Abstract
Hepatitis C virus (HCV)-specific CD8 cell exhaustion may represent a mechanism of HCV persistence. The inhibitory receptor PD-1 has been reported to be up-regulated in exhausted CD8 cells. Therefore, we studied PD-1 expression longitudinally during acute HCV infection. Most HCV-specific CD8 cells expressed PD-1 at the time of acute illness, irrespective of the final outcome. PD-1 expression declined with the acquisition of a memory phenotype and recovery of an efficient CD8 cell function in resolving HCV infections, whereas high levels were maintained when HCV persisted and HCV-specific CD8 cells remained dysfunctional. Blocking PD-1/PDL-1 interaction with an anti-PDL-1 antibody improved the capacity of expansion of virus-specific CD8 cells.
Adaptive immunity has a central role in the pathogenesis of hepatitis C virus (HCV) infection, and understanding the behavior of the T-cell response is crucial for the design of effective strategies to control HCV.
At the acute stage of HCV infection, HCV-specific CD8 cells have been reported to be poorly functional irrespective of the final outcome of the disease (6, 11, 13-15). However, at this stage, virus-specific CD8 cells are expected to be predominantly effectors, which should be able to express efficiently antiviral effector functions ex vivo, based on the accepted models of CD8 cell differentiation following exposure to viral pathogens (16-17). The reasons why HCV-specific CD8 cells do not efficiently exert effector functions at the time of acute hepatitis C and why this functional impairment persists when HCV infection becomes chronic are not well understood.
A possible mechanism responsible for this behavior of the HCV-specific CD8 response is exhaustion, which might be sustained initially by the rapid kinetics of HCV replication and spread following infection and later on by the persistent exposure of CD8 cells to high antigen concentrations. Many recent studies indicate that the PD-1/PD-1 ligand (PD-L1) pathway can play a role in T-cell exhaustion occurring when T cells are chronically exposed to high antigen loads and that blockade of PD-1/PDL-1 interaction can allow restoration of exhausted CD8 cells (1, 3, 4, 10, 12). Thus, high expression of the inhibitory PD-1 receptor seems to be a signature of functional CD8 cell exhaustion.
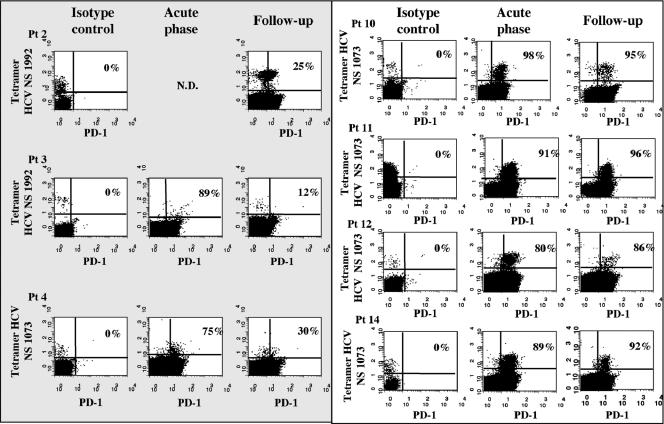
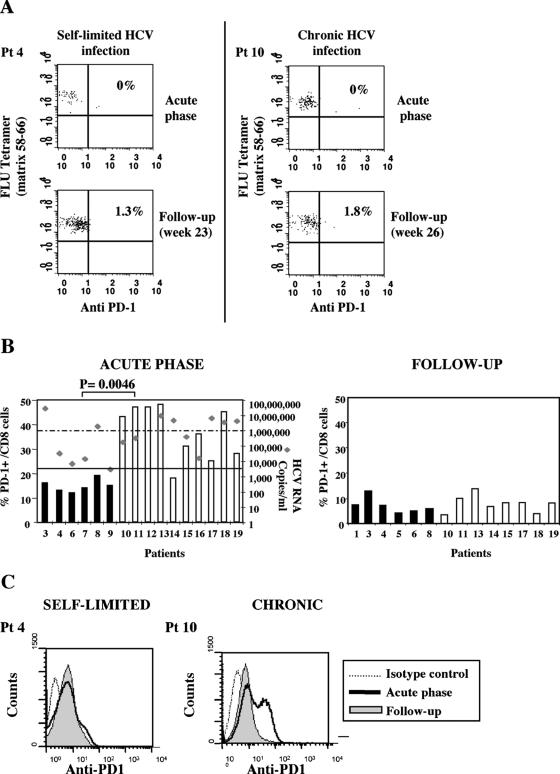
To define the role of PD-1 and CD8 cell exhaustion in HCV infection, we analyzed longitudinally throughout a follow-up ranging from 1 to 56 weeks the expression of PD-1 for 19 patients with acute hepatitis C, 9 of whom had a self-limited infection and 10 of whom had a chronic evolution of infection (Table 1). Analysis was initially performed by flow cytometry on tetramer-positive CD8 cells specific for NS3 1073-1081 or NS4B 1992-2000 for 10 HLA-A2+ patients. At the time of acute illness when patients were seen for the first time in the clinic, most circulating tetramer-positive HCV-specific CD8 cells expressed PD-1, both in self-limited and in persistent infections (Fig. 1), and displayed predominantly an effector memory phenotype (data not shown). Negative PD-1 expression on influenza virus-specific CD8 cells derived from the same patients with acute hepatitis (Fig. 2A) suggested selective expression of PD-1 by HCV-specific CD8 cells. In line with this finding, only a limited fraction of total CD8 cells from patients with resolving infection were PD-1 positive at the time of acute illness, as recently reported for acute hepatitis B virus infection (Fig. 2B) (2). However, a significantly greater percentage of the total CD8 cell pool was PD-1 positive for patients with a chronically evolving infection (Fig. 2B and C), even if PD-1 was analyzed for all patients at the time they were first referred to the clinic. This different degree of PD-1 expression was not simply due to different levels of CD8 activation, because other activation markers, such as HLA-DR and CD69, were similarly expressed at the time of clinical presentation by CD8 cells derived from the two categories of patients (data not shown).
TABLE 1.
Clinical and virological characteristics of cells from patients with acute HCV infectiona
| Patient no. | Level of ALT | Titer of HCV RNA | Viral genotype | Expression of HLA-A2 | Outcome |
|---|---|---|---|---|---|
| 1 | 876 | 3,200 | 1b | pos | Self-limited |
| 2 | 751 | 543,653 | 3 | pos | Self-limited |
| 3 | 874 | 37,507,596 | 3 | pos | Self-limited |
| 4 | 430 | 34,413 | 1b | pos | Self-limited |
| 5 | 1,701 | ND | 3 | pos | Self-limited |
| 6 | 1,150 | 15,024 | ND | neg | Self-limited |
| 7 | 1,616 | 7,203 | ND | neg | Self-limited |
| 8 | 212 | 2,011,899 | 1 | neg | Self-limited |
| 9 | 940 | <3,200 | 2 | neg | Self-limited |
| 10 | 2,219 | 185,463 | 1b | pos | Chronic |
| 11 | 1,462 | 340,025 | 1b | pos | Chronic |
| 12 | 324 | ND | 1 | pos | Chronic |
| 13 | 513 | 4,858,409 | 3 | pos | Chronic |
| 14 | 1,060 | 11,652,683 | 1b | pos | Chronic |
| 15 | 397 | 411,425 | 1b | neg | Chronic |
| 16 | 964 | 16,424 | 3 | neg | Chronic |
| 17 | 875 | 6,775,737 | 3 | neg | Chronic |
| 18 | 459 | 3,722,564 | 3 | neg | Chronic |
| 19 | 1,214 | 4,375,448 | 2C | neg | Chronic |
ALT, U/liter; HCV RNA, copies/ml; pos, positive; neg, negative; ND, not determined.
FIG. 1.
Analysis of PD-1 expression in HCV-specific CD8 cells. The dot plots show the percentages of tetramer-positive/PD-1-positive cells at two different time points corresponding to the acute phase of infection and to a later time point (24 to 30 weeks of follow-up after clinical presentation) in patients with self-limited (shaded area) or chronic evolution of (white area) disease. Flow cytometry analysis, gating on total CD8 cells, was performed with specific anti-PD1 or with isotype control immunoglobulin G1 antibodies (PD-1 fluorescein isothiocyanate; Becton Dickinson Biosciences, San Diego, CA) on HCV-specific CD8 cells detected by tetramer staining with two phycoerythrin-labeled tetrameric peptide-HLA class I complexes (Proimmune LTD, Oxford, United Kingdom), containing the HCV peptides NS3 1073-1081 (CINGVCWTV) and NS4B 1992-2000 (VLSDFKTWL). The study was approved by the Ethical Committee of the Azienda Ospedaliero-Universitaria di Parma, and all subjects gave written informed consent. N.D., Not determined; Pt, patient.
FIG. 2.
PD-1 expression in influenza virus-specific and total CD8 cells. (A) Representative dot plot analysis of PD-1 expression analyzed on FLU-specific tetramer-positive cells (influenza virus epitope matrix GILGFVFTL) for two representative patients with different evolution of hepatitis C virus infection at two different time points. Pt, patient. (B) Percentages of PD-1-positive cells among the total CD8+ subset detectable during the acute phase of HCV infection and 6 to 12 months of follow-up for patients with self-limited (black bars) or chronically evolving acute (white bars) hepatitis. Each rhomb in the left panel depicts the viremia levels detected for each patient at the time of PD-1 analysis. Statistical analysis was performed by the Mann-Whitney test. (C) Fluorescence intensities of PD-1 expressed by total CD8+ cells for patients with different outcomes of the disease.
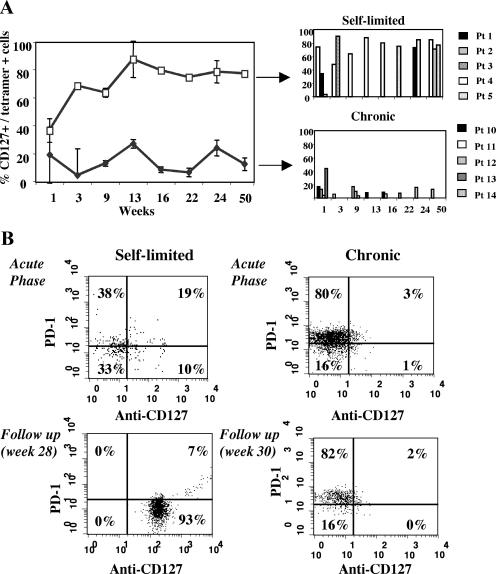
PD-1 expression was then evaluated after the acute stage of hepatitis C, when effector CD8 cells are expected to undergo a progressive differentiation into long-lived CD127+ memory cells if infection is successfully controlled but not if the virus persists (5, 7-9). In keeping with this prediction, HCV-specific CD8 cells progressively acquired the expression of CD127 in patients with self-limited hepatitis, whereas in patients with chronic evolution of the disease, CD127 remained predominantly negative during the follow-up (Fig. 3A and B).
FIG. 3.
Longitudinal evolution of CD127 expression on HCV tetramer-positive CD8 cells. (A) Percentage of tetramer-positive/CD127+ CD8 cells during a 50-week follow-up from the time of clinical presentation in patients with self-limited (white squares and upper panel) and chronically evolving (black triangle and lower panel) acute hepatitis C. The right panels illustrate CD127 expression for each individual patient at the different time points analyzed. (B) Dot plots of CD127/PD-1 expression analyzed at two different time points on HCV tetramer-positive cells (NS3 1073-1081) for two representative patients with different outcomes of acute HCV infection.
Acquisition of a memory phenotype in resolving HCV infection was associated with a decline in PD-1 expression. Four to six months after clinical presentation, PD-1 was expressed in a limited percentage of HCV-specific CD8 cells from patients who succeeded in resolving infection, ranging from 18 to 30% of tetramer-positive cells (Fig. 1). In contrast, in patients with chronically evolving hepatitis, PD-1 was still expressed after a similar interval of time from clinical presentation in virtually all tetramer-positive cells (Fig. 1), which maintained an effector memory phenotype (data not shown). The difference in PD-1 expression by HCV-specific CD8 cells between the two categories of patients was statistically significant (P = 0.008). As in the acute stage of infection, control influenza virus-specific CD8 cells derived from resolving and persisting infections were PD-1 negative (Fig. 2A). Moreover, the proportion of PD-1-positive CD8 cells among the total CD8 pool declined in chronically evolving infections compared to that in the acute phase of illness, acquiring a level of expression comparable to that of resolved infections (Fig. 2B and C). Decline of PD-1 on HCV-specific CD8 cells is in line with previous observations (14, 15), indicating that after the acute phase of infection, a recovery of the CD8 cell function is detectable for patients with self-limited hepatitis in terms of gamma interferon and interleukin 2 production. On the other hand, persistent impairment of the CD8 function in chronic patients is associated with persistent expression of PD-1, supporting the possibility of exhaustion as a crucial mechanism of CD8 cell inactivation in HCV infection.
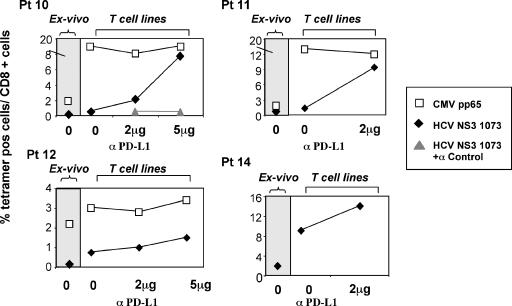
To assess whether the elevated expression of PD-1 really affects the HCV-specific CD8 cell function, signaling through PD-1 was inhibited by blocking PD-1 interaction with its ligand by an anti-PDL-1 antibody. Adherent mononuclear cells purified from peripheral blood mononuclear cells of patients with chronically evolving infection were incubated with anti-PDL-1 or control antibody. The capacity of CD8+ cells to expand upon peptide-specific stimulation was tested by tetramer staining, analyzing the frequency of HCV-specific CD8 cells before and after 10 days of peptide stimulation. As shown in Fig. 4, PDL-1 blockade enhanced HCV-specific proliferation in a dose-dependent manner. Indeed, the capacity of expansion upon peptide stimulation was very poor, because the frequency of HCV-specific tetramer-positive cells after 10 days of in vitro culture differed slightly from the frequency detectable ex vivo. The inhibition of PD1/PDL-1 engagement by the addition of anti-PDL-1 to the culture restored the capacity of HCV-specific CD8 cells to expand efficiently. This functional restoration was detectable for all patients tested, although different levels of improvement were observed for individual patients. This effect of PD-1/PDL-1 blockade was not observed with cytomegalovirus (CMV)-specific CD8 cells, which expressed low levels of PD-1 and were not impaired in their capacity to proliferate.
FIG. 4.
Effect of anti-PDL-1 antibody on peptide-induced proliferation of HCV-specific CD8 cells. Frequencies of tetramer-positive (tetramer-pos) CD8 cells are illustrated ex vivo (gray area) and upon specific peptide stimulation of T-cell lines (white area) in the absence or in the presence of different anti-PDL-1 concentrations. For these experiments, adherent mononuclear cells isolated from peripheral blood mononuclear cells of patients with chronically evolving infection were incubated for 45 min with different concentrations (2 or 5 μg/ml) of anti-PD-L1 (e-Bioscience, Boston, MA) or control antibody (negative control for mouse immunoglobulin G1 Ab-1; Fremont, CA). Cells were then washed and coincubated for 10 days with nonadherent cells and specific peptides (HCV NS3 1073-1081 or CMV pp65) at the final concentration of 1 μg/ml (interleukin 2 was added on day 3). White squares (□) indicate tetramer-positive cells specific for CMV pp65 (NLVPMVATV); black rhombs (⧫) indicate tetramer-positive cells specific for HCV (CINGVCWTV); gray triangles ( ) indicate HCV NS3 1073 tetramer-positive CD8 cells stimulated with the specific peptide in the presence of a control isotype antibody.
In conclusion, PD-1 is expressed at a high level on HCV-specific CD8 cells during the acute phase of infection, irrespective of the subsequent outcome to recovery or viral persistence. This is in keeping with PD-1 expression on early effector CD8 cells in both acute self-limiting and persistent lymphocytic choriomeningitis virus infection in mice (1). At the time of acute illness, in chronically evolving HCV infection, a greater proportion of the total CD8 cell population was PD-1 positive than was observed with resolving HCV infections. At this time, alanine aminotransferase levels were comparable in self-limited and persisting infections, while viremia was generally higher for the latter group of patients (Table 1). The different HCV RNA titers raise the possibility that patients with different outcomes are studied at different stages of acute infection. Nevertheless, patients with self-limited infection with a level of viremia similar to those of patients with chronic evolution (patients 3 and 8) showed low PD-1 expression, and conversely, cells from patient 16, with levels of viremia below 105 and chronic evolution of the disease, displayed high PD-1 expression. All these observations may be consistent with the possibility that both groups of patients were studied at a similar time of infection but viremia levels were different because of different viral kinetics associated with resolution or persistence of the virus. Thus, the different PD-1 profiles of total CD8 cells may actually represent a relevant difference of acute-phase response between self-limited and evolving infections, and PD-1 expression by total CD8 cells may represent a promising early marker of disease progression.
After the acute stage of infection, the expression of PD1 by HCV-specific CD8 cells declined in self-limited infections concurrently with CD8 differentiation towards a memory CD127+ phenotype, as observed also in acute hepatitis B virus infection (2). For patients with chronic evolution of infection, HCV-specific CD8 cells maintained high levels of PD-1 and remained functionally impaired, with no switch from an effector to a memory phenotype. Restoration of antiviral CD8 function concurrent with PD-1 decline in self-limited HCV infection and persistence of CD8 impairment concomitant with persistent expression of high levels of PD-1 by HCV-specific CD8 cells support a role for the PD-1/PD-L1 pathway in modulating CD8 function under conditions of sustained high levels of HCV antigen stimulation. This is confirmed by the observation that blockade of PD1/PDL-1 interaction enhances proliferation of HCV-specific CD8 cells. This functional restoration was observed with HCV-specific but not with CMV-specific CD8 cells, in keeping with the low PD-1 expression on CMV-specific CD8 cells (data not shown). The possibility of restoring the CD8 function by inhibiting the PD-1/PDL-1 pathway needs to be further investigated with patients with a long-lasting condition of chronic hepatitis by also testing cytokine production and cytotoxic activity. Nevertheless, the data of this report strongly suggest that this mechanism can be a therapeutic target to revert T-cell dysfunction in chronic hepatitis C.
Acknowledgments
This study was supported by grant RBNE013PMJ_006 from FIRB (Fondo per gli Investimenti della Ricerca di Base), MIUR (Ministry of Education, University and Research), by grant no. 120 (Progetto di Ricerca Finalizzato), Ministry of Health, Italy, by VIRGIL EC grant QLK2-CT-2002-00700, by Schering-Plough SpA, Milan, Italy, and by Fondazione Cassa Risparmio di Parma, Parma, Italy.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 2.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, J. A., D. M. Dorfman, F. R. Ma, E. L. Sullivan, O. Munoz, C. R. Wood, E. A. Greenfield, and G. J. Freeman. 2003. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170:1257-1266. [DOI] [PubMed] [Google Scholar]
- 4.Cai, G., A. Karni, E. M. Oliveira, H. L. Weiner, D. A. Hafler, and G. J. Freeman. 2004. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol. 230:89-98. [DOI] [PubMed] [Google Scholar]
- 5.Fuller, M. J., D. A. Hildeman, S. Sabbaj, D. E. Gaddis, T. E. Tebo, L. Shang, P. A. Goepfert, and A. J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174:5926-5930. [DOI] [PubMed] [Google Scholar]
- 6.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 9.Lang, K. S., M. Recher, A. A. Navarini, N. L. Harris, M. Lohning, T. Junt, H. C. Probst, H. Hengartner, and R. M. Zinkemagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738-745. [DOI] [PubMed] [Google Scholar]
- 10.Latchman, Y. E., S. C. Liang, Y. Wu, T. Chernova, R. A. Sobel, M. Klemm, V. K. Kuchroo, G. J. Freeman, and A. H. Sharpe. 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA 101:10691-10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki, T., and T. Honjo. 2006. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 27:195-201. [DOI] [PubMed] [Google Scholar]
- 13.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbani, S., C. Boni, G. Missale, G. F. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76:12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbani, S., B. Amadei, P. Fisicaro, D. Tola, A. Orlandini, L. Sacchelli, C. Mori, G. Missale, and C. Ferrari. 2006. The outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126-139. [DOI] [PubMed] [Google Scholar]
- 16.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]