyses.
Statistical analyses and graphical presentations were computed with GraphPad Prism, using nonparametric Wilcoxon rank sum tests for distributions and a Wilcoxon chi-square test for Kaplan-Meier survival analysis. P values of <0.05 were considered significant. A Spearman correlation test was performed to analyze the association between the cytokine responses.
RESULTS
Thirty Mamu-A*01− rhesus monkeys were vaccinated with a plasmid DNA prime/rAd boost regimen (16). Four groups of six animals per group received different experimental vaccination regimens that included plasmid DNA and rAd or rAd constructs alone expressing various combination of SIVmac239 env, gag, and pol; six control animals received sham immunogens. The monkeys were then challenged by the intravenous route with SIVmac251 and monitored for 850 days to evaluate their clinical, immunologic, and virologic status.
Survival was not associated with the preservation of total CD4+ T cells or plasma viral RNA levels assessed 203 days following SIVmac251 challenge.
We previously reported that total peripheral blood CD4+ T-cell counts and viral loads measured on day 112 postchallenge were not associated with survival in this cohort of monkeys (16). To assess the duration of this effect, we measured plasma viral RNA levels and total CD4+ T-cell counts 203 days following SIVmac251 challenge. The data for the 29 animals still alive at that time were divided into thirds based on magnitudes of absolute peripheral blood CD4+ T-cell counts or plasma viral RNA levels. No statistically significant differences in survival were seen between these groups of monkeys, although monkeys with low viral loads had a trend toward longer survival than that of monkeys with high viral loads (P = 0.09) (data not shown). Thus, the usual virologic and immunologic parameters that are monitored to predict clinical outcomes in the setting of HIV type 1 (HIV-1)/SIV infection did not have long-term prognostic value for the vaccinated monkeys.
Survival was associated with the preservation of Gag-specific CD4+ and CD8+ T-cell responses following SIVmac251 challenge.
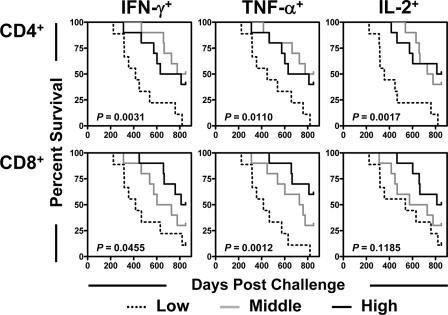
We previously reported that survival following challenge in this cohort of monkeys was associated with the preservation of peripheral blood central memory CD4+ T-cell counts and that Gag-specific T-cell responses were better maintained in the vaccinated than in the control monkeys (16). We sought further to determine whether the magnitudes of these monkeys' SIV-specific T-cell responses might predict their survival (Fig. 1). Peripheral blood lymphocytes from the 29 remaining monkeys obtained at 203 days postchallenge were exposed to a pool of overlapping peptides spanning the Gag protein, and the fractions of CD4+ or CD8+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining. The data for the monkeys were then divided into thirds based upon the magnitudes of their peripheral blood Gag-specific CD4+ or CD8+ T-cell responses, and each group was evaluated for survival (Fig. 1). A highly significant survival advantage was apparent for the monkeys with the highest SIV Gag-specific CD4+ T-cell responses. Importantly, this association was demonstrable for Gag peptide-stimulated production of IFN-γ, TNF-α, and IL-2. Moreover, survival was also longest in the monkeys with the highest frequencies of Gag-specific CD8+ T-cell responses. This association was statistically significant for Gag peptide-stimulated production of IFN-γ and TNF-α, and a trend toward an association was seen for Gag peptide-stimulated IL-2 production. These results suggest that the mechanism by which preserved total central memory CD4+ T lymphocytes confer protection against progressive disease is through the potentiation of SIV-specific cell-mediated immunity.
FIG. 1.
Survival following challenge was associated with the preservation of Gag-specific CD4+ and CD8+ T-lymphocyte responses. T lymphocytes obtained 203 days after challenge were stimulated with a pool of overlapping Gag peptides. The fraction of CD4+ or CD8+ T lymphocytes producing IFN-γ, TNF-α, or IL-2 was assessed by intracellular cytokine staining. Data for monkeys were divided into thirds based on the magnitudes of Gag-specific CD4+ T-lymphocyte or Gag-specific CD8+ T-lymphocyte responses. Comparisons of survival for these groups of monkeys were done using a log-rank test.
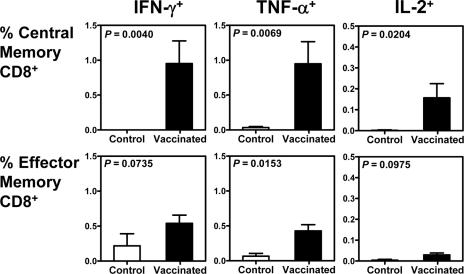
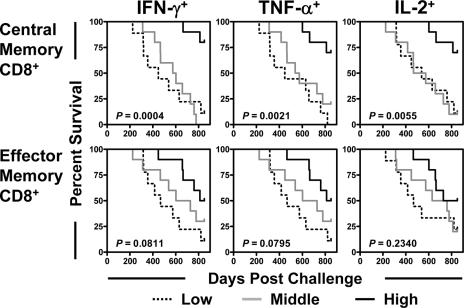
Prior vaccination was associated with the preservation of Gag-specific central memory CD8+ T cells following SIVmac251 challenge.
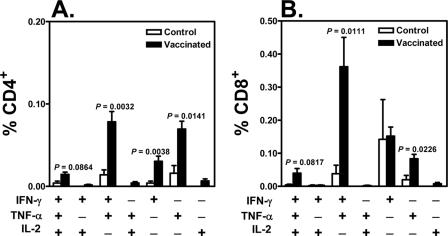
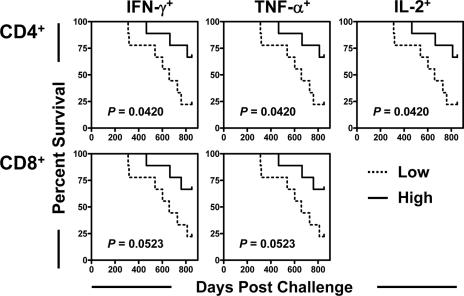
Studies have shown that central memory CD8+ T cells are particularly important in the control of disease progression in a number of murine virus infection models (29). We therefore sought to evaluate the clinical ramifications of the preservation of specific subsets of SIV-specific CD8+ T lymphocytes in these monkeys. Total memory CD8+ T cells were divided into central and effector memory cells on the basis of their expression of CD28 and CD95 (20, 28). The magnitude of each memory CD8+ T-cell subset that produced IFN-γ, TNF-α, or IL-2 upon Gag peptide stimulation was assessed by intracellular cytokine staining. Interestingly, the SIV Gag-specific central memory CD8+ T-cell response was better preserved in the vaccinated than in the control monkeys, while the IFN-γ- and IL-2-producing SIV Gag-specific effector memory CD8+ T-cell responses were comparable in magnitude in the peripheral blood of vaccinated and control monkeys (Fig. 2). More importantly, a clear survival advantage was apparent for the monkeys with the highest Gag-specific central memory CD8+ T-cell responses (Fig. 3). Only a trend was observed between the magnitudes of Gag-specific effector memory CD8+ T-cell responses and survival. These findings suggest that SIV-specific central memory CD8+ T cells play a critical role in mediating protection against disease progression in vaccinated, infected monkeys.
FIG. 2.
Prior vaccination was associated with the preservation of Gag-specific central memory CD8+ T-lymphocyte responses following SIV challenge. T lymphocytes obtained 203 days after challenge were stimulated with a pool of overlapping Gag peptides. Total memory CD8+ T lymphocytes were divided into central memory and effector memory subpopulations based on their expression of CD28 and CD95. The fraction of each memory CD8+ T-lymphocyte subset producing IFN-γ, TNF-α, or IL-2 was assessed by intracellular cytokine staining. Differences between control and vaccinated monkeys were analyzed using the Wilcoxon rank sum test.
FIG. 3.
Survival was associated with the preservation of Gag-specific central memory CD8+ T lymphocytes but not Gag-specific effector memory CD8+ T lymphocytes following SIV challenge. Data for monkeys were divided into thirds based on the magnitudes of Gag-specific central memory CD8+ T-lymphocyte or Gag-specific effector memory CD8+ T-lymphocyte responses after challenge. Comparisons of survival for these groups of monkeys were done using a log-rank test.
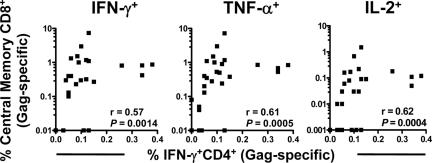
Association of SIV-specific CD4+ T-cell responses and maintenance of Gag-specific central memory CD8+ T cells following SIVmac251 challenge.
There is accumulating evidence that CD4+ T-cell help is crucial for the maintenance of virus-specific central memory CD8+ T-cell responses (10, 12, 26, 27). To examine whether an association exists between the magnitudes of these immune responses in the present study, correlations were assessed between the preservation of SIV Gag-specific IFN-γ-producing CD4+ T cells and the frequencies of Gag-specific central memory CD8+ T cells monitored by IFN-γ, TNF-α, or IL-2 production. In fact, positive correlations were demonstrated between the magnitudes of each of these SIV-specific central memory CD8+ T-cell cytokine responses and the preservation of SIV-specific CD4+ T-cell help (Fig. 4). These associations are consistent with the possibility that the preserved central memory CD4+ T cells in the vaccinated monkeys contribute to prolonged survival by maintaining potent virus-specific central memory CD8+ T-cell responses.
FIG. 4.
Association of Gag-specific CD4+ T lymphocytes with Gag-specific central memory CD8+ T lymphocytes following SIV challenge. Data on each of the control or vaccinated monkeys are indicated by individual filled squares. The relationships between the frequencies of Gag-specific IFN-γ-producing CD4+ T lymphocytes and the frequencies of Gag-specific central memory CD8+ T lymphocytes, as determined by IFN-γ, TNF-α, or IL-2 production, were evaluated using the Spearman correlation test.
Gag peptide-stimulated cytokine production by T cells from control and vaccinated monkeys following SIV challenge.
Recent studies have suggested that polyfunctional HIV-specific CD8+ T cells are important in the control of disease progression in infected individuals (4). We therefore used polychromatic flow cytometry to simultaneously assess Gag peptide-stimulated production of three cytokines to determine whether the quality of Gag-specific T-cell responses is important for protection against disease progression in vaccinated, infected monkeys. Total SIV Gag-specific CD4+ T cells or CD8+ T cells were divided into seven distinct populations based on their production of IFN-γ, TNF-α, and IL-2 (Fig. 5). In the control animals, IFN-γ was the only cytokine readily detected in Gag-specific CD8+ T cells. Gag peptide exposure stimulated little TNF-α and no IL-2 production by these T cells. However, the qualities of the CD8+ T-cell responses of the vaccinated monkeys were quite different. Some CD8+ T cells produced all three cytokines. A large population of Gag-specific CD8+ T cells were IFN-γ+ TNF-α+ IL-2−, and the difference in magnitude of this lymphocyte subpopulation between the control and vaccinated monkeys was significant (P = 0.011). Furthermore, IFN-γ and TNF-α production dominated the Gag-specific CD4+ T-cell responses. IL-2 production by Gag-specific CD4+ T cells was detectable in the vaccinated but not in the control monkeys. Thus, cells producing IFN-γ, TNF-α, and IL-2, either individually or in any combination, were consistently better preserved in the vaccinated than in the control monkeys.
FIG. 5.
Gag peptide-stimulated cytokine production by T cells from control and vaccinated monkeys following SIV challenge. T lymphocytes obtained 203 days after challenge were stimulated with a pool of overlapping Gag peptides. Total SIV Gag-specific CD4+ T cells or CD8+ T cells were divided into seven distinct populations based on their production of IFN-γ, TNF-α, and IL-2. Differences in magnitudes of responses between control and vaccinated monkeys were analyzed using the Wilcoxon rank sum test.
Association between vaccine-elicited SIV Gag-specific T-cell responses and survival.
Finally, we evaluated the contribution of vaccine-elicited immunity to survival in these monkeys. We previously showed that the potency of vaccine-induced CD8+ and CD4+ T-cell responses to SIV, as measured by IFN-γ production, was associated with survival after challenge (16). We wanted to determine whether additional qualitative aspects of this vaccine-elicited cellular immune response might contribute to vaccine protection. To evaluate this possibility, peripheral blood lymphocytes from the 18 monkeys that had received a Gag immunogen were obtained 4 weeks following the rAd booster immunization. The fraction of peripheral blood CD4+ or CD8+ T cells producing IFN-γ, TNF-α, or IL-2 following exposure to a pool of Gag peptides was assessed by intracellular cytokine staining. The data for the 18 monkeys that received Gag immunogens were then divided into halves based on magnitudes of vaccine-elicited Gag-specific CD4+ T-cell or Gag-specific CD8+ T-cell responses, and survival postinfection was assessed. A survival advantage was apparent for the monkeys with the highest frequencies of vaccine-elicited cellular immune responses, whether those responses were peptide-stimulated production of IFN-γ, TNF-α, or IL-2 (Fig. 6). Too few vaccine-elicited IL-2-producing SIV Gag-specific CD8+ T cells were detected to evaluate the predictive ability of that response. Therefore, the magnitudes of the cellular immune responses generated by vaccination were associated with survival following virus infection. Extending the previously reported observations on the contribution of vaccine-elicited cellular IFN-γ responses to survival after challenge, we show that this association was observed for vaccine-elicited CD4+ and CD8+ T cells and for the production of a variety of cytokines.
FIG. 6.
Association between vaccine-elicited Gag-specific T-cell responses and survival. The fraction of CD4+ or CD8+ T lymphocytes producing IFN-γ, TNF-α, or IL-2 in response to a pool of Gag peptides was assessed by intracellular cytokine staining before challenge. The 18 monkeys that received the Gag immunogen were divided into halves based on the magnitudes of their peak vaccine-elicited Gag-specific CD4+ T-lymphocyte or Gag-specific CD8+ T-lymphocyte responses. Comparisons of survival for these groups of monkeys were done using a log-rank test.
DISCUSSION
Although it is now clear that the induction of CD8+ T-lymphocyte responses is associated with the containment of HIV/SIV replication (5, 11, 23), how these cells mediate this antiviral effect in vivo has not yet been defined fully. Thus, while CTL killing is important for the long-term control of human T-lymphotropic virus type 1 and CD8+ T-lymphocyte production of IFN-γ is critical for hepatitis B virus containment, the phenotypic and functional profiles of the T-cell populations that control primate lentivirus replication in vivo remain poorly characterized.
The present study defines the virus-specific cellular immune correlates of protection against primate immunodeficiency viruses in the SIV-macaque model. Because we monitored a large cohort of monkeys for 850 days after infection, we were able to use survival as an end point in this study. Moreover, by evaluating vaccinated monkeys that were subsequently challenged with pathogenic virus, we were able to assess the contributions of virus-specific immune functions to protection in two different settings. We characterized the contribution of immune responses generated through vaccination prior to infection as well as the contribution of immune responses generated in the face of ongoing viral replication.
The vaccination regimens elicited high-frequency SIV-specific cellular immune responses, with greater responses detected in the monkeys that received the DNA prime-rAd boost regimen than in those that received only rAd immunizations (16). After virus challenge, there were no statistically significant differences in cellular immune responses between the various groups of experimentally vaccinated monkeys. In fact, animals vaccinated with the Env constructs alone had robust Gag responses. We presume that the Env vaccine protected the monkeys from memory CD4+ T-cell loss, thus preserving CD4 help for the generation of Gag-specific CD8+ T-cell responses following SIV infection. All 24 experimentally vaccinated monkeys were treated as a single group and were compared to the 6 sham-vaccinated control monkeys, as shown in Fig. 2 and 5.
Little work has been done previously to define the quality of memory T cells required for protection against primate immunodeficiency virus-induced disease. Since central memory CD8+ T cells have been implicated in protection against disease in murine infection models (29), we hypothesized that the same may be true for SIV-infected rhesus monkeys. In this study, we show that survival was associated with vaccine-induced CD8+ T cells and was also associated with the preservation of Gag-specific central memory, but not effector memory, CD8+ T-cell responses following SIV challenge. The protection conferred by virus-specific central memory CD8+ T cells may be explained by their proliferative capacity upon encountering antigen. Central memory CD8+ T cells can recognize viral antigen and proliferate to generate a large pool of secondary effector cells. Such secondary effector cells may then mediate anti-SIV activity. Whatever mechanism is used, the present findings suggest that the SIV-specific central memory CD8+ T cells play a critical role in protecting vaccinated, infected monkeys against disease progression.
Given the importance of CD4+ T lymphocytes in potentiating the function of CD8+ T lymphocytes (10, 12, 26, 27), it was important to evaluate the contribution of CD4+ T lymphocytes to the containment of SIV replication and the prolongation of survival in this cohort of monkeys. In fact, we previously reported that survival following challenge in this cohort of monkeys was associated with preserved peripheral blood central memory CD4+ T-cell counts. Moreover, we also showed that survival was associated with vaccine-elicited SIV Gag-specific CD4+ T-cell IFN-γ responses before challenge (16). We built upon these observations in the present study by showing that preserved SIV-stimulated CD4+ T-cell production of IFN-γ, TNF-α, and IL-2 was associated with prolonged survival in the infected monkeys. Importantly, we also show an association between the magnitude of SIV-specific central memory CD8+ T-cell cytokine responses and the preservation of SIV-specific CD4+ T-cell help. These findings underscore the critical importance of virus-specific CD4+ T-cell help in the generation and maintenance of long-lived virus-specific central memory CD8+ T cells.
Recent studies have shown that HIV-1-infected clinical nonprogressors maintain virus-specific CD8+ T cells with a larger functional repertoire than that of cells from clinical progressors (4). In the present study, we show that the functional profile of SIV Gag-specific CD8+ T cells from control vaccinated monkeys was largely limited to the expression of IFN-γ, while the virus-specific CD8+ T cells of vaccinated monkeys retained the capacity to produce IFN-γ, TNF-α, and IL-2. These findings suggest that the preservation of multifunctional populations of virus-specific T cells will be associated with protection against disease progression in vaccinated, infected monkeys. To what extent the preservation of the multifunctionality of the virus-specific T lymphocytes represents a cause or a consequence of the improved clinical status of the vaccinated monkeys remains to be determined.
Acknowledgments
This work was supported in part by funds from the intramural research program of the Vaccine Research Center, NIAID, NIH, and by Harvard Medical School CFAR grant AI060354.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Acierno, P. M., J. E. Schmitz, D. A. Gorgone, Y. Sun, S. Santra, M. S. Seaman, M. H. Newberg, J. R. Mascola, G. J. Nabel, D. Panicali, and N. L. Letvin. 2006. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 176:5338-5345. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, B. Combadiere, and P. Debre. 2004. Therapeutic vaccines for chronic infections. Science 305:205-208. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 7.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 11.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzavecchia, A., and F. Sallusto. 2005. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 17:326-332. [DOI] [PubMed] [Google Scholar]
- 14.Letvin, N. L. 2005. Progress toward an HIV vaccine. Annu. Rev. Med. 56:213-223. [DOI] [PubMed] [Google Scholar]
- 15.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227-255. [DOI] [PubMed] [Google Scholar]
- 18.Nabel, G., W. Makgoba, and J. Esparza. 2002. HIV-1 diversity and vaccine development. Science 296:2335. [DOI] [PubMed] [Google Scholar]
- 19.Nabel, G. J. 2002. HIV vaccine strategies. Vaccine 20:1945-1947. [DOI] [PubMed] [Google Scholar]
- 20.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 22.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 24.Seaman, M. S., L. Xu, K. Beaudry, K. L. Martin, M. H. Beddall, A. Miura, A. Sambor, B. K. Chakrabarti, Y. Huang, R. Bailer, R. A. Koup, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J. Virol. 79:2956-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 26.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 27.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun, Y., J. E. Schmitz, P. M. Acierno, S. Santra, R. A. Subbramanian, D. H. Barouch, D. A. Gorgone, M. A. Lifton, K. R. Beaudry, K. Manson, V. Philippon, L. Xu, H. T. Maecker, J. R. Mascola, D. Panicali, G. J. Nabel, and N. L. Letvin. 2005. Dysfunction of simian immunodeficiency virus/simian human immunodeficiency virus-induced IL-2 expression by central memory CD4+ T lymphocytes. J. Immunol. 174:4753-4760. [DOI] [PubMed] [Google Scholar]
- 29.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]