Abstract
This article focuses on human Borna disease virus (BDV) infections, most notably on the development of valid diagnostic systems, which have arisen as a major research issue in the past decade. The significance of a novel modular triple enzyme-linked immunosorbent assay that is capable of specifically measuring anti-BDV antibodies as well as major structural proteins N (p40) and P (p24) in the blood, either as free antigens in the plasma or as antibody-bound circulating immune complexes (CICs), is explained. The impact of CICs and plasma antigen, which indicate periods of antigenemia in the course of BDV infection, along with other infection markers that are still in use is discussed. The review further provides new insight into possible links of BDV to human diseases, summarizing cross-sectional and longitudinal data which correlate acute depression with the presence and amount of antigen and CICs. Moreover, BDV prevalence in healthy people is reevaluated, suggesting that this was previously underestimated. Antiviral efficacy of amantadine, in vivo and in vitro, is outlined as well, with emphasis on wild-type (human and equine) versus laboratory strains. Finally, the pros and cons of the association of BDV with human disease, as detailed in the literature, are critically discussed and related to our data and concepts. This article supports existing correlative evidence for a pathogenic role of BDV infection in particular human mental disorders, in analogy to what has been proven for a variety of animal species.
INTRODUCTION
A sense of wellness and mental health reflects an evolutionarily adapted neurotransmitter balance in human and other mammalian brains, which certainly comprises more than the absence of illness. In part depending upon hereditary factors, mood swings in humans can go beyond physiological amplitudes and thereby be diagnosed as mood disorders, such as mania and depression. Psychopharmacological studies have shown that these disorders correlate with neurotransmitter imbalances, the cause of which remains unsolved.
Many agents that preferentially infect the nervous system, causing neurological diseases or at least abnormal mental status, have been identified (35). However, only Borna disease virus (BDV) has been linked specifically with dysfunctioning of evolutionarily old brain structures. Unlike the closely related rabies virus, which afflicts limbic structures of the brain and usually destroys them, BDV is noncytolytic, with many specific properties eliciting functional disturbances in the brain resembling those in mood disorders (9).
In both natural and experimental BDV infections, the disease process manifests as a broad spectrum of symptoms, ranging from subtle to severe alterations in normal behavior. Occasionally, severe neurological forms can lead to a fatal outcome (43). Significant antigen accumulation is found in preferred sites of the neuronal network in limbic structures of the brain, and an inflammatory response appears to correlate with a variety of clinical syndromes (28).
General knowledge about animal BDV infections, including aspects of comparative anatomy, physiology, and neuropathology, has guided research on human infection and led to worldwide efforts linking BDV with human behavioral disorders. However, until recently, gaps in the understanding of BDV pathogenesis in humans triggered ongoing controversies regarding an etiopathogenic relationship (38). In addition to issues of clinical significance, the establishment of human infections has been continuously debated, mainly due to insufficient diagnostic systems. In several reports BDV parameters assessed in human patients have been suggested to represent false-positive results (1, 54). In fact, for almost a decade, antibodies to BDV components had been the only markers and were found not only in patients with neuropsychiatric disease but also in immunocompromised and healthy individuals (5).
BDV antigens and nucleic acid have been measured in peripheral blood mononuclear cells (PBMCs) from psychiatric patients by novel techniques (3, 4). PBMCs from severely depressed patients subsequently served as the source of the first BDV isolates from humans by long-term cocultivation procedures (6). A putative human isolate of BDV was also obtained from granulocytes (46), and the first human brain isolate from a schizophrenic patient has recently been reported (44). We believe the evidence has sufficiently confirmed human infection with BDV, but a causal link with psychiatric diseases no doubt remains difficult to prove and may only be deduced from broad interdisciplinary studies, as we shall outline in this review.
We are aware that the hypothesis of a mainly infectious cause of major mental disorders both means a change of paradigm in psychiatry and implies an important impact on public health systems. As long as a final proof is pending, debate will be both an expected and necessary part of research (1, 12, 54). Premature conclusions will only add to confusion rather than helping patients and doctors, who are seeking appropriate diagnosis and alternative therapeutic options (7, 9). In the light of Brundtland's recent statement that “within the next 20 years, depression will (become) the second cause of global disease burden” (11), rigorous analysis to prove or refute a viral etiology for mental disorders seems urgent.
Thus, here we review the essential findings regarding human BDV infection to date, noting the ubiquity of this infection (20). Recent significant progress in diagnosing BDV infection is presented, with a focus on markers that are keys to understanding the course of infection, thereby shedding light on clinical aspects of disease and therapy.
HISTORY OF BDV RESEARCH IN HUMANS
Based on early work with human antibodies in Giessen, Germany, in the 1970s (20) (Ludwig, Becht, and Rott, unpublished data), the significance of using serologic markers to detect BDV infections in psychiatric patients was demonstrated (49), followed by many confirmatory contributions worldwide (5). Detection of virus components (BDV RNA or proteins) in human blood samples together with BDV isolates from white blood cells recovered by our group in Berlin (3, 4, 6) marked further milestones. These reports were later challenged by Japanese, German, and American groups (9), followed by the detection of BDV RNA in human brains by de la Torre's and Lipkin's groups (15, 50) and a human isolate recovered from brain tissue in Japan (44).
Fruitful trans-Atlantic cooperation led to essential basic molecular studies on animal and human reference strains (10, 14), which influenced the subsequent intensive searches for human viral RNA in PBMCs and brains. Clinical research, particularly in patients with mood disorders, has been further stimulated by the discovery of the antiviral drug amantadine, despite controversies about drug efficacy due to resistant laboratory strains (7, 9). Our recent discovery of circulating immune complexes (CICs) and antigen (proteins N and P) in plasma as characteristic markers of activated BDV infection states (8) suggests a new direction in clinical research on BDV infections in humans and offers future approaches for epidemiological studies. Over the years, Ikuta and coworkers have contributed greatly to establishing and confirming the presence of human and animal BDV infections in Asian regions (32). Staeheli's and Carbone's groups (12, 54) as yet still hesitate to accept human BDV infections, but nevertheless have contributed to the dialogue regarding the significance of human BDV research.
LABORATORY PARAMETERS AND THEIR DIAGNOSTIC RELEVANCE
The biological (41, 42) and molecular (10, 14) properties of BDV, in particular its replication in the cell nucleus, have led to the classification of a new family, Bornaviridae, of the order Mononegavirales. Detailed data regarding the reference virus (highly adapted laboratory strain) and its genome, proteins, and replication mechanisms have recently been published by the International Committee on the Taxonomy of Viruses (16). With knowledge about the structural elements of the virus and modern laboratory parameters, detection of proteins that are expressed during infection has become central in importance to our recognition of BDV in human specimens and understanding the etiologic role of BDV infection.
BDV infection in humans was first detected by serological methods, mainly immunofluorescence assays (IFAs). IFA with fixed antigen accumulating in the nucleus of infected cells in the mid-1980s became an indispensable tool for monitoring and titrating antibodies (5, 49). Earlier techniques such as complement fixation and Ouchterlony tests, which measured either antibodies or precipitated antigen, were additionally helpful but insensitive tools (39).
In the mid-1990s, BDV core proteins N and P (3) and nucleic acid (4) were detected in PBMCs, leading to worldwide prevalence studies in patients (13, 33). However, controversy ensued because of the heterogeneous results with respect to the lack of correlation between antibodies and RNA-positive cells, the low and discontinuous antibody titers in patients, and, at least in some studies, a similarly low antibody prevalence in controls and patients (9, 32, 38, 43). Investigators who had consistently doubted the possibility of human BDV infection were encouraged to dismiss a clinical role for this agent (12). The recent discovery of CICs, which explain declining antibody levels, and the relationship between plasma antigenemia of BDV products and severity of depression (8) have now overcome major doubts about the infection of humans and appear to resolve current diagnostic heterogeneity.
Sensitivity and Specificity of Antibody Testing
Most research laboratories continue to use immunofluorescence-based assays to detect the serum antibody response to human BDV infections. Antibodies preferentially directed against the major immunogenic components (N and P proteins) can be titered on acetone-fixed cells, preferably of rabbit origin (30, 39). Modification of IFA with monoclonal anti-N antibodies in a double-staining protocol further improved specificity (5), and focus immunoassays and cell enzyme-linked immunosorbent assay (ELISA) enabled faster monitoring of animal and human antibodies against BDV (42). However, all of these assays depend on subjective endpoint titration and are time-consuming. While electrochemiluminescence immunoassays (ECLIA) enabled the amount of antibodies to be determined more precisely, they did not provide any new insights into human BDV infections (24, 58).
Worldwide large-scale studies of human serum samples with IFA, also from our group, gave comparative information on infections in healthy people versus a higher frequency of antibodies in psychiatric patient groups (5).
Based on IFA modified by treatment with 3 M urea, Allmang et al. (1) recently concluded that human anti-BDV antibodies were of low avidity, while antibodies of experimentally infected animals demonstrated high avidity, raising doubts about the specificity of human antibodies to BDV. However, defined panels of serum samples from infected humans and naturally infected animals used in avidity experiments with 6 M urea, as tested by ELISA with native antigen in a double-sandwich format, revealed low- and high-avidity antibodies in both species (Bode, Stoyloff, and Ludwig, unpublished data), consistent with findings in other persistent virus infections.
ELISA.
Numerous approaches have already been used to detect human anti-BDV antibodies, with variable success. All of these ELISAs were based on recombinant proteins (N, P, or M protein) to measure antibodies (12, 21, 38). The overall surprisingly low sensitivity of recombinant ELISAs for anti-BDV antibodies might explain the heterogeneity of data from different studies.
Western blots serve as confirmatory tests in the diagnosis of many virus infections and have been useful in identifying antibodies against the N and P proteins in serum (5, 12, 23) and cerebrospinal fluid (CSF) (17, 43). However, Western blots have not come to the forefront of human BDV diagnosis, because they are less sensitive than new-generation ELISAs with native viral antigens (6, 8). Recent epitope-mapping experiments for BDV N and P proteins (Willemsen and Bode, unpublished data) revealed results concordant with ELISAs with native antigens and thus supported the significance of native conformational epitopes as efficient targets to detect anti-BDV antibodies.
The ELISA developed by our group (8) is based on a double-sandwich format with antibody-stabilized monoclonal antibodies recognizing conformational epitopes of BDV N (p40) and P (p24) proteins, the major immunogenic components in BDV infections (39, 42, 43). The monoclonal antibodies bind to native BDV antigen prepared from unpurified infected horse brain or persistently infected tissue culture cells. Antibodies (immunoglobulin G) in animal and human serum samples (initial dilution, 1:100) are captured by these antigens and measured by conventional alkaline phosphatase-labeled species-specific second-antibody conjugates (8). At present, this assay represents the method of choice for detecting BDV-specific antibodies (9) due to its high sensitivity and specificity (further confirmed by epitope mapping), and therefore it appears to us that it will become the gold standard test.
Indeed, comparison of data assessing antibody levels from an ELISA with recombinant N protein versus ELISA with our native BDV antigen (applying 30 coded reference samples from either healthy and ELISA- and IFA-negative or ELISA- and IFA-positive Borna disease-afflicted horses) revealed that 50% of the recombinant protein results tested false-positive (Pawlita and Bode, unpublished data). Our view of three additional reports with antibody-positive psychiatric patient groups (21, 25, 58) leaves the interpretation that sensitive antibody assays may only be achieved with native antigens.
Antigenemia and Immune Complexes
The antibody ELISA as well as the antigen and immune complex (CIC) ELISA described below is part of a triple ELISA that commonly uses antibody-stabilized BDV-specific monoclonal antibodies as a basic immune module. This setup led to the first demonstration of N and P proteins in human and animal samples, as monomers or heteromers (N/P) probably attached to host transporter proteins, in serum samples (8) and CSF samples (17) of psychiatric patients. Those BDV-specific antigens also have been shown to be present in CSF samples with Western blots (see Fig. 14 in reference 43).
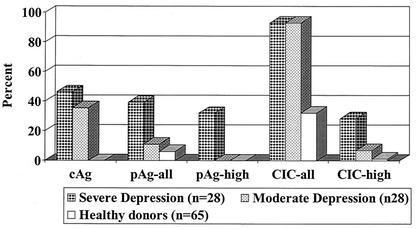
Large amounts of plasma antigen were found in a cohort of severely depressed patients (n = 39; see also Fig. 1 and Tables 1 and 2 in reference 8) during an acute crisis. When a subcohort of these patients (n = 28) was compared with an age-, gender-, and diagnosis-matched subcohort of moderately depressed outpatients, it turned out that the frequency and extent of antigenemia were significantly different (Fig. 1). The severity of symptoms appeared to correlate with the concentration and duration of antigenemia (Fig. 2), an observation made in longitudinal studies of both patients with acute major or bipolar depression (8) and some patients with chronic obsessive-compulsive disorder (Dietrich and Bode, unpublished data). In contrast, the presence of antigen in plasma occurs rarely and mainly at low levels in “healthy” individuals (n = 65) (Fig. 1) (see also Table 2 in reference 8). Thus, plasma antigenemia (predominantly in the presence of large amounts of CICs; see below) indicates an acute and productive but transient phase of infection (for details, see references 8 and 9). The assay to capture plasma antigen represented a breakthrough in Borna virus diagnostics, because free antigen present in body fluids during infection with a low-level replicating agent is an unexpected, completely novel finding.
FIG. 1.
Prevalence and duration of BDV antigen markers in patients with major depressive disorder or bipolar disorder during acute depression. Prevalence of three antigen markers, cAg (antigen in PBMCs), pAg (antigen in plasma), and CICs (circulating immune complexes) in representative age- and gender-matched major depressive disorder and bipolar disorder patient cohorts versus healthy controls; for plasma antigen and CIC, the prevalence of patients with large amounts (plasma antigen high, CIC high) is shown separately (data modified from Table 2 in reference 8). Note that only severely depressed patients showed high plasma antigen values.
TABLE 1.
Published opinions on the correlation of human BDV infection and diseasea
| Reference | Antibodies
|
RNA
|
Antigen
|
CICs, serum | Virus
|
BDV related to disease | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | PBMCs | Brain | PBMCs, serum | CSF | Blood | Brain | |||
| Rott et al. (49) | + | + | − | Mainly supportiveb (mild encephalitis) | ||||||
| Stitz, Planz | + | ? | Indeterminate | |||||||
| Staeheli et al. | ? | − | +c | Mainly against | ||||||
| Bode, Ludwig, et al. | + | + | + | + | + | + | + | Clearly supportive (mood disorders) | ||
| de la Torre et al. | + | + | + | +d | +d | Indeterminate | ||||
| Lipkin et al. | + | + | Indeterminate | |||||||
| Carbone et al. | + | Mainly against | ||||||||
| Ikuta et al. (32) | + | + | + | +d | Indeterminate | |||||
| Yamaguchi et al. (58) | + | + | Mainly against | |||||||
| Chen et al. (13) | + | + | Indeterminate | |||||||
TABLE 2.
Value of current diagnostic tools to detect and evaluate human BDV infectiona
| Test | Sample | Marker | Antigen | Specificity of method | Sensitivity of method | Indicative of infection | Indicative of disease | Quanti- tative | Convenience | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Double diffusion | Serum | Antibodies | Native | ++ | + | + | − | No | Simple | 39 |
| Complement fixation | Serum | Antibodies | Native | ++ | + | + | − | Yes | Simple | 39 |
| Immunofluorescence | Serum | Antibodies | Altered by fixation | +++ | ++ | ++ | − | Yes | Laborious | 5, 30, 39, 49 |
| CSF | +++ | − | +++ | ++ | Yes | Laborious | 17, 42, 57 | |||
| Cell ELISA | Serum | Antibodies | Altered by fixation | ++ | + | + | − | Yes | Simple | 42 |
| ECLIA | Serum | Antibodies | Recombinant | +++ | ++ | ++ | − | Yes | Simple | 58 |
| Western blot | Serum | Antibodies antigens | Denatured | +++ | + | ++ | − | No | Difficult | 5, 12, 23 |
| CSF | +++ | − | +++ | +++ | No | Difficult | 17, 43 | |||
| Recombinant ELISA | Serum | Antibodies | Recombinant | ++ | ++ | ++ | − | Yes | Simple | 12, 32, 38 |
| Antibody ELISA | Serum | Antibodies | Native | ++++ | ++++ | +++ | − | Yes | Simple | 6, 8 |
| Flow cytometry | PBMCs | Antigens | Mild fixation | +++ | ++ | ++ | ++ | Yes | Difficult | 3, 4, 6 |
| Cell antigen ELISA | PBMCs | Antigens | Native | ++++ | + | ++ | ++ | Yes | Simple | 6, 8, 22a |
| Plasma antigen ELISA | Serum | Antigens | Native | ++++ | ++ | +++ | ++++ | Yes | Simple | 8 |
| CSF | Antigens | Native | ++++ | + | +++ | ++++ | Yes | Simple | 17 | |
| CIC ELISA | Serum | CICs | Native | ++++ | ++++ | ++++ | ++++ | Yes | Simple | 8 |
| RT-PCR | PBMCs | RNA | ++++ | +++ | +++ | − | No | Difficult | 4, 12, 32, 43, 57a | |
| Brain | RNA | ++++ | ++ | +++ | − | No | Difficult | 13a, 15, 50 | ||
| RT-PCR | Serum | RNA | ++++ | + | +++ | ++ | No | Difficult | 8 | |
| Isolation | PBMCs | Virus | ++++ | − | ++++ | +++ | No | Very difficult | 6 | |
| Brain | Virus | ++++ | − | ++++ | +++ | No | Difficult | 44 |
We rank the test systems as being of poor (−), low (+), moderate (++), high (+++), and excellent (++++) diagnostic value.
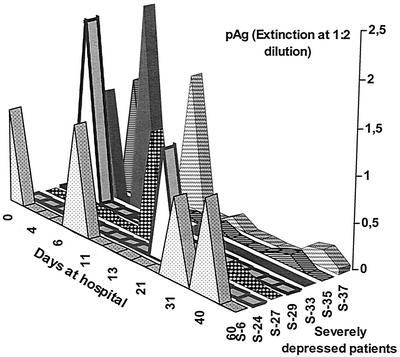
FIG. 2.
Duration of antigenemia in seven individual patients with acute severe depression (these patients were selected from group I in Table 2 of reference 8) presenting with high levels of plasma antigen (pAg) over a period of several weeks.
Unlike human patients, a minority of BDV-infected horses can cope with high levels of antigenemia, and the animals may remain healthy for several months. However, longitudinal observation of individual animals revealed an increased morbidity risk if large amounts of plasma antigen were maintained over several months. Indeed, most of the horses with symptoms of Borna disease presented with strong antigenemia (Ludwig and Bode, unpublished data).
A plausible consequence of specific antigen and antibodies in serum is the formation of CICs. This phenomenon, however, was first discovered inadvertently, prior to the detection of antigenemia, when the antibody ELISA was established.
The possibility of involvement of CICs was initially suggested by the confusing reactivity of many serum samples from experimentally infected rabbits with both BDV-infected and control cell suspensions in ELISA tests for antibody detection. The existence of CICs in serum samples of infected species has since been described for humans and horses (8) and has also been shown in cattle, cats, and rabbits (unpublished data). Provided that both antibodies and CICs are present, the antigen component of the immune complex would bind to the BDV monoclonal antibodies, and the complex is visualized via its antibody part and species-specific conjugate, leading to a positive reaction when no test antigen (infected control cells) has been added. Based on this discovery, we used the above BDV monoclonal antibodies as the basic module for a triple ELISA (8) that measures CICs, plasma antigen, and antibodies in the same plasma or serum sample. The BDV specificity of CICs was demonstrated in Western blots after precipitation with polyethylene glycol or isolation on protein G-Sepharose columns (see Fig. 3 in reference 8). The immune complexes consist of N and P monomers or dimers and most probably N-P heteromers bound to immunoglobulin G antibodies and are very stable, like the antigens. Molecular studies on antigen-antigen interactions and their biochemical characterization are ongoing.
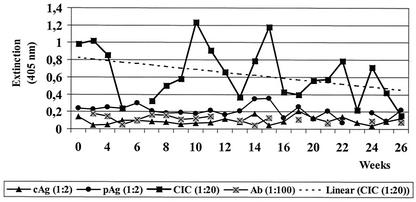
FIG. 3.
BDV CIC levels in a patient with chronic depression. Six-month follow-up of a representative patient (male, age 59 years), showing continuously changing CIC values as the predominant infection marker (reprinted with slight modifications from reference 8 with permission). See Fig. 1 legend for abbreviations. Ab, antibodies. The linear regression line for CIC values shows almost no change over 6 months.
After analysis of hundreds of human and horse serum samples, we consider the optimum initial serum dilutions to be 1:2 for testing plasma antigen, 1:20 for testing CICs, and 1:100 for testing serum antibodies. At these dilutions, the detection limit for each of the BDV-specific components is reached at an extinction value of 0.1 (8). This is a low cutoff value compared to that of several ELISAs used in other virus systems, which provides a significant signal-to-noise ratio in the BDV test system, resulting in high sensitivity.
Testing several thousand human and equine samples over the past 3 years, the CIC assay and the complete test triplet have been evaluated thoroughly for their ability to detect BDV markers and for correlation of results with disease parameters. Presently, our unique BDV database and sample bank consist of approximately 20,000 specimens. Human samples were derived mainly from follow-up visits of psychiatric patients from different clinical studies. The horse samples come from animals with suspected clinical symptoms of Borna disease as well as healthy animals from stables and breeding stocks with clinical cases (L. Bode and H. Ludwig, unpublished data). The CIC assay permits a quantitative analysis of CIC levels. As detailed elsewhere (8), CICs represent the prevailing infection marker and are found in more than 90% of patients with a major depressive disorder or bipolar disorder during acute depression. Screening blood samples solely for CICs may be sufficient to confirm BDV infection (9). Based only on CIC tests, about 20 to 30% were identified as healthy human carriers (8), and for healthy horses, the prevalence rates roughly double, between 30 and 60%. The prevalence of antibodies was commonly or regularly not higher than that of CICs in either healthy humans or horses (Bode et al., unpublished data).
Long-term follow-up studies of patients are particularly important in major affective disorders because the patients' condition alternates between disease and good health. Interestingly, regular blood monitoring by the triple ELISA (antibodies, CICs, and plasma antigen) demonstrated the immunological dynamics of infection, which allowed interpretation of the patient's status at each time point. During a productive phase, the appearance of plasma antigen parallels the disease process until antibodies bind the antigens to form CICs. The complexes are measurable for weeks or months, a common finding in patients followed longitudinally (Fig. 3). Since we have no information as yet on the turnover rate of BDV CICs, we assume that they are continuously built from released free plasma antigen and the corresponding antibodies. Nevertheless, changes in the relative amounts of CIC and plasma antigen over time can be monitored precisely and even allow a prognostic estimate, e.g., large plasma antigen amounts correlated significantly with the severity of depression in major depressive disorder and bipolar disorder patients (8), whereas decline of these markers coincided with clinical improvement. Continuously high levels of CICs and plasma antigen, detected in a minority (high producers) of severely ill (obsessive-compulsive disorder) patients and paralleling their dramatic condition, appear to signal a more negative prognosis (Dietrich and Bode, unpublished data).
The frequency and relative stability of BDV CICs make them currently the best available screening marker for BDV infection. Thus, the CIC assay is an indispensable tool in BDV diagnostics for both clinical studies and epidemiological approaches. The latter has been neglected in recent years but is now progressing with the new assays (Bode and Ludwig, unpublished data). Interestingly, preliminary investigations with the CIC and plasma antigen assays to test several hundred human and horse serum samples from the Czech Republic, the United Kingdom, and Australia (always in a double-blind manner) revealed similarities and differences for BDV infections in different countries (Kabickova, Higham, Flower, and Bode, personal communications).
Independent evaluations made by BDV researchers from Leipzig, Tübingen, Freiburg, and Frankfurt, Germany, in 2000 during a workshop at the Robert Koch Institute in Berlin following the instructions of our protocols (see reference 8) confirmed that our ELISAs were robust and easy to handle and gave reproducible results (unpublished data). Overall, the combined determination of CICs, plasma antigen, and antibodies represents a considerable step forward in BDV diagnosis. The discovery of CICs after years of controversy due to low antibody titers or “disappearing” antibodies explains many previous discrepancies (1, 2, 12, 21, 24, 32, 38, 46, 58). Moreover, the existence of BDV CICs and antigenemia has provided insight into the dynamics of a poorly understood equilibrium of plasma antigen, antibodies, and CICs during persistent BDV infection and its impact on clinical features (9).
Demonstration of BDV RNA
BDV markers, specifically RNA in PBMCs of major depressive disorder patients and one obsessive compulsive disorder patient, were first demonstrated by our group in the mid-1990s by nested reverse transcription (RT)-PCR (4, 6). In the following years, and based on increasing knowledge about BDV RNA and its high genomic conservation across mammalian species (53), viral RNA has been detected in PBMCs from diseased and healthy humans by different groups around the world (9, 12, 32, 38, 43). Despite the caveat that granulocytes represent potential contaminants of PBMCs (46), the reported isolation of Borna virus from granulocytes focused the search for BDV-specific RNA on this white blood cell fraction (38, 46). The ability of granulocytes to incorporate antigen-antibody complexes and possibly ribonucleoproteins floating in the blood could explain the above results (46). Nevertheless, most investigators agree that BDV in the peripheral circulation is carried by PBMCs (3-6, 8, 9, 12-14, 24, 32, 33, 43, 45, 51, 52, 54, 57, 57a).
The value of demonstrable BDV RNA by different RT-PCR techniques has been reviewed extensively (9, 12, 32, 38, 43). Table 1 summarizes the basic opinions among different research groups. Most researchers have reported a higher prevalence of RNA-positive PBMCs in psychiatric patients than in “healthy” people, but the positive rates differed among laboratories. Positive RT-PCRs in clinically asymptomatic patients and healthy controls do not refute the role of BDV in human disease. The discrepancy between RNA-positive cells and antibodies in the corresponding plasma sample can be reconciled in light of data obtained with the CIC tests (9, 43).
Despite some reports on the absence of BDV RNA in psychiatric patients (for reviews, see references 9 and 38), the occurrence of BDV RNA in human samples is generally accepted and is furthermore supported by the finding of BDV RNA in brain samples taken at autopsy (13a, 15, 44, 50). Debate on this issue regarding the possibility that the BDV RNA was the result of contamination has diminished, and the specificity of those specimens seems to be generally accepted (9, 43). All research groups are aware of the possibility that the finding of BDV RNA might indicate contamination of human and animal samples, but this potential can be minimized by using improved safety conditions (51, 57, 57a). Overall, arguments questioning human BDV infection based on contamination (38) have faded.
Sensitive RT-PCR techniques and real-time RT-PCR will be very significant tools for future molecular epidemiological studies, as will be sequencing of the amplicons. Those techniques are indispensable for tracing different BDV strains and helpful in analyzing the geographic distribution of BDV and will also elucidate intraspecies (and possibly interspecies) transmission routes and sources of this virus. Construction of phylogenetic trees based on sequences of single BDV genes will be a powerful tool in this respect. However, unlike other virus infections, where the presence of nucleic acid in blood products represents the best diagnostic tool, BDV infections in patients do not appear to be reliably diagnosed by RT-PCR because of the known small number of infected PBMCs and BDV's low replication rate. Therefore, a negative RT-PCR result does not exclude infection.
Furthermore, RNA is more sensitive to chemical disintegration than are proteins. This would explain why detection of proteins in this virus system, where core proteins are overexpressed (16), is measurable even when BDV RNA is not detected. This makes our established ELISA test battery with N and P protein as indicators (8) considerably more advantageous than testing for RNA. Conversely, detection of BDV RNA in a plasma sample does not confirm an etiologic role for the virus in a patient. Although we recently showed that viral RNA can be amplified even from cell-free plasma samples, if strong antigenemia is present in patients during a clinical state of acute depression (8), the search for BDV-specific nucleic acid in animal and human patient blood products seems unlikely to be of great diagnostic value.
Our viewpoint of the relative usefulness of current techniques to evaluate human BDV infection is presented in Table 2. Considering the relevant literature and our experience with most of these test systems, as well as the evidence accumulated from at least 10,000 samples (humans and horses) completely analyzed by the triple ELISA, we have come to the following conclusions. First, native antigens are of paramount diagnostic value, and second, the established method of testing plasma antigen, CICs, and antibodies is simple and allows a quick and prognostically valuable estimation of acutely or chronically activated processes. Virus isolation is a definitive proof of infection but is less indicative of an etiologic relationship to clinical disease.
Human Borna Virus Strains
To date, only six isolates recovered from human white blood cells or brain have been described. The sources were PBMCs from two bipolar, one obsessive-compulsive disorder, and one chronic fatigue syndrome patient (the latter also presenting with depression) (6, 14). The fifth isolate (named RW98) was reportedly obtained from granulocytes of a schizophrenic patient (46). However, the investigators recently withdrew their finding, attributing detection of the virus to laboratory contamination. When they retested the same patient, the BDV RNA amplicons had nucleotide sequences different from those of RW98 and reference laboratory strains (47). Furthermore, a sixth isolate recovered from the brain of a Japanese schizophrenic patient has been reported (44). The properties of all these isolates have been reviewed (9). We have repeatedly noted that the human strains show clear differences from the animal strains (6), including nucleic acid and nucleotide identity of isolates with the corresponding PBMCs of the patients (14, 44), as well as sequences that demonstrated their uniqueness and specificity. Furthermore, all human isolates are sensitive to amantadine treatment in vitro, in contrast to laboratory strains (9). In light of these arguments, repeatedly expressed concerns about laboratory contamination (54) seem unjustified (except for reference 46) and were recently refuted (see references 9 and 43 and the report on the meeting of the Public Health Laboratory Service of the United Kingdom in Rhonda/Cardiff in 2000 for additional discussion).
BDV can frequently be isolated from the brain of the few horses that die naturally from Borna disease, while isolation from PBMCs of diseased horses is less efficient (8 to 10 blind passages in human oligodendroglial cells are required) (43). In diseased humans, the virus is more difficult to disentangle from its lymphoid (6) or nervous system (44) origin through long-term adaptation to oligodendroglial cells. This explains why only groups experienced in performing multiple passages per sample in tissue culture (at least 20) succeeded in isolating the human virus from patients and why so few isolates exist to date (see also reference 9). Finally, a large number of defined follow-up samples seem to be required for isolation of BDV. This depends on long-term cooperation with experienced psychiatrists.
PREVALENCE OF BDV INFECTION IN PSYCHIATRIC PATIENTS AND HEALTHY INDIVIDUALS
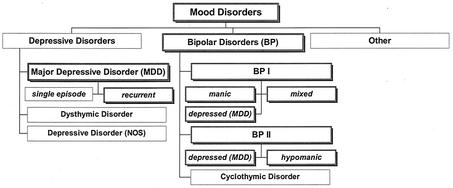
Half of the mood disorders defined according to the “Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association,” 4th ed. (DSM IV), are thought to be of “endogenous” origin with unknown causes, most probably driven by an imbalance in the neurotransmitter network (26). These serious conditions (bipolar disorder and major depressive disorder [DSM IV, no. 296.xx]) (see also Fig. 4) are present in 1 to 5% of the population throughout life, commonly following a relapsing episodic course. Thus, some one hundred million people worldwide suffer chronically from alternating between mania and depression or from recurrent depressive episodes (26).
FIG. 4.
Scheme of mood disorders. This classification is based on DSM IV; shadowed frames indicate those disorders (major depressive disorder [MDD] and bipolar [BP] disorders I and II; DSM IV, no. 296.xx), for which almost all patients were found to be infected (>90%) as deduced from the presence of CICs in blood when tested during an acute depressive episode. NOS, not otherwise specified.
With regard to BDV infections, several reviews provide detailed information on this issue (5, 9, 32, 38, 43). After 1990, studies were focusing mainly on patients with major affective disorders and schizophrenia (DSM IV, no. 296.xx and 295.xx). Related conditions such as obsessive-compulsive disorder (DSM IV, no. 300.3) and chronic fatigue syndrome have gained greater attention as well.
The heterogeneity in the reported data, namely, findings ranging from high frequencies of BDV RNA to no BDV RNA in psychiatric patients and from no significant levels to large amounts of antibodies correlating with disease (32), appears to be accounted for by the use of different methods for BDV antibody and RNA detection as well as insufficient clinical differentiation within subtypes of major psychiatric disorders. Moreover, most studies have applied a cross-sectional design, one sample per patient, with clinically undefined times of blood collection and no periodic monitoring of the course of disease.
In affective disorders, the point prevalence of BDV may range from 2 to 100% depending on the time point and diagnostic parameter used. Our first analysis of psychiatric patients and control individuals by IFA indicated a low seropositive rate of 2% (5), which is in the range of that found in studies by others (49), due to investigation of outpatients mainly when they were in the well state. In contrast, follow-ups by the same method with serum samples from the same patients indicated that 20% were positive during acute depression (5). Therefore, most of our clinical studies were designed as longitudinal investigations.
Among patients in the acute stage, 40 to 50% had BDV antigen-positive PBMCs in the group of severe cases (4, 8, 9, 22a), and the probability of isolating virus increased considerably with increased disease severity (6). Most of the acutely depressed major depressive disorder and bipolar disorder patients were CIC positive (>90%), and the severity of symptoms correlated with high plasma levels of CIC and antigen (Fig. 1). Among patients of all clinical categories of depression in the study, about 50% were CIC positive (8).
We are currently studying patients with obsessive-compulsive disorder, which is a disabling syndrome in 0.5 to 1.0% of the population. It often follows a chronic course and shows 50% comorbidity with major depressive disorder. Of the 40 obsessive-compulsive disorder patients followed longitudinally so far, a considerable proportion were found to express BDV infection markers (CICs, plasma antigen, or antibodies) in their blood. The duration and levels of antigenemia and CICs paralleled severe long-lasting courses of this disorder, indicating a chronically productive infection state (Bode, Dietrich, et al., unpublished data). However, in classical major depressive disorder and bipolar disorder patients, the periodicity of antigenemia was predominant; while lasting for weeks, antigenemia peaked during the clinical episode (Fig. 2).
The diagnostic value of novel BDV laboratory parameters became clear in patient groups with heterogeneous clinical conditions, such as chronic fatigue syndrome. Preliminary experiments revealed no serum BDV antibodies, but later follow-up studies allowed isolation of virus from an American chronic fatigue syndrome patient (9) and detection of activated infection (CICs and plasma antigen) in about one-third of such patients (with depression comorbidity) (Bode and Komaroff, unpublished data). These results indicate that the virus persists; thus, long-term antiviral treatment may be beneficial to ameliorate symptoms, as seen in a 5-year follow-up of a patient (Fig. 5).
FIG. 5.
BDV infection in chronic fatigue syndrome and antiviral treatment. Five-year follow-up of a chronic fatigue syndrome patient with high initial antigenemia, showing a slow but continuous decrease (see linear regression line) of plasma antigen during amantadine treatment started in month 14. This was paralleled by partial but sustained clinical improvement, i.e., expressed by restored ability for daily work but still with reduced energy compared to previous skills. For abbreviations, see the legends to Fig. 1 and 3.
For the heterogeneous clinical entity of chronic fatigue syndrome, BDV prevalence, based on detection of RNA, ranges from 0% in Swedish patients (21) to >80% in Japanese family clusters (32). The single patient analyzed by an Austrian group was also positive for BDV RNA (45). In our opinion, these data suggest the potential value of including chronic fatigue syndrome and related conditions in future epidemiological investigations to unravel the potential influence of BDV infection in certain disease subtypes.
The presence and expression of BDV in human brain, analogous to findings in naturally and experimentally infected animals (43), have been a matter of debate. However, in samples obtained at autopsy from American and European brain banks, PCR-amplifiable BDV-specific RNA (found in 53% and 40% of samples, respectively) was derived only from patients with schizophrenia and bipolar disorder and not from cases of neurological diseases (50). By contrast, the presence of BDV RNA in the brain does not necessarily reflect an active state of viral replication. In addition to the report of a Japanese brain isolate (44), the only study indicating BDV activity in the human brain correlating with disease was our report of BDV antigens in CSF samples from patients with major depressive disorder but not with other psychiatric disorders (17). In some of the cases, these viral antigens could also be demonstrated in Western blots (43).
The question of BDV infections' remaining clinically silent in healthy humans had already been addressed in the early 1990s with IFAs. Several research groups reported a seroprevalence of 2 to 3% (for details, see reference 5). Data on BDV RNA obtained from PBMCs supported the notion of a carrier state in up to 5% of the population, but data on antibodies and RNA from PBMCs of corresponding samples did not match (32, 43). When immune complexes were introduced as a marker, the rate of infected but asymptomatic humans increased to 20 to 30%, as noted earlier, suggesting a rate of silent carriers 10- to 20-fold higher (8; Bode and coworkers, unpublished data) than previously suggested from IFA serology (5) and RNA amplified from human brain suspensions (32, 38).
Our data are presently based on two groups of 100 randomly selected blood donors each, with 20 to 30% CIC and 2 to 4% IFA positive, as well as 133 healthy volunteers from randomly selected families, with 26.3% CIC positive. Samples were obtained at different time points (1998 and 2000 to 2001) and places (Berlin and Leipzig). A recent collaborative study on 225 Australian blood donors from the area of New South Wales (age, early 40s; gender, randomly selected) confirmed an infection prevalence of around 33% (Flower, Kamjieh, Bode, and Ludwig, unpublished data). In the Berlin blood donor group, the gender ratio was 74.5% males to 25.5% females, which is almost the reverse of the gender ratio observed for depressed patients (2:1, females to males). However, this ratio is somewhat compensated for by the healthy volunteer group, with a ratio of 35.3% males to 64.7% females. In terms of mean (± standard deviation) age, neither the blood donors (41.5 ± 12 years) nor the volunteers (31 years; data not available) matched the age of the group of patients with a long history of recurrent affective disorders (50 ± 10 years) (8; Bode and coworkers, unpublished data).
Selection of an appropriate control group always presents a problem, particularly for a comparison with psychiatric patients, because in addition to age and gender mismatches, standardized health status questionnaires rather than mental-health research data are usually not available. Blood donors are tested for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infections, but from a gender and socioeconomic standpoint, the control group is far from being representative of the “normal” population. Based on reports of an elevated infection rate with BDV among healthy relatives of patients (13, 32), a potentially higher risk of acquiring BDV infection should be envisioned in the healthy volunteers if a member of the family or a group tests positive for BDV (13, 32, 38).
Despite these concerns in past and present control cohorts studied for BDV infection, it is generally accepted that these individuals are significantly less frequently positive than psychiatric patients for the viral parameters measured (5, 12, 32, 38).
With respect to epidemiological considerations, a rather high prevalence of BDV infection in the healthy population would imply a relatively low pathogenicity of the agent, suggesting that the majority of infections have little or no morbidity risk. This contrasts with the close to 100% infection rate in well-defined major depressive disorder and bipolar disorder patients in the acute phase (8), which supports a relationship between BDV infection and disease. In line with our previous discussions on known inherited vulnerability and BDV infection (6), frequent activation of this agent occurring in those patients may be a consequence of genetic predisposition. Thus, BDV may become a major environmental factor, among others (26), contributing to frequency, severity, and duration of disease episodes.
Unlike schizophrenia, major affective disorders (major depressive disorder and bipolar disorder) have become increasingly linked with activated BDV infection. Again, the importance of diagnostic methods with respect to their different sensitivities and diagnostic values must be emphasized. Simple summaries of heterogeneous prevalence data have added to the confusion rather than providing clues on how to interpret the findings (12, 32). However, a search of the BDV literature makes it clear that the absence of neither antibodies nor RNA in blood cells can exclude infection, since antibodies may bind to plasma antigen, forming CICs (8), and RNA may be present in only 1 per 105 PBMCs (51). Unlike viral antigen and CICs, free antibodies and RNA have no meaning in terms of a currently productive infection state. Antibodies may only indicate a “latent” state or previous infection. In contrast, the new markers (plasma antigen and CIC) in our triple ELISA provide valuable tools to overcome the current diagnostic problems, facilitating the comparison of multinational studies which has been lacking.
IMPACT OF ANTIVIRAL THERAPY
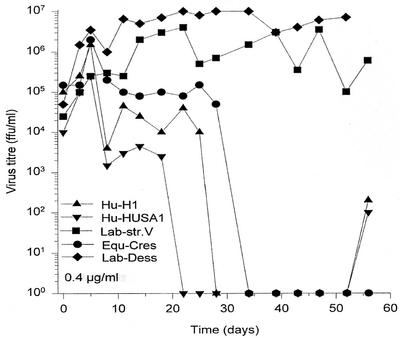
We recently described the in vitro and in vivo antiviral effect of a 30-year-old drug, amantadine sulfate, against a human BDV isolate. A severely depressed infected bipolar patient improved dramatically under low-dose amantadine therapy, in parallel with the disappearance of viral markers in the blood (7). The prospect of a new low-risk therapeutic option for BDV-infected patients whose depression had already become resistant to conventional antidepressants aroused much interest, even in the public sectors. Controversies arose when amantadine resistance of BDV laboratory strains was misleadingly assumed to cover all Borna viruses (for additional references and further details, see references 43 and 9). Our in vitro studies with two long-term laboratory-adapted strains in fact confirmed their insensitivity to amantadine. However, seven natural human and equine isolates treated with four amantadine concentrations between 0.2 and 1.2 μg/ml revealed a clear dose-dependent inhibition profile, as was published for one of these human strains (7). An in vitro dose of 0.4 μg of amantadine/ml corresponds to the well-tolerated blood level reached by a low oral dose of 200 mg daily, which was successfully applied in our open clinical trials (18, 22). In vitro, this dose led to a decrease in infectious virus of about 104 focus-forming units/ml after 2 to 3 weeks (Fig. 6). Two 60-day series of in vitro inhibition-of-replication experiments analyzing the titer drop in infected oligodendroglial cells confirmed the resistance of laboratory strains and the sensitivity of wild-type virus (Stoyloff and Bode, unpublished data).
FIG. 6.
In vitro antiviral treatment of BDV-infected human oligodendroglia cells. Persistently infected oligodendroglia cells were split every 3 days and maintained in medium containing amantadine (0.4 μg/ml, therapeutic blood level doses). At the indicated time points, infectivity in these cells was titered on young rabbit brain cells (for details, see reference 7). Note the time-dependent infectivity decrease for the sensitive wild-type strains, two human isolates (Hu-H1 [8] and Hu-HUSA1, isolated from an American chronic fatigue syndrome patient [9]), and one equine isolate (Equ-Cres, isolated from PBMCs of a horse with Borna disease) versus stable titers in two resistant laboratory strains (strains V and Dessau).
We had already shown that amantadine not only inhibits the replication of wild-type BDV in cells in vitro but also prevents infection of naïve cells and that both activities are dose dependent (7, 9). The ID50 (50% infection-inhibitory dose) of the most sensitive human strain differs by six log10 units from that of a resistant laboratory strain (<0.001 μg versus >1,000 μg of amantadine/ml) (9). The molecular mechanism of inhibition, however, remains unknown, and the putative mutations involved in the resistance of laboratory strains await genetic analysis. Laboratory strains are the product of multiple in vivo and in vitro cross-species passages, sometimes including prior infection of different animal species to adapt the virus to replicate in the appropriate cells (42).
About 60 BDV-infected patients with acute major depressive disorder or bipolar disorder depression were included in two independent open trials. Patients were treated daily with a mean oral dose of 200 mg of amantadine for 12 weeks, and the majority showed a significant and rapid clinical response after an average of 3 weeks (18, 22). Of the responders, 68% (17 of 25) in the Hanover study (18), 70.6% showed no depression at all, and 29.4% had a >50% decrease in symptoms, according to the widely used 21-item Hamilton rating scale for depression. In the Berlin study (22), 63.3% (19 of 30) of the patients showed a significant decrease in depressive symptoms, as measured by an at least 40% reduction in points on the Montgomery-Asberg depression rating scale. Remarkably, this considerably favorable effect of amantadine therapy, comparable to that of the Hanover study, could be achieved in patients who had been recruited due to poor or even complete lack of response (17 of 30, 56.7%) to any antidepressant for more than 1 year (22).
Such encouraging responder rates were confirmed by a placebo-controlled double-blind study with 33 BDV-infected patients for whom amantadine was found to be significantly superior to placebo after 7 weeks (87.5% versus 29.4%; P = 0.003), according to the Hamilton scale. The clinical responder rate after 14 weeks (6 weeks of treatment plus 1 week of wash-out, 6 weeks of placebo plus 1 week of wash-out, or vice versa) reached 74.2% (23 of 31 patients), similar to the rates achieved in the above-mentioned open trials. Moreover, preliminary analysis of a posttherapy follow-up (at least 1 year) of patients from this double-blind study suggested a long-term benefit of anti-BDV treatment despite the very short single therapy period of 6 weeks (Dietrich, Emrich, Bode, Ludwig, and coworkers, unpublished data).
The initial laboratory sign of antiviral efficacy in the treated patients is the disappearance of antigen from plasma and PBMCs. However, the long-term profile of BDV CICs seemed to be the most sensitive marker, indicating whether and to what extent antigen-productive phases could be abolished. Importantly, in the clinical studies mentioned above, but the initial antigen load was mainly moderate or low. In individual patients, especially those presenting with chronically severe clinical conditions, an extremely high level of antigenemia and CICs is possible, and amantadine therapy prevents a further increase but may not be capable of achieving a significant decrease at a given well-tolerated dose (Bode and Dietrich, unpublished data). Nevertheless, even high initial antigen loads tend to decline over the years in patients treated with a continuous antiviral regimen (Fig. 5). Continuous reduction of plasma antigen levels was demonstrated in the 5-year follow-up of a chronic fatigue syndrome patient who partially improved under treatment and was able to carry out daily activities despite residual fatigue (Fig. 5) (Bode and Ludwig, unpublished data).
ARGUMENTS FOR AND AGAINST ETIOPATHOGENIC SIGNIFICANCE
The issues of BDV infection and BDV-related disease must be strictly separated because a considerable number of silent infections exist in humans and other animal species. The etiopathogenic relationship between virus and human disease remains the major question. However, unlike some other viral diseases in which blood parameters regularly parallel defined clinical manifestations, persistent BDV infection in humans is characterized on the one hand by small amounts of CICs with or without free antibodies in symptomless virus carriers, and on the other hand by significant expression of viral markers (plasma antigen which leads to CICs) in acute mood disorders. A skeptical view of this virus as an etiologic agent of central nervous system disease in humans is no surprise, since sources and routes of infection are still unknown and previous diagnostic markers have not allowed conclusive statements. Moreover, avidity studies questioning human antibodies (1, 2a), alternative views on the clinical relevance of BDV infection (12), and the ambiguous interpretations of a virus-disease relationship (24) have further clouded the question of a role for BDV in human diseases despite efforts to clarify this issue (9, 37, 38) and despite fulfillment of Koch's postulates with human isolates inoculated into animals (6).
Our observations (9, 43; this article) that make an etiopathogenic relationship likely include (i) temporal relationship of infection markers and disease, (ii) frequent presence of antigenemia and CICs in acute depressive episodes, (iii) high levels of plasma antigen and CICs correlating with severity of psychiatric symptoms, (iv) long-term benefit of amantadine treatment in infected depressed patients, (v) virus isolation from PBMCs of severely diseased patients with high antigenemia, and (vi) multiple analogies of clinical and other parameters between animal models and infected humans.
Arguments against the clinical significance of BDV in humans include (i) lack of BDV RNA in PBMCs of some psychiatric patient cohorts, (ii) genomic BDV RNA questioned as contamination due to a low percentage of genetic divergence compared to laboratory strains, (iii) incongruence of BDV-specific amplicons and the presence of antibodies, (iv) questionable activity of human antibodies to BDV in avidity studies (2a), (v) irreproducibility of amantadine inhibition effects with laboratory strains, and (vi) difficulty in directly linking BDV infection with human disease.
Our Opinion
A recent reappraisal of BDV's role in neuropsychiatric diseases (38) remains open to criticism in light of the current state of knowledge in this field (9; this review). The same authors (38) have confirmed (50) previous findings of BDV RNA in human brain specimens (15) but expressed doubt concerning infection in the blood despite overwhelming evidence found by many groups worldwide. The suggestion to search for infected blood cells (e.g., granulocytes) other than PBMCs (38) does not seem to pave the way for a better understanding of BDV infection outside the brain (see also reference 24). Furthermore, recombinant proteins appear to be of limited value for detection of anti-BDV antibodies (21, 38). A recent study, however, with baculovirus-expressed p40 antigen as the target to detect horse antibodies to BDV revealed some hints that this expression system might be superior to other recombinant approaches (59). Improved RT-PCR techniques (51, 57, 57a) have now negated concerns about potential cross-contamination (38). There is a definite need for multicenter studies that incorporate novel BDV parameters, particularly the CIC test (as proposed in this review).
The current focus on measurement of humoral infection markers seems likely to predominate over any diagnostic approaches using cell-mediated immunity parameters (24) in the future. Especially, because the experience with small-animal models cannot simply be superimposed on humans (9, 31, 43), the time has come to quantify the amount of native and antibody-complexed BDV antigen in blood plasma along with longitudinal monitoring. Whether or not BDV antigenemia has a similar or different impact on disease compared with other persistent virus infections (e.g., human immunodeficiency virus and hepatitis B virus), the new infection markers should provide new insights into BDV infection and pathogenesis.
SPECULATIONS AND OUTLOOK
BDV has been classified (16) and shown to infect many species, including humans (9, 32, 38, 43). The incredible pathogenic flexibility of this virus (40), depending on the developmental state of the organism, has been shown experimentally (38, 43). However, the same must be proven in natural infections before claiming that inhibition of nerve outgrowth (36), interference with developmental cell growth and synaptic pathology (25, 31, 48), learning deficiencies (19), and behavioral changes (9, 43, 48, 55, 56) due to neurotransmitter imbalances (27, 37) or to inflammatory reactions (28, 31) occur in humans. Even more interesting would be the demonstration of an effect of BDV on neurotransmitter balance and action in humans, as studied in experimental animals (29, 31, 37), since this would provide insight into neuronal activity in the human limbic system.
Presently, we can only speculate on how BDV, as based on its preference for limbic structures, its high genetic stability, its complicated splicing machinery, and many other biological properties, might (directly or indirectly) alter neurotransmitter balances in the brains of manic, depressed, or obsessive-compulsive patients (26) or influence the emotion and behavior of these patients (34). Is BDV a “mood” virus? Unraveling the spectrum of BDV's pathogenicity will still require unusual efforts and unconventional approaches to further strengthen its “correlational evidence” for causing disease in humans as definitely proven in animals.
Many viral pathogens are hazardous to human health, compromising it by organ-specific destruction. Borna virus is the only agent known to selectively interfere with behavior in many animal species, depending on the time in their life span of infection. From natural infections in horses and cats, a broad spectrum of transient changes ranging from subtle behavioral alterations to severe neurological disease have been directly linked to BDV infection. From all we know about human mental health and wellness, BDV is the only candidate agent providing unique properties consistent with both episodic courses of dysfunction and complete recovery and with subclinical persistence, depending on host vulnerability or resistance.
Acknowledgments
The two authors contributed equally to this article.
We are grateful to clinical psychiatrists in the front line, namely, D. E. Dietrich, H. Emrich, R. Ferszt, E. Severus, our neuropathologist G. Gosztonyi, the biochemist R. Stoyloff, and the technical assistants Patrizia Reckwald and Tine Leiskau, who helped to create the data and views outlined in this focus article. We thank Martina Hoffmann for editorial help and appreciate the comments and suggestions from the reviewers.
Long-term grants from the Deutsche Forschungsgemeinschaft and European Community supported producing the basic data.
REFERENCES
- 1.Allmang, U., M. Hofer, S. Herzog, K. Bechter, and P. Staeheli. 2001. Low avidity of human serum antibodies for Borna disease virus antigens questions their diagnostic value. Mol. Psychiatry 6:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Bechter, K. 1998. Borna Disease Virus, mögliche Ursache neurologischer Störungen des Menschen. Monographien aus dem Gesamtgebiet der Psychiatrie. Steinkopff, Darmstadt, Germany.
- 2a.Billich, C., et al. 2002. Biol. Psychiatry 51:979-987. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L., F. Steinbach, and H. Ludwig. 1994. A novel marker for Borna disease virus infection. Lancet 343:297-298. [DOI] [PubMed] [Google Scholar]
- 4.Bode, L., W. Zimmermann, R. Ferszt, F. Steinbach, and H. Ludwig. 1995. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat. Med. 1:232-236. [DOI] [PubMed] [Google Scholar]
- 5.Bode, L. 1995. Human infections with Borna disease virus and potential pathogenic implications. Curr. Top. Microbiol. Immunol. 190:103-130. [DOI] [PubMed] [Google Scholar]
- 6.Bode, L., R. Dürrwald, F. A. Rantam, R. Ferszt, and H. Ludwig. 1996. First isolates of infectious human Borna disease virus from patients with mood disorders. Mol. Psychiatr. 1:200-212. [PubMed] [Google Scholar]
- 7.Bode, L., D. E. Dietrich, R. Stoyloff, H. M. Emrich, and H. Ludwig. 1997. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet 349:178-179. [DOI] [PubMed] [Google Scholar]
- 8.Bode, L., P. Reckwald, W. E. Severus, R. Stoyloff, R. Ferszt, D. E. Dietrich, and H. Ludwig. 2001. Borna disease virus-specific circulating immune complexes, antigenemia, and free antibodies—the key marker triplet determining infection and prevailing in severe mood disorders. Mol. Psychiatr. 6:481-491. [DOI] [PubMed] [Google Scholar]
- 9.Bode, L., and H. Ludwig. 2001. Borna disease virus—a threat for human mental health? p. 269-310. In G. L. Smith, W. L. Irving, J. W. McCauley, and D. J. Rowlands (ed.), New challenges to health: the threat of virus infection. Society for General Microbiology, Cambridge University Press, Cambridge, England.
- 10.Briese, T., A. Schneemann, A. J. Lewis, Y.-S. Park, S. Kim, H. Ludwig, and W. I. Lipkin. 1994. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 91:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brundtland, G. H. 2001. Message from the Director General. In Mental health: new understanding, new hope: the World Health Report 2001. World Health Organization, Geneva, Switzerland.
- 12.Carbone, K. 2001. Borna disease virus and human disease. Clin. Microbiol. Rev. 14:513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C.-H., Y.-L. Chiu, C.-K. Shaw, M. T. Tsai, A. L. Hwang, and K.-J. Hsiao. 1999. Detection of Borna disease virus RNA from peripheral blood cells in schizophrenic patients and mental health workers. Mol. Psychiatr. 4:566-571. [DOI] [PubMed] [Google Scholar]
- 13a.Czygan, M., et al. 1999. J. Infect. Dis. 180:1695-1699. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre, J. C., L. Bode, R. Dürrwald, B. Cubitt, and H. Ludwig. 1996. Sequence characterization of human Borna disease virus. Virus Res. 44:33-44. [DOI] [PubMed] [Google Scholar]
- 15.de la Torre, J. C., D. Gonzalez-Dunia, B. Cubitt, M. Mallory, N. Mueller-Lantzsch, F. A. Grässer, L. A. Hansen, and E. Masliah. 1996. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology 223:272-282. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre, J. C., L. Bode, K. M. Carbone, B. Dietzschold, K. Ikuta, W. I. Lipkin, H. Ludwig, J. A. Richt, P. Staeheli, and L. Stitz. 2000. Family Bornaviridae, p. 531-538. In M. H. V. van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy. Academic Press, London, England.
- 17.Deuschle, M., L. Bode, I. Heuser, J. Schmider, and H. Ludwig. 1998. Borna disease virus proteins in cerebrospinal fluid of patients with recurrent depression and multiple sclerosis. Lancet 352:1828-1829. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich, D. E., L. Bode, C. W. Spannhuth, T. Lau, T. J. Huber, B. Brodhun, H. Ludwig, and H. M. Emrich. 2000. Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial. Bipolar Disorders 2:65-70. [DOI] [PubMed] [Google Scholar]
- 19.Dittrich, W., L. Bode, H. Ludwig, M. Kao, and K. Schneider. 1989. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol. Psychiatr. 26:818-828. [DOI] [PubMed] [Google Scholar]
- 20.Dürrwald, R., and H. Ludwig. 1997. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. J. Vet. Med. B 44:147-184. [DOI] [PubMed] [Google Scholar]
- 21.Evengard, B., T. Briese, G. Lindh, S. Lee, and W. I. Lipkin. 1999. Absence of evidence of Borna disease virus infection in Swedish patients with chronic fatigue syndrome. J. Neurovirol. 5:495-499. [DOI] [PubMed] [Google Scholar]
- 22.Ferszt, R., K.-P. Kühl, L. Bode, W. E. Severus, B. Winzer, A. Berghöfer, G. Beelitz, B. Brodhun, B. Müller-Oerlinghausen, and H. Ludwig. 1999. Amantadine revisited: an open trial of amantadine sulfate treatment in chronically depressed patients with Borna disease virus infection. Pharmacopsychiatry 32:142-147. [DOI] [PubMed] [Google Scholar]
- 22a.Ferszt, R., et al. 1999. Pharmacopsychiatry 32:93-98. [DOI] [PubMed] [Google Scholar]
- 23.Fu, Z. F., J. D. Amsterdam, M. Kao, V. Shankar, H. Koprowski, and B. Dietzschold. 1993. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by Western immunoblot technique. J. Affect. Disord. 27:61-68. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda, K., K. Takahashi, Y. Iwata, N. Mori, K. Gonda, T. Ogawa, K. Osonoe, M. Sato, S.-I. Ogata, T. Horimoto, T. Sawada, M. Tashiro, K. Yamaguchi, S.-I. Niwa, and S. Shigeta. 2001. Immunological and PCR analyses for Borna disease virus in psychiatric patients and blood donors in Japan. J. Clin. Microbiol. 39:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales-Dunia, D., M. Watanabe, S. Syan, M. Mallory, E. Masliah, and J. C. de la Torre. 2000. Synaptic pathology in Borna disease virus persistent infection. J. Virol. 74:3441-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin, F. K., and K. R. Jamison. 1990. Manic-depressive illness. Oxford University Press, New York, N.Y.
- 27.Gosztonyi, G., and H. Ludwig. 1984. Neurotransmitter receptors and viral neurotropism. Neuropsychiatr. Clin. 3:107-114. [Google Scholar]
- 28.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 29.Gosztonyi, G., and H. Ludwig. 2001. Interactions of viral proteins with neurotransmitter receptors may protect or destroy neurons. Curr. Top. Microbiol. Immunol. 253:121-144. [DOI] [PubMed] [Google Scholar]
- 30.Herzog, S., and R. Rott, R. 1980. Replication of Borna disease virus in cell cultures. Med. Microbiol. Immunol. 168:153-158. [DOI] [PubMed] [Google Scholar]
- 31.Hornig, M., M. V. Solbrig, N. Horscroft, H. Weissenböck, and W. I. Lipkin. 2001. Borna disease virus infection of adult and neonatal rats: models for neuropsychiatric disease. Curr. Top. Microbiol. Immunol. 253:157-177. [DOI] [PubMed] [Google Scholar]
- 32.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7d:470-495. [DOI] [PubMed] [Google Scholar]
- 33.Iwata, Y., K. Takahashi, X. Peng, K. Fukuda, K. Ohno, T. Ogawa, K. Gonda, N. Mori, S.-I. Niwa, and S. Shigeta. 1998. Detection and sequence analysis of Borna disease virus p24 RNA from peripheral blood mononuclear cells of patients with mood disorders or schizophrenia and of blood donors. J. Virol. 72:10044-10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamison, K. R. 1993. Touched with fire. Manic-depressive illness and the artistic temperament. Free Press, New York, N.Y.
- 35.Johnson, R. T. 1998. Viral infections of the nervous system, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 36.Kamitani, W., Y. Shoya, T. Kobayashi, M. Watanabe, B.-J. Lee, G. Zhang, K. Tomonaga, and K. Ikuta. 2001. Borna disease virus phosphoprotein binds a neurite outgrowth factor, amphoterin/HMG-1. J. Virol. 75:8742-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipkin, W. I., K. M. Carbone, M. C. Wilson, C. S. Duchala, O. Narayan, and M. B. A. Oldstone. 1988. Neurotransmitter abnormalities in Borna disease. Brain Res. 475:366-370. [DOI] [PubMed] [Google Scholar]
- 38.Lipkin, I. W., M. Hornig, and T. Briese. 2001. Borna disease virus and neuropsychiatric disease—a reappraisal. Trends Microbiol. 9:295-298. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig, H., and H. Becht. 1977. Borna disease—a summary of our present knowledge, p. 75-83. In V. ter Meulen and M. Katz (ed.), Slow virus infections of the central nervous system: investigational approaches to etiology and pathogenesis of these diseases. Springer, New York, N.Y.
- 40.Ludwig, H., W. Kraft, M. Kao, G. Gosztonyi, E. Dahme, and H. Krey. 1985. Borna-Virus Infektion (Borna-Krankheit) bei natürlich und experimentell infizierten Tieren: Ihre Bedeutung für Forschung und Praxis. Tierärztl. Praxis 13:421-453. [PubMed] [Google Scholar]
- 41.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 42.Ludwig, H., K. Furuya, L. Bode, N. Klein, R. Dürrwald, and D. S. Lee. 1993. Biology and neurobiology of Borna disease viruses (BDV), defined by antibodies, neutralizability and their pathogenic potential. Arch. Virol. Suppl. 7:111-133. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig, H., and L. Bode. 2000. Borna disease virus: new aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Techn. Off. Int. Epizoot. 19:259-288. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura, Y., H. Takahashi, Y. Shoya, T. Nakaya, M. Watanabe, K. Tomonaga, K. Iwahashi, K. Ameno, N. Momiyama, H. Taniyama, T. Sata, T. Kurata, J. C. de la Torre, and K. Ikuta. 2000. Isolation of Borna disease virus from human brain tissue. J. Virol. 74:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowotny, N., and J. Kolodziejek. 2000. Demonstration of Borna disease virus nucleic acid in a patient with chronic fatigue syndrome. J. Infect. Dis. 181:1860-1862. [DOI] [PubMed] [Google Scholar]
- 46.Planz, O., C. Rentzsch, A. Batra, T. Winkler, M. Büttner, H.-J. Rziha, and L. Stitz. 1999. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J. Virol. 73:6251-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Planz, O., H.-J. Rziha, and L. Stitz. 2003. Genetic relationship of Borna disease virus isolates. Virus Genes 26:25-30. [DOI] [PubMed] [Google Scholar]
- 48.Pletnikov, M. V., S. A. Rubin, K. Vasudevan, K., T. H. Moran, and K. M. Carbone. 1999. Developmental brain injury associated with abnormal play behaviour in neonatally Borna disease virus-infected Lewis rats: a model of autism. Behav. Brain Res. 100:43-50. [DOI] [PubMed] [Google Scholar]
- 49.Rott, R., S. Herzog, B. Fleischer, A. Winokur, J. Amsterdam, W. Dyson, and H. Koprowski. 1985. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 228:755-756. [DOI] [PubMed] [Google Scholar]
- 50.Salvatore, M., S. Morzunov, M. Schwemmle, and W. I. Lipkin. 1997. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorders. Bornavirus Study Group. Lancet 349:1813-1814. [DOI] [PubMed] [Google Scholar]
- 51.Sauder, C., and J. C. de la Torre. 1998. Sensitivity and reproducibility of RT-PCR to detect Borna disease virus (BDV) RNA in blood: implications for BDV epidemiology. J. Virol. Methods 71:229-245. [DOI] [PubMed] [Google Scholar]
- 52.Sauder, C., A. Müller, B. Cubitt, J. Mayer, J. Steinmetz, W. Trabert, B. Ziegler, K. Wanke, N. Mueller-Lantzsch, J. C. de la Torre, and F. A. Grässer. 1996. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J. Virol. 70:7713-7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider, P. A., T. Briese, W. Zimmermann, H. Ludwig, and W. I. Lipkin. 1994. Sequence conservation in field and experimental isolates of Borna disease virus. J. Virol. 68:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwemmle, M., C. Jehle, S. Formella, and P. Staeheli. 1999. Sequence similarities between human bornavirus isolates and laboratory strains question human origin. Lancet 354:1973-1974. [DOI] [PubMed] [Google Scholar]
- 55.Solbrig, M. V., J. H. Fallon, and W. I. Lipkin. 1995. Behavioral disturbances and pharmacology of Borna disease. Curr. Top. Microbiol. Immunol. 190:93-99. [DOI] [PubMed] [Google Scholar]
- 56.Sprankel, H., K. Richarz, H. Ludwig, and R. Rott. 1978. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med. Microbiol. Immunol. 165:1-18. [DOI] [PubMed] [Google Scholar]
- 57.Vahlenkamp, T. W., H. K. Enbergs, and H. Müller. 1999. Presence of Borna disease virus (BDV) RNA in cells of the peripheral blood. Vet. Microbiol. 76:229-244. [DOI] [PubMed] [Google Scholar]
- 57a.Vahlenkamp, T. W., A. Konrath, M. Weber, and H. Müller. 2002. Persistence of Borna disease virus in naturally infected sheep. J. Virol. 76:9735-9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi, K., T. Sawada, T. Naraki, R. Igata-Yi, H. Shiraki, Y. Horii, T. Ishii, K. Ikeda, N. Asou, H. Okabe, M. Mochizuki, K. Takahashi, S. Yamada, K. Kubo, S. Yashiki, R. W. Waltrip II, and K. M. Carbone. 1999. Detection of Borna disease virus-reactive antibodies from patients with psychiatric disorders and from horses by electrochemiluminescence immunoassay. Clin. Diagn. Lab. Immunol. 6:696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yilmaz, H., C. R. Helps, N. Turan, A. Uysal, and D. A. Harbour. 2002. Detection of antibodies to Borna disease virus (BDV) in Turkish horse serum samples with recombinant p40. Arch. Virol. 147:429-435. [DOI] [PubMed] [Google Scholar]