Abstract
Despite effective antibiotic therapy, bacterial meningitis is still associated with high morbidity and mortality in both children and adults. Animal studies have shown that the host inflammatory response induced by bacterial products in the subarachnoid space is associated with central nervous system injury. Thus, attenuation of inflammation early in the disease process might improve the outcome. The feasibility of such an approach is demonstrated by the reduction in neurologic sequelae achieved with adjuvant dexamethasone therapy. Increased understanding of the pathways of inflammation and neuronal damage has suggested rational new targets to modulate the host response in bacterial meningitis, but prediction of which agents would be optimal has been difficult. This review compares the future promise of benefit from the use of diverse adjuvant agents. It appears unlikely that inhibition of a single proinflammatory mediator will prove useful in clinical practice, but several avenues to reprogram a wider array of mediators simultaneously are encouraging. Particularly promising are efforts to adjust combinations of cytokines, to inhibit neuronal apoptosis and to enhance brain repair.
INTRODUCTION
Sixty years after their discovery, antibiotics remain virtually the only weapons against bacterial meningitis (126). Antibiotic therapy has changed meningitis from a uniformly fatal disease to a frequently curable one. However, the outcome is still often unfavorable, with a mortality of 5 to 10% and permanent neurologic sequelae occurring in 5 to 40% of survivors, depending on patient age and pathogen (5, 9, 31, 44). Recent scientific progress has resulted in an effective polysaccharide-protein conjugate vaccine against Haemophilus influenzae that has reduced the current incidence of bacterial meningitis to ≈4.0 per 100,000 population in most developed countries (140, 161). There are also effective conjugate vaccines against Streptococcus pneumoniae and Neisseria meningitidis type C (87, 145). Maternal immunization with group B streptococcal (Streptococcus agalactiae) conjugate vaccine may present a feasible strategy to reduce neonatal group B streptococcal disease incidence (14, 103). Development of a group B meningococcal conjugate vaccine has been hindered by the potential risks of autoantibodies that cross-react with glycosylated host antigens. New vaccine candidates discovered during the sequencing of the group B meningococcal genome offer promise of an effective and safe vaccine against group B N. meningitidis, which would result in a significant further reduction in morbidity and mortality from bacterial meningitis (52). At present, however, physicians depend on early antibiotic therapy in the remaining cases of meningitis to avert dismal outcomes (6, 127, 166).
Two-thirds of meningitis-related deaths are attributable primarily, or at least in part, to central nervous system complications; the remainder result from systemic complications, such as septic shock (113). Increased insight into the pathophysiology of bacterial meningitis has shown that the inflammatory host response to bacterial products continues after the bacteria are killed with antibiotics. This host response regrettably affects host tissues and contributes significantly to central nervous system injury (83, 90, 97, 137). Thus, the addition of anti-inflammatory or neuroprotective agents would be expected to protect against tissue injury associated with meningeal inflammation. While this is a logical hypothesis, real-life experience with modulation of the host inflammatory cascade, such as in clinical sepsis trials, has yielded mixed results and demonstrated that the setting is so complex that predicting the benefit of any intervention is very tricky (39).
Adjuvant use of corticosteroids in clinical trials in bacterial meningitis has demonstrated that there is an opportunity to protect against tissue injury and thereby improve outcome. Corticosteroids have been used against bacterial meningitis since the 1950s, even though initial clinical studies with methylprednisolone failed to demonstrate any benefit in children with meningitis; it is important to note that no mention was made of hearing evaluation in these patients. As concluded in a recent meta-analysis, adjuvant dexamethasone therapy, which is more potent than methylprednisolone, in children with H. influenzae type b meningitis significantly reduces neurologic sequelae, most notably severe hearing loss (number needed to treat, 12) (89). Extending the efficacy of corticosteroids to adults and to meningitis due to other pathogens has remained much debated, however, because the results from several small trials showed no clear benefit of this intervention.
Following the introduction of the H. influenzae type b vaccine, the clinical use of corticosteroids in bacterial meningitis became more controversial, and additional clinical trials of sufficient size were warranted to resolve this issue (30). A recently completed, large clinical trial in adult patients shows that early dexamethasone treatment initiated before or with the first dose of antibiotics improves outcome (25). The effect was especially strong in patients with pneumococcal meningitis (number needed to treat 4 to 10). Adjuvant dexamethasone treatment did not result in an increased risk of serious adverse events, such as gastrointestinal bleeding. Studies in the developing world, where presentation to hospital is often delayed, showed that adjuvant steroid treatment could not improve outcome in this setting (96, 123). These results indicate that corticosteroids are only beneficial when administered early in the course of the disease.
The clinical efficacy of corticosteroids is proof of the principle that reduction of injury to the central nervous system can be achieved by modulation of the host response. Other avenues of benefit may also exist. For example, although tissue injury, such as disruption of the blood-brain barrier, cerebral edema, and mild neuronal injury, always occurs in meningitis, some patients survive the disease and recover fully. This illustrates the natural capacity of the body to repair injured tissues, at least in part. Strengthening of the body's repair systems may present a second major avenue to achieving benefit and reducing permanent tissue damage in the central nervous system. The molecular dissection of cell maintenance and repair mechanisms has given important leads to therapies that might enhance the body's innate repair response.
CELLULAR DYSFUNCTION IN MENINGITIS
Endothelium of the blood-brain barrier
The cerebral microvascular endothelium has unique ultrastructural properties, such as continuous intercellular tight junctions and a slow rate of fluid endocytosis, that enable it to function as a high-resistance barrier to circulating macromolecules, also called the blood-brain barrier. Disruption of the blood-brain barrier is a hallmark event in the pathophysiology of bacterial meningitis. Disturbance of cerebral endothelial function results in the development of vasogenic cerebral edema in 30% of cases and cerebral herniation in 6 to 8% of clinical cases in some series (113). Cerebral edema and resultant increased intracranial pressure impair tissue perfusion and are associated with a high risk of death or severe neurologic sequelae. Bacterial toxins in blood or cerebrospinal fluid (CSF), such as gram-negative lipopolysaccharide (LPS), gram-positive peptidoglycan, and cytotoxins, engage Toll-like receptors of endothelia and activate their downstream signaling cascades. The endothelial cells then release mediators, such as tumor necrosis factor alpha (TNF-α), nitric oxide, and matrix metalloproteinase-2 (MMP-2), which increase endothelial permeability (63, 76, 125). The endothelium expresses multiple leukocyte adhesion molecules and presents chemotactic factors such as interleukin-8 (IL-8) when activated by inflammatory mediators. This combination promotes neutrophil adherence and transendothelial migration. Upregulation of tissue factor on the endothelial surface induces a procoagulant state and stimulates thrombus formation. Cerebrovascular events such as thrombosis and hemorrhage are frequently found in autopsy cases (113).
Endothelial activation and vascular inflammation have a negative impact on cerebral blood flow. Vasculitis characterized by subintimal infiltration of cerebral blood vessels by neutrophils causes narrowing of the vascular lumen and vasospasms (112, 113). Release of vasoconstrictive agents, such as the endothelins, and vasodilatory agents, such as nitric oxide, causes loss of autoregulation of cerebral perfusion pressure. In the face of systemic hypotension, these events further decrease cerebral perfusion (121).
The Neuron
In bacterial meningitis, hypoxia, neurotoxic bacterial products, and host mediators combine to cause neuronal injury. Whether the damage is bacterial or leukocyte derived, the final toxic element is often the free radical. These oxidants, including reactive oxygen intermediates and reactive nitrogen intermediates, have direct toxic effects on neurons (53). Activation of apoptotic and necrotic cell death pathways causes neuronal loss that may result in permanent neurologic sequelae or even death (63, 76, 77, 99, 114, 125, 151, 153). Neuronal apoptosis in the granular layer of the hippocampal dentate gyrus and necrosis of pyramidal cells in the hippocampus are frequently found in the brains of patients who died from bacterial meningitis. Studies applying magnetic resonance imaging in survivors of bacterial meningitis often demonstrate hippocampal atrophy (37).
The cascade of pathophysiologic events during bacterial meningitis is summarized in Table 1. Focal damage in other brain regions such as the neocortex and brain stem is less common. Long-term sequelae due to these processes of neuronal death include deafness, intellectual and cognitive impairment ranging from severe intellectual disability to educational deficits and behavioural problems, and less commonly epilepsy, spasticity, or focal neurologic deficits. (9, 12, 15, 24, 44, 140).
TABLE 1.
Phases in the pathophysiology of bacterial meningitis
| Time (h postinfection) | Pathophysiologic events | Clinical symptoms |
|---|---|---|
| 0 | Bacteria and bacterial products accumulate in CSF | None |
| 4 | Release of inflammatory mediators and cytokines | Fever |
| 8-24 | Blood-brain barrier disruption; development of cerebral edema; transendothelial migration of leukocytes; more proinflammatory and toxic mediators; followed by impaired cerebral blood flow, elevated intracranial pressure, and vasculitis | Elevated CSF protein; meningism/neck stiffness; elevated CSF leukocytosis; possible systemic complications; followed by altered mental status, focal symptoms, and seizures |
| 24-48 | Neuronal injury | Focal symptoms; hearing loss; paralysis; cognitive impairment; death |
POTENTIAL INTERVENTIONS
Protect against Injury
Neutralize bacterial toxins.
Clinical outcome in bacterial meningitis is directly related to concentrations of bacteria and bacterial antigens in the CSF (34, 90, 137). Because the CSF contains few humoral and cellular defenses, bacteria multiply freely before being detected, releasing toxic components such as lipopolysaccharide (LPS), peptidoglycan, and teichoic acid, which elicit a strong inflammatory response. Bacterial hemolysins and cytotoxins have direct lethal effects on host tissues. Bacterial lysis associated with killing by bactericidal antibiotics releases a pulse of toxic bacterial components comparable to the Jarisch-Herxheimer phenomenon in syphilis (38, 155). One should be aware, however, that the amount of toxins released from untreated bacteria that continue to grow is considerably greater (38). One strategy to minimize exposure of the brain to bacterial toxins is to minimize the release of toxic bacterial compounds. It is possible to lower the amount of bacterial toxins released by choosing bactericidal antibiotics that act by inhibiting RNA or protein synthesis or DNA replication (rifamycins, macrolides, clindamycin, ketolides, and quinolones) (16, 100). However, assessment of the effectiveness of different antibiotic therapies is challenging, and at present insufficient data are available to recommend a change in empirical antibiotic therapy (67, 126).
Neutralization of proinflammatory bacterial products is a second logical strategy to prevent harmful effects. However, in practice the potential of this approach is limited because no single agent neutralizes both gram-positive and gram-negative bacterial products. Preliminary studies evaluating antibody neutralization of the gram-positive pneumococcal cell wall have not been followed by successful clinical studies (153). Neutralization of the lipid A moiety of LPS in gram-negative bacterial meningitis by intrathecal administation of neutralizing antibodies, polymyxin B, or recombinant fragments of bactericidal/permeability-increasing protein (rBPI) (an endogenous LPS-neutralizing protein) has been studied in several animal models of bacterial meningitis. All these agents had only limited efficacy when administered at clinically relevant timepoints in live-infection models (150; I. Lutsar, I. R. Friedland, H. Jafri, L. Wubbel, W. Ng, F. Ghaffar, and G. H. McCracken, Jr., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr S117-B B-38, 1998). Therefore, this type of adjuvant treatment is likely to fail in clinical practice, most probably because the treatment is given too late following the unleashing of the inflammatory cascade. This conclusion seems to be supported by the disappointing results of clinical trials evaluating LPS neutralization in gram-negative sepsis (43, 79, 174).
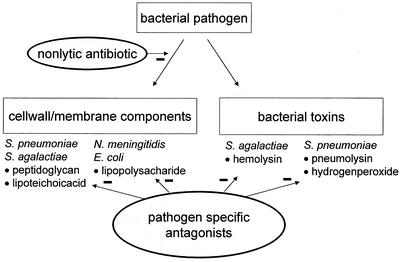
For most pathogens, the bacterial toxins that trigger host cell apoptotic responses have not been identified. Studies with pneumococcus have identified two toxins responsible for permanent loss of neurons in the hippocampus. Pneumolysin, a pore-forming toxin, and hydrogen peroxide produced by the bacterium induce apoptosis by the release and translocation of mitochondrial apoptosis-inducing factor (19). For group B streptococcus, an important pathogen in neonatal meningitis, hemolysin has recently been identified as the toxin that triggers host cell apoptosis (129). Since these toxins do not have structural homology, a strategy to neutralize them has not been forthcoming. Targeting of bacterial toxins is summarized in Fig. 1.
FIG. 1.
Targeting of bacterial cell wall, cell membrane components, and toxins to prevent injury. Use of nonlytic antibiotic agents may minimize the release of toxic components following initiation of antibiotic therapy. Different organisms and different bacterial substances require separate neutralizing antagonists, making a uniform strategy difficult to design.
Reprogram the inflammatory response.
The highly evolved systems of innate and adaptive immunity generally allow the host to detect microbial invaders and to initiate an inflammatory response to dispose of the invaders without destroying host tissues. However, in central nervous system infection, because neuronal cells have little regenerative capacity, damage to host tissues as a side effect of the inflammatory response is more prominent. Experimental work has demonstrated that the presence of dead bacterial material in the CSF elicits such a strong inflammatory response that severe neuronal injury or death may result. Since modern antibiotic therapy ensures rapid killing of invading bacteria in clinical patients, some components of the inflammatory response may no longer serve a critical role in an effective inflammatory response. When making a comparison to warfare, it is like bombing a city and destroying it once the enemy has already been defeated. Obviously this does not mean the whole sophisticated immune system has become useless. Just attenuating or reducing the immune response will delay effective clearance of bacterial debris and tissue healing. However, within the context of modern bactericidal antibiotic therapy, reprogramming the inflammatory response, leaving some helpful components in place and changing other harmful components of the cascade, may improve outcome. What exact balance in the inflammatory response constitutes “good” versus “bad” is hard to predict.
(i) Reset the cytokine balance.
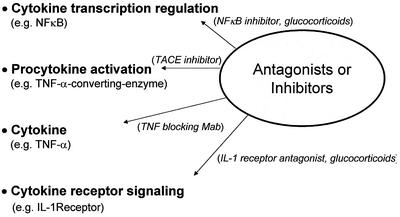
Cytokines are key regulators of the immune response, switching on and off whole cascades of events. These cytokines are released by macrophages, microglia, astrocytes, ependyma, and endothelia. Four major avenues exist to intervene in the inflammatory response at the cytokine level: inhibition of transcription of the cytokine gene, blockage of the cytokine itself or by cleavage to activate the procytokine, and antagonism of the cytokine receptor and its downstream signaling pathways, as summarized in Fig. 2. The maximum benefit of interference with any cytokine can be predicted by studies in knockout mice lacking the specific cytokine gene or transgenic mice displaying altered expression of a certain mediator.
FIG. 2.
Interventions aimed at cytokines may target different levels: regulation of cytokine production via interference with transcriptin factors, interference with procytokine activation by proteolytic enzyme inhibition, direct blocking of the cytokine, and interference with the cytokine receptor or signaling pathway.
Neutralizing cytokine antibodies and endogenous cytokine inhibitors have been tested in animal models as adjuvant agents. TNF-α and IL-1β antagonists (antibodies) reduced meningeal inflammation in most animal models when instilled into the CSF space at the beginning of the infection (128, 135, 149). Endogenous inhibitors of cytokines, such as recombinant IL-1 receptor antagonist and soluble TNF receptor, were effective against cytokine-induced meningitis but failed to have a significant effect against LPS-induced meningitis. This important finding highlights the functional overlap between mediators (104) that frustrates the design of simple therapeutic interventions. This could perhaps have been predicted by results in knockout mouse models. In a live pneumococcal meningitis model without antibiotic therapy, knockout mice deficient in TNF-α (but still expressing TNF-β) did not show any decrease in meningeal inflammation and experienced greater growth of Streptococcus pneumoniae in the bloodstream, yet studies in TNF receptor-deficient mice did show decreased meningeal inflammation but no benefit in terms of reducing neuronal injury (172). These opposite results again demonstrate the functional overlap between different mediators and the probable conclusion that individual targets within the cytokine cascade are not currently a fruitful area of pursuit in the development of adjuvant therapies for meningitis.
An alternative target for cytokine inhibition may be found in enzymes involved in the activation of procytokines. TNF-α is produced as membrane-associated pro-TNF-α and cleaved to its active soluble form by TNF-α-converting enzyme, a metalloproteinase closely related to matrix metalloproteinases. Experiments in the neonatal rat model of bacterial meningitis showed significantly downregulated CSF levels of TNF-α by combined inhibition of matrix metalloproteinases and TNF-α-converting enzyme with a broad-spectrum hydroxamic acid-based metalloproteinase inhibitor (BB-1101). In addition, the incidence of seizures, mortality, and neuronal injury was significantly decreased (71). Current inhibitors of TNF-α-converting enzyme have a low potency, necessitating the use of high doses; however, it is expected that modifications will result in improved agents (10).
Similarly, IL-1β is formed by the cleavage of its precursor pro-IL-1β by caspase-1. Treatment of rabbits and rats with experimental pneumococcal meningitis with the caspase-1 inhibitor z-VAD-fmk (benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) resulted in a reduced inflammatory response, with lower fever, CSF white blood cell counts, and intracranial pressure, fewer cerebrovascular changes, and less neuronal apoptosis. This response is probably affected at least partially by reduced IL-1β activation (64). Caspase-1 is further involved in activation of the apoptotic cell death pathway (17).
Although disruption of the activity of individual cytokines has been disappointing in gaining neurological benefit in meningitis, several avenues have suggested that broader-spectrum interventions have promise. These approaches include enhancing the native anti-inflammatory cascade, inhibiting several cytokines by single agents, and blocking global transcription factors. Anti-inflammatory cytokines such as IL-10 and transforming growth factor beta downregulate the host inflammatory response. Therapeutic administration of IL-10 has been studied in animal models. Early intravenous administration of IL-10 was consistently effective in modulating meningeal inflammation (59, 105), and transforming growth factor beta inhibited cerebrovascular changes and brain edema in bacterial meningitis (115). Since these downregulators have effects on many positive inflammatory cascades, they offer more potential for benefit in a true-life, clinical setting.
This concept can be modeled pharmacologically in the case of thalidomide. In addition to its well-known sedative and teratogenic effects, thalidomide possesses significant and unique anti-inflammatory activities. It inhibits TNF-α and IL-8 production by LPS-stimulated monocytes but does not interfere with the production of IL-6 and IL-1β. Experiments have shown that thalidomide can reduce peak CSF TNF-α levels by 30 to 50% in conjunction with sustained inhibition of CSF pleiocytosis (21). In animal models of tuberculous meningitis, thalidomide greatly enhanced survival and decreased brain pathology (152). However, a clinical trial of thalidomide as an adjuvant treatment of tuberculous meningitis was halted prematurely because of concern about adverse effects. A 6-month outcome analysis of the 47 children included in the study showed a worse outcome for children receiving adjuvant thalidomide therapy (138, 139).
Intervention at the transcription level regulates the expression of many cytokines at once. NF-κB is an important transcription factor, first recognized for its role in the immune system. Various anti-inflammatory drugs including corticosteroids and anti-inflammatory cytokines such as IL-10 inhibit the NF-κB pathway. In resting cells, the NF-κB heterodimer remains in the cytoplasm, where it is associated with inhibitory IκB proteins. Stimuli from outside the cell activate IκB via phosphorylation and induce its proteolytic degradation, leading to translocation of NF-κB to the nucleus, which results in a concerted activation of target genes. Pharmacological manipulation of NF-κB activation was recently investigated in experimental meningitis-associated central nervous system complications and clinical symptoms. Two agents were tested, the NF-κB inhibitor N-acetyl-leucinyl-leucinyl-norleucinial, which interferes with IκB proteolysis, and BAY 11-7085, which inhibits IκB phosphorylation. Both agents improved clinical status and reduced the increase in intracranial pressure, blood-brain barrier permeability, CSF pleiocytosis, and changes in vascular status (58). Importantly, inhibition of NF-κB may protect against neural cell death (136). Given the broad involvement of NF-κB in cell regulation, it is expected that systemic inhibition will also affect nonimmune cells. The present challenge is to examine which components of the NF-κB signaling pathway can be inhibited without causing unacceptable side effects.
It must be appreciated that single mediators often induce many effects and that there is considerable functional overlap between different mediators. This explains why inhibition of one mediator, once the concentration of a second mediator is above a critical level, cannot prevent the progression of inflammation (128). It is therefore unlikely that inhibition of a single proinflammatory mediator will prove useful in clinical practice.
(ii) Noncytokine mediators.
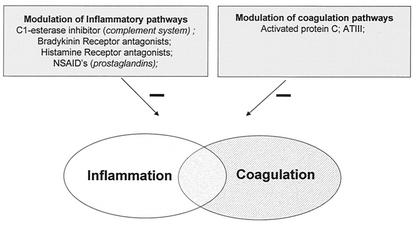
Inflammation activates the coagulation cascade. Not surprisingly, therefore, advanced bacterial meningitis is associated with thrombosis and ischemia. Thus, modulation of inflammatory or coagulation pathways may have a broader impact than suspected from the primary target, as illustrated in Fig. 3. Several clinical trials testing the therapeutic effect of different anticoagulant agents in sepsis have been performed following promising increases in survival in animal studies. One of these agents, recombinant activated protein C, significantly reduced mortality in adult patients with severe sepsis (13, 39). Activated protein C, a component of the natural anticoagulant system, is a potent antithrombotic serine protease with substantial anti-inflammatory properties. However, sepsis trials testing other anticoagulants such as antithrombin III have all failed (168).
FIG. 3.
Interference with inflammatory pathways may target a whole spectrum of noncytokine mediators. Several mediators influence both the inflammatory and coagulation cascades. NSAID's. nonsteroidal anti-inflammatory drugs. ATIII, antithrombin III.
Why activated protein C was effective where antithrombin III failed puzzles many people in the field. Sepsis therapy with protein C is not perfect, however. Because of the anticoagulant activity of activated protein C, the administration of this drug might increase the risk of hemorrhage, and further studies to assess safety in different categories of sepsis patients will be needed. Activated protein C may pose too big a risk in meningitis patients because intracranial bleeding is associated with such poor outcome.
Several plasma enzyme systems are activated during inflammation and have been inhibited in experimental models. These include the complement system (P. G. J. Zwijnenburg, T. van der Toll, C. E. Hack, S. J. H. van Deventer, J. J. Roord, and A.M. van Furth, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr 1600, 1999), the vasoactive nonapeptide bradykinin (82), and the vasoactive amine histamine (170). At present, the results of these studies are inconclusive. Complement inhibition might limit endothelial injury, and results from a clinical trial with C1-esterase inhibitor in sepsis show some improvement in clinical status, but the data are still limited (22, 57). Bradykinin and histamine are likely to be too early in the cascade of mediators in bacterial meningitis to be of clinical significance as therapeutic targets.
During inflammation, cytokines stimulate phospholipase A2, which acts on membrane phospholipids, releasing arachidonic acid and glycerophosphocholine. Arachidonic acid is metabolized to cyclooxygenase products (prostaglandins and thromboxanes) and lipooxygenase products (leukotrienes). Inhibition of the lipooxygenase pathway of arachidonic acid metabolism (leukotrienes) by nordihydroguaiaretic acid did not significantly decrease meningeal inflammation in an animal model of meningitis. In contrast, inhibition of cyclooxygenases (prostaglandins and thromboxanes) by the nonsteroidal anti-inflammatory drug oxindanac was effective and even reduced permanent sequelae and death in rabbits. The cyclooxygenase inhibitors indomethacin and diclofenac sodium also reduced inflammation, but not as effectively (155, 159). Oxindanac was more effective than corticosteroids in experimental meningitis in rabbits; however, to our knowledge, the drug has not been clinically developed, possibly because of the risk of side effects (154).
(iii) Leukocyte attraction, activation, and adhesion.
Should we stop leukocytes? By analogy to other sites of infection, one would intuitively infer a beneficial role for neutrophils. However, early experimental work has suggested detrimental effects of the leukocyte response and inflammatory exudate in the CSF space. Moreover, the absence of humoral immune components in the CSF prevents opsonization of bacteria and subsequent effective phagocytosis and killing by neutrophils. Indeed, in vivo comparison of leukopenic with nonleukopenic rabbits demonstrated that neutrophils fail to stop bacterial multiplication in the CSF (32). The mean duration of survival after intracisternal inoculation of pneumococci was actually longer in leukopenic dogs than in normal nonleukopenic controls (62 versus 47 h) (111). Still, the presence of neutrophils limited the magnitude of bacteremia as a consequence of meningitis, which may have clinical significance, since bacteremia is associated with a worse outcome (34).
A large in vivo study by Giampaolo et al. examining the interrelationships of sequential CSF bacterial titers and leukocyte counts in nonneutropenic rabbits produced somewhat contradictory results (40). In this study, a high CSF leukocyte count on day 1 prior to antibiotic therapy correlated negatively with the bacterial titer in the CSF, suggesting reduction of bacterial multiplication by CSF leukocytes. This study also showed an association of higher CSF leukocyte counts prior to therapy with greater chances of survival. These latter results are similar to the findings in clinical case series, in which bacterial meningitis patients with an initial low CSF leukocyte count, before initiation of antibiotic therapy, generally have a worse outcome than patients with initially higher CSF leukocyte counts (66, 166). However, the study by Giampaolo et al. also showed that continued CSF leukocyte elevation after initiation of antibiotic therapy was associated with a greater chance of death, indicating detrimental effects of CSF pleiocytosis in the context of effective antibiotics.
Previous experimental studies have demonstrated that the number of leukocytes in the meninges immediately adjacent to the cerebral cortex contributes significantly to death (88). Early in vitro studies demonstrated direct cytotoxic effects of leukocytes on cerebral cortex tissue (36). On autopsy, cortical leukocyte infiltration and ventricular empyema are frequently found (113). Since the combined evidence suggests that neutrophils are not very efficient in bacterial phagocytosis and killing in the CSF environment and that persistent CSF pleiocytosis following initiation of antibiotic therapy is associated with a worse outcome, inhibition of leukocyte recruitment into the CSF compartment when initiating antibiotic therapy seemed worthy of exploration. Three avenues to decreasing white blood cell influx into the CSF have been examined: inhibition of attraction, adherence, and activation, as summarized in Table 2.
TABLE 2.
Selected strategies to target leukocyte influx
| Phase of leukocyte influx | Target | Agent class |
|---|---|---|
| Leukocyte attraction (chemotaxis) | Chemokines, e.g., IL-8, MCP-1 | Monoclonal antibodies |
| Leukocyte activation | cAMP production up | A2A adenosine receptor agonist |
| cAMP degradation down | Phosphodiesterase inhibitors, e.g., pentoxifylline | |
| Leukocyte adhesion and migration | Selectin class of adhesins | Carbohydrate antagonist, e.g., fucoidin |
| Integrin class of adhesins | Monoclonal antibodies, peptides |
The process of leukocyte attraction to the site of infection is regulated by chemotactic substances such as complement factor C5a, platelet-activating factor, and chemotactic cytokines called chemokines. The chemokines are subdivided into several families according to structural features related to the relative positions of their cysteine residues. Chemokine receptors belong to the seven-membrane-spanning G-coupled protein receptor family (85). Chemokines are produced at sites of inflammation and are then presented at the luminal side of endothelial cells, sometimes on surface heparan sulfates (92). The CSF of patients with bacterial meningitis contains detectable levels of the C-X-C chemokines (IL-8) and C-C chemokines (monocyte chemotactic protein 1 [MCP-1], macrophage inflammatory proteins MIP-1α and MIP-1β) (144). In experimental models of meningitis, systemic neutralization of IL-8 had a sustained effect, and neutralization of MCP-1 reduced macrophage infiltration, whereas systemic neutralization of MIP-1α or MIP-2 with antibody temporarily reduced neutrophil influx into the CSF. Intrathecal neutralization of IL-8 had no effect on immune cell recruitment (27, 28, 102). Because of multiplicity and overlap between chemokines, it will be difficult to make a good formula to stop attraction of leukocytes.
Processes such as integrin-mediated leukocyte adhesion and release of mediators require leukocyte activation. High levels of the intracellular second-messenger cyclic AMP (cAMP) prevent leukocyte activation. Drugs can influence intracellular cAMP levels either by increasing the synthesis of intracellular cAMP or by preventing the breakdown of cAMP. The level of cAMP can be increased by stimulation of inhibitory leukocyte receptors such as the adenosine receptor A2A. Indeed, treatment with an A2A adenosine receptor agonist (WRC-0470) inhibited pleiocytosis and reduced blood-brain barrier disruption in a rat model of LPS-induced meningitis (146). Degradation of intracellular cAMP can be inhibitid by phosphodiesterase inhibitors, such as the type IV phosphodiesterase inhibitor rolipram. In addition, the phophodiesterase inhibitor pentoxifylline (a methylxanthine derivative) reduced inflammation in the subarachnoid space in several models of bacterial meningitis but had no significant effect on blood-brain barrier permeability or neuronal damage (106, 133, 175). New xanthine derivatives such as lisofylline, which have a similar mode of action, are less toxic, making their clinical use more feasible (165). Newly discovered regulators of neutrophil activation, such as inosine monophosphate, may lead to the development of other effective inhibitors of neutrophil activation (124). Since these drugs will affect leukocytes both in the bloodstream and in the CSF, this seems a promising strategy.
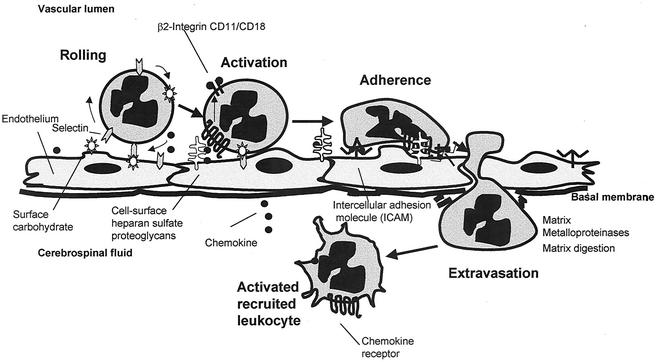
Leukocyte migration into the CSF involves the interaction of leukocytes with the vascular endothelium via several sets of surface adhesion molecules. The process of leukocyte transmigration is illustrated in Fig. 4.
FIG. 4.
Process of neutrophil transendothelial migration. Initially the neutrophil is slowed and rolls down the endothelium, following thethering by an interaction of endothelial E-selectin and P-selectin with the leukocyte CD15 sialyl Lewis-X carbohydrate moieties and an interaction of L-selectin with endothelial cell carbohydrate moieties. Chemokine binding to receptors on leukocytes activates leukocyte adhesins of the integrin family, such as the β2αM integrin CD18/CD11b. Simultaneously, mediators such as IL-8 upregulate the endothelial adhesins of the immunoglobulin superfamily (e.g., intercellular adhesin molecule 1 [ICAM-1]) which bind CD18 on the leukocyte, initiating diapedesis into the cerebrospinal fluid. Matrix metalloproteinases released by the leukocyte digest intercellular tight junctions and the basal membrane during diapedesis.
Selectin-mediated rolling of leukocytes is essential for the transendothelial migration of neutrophils, as elegantly demonstrated in selectin-deficient mice (148). Several proinflammatory mediators, such as histamine, stimulate the upregulation of endothelial selectins and promote rolling of leukocytes. Interference with activation of the histamine (H1) receptor in a rat model of bacterial meningitis temporarily inhibited leukocyte rolling in the early phase of meningitis (170). In addition, several carbohydrate agents prevent the selectin-mediated rolling of leukocytes and inhibit neutrophil migration into the CSF in models of meningitis. These agents include heparin (171), the polysaccharide fucoidin (3, 42), and the polysaccharide glucuronoxylomannan from the capsule of the yeast Cryptococcus neoformans. Glucuronoxylomannan decreased TNF-α levels in the CSF, an effect not studied for the other polysaccharide agents (80). Though rolling is the initial phase of leukocyte transmigration, inhibition of rolling by fucoidin at the time of antibiotic treatment still affected CSF neutrophil numbers, suggesting that treatment with fucoidin may be relevant in the late phase of the disease, which is the important phase clinically (41).
The β2-integrin (CD11a/CD18; CD11b/CD18) and intercellular adhesion molecule-1 (ICAM-1)-dependent phase of leukocyte endothelial transmigration can be inhibited by antibodies or peptides blocking the CD18 complex or ICAM-1 (130, 131, 157, 169). Blockage of CD18-mediated adhesion in an animal model prevented CSF pleiocytosis and protected against enhanced blood-brain barrier permeability and brain edema without changing the efficacy of bacterial killing by ampicillin. Antibodies against leukocyte adhesion molecules have already been used successfully in clinical trials as treatment for primarily lymphocyte-dependent illnesses, including transplant rejection and inflammatory bowel disease (23, 45). An additional target for intervention may be the structural tight junction components. Antibodies against the cerebral endothelial tight junction component junctional adhesion molecule inhibited leukocyte recruitment in a model of cytokine-induced meningitis in mice (26) but not in a model of infectious meningitis. Moreover, in the latter model, complement-mediated endothelial damage induced by the antibody treatment was observed (69). Since the effects of activated leukocytes in the CSF are very wide-ranging, further evaluation of strategies to interfere with leukocyte actions still seems worthwhile.
Conserve cellular function.
(i) Blood-brain barrier and increased intracranial pressure.
In bacterial meningitis, the pathophysiologic changes are much broader than the above-mentioned direct toxic effects of bacteria, release of proinflammatory mediators and activation of leukocytes. As a result of the inflammatory response, many cell types, including endothelium, smooth muscle cells, and neurons, dysfunction and contribute to aggravation of the condition, as summarized in Table 3. This vicious circle poses a major risk for death or neuronal damage. One cause of an unfavorable outcome of bacterial meningitis is cerebral edema and increased intracranial pressure. At present, the only clinical treatments for cerebral edema and elevated intracranial pressure are corticosteroids, infusions with hyperosmolar mannitol solutions, and mechanical hyperventilation. All these treatment strategies are controversial. Although corticosteroids reduce tumor-associated edema, it is unclear whether their beneficial effect in bacterial meningitis is related to edema reduction. Mannitol has been shown to reduce intracranial pressure in experimental models of meningitis and has been used in the clinic (84, 93, 147). However, its use is debated because of possible paradoxical effects (46, 55). Similarly, mechanical hyperventilation will reduce cerebral blood flow and thus intracranial pressure but may inadvertently worsen cerebral ischemia (7).
TABLE 3.
Cellular dysfunction contributes to exacerbation of pathophysiology
| Dysfunctional cell type | Pathophysiologic event | Effect |
|---|---|---|
| Endothelium | Disruption of bood-brain barrier | Vasogenic edema, increased intracranial pressure, compromised cerebral bloodflow, cerebral herniation |
| Procoagulant state | (Micro)thrombi, ischemia | |
| Smooth muscle | Loss of cerebral autoregulation | Cerebral hypo-/hypertension |
| Vasoconstriction | Cerebral ischemia | |
| Neuron | Increased release of excitatory amino acids | Excessive stimulation, metabolic disturbances, cellular edema |
Vasogenic edema is caused by the leakage of plasma proteins into tissue following disruption of the blood-brain barrier. Many of the inflammatory mediators mentioned above contribute to this disruption of the blood-brain barrier. Vasoactive mediators such as vascular endothelial growth factor (VEGF) are released in response to bacterial toxins and proinflammatory mediators (163). VEGF is intrathecally released from invading neutrophils in the CSF in bacterial meningitis (164). VEGF induces the formation of transcellular canals called vesiculo-vacuolo organelles and causes loss of intercellular tight junctions (35, 167). Blocking VEGF reduces cerebral edema in experimental cerebral ischemia, but the role of VEGF in blood-brain barrier disruption in bacterial meningitis awaits further evaluation (162).
Furthermore, degrading enzymes such as the matrix metalloproteinases are implicated in disruption of endothelial tight junctions and the blood-brain barrier during leukocyte diapedesis. Matrix metalloproteinases are a family of zinc-dependent endopeptidases that are released in inactive form and are regulated by endogenous tissue inhibitors of metalloproteinases. Disruption of the blood-brain barrier in experimental meningitis can be modulated by administration of matrix metalloproteinase inhibitors such as batimastat (BB-94) and hydroxamic acid-type inhibitors (GM-6001 and BB-1101) (78, 109). Broad-spectrum hydroxamic acid-based inhibitors simultaneously inhibit proteolytic activation of pro-TNF, thereby inhibiting the progression of inflammation (75).
In addition to vasogenic edema, interstitial edema resulting from decreased CSF outflow and cellular edema due to cellular injury contribute to brain edema. Intracellular calcium is an important mediator of inflammatory cellular responses, and calcium channel antagonists have been demonstrated to modulate the production of cytokines and reactive oxygen intermediates and reduce cellular injury. In an experimental pneumococcal meningitis model, nimodipine, a calcium channel blocker, reduced intracranial pressure. In patients with subarachnoid hemorrhages, nimodipine reduced the proportion of ischemic neurologic deficits and improved overall outcome (33). However, nimodipine failed to protect the brain from ischemic stroke in the clinical setting (47). Because nimodipine also induces cerebral vasodilation, which lowers cerebral perfusion pressure, hypotension and shock are regarded as contraindications for its use. Possible effects on cerebral blood flow in meningitis require further experimental evaluation (108).
(ii) Protecting cerebral blood flow.
In bacterial meningitis, the cerebral blood flow initially increases as a result of vasodilating neuropeptide release and then steadily decreases due to vasoconstriction and the pressure of surrounding cerebral edema (7, 118, 158). In the early phase of pneumococcal meningitis, dilation of pial vessels and increased blood flow are initiated by endogenous vasodilating neuropeptides such as substance P. Treatment with the substance P antagonist spantide reduced pial arteriolar dilatation (120). The vasodilatator effects of substance P seem to be mediated, at least in part, by nitric oxide production. Early pial vasodilatation is associated with stimulation of endothelial inducible nitric oxide synthase and neuronal nitric oxide synthase activity. Nitric oxide freely diffuses into the cytosol and stimulates guanylate cyclase, which transforms guanylate triphosphate into cyclic guanylate monophospate, raising the intracellular cyclic GMP levels and causing relaxation of smooth muscle cells. Nitric oxide antagonists (7-nitroindazole and N-nitro-l-arginine) prevent early pial vasodilatation in experimental models, which suggests that nitric oxide is a potential therapeutic target (107). However, the early timing of inhibitor administration is crucial, because administration of the inhibitor later in the disease process exacerbates hypoperfusion (72, 142).
During bacterial meningitis, cerebral autoregulation is lost, and systemic changes in mean arterial pressure influence cerebral blood flow (95). This loss of cerebral autoregulation is due at least in part to cerebral arteriolar dilatation and can be restored by hyperventilation (94). Decreased blood flow is mediated by vasoconstrictive agents such as endothelins, the levels of which are significantly elevated in the CSF of patients with bacterial meningitis (60). The vasoconstrictive effects of endothelins may contribute to ischemic neuronal injury, because an endothelin antagonist (bosentan) significantly reduced neuronal injury in experimental meningitis. The protective effect of bosetan was independent of an effect on nitric oxide production (121). These results should be confirmed in further experiments, especially because bosetan treatment was associated with a substantial increase in spontaneous deaths, even though the increase in mortality did not reach statistical significance. Studies with endothelin B receptor-deficient rats and autopsy studies of humans and rabbits suffering pneumococcal meningitis indicate that endothelin may also function to increase neuronal survival (29).
Cerebral blood flow can be further compromised by the activation of platelets and the induction of a procoagulant state on the endothelial surface. Cerebrovascular complications and focal ischemia are frequently found on autopsy (113). Decrease in cerebral blood flow is accompanied by a steady increase in intracranial pressure and CSF lactate concentration, which is a sign of deleterious metabolic changes. In clinical practice, fluid restriction in bacterial meningitis patients for fear of of inappropriate antidiuretic syndrome has long been the standard of care. Experimental work on the effects of the hydration status on cerebral blood flow and CSF lactic acidosis has demonstrated better outcome with no fluid restriction (160). This important conclusion is supported by some small clinical studies (122, 143).
(iii) Neurotoxicity and intrinsic cellular death pathways.
Oxidants such as reactive oxygen species and reactive nitrogen intermediates are terminal mediators of brain damage in bacterial meningitis (62). These highly reactive instable molecules are cytotoxic through their inactivation of enzymes, damage to membrane ion transporters, and initiation of lipid peroxidation (76). In addition, they cause damage to DNA, causing single- and double-strand breaks, which activate poly(ADP-ribose) polymerase 1. Excessive activation of poly(ADP-ribose) polymerase 1 causes futile consumption of intracellular energy stores, cellular dysfunction, and eventually necrotic cell death (65).
Experimental and endogenous antioxidants (α-phenyl-tert-butyl, superoxide dismutase, catalase, the 21-aminosteroid U74389F, and uric acid) (54, 73, 81, 83, 116, 117, 119) and several clinically used antioxidants (N-acetylcysteine, desferoxamine, and trylizad mesylate) (8) reduce brain edema, cortical damage, and changes in cerebral blood flow in experimental meningitis. Assessment of the effect on hippocampal neurons produced conflicting results, varying from reduction of apoptosis by α-phenyl-tert-butyl in group B streptococcal meningitis to aggravated apoptosis and learning deficits with the same agent in pneumococcal meningitis (73, 81). Inhibition of poly(ADP-ribose) polymerase 1 by 3-aminobenzamide also reduces central nervous system complications (65). Interference with reactive oxygen species and reactive nitrogen intermediates as terminal mediators of meningitis-associated central nervous system cell damage holds much promise for future therapeutic interventions. When agents prove protective for brain function in clinical trials of cerebral ischemia, they will be excellent candidates as adjunctive therapy in bacterial meningitis.
Inflammation-induced production of excitatory amino acids (glutamate, aspartate) causes neuronal damage due to excessive stimulation. The excitatory amino acids activate cells by opening receptor-operated calcium channels, resulting in elevated intracellular calcium levels and metabolic changes which lead to cellular edema and neuron death. Stimulation of excitatory amino acid receptors (such as the NMDA [N-methyl-d-aspartate] receptors) contributes to inflammation by activation of inflammatory gene expression (50). In the neonatal rat group B streptococcus meningitis model, kynurenic acid, an NMDA receptor antagonist, significantly reduced neuronal injury in the cortex and hippocampus compared to that seen in control animals (74). In an experimental model of pneumococcal meningitis, an NMDA antagonist (HU-211) reduced brain edema and blood-brain barrier impairment even when given together with antibiotics as late as 18 h after infection (11). However, NMDA blockade could not reduce ischemic or traumatic brain damage in several animal models, and depending on the timing of inhibition, NMDA blockage may even increase neuronal cell death (49). Moreover, the results of clinical trials evaluating the supposed protective efficacy in ischemic brain damage have been disappointing (70). Blockade of another glutamate receptor subtype, the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor) to date seems very promising in experimental studies but has not yet been tested in experimental meningitis or clinical trials in other neurologic diseases (49).
Nonpharmacological therapies may also effectively improve the outcome of bacterial meningitis. For example, hypothermia, which is clinically applied in neurosurgery to lower metabolism in the brain and to make the brain less vulnerable to neuronal damage, has been tested in an experimental meningitis model. Hypothermia successfully reduced meningitis-induced changes, particularly the increased intracranial pressure (2). However, the differential effects of hypothermia on outcome demand further investigation. Inflammatory mediators in the CSF trigger the activation of caspases, a specific class of proteases involved in cell survival. Activation of caspases ultimately results in neuronal apoptosis. Bacterial meningitis may cause apoptotic cell death in specific areas of the brain, as evidenced by the presence of apoptotic neurons in the hippocampus at autopsy (101, 176). This neuronal loss may explain some of the learning and memory difficulties seen in survivors of meningitis.
In animal experiments, treatment with the caspase inhibitor z-VAD-fmk greatly reduced the extent of meningitis-associated apoptosis (17). Caspase antagonist therapy still reduced neuronal damage and CSF white blood cell counts when started 8 h postinfection, but since the largest increase in CSF white blood cell counts in a rabbit model of pneumococcal meningitis occurred from 12 h postinfection onward, further evaluation of the window of opportunity is needed (156). Other investigators have shown the efficacy of z-VAD-fmk when administered up to 6 h after infection in a rat model of pneumococcal meningitis (64). The relevance of caspase-1 for both inflammation and apoptosis in bacterial meningitis has been studied in a caspase-1-deficient knockout mouse model of meningitis. However, the results are conflicting (64; M. V. Mering, A. Wellmer, U. Michel, and R. Nau, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr 431, 2000).
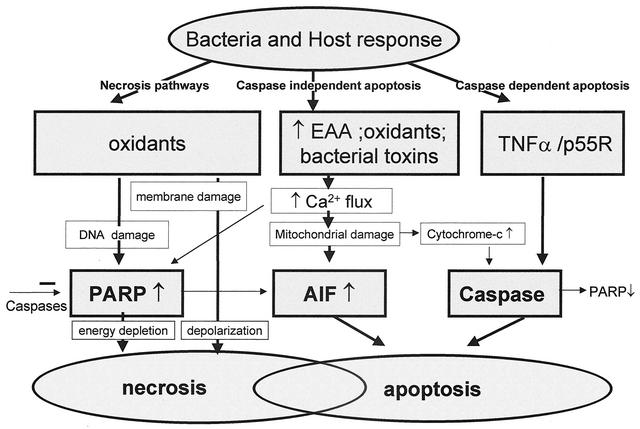
Since neuronal apoptosis is the final common pathway of many damaging factors, the components of the apoptosis pathway are attractive novel targets for adjuvant neuroprotective therapy (20). It must be kept in mind, however, that there are many pathways to apoptosis, both caspase dependent and independent. Thus, the involvement of the individual pathways must be compared in order to choose agents with the most potential benefit. For instance, in pneumococcal infection, an equal contribution of caspase-dependent and -independent pathways suggests that at least two inhibitors would be needed (18). The different neuronal cell death pathways and their interactions are summarized in Fig. 5.
FIG. 5.
Neuronal cell death pathways may be divided into necrotic pathways, caspase-independent apoptotic pathway, and caspase-dependent apoptotic pathway. Bacterial and host oxidants cause damage to cell membranes via lipid peroxidation, leading to loss of membrane integrity and depolarization and finally necrotic cell death. Oxidants also cause DNA damage, resulting in the energy-consuming activation of the poly(ADP-ribose) polymerase (PARP). When DNA repair is futile because of the magnitude of the damage, massive energy depletion will cause necrotic cell death. Oxidants, high concentrations of excitatory amino acids (EAA), and bacterial toxins such as the pneumolysin of S. pneumoniae or the hemolysin of S. agalactiae all cause increased cytosolic free calcium levels. This may result in PARP activation and contribute to necrotic death but primarily causes damage to the mitochondrial outermembranes with PARP-dependent release of apoptosis-inducing factor (AIF). Free cytosolic apoptosis-inducing factor will move into the nucleus and cause chromatin condensation and apoptotic cell death. Release of inflammatory mediators such as TNF-α in response to the invading bacterial pathogens will result in activation of caspases. This results in activation of the apoptotic pathway and will also inhibit necrosis through inactivation of PARP. Release of cytochrome c from mitochondria following mitochondrial damage in response to increased cytosolic free calcium levels also causes activation of caspases and apoptotic death. As indicated in the figure, several crossroads exist between the different death pathways. In many cases, both necrotic and apoptotic cell death may be revealed on histologic examination.
Enhance Repair
Endothelial recovery.
When inflammation subsides, restoration of blood-brain barrier integrity is important because continuing vasogenic edema and leakage of potentially harmful molecules such as excitatory amino acids from the blood into the CSF and cerebral tissue will sustain metabolic disturbances in the brain. The mechanisms responsible for endothelial repair have long remained elusive. Until recently, vascular endothelial growth factor (VEGF), a hypoxia-inducible, angiogenic factor, was the only proven specific and critical factor for endothelial growth and blood vessel formation. The advance in genetic techniques has resulted in the discovery of a whole series of new growth factors acting on the vascular endothelium, including new members of the VEGF family, four members of the angiopoietin family, and at least one member of the ephrin family (173). Furthermore, it has become clear that many other growth factors that are not vascular endothelium specific are important for blood vessel growth and repair, such as transforming growth factor beta.
A challenging new concept in the understanding of blood vessel formation is the recruitment of bone marrow-derived endothelial progenitor cells to sites of angiogenesis (86). The possible role of these cells in repairing damaged and leaky vessels following inflammation and possibly ischemia has to be studied further. Experimental work has demonstrated how angiopoietin 1 administration protects the adult vasculature from damage and leaks induced by inflammation and VEGF. The role of signaling mechanisms from astrocytes or other cells in maintaining and restoring the defined characteristics of the blood-brain barrier are still poorly understood and require further study (48, 110, 132). It is expected that these new insights will result in therapeutics that improve vascular repair, which might limit the development of cerebral edema and cerebrovascular complications in the context of bacterial meningitis.
Neurotrophic factors.
Neurotrophic growth factors may have therapeutic potential to protect brain tissue from damage. For instance, receptors for VEGF are present on neuronal cells. VEGF promotes survival of neuronal cells in culture and stimulates axonal outgrowth and survival of mouse dorsal root ganglions in vivo, but also protects neuronal cells from the effects of hypoxia (51). Many of the neurotrophic growth factors have additional critical roles for many other systems. For instance, VEGF may also assert negative effects, since it may induce vascular permeability and vasogenic edema (162). VEGF can be detected in the CSF of bacterial meningitis patients, suggesting that it may have a biological role in bacterial meningitis (164).
In general, proinflammatory cytokines are neurotoxic. Some anti-inflammatory cytokines, such as transforming growth factor beta, possess neurotrophic functions. Transforming growth factor beta 2 has additional beneficial effects, since it inhibits cerebrovascular changes and brain edema in bacterial meningitis (115).
The functions and therapeutic potential of neurotrophic and neuroprotective factors have drawn much research attention in recent years (1). However, the list of neurotrophic growth factors is long, and it remains an enormous challenge to determine the exact effects and potential of the individual factors and their interactions, and much research is needed before therapeutic applications can be expected.
Neuronal regeneration.
Recently, it has become evident that, even in adults, neurons are continuously born from endogenous stem cells and added to the dentate gyrus throughout life. This process is regulated by several growth factors important in neuronal development, e.g., epidermal growth factor and Sonic hedgehog (68). Hippocampal pyramidal cells can be replaced by endogenous progenitors which migrate into the hippocampus to generate new neurons, although this potential for regeneration declines with age (56, 98, 141). The dentate gyrus and hippocampus are the primary sites of neuron loss in clinical meningitis. In animal experiments, intraventricular infusion of growth factors (e.g., fibroblast growth factor 2 and epidermal growth factor) augments the regenerative response in the hippocampus following ischemia, providing hope for the concept that this endogenous regenerative potential may be applied therapeutically to ameliorate hippocampal atrophy (98). Other animal experiments have shown how an enriched environment providing adequate stimulation improves hippocampal neurogenesis and behavioral performance (56). Potentially, this source of neuronal self-renewal may one day be fully employed. Several strategies to enhance the body's repair mechanisms are summarized in Table 4.
TABLE 4.
Strategies to enhance repair
| Cellular target | Aim | Potential agents |
|---|---|---|
| Endothelium | Restore blood-brain barrier | Endothelial growth factors, e.g., angiopoietin |
| Limit vasogenic edema | ||
| Protect composition of CSF and interstitial fluid in brain | ||
| Neurons | Stimulate recovery | Growth factors, e.g., transforming growth factor beta, VEGF |
| Prevent neuron loss | ||
| Replace dead neurons | Growth factors, e.g., epidermal growth factor, fibroblast growth factor 2 | |
| Stimulate recruitment of endogenous progenitor cells |
FUTURE PROSPECTS
Considering ways to improve outcome in bacterial meningitis, the experience gained in sepsis and stroke research has to be taken into account. The retrospective evaluation of several unsuccessful clinical trials has taught us the value of thorough preclinical evaluation. When all available preclinical data are reexamined, it seems that some of the drugs that failed in clinical trials for stroke or sepsis did not have a reasonable chance to succeed when these trials were initiated. Since well-designed clinical trials to evaluate the effect of adjuvant agents in bacterial meningitis require an enormous effort from many people, it is of utmost importance that drugs proposed for such trials undergo sufficient preclinical evaluation to select the most promising candidates (30). Researchers need to be aware of the limitations of different animal models, and methods to assess data from several animal experiments are available (61, 91, 134). Guidelines of adequate preclinical drug evaluation such as those developed for stroke research are relevant to prevent futile trial efforts in bacterial meningitis, which may damage the execution of more promising future trials (4).
Knowledge of the effects of timing during the course of disease and the optimal dose agents often seems to be crucial to reaching a beneficial effect. As mentioned above, many mediators may be beneficial early in the disease process or at low doses but may have detrimental effects later or in higher doses. For instance, corticosteroids protect clinically against unfavorable outcome when administered early but have no effect when administered later during the course of disease (89, 96, 123). Successes in related conditions, such as adjuvant treatment with activated protein C for severe sepsis, should not be simply assumed to apply to bacterial meningitis as well (13).
Unlike antibiotic therapy, the effectiveness of adjuvant therapy may be very different in sepsis or meningitis. It is important to appreciate the differences in pathology between these different diseases, which are likely to translate to different risk-benefit profiles for individual agents. In the case of activated protein C, the risk of intracranial bleeding in bacterial meningitis may outweigh other beneficial effects, such as a decrease in ischemic events. However, clinical experience with protection against injury or enhancement of cellular repair in sepsis and stroke may help select agents to evaluate for bacterial meningitis. For some substances, such as neuroprotective agents, it is probably best to first await successes in clinical stroke trials, which allow faster patient recruitment.
Based on the compiled experimental evidence, it seems unlikely that inhibition of a single proinflammatory mediator will prove useful in clinical practice. Encouraging results have been found with several avenues that simultaneously reprogram a broader range of mediators. Particularly exciting are the adjustment of cytokine combinations such as effectuated by inhibition of the NF-κB signal pathway and the results with leukocyte activation inhibitors. Equally promising are strategies to interfere with oxidant-mediated damage processes, to inhibit neuronal apoptosis, and to enhance endothelial and neuron repair.
Acknowledgments
This work was supported in part by a stipend from the Medical Branch of the Dutch Sciences Organization (MW-NWO AGIKO, number 920-03-077) to M.v.d.F.. by a grant from NIH R01 AI27913 to E.I.T., and by the American Lebanese-Syrian Associated Charities.
REFERENCES
- 1. Abe, K. 2000. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 20:1393-1408. [DOI] [PubMed] [Google Scholar]
- 2. Angstwurm, K., S. Reuss, D. Freyer, G. Arnold, U. Dirnagl, R. R. Schumann, and J. R. Weber. 2000. Induced hypothermia in experimental pneumococcal meningitis. J. Cereb. Blood Flow Metab. 20:834-838. [DOI] [PubMed] [Google Scholar]
- 3. Angstwurm, K., J. R. Weber, A. Segert, W. Burger, M. Weih, D. Freyer, K. M. Einhaupl, and U. Dirnagl. 1995. Fucoidin, a polysaccharide inhibiting leukocyte rolling, attenuates inflammatory responses in experimental pneumococcal meningitis in rats. Neurosci. Lett. 191:1-4. [DOI] [PubMed] [Google Scholar]
- 4. Anonymous. 1999. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30:2752-2758. [DOI] [PubMed] [Google Scholar]
- 5.Arditi, M., E. O. Mason, J. S. Bradley, T. Q. Tan, W. J. Barson, G. E. Schutze, E. R. Wald, L. B. Givner, K. S. Kim, R. Yogev, and S. L. Kaplan. 1998. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics 102:1087-1097. [DOI] [PubMed] [Google Scholar]
- 6.Aronin, S. I., P. Peduzzi, and V. J. Quagliarello. 1998. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann. Intern. Med. 129:862-869. [DOI] [PubMed] [Google Scholar]
- 7.Ashwal, S., L. Tomasi, S. Schneider, R. Perkin, and J. Thompson. 1992. Bacterial meningitis in children: pathophysiology and treatment. Neurology 42:739-748. [DOI] [PubMed] [Google Scholar]
- 8.Auer, M., L. A. Pfister, D. Leppert, M. G. Tauber, and S. L. Leib. 2000. Effects of clinically used antioxidants in experimental pneumococcal meningitis. J. Infect. Dis. 182:347-350. [DOI] [PubMed] [Google Scholar]
- 9.Baraff, L. J., S. I. Lee, and D. L. Schriger. 1993. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr. Infect. Dis. J. 12:389-394. [DOI] [PubMed] [Google Scholar]
- 10.Barlaam, B., T. G. Bird, C. Lambert-Van Der Brempt, D. Campbell, S. J. Foster, and R. Maciewicz. 1999. New alpha-substituted succinate-based hydroxamic acids as TNFα convertase inhibitors. J. Med. Chem. 42:4890-4908. [DOI] [PubMed] [Google Scholar]
- 11.Bass, R., D. Engelhard, V. Trembovler, and E. Shohami. 1996. A novel nonpsychotropic cannabinoid, HU-211, in the treatment of experimental pneumococcal meningitis. J. Infect. Dis. 173:735-738. [DOI] [PubMed] [Google Scholar]
- 12.Berg, S., B. Trollfors, S. Hugosson, E. Fernell, and E. Svensson. 2002. Long-term follow-up of children with bacterial meningitis with emphasis on behavioural characteristics. Eur. J. Pediatr. 161:330-336. [DOI] [PubMed] [Google Scholar]
- 13.Bernard, G. R., J.-L. Vincent, P.-F. Laterre, S. P. LaRosa, J.-F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, C. J. Fisher, Jr., and the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (POWESS) Study Group. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-707. [DOI] [PubMed] [Google Scholar]
- 14.Berner, R. 2002. Group B streptococci during pregnancy and infancy. Curr. Opin. Infect. Dis. 15:307-313. [DOI] [PubMed] [Google Scholar]
- 15.Bohr, V., O. B. Paulson, and N. Rasmussen. 1984. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch. Neurol. 41:1045-1049. [DOI] [PubMed] [Google Scholar]
- 16.Bottcher, T., J. Gerber, A. Wellmer, A. V. Smirnov, F. Fakhrjanali, E. Mix, J. Pilz, U. K. Zettl, and R. Nau. 2000. Rifampin reduces production of reactive oxygen species of cerebrospinal fluid phagocytes and hippocampal neuronal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 181:2095-2098. [DOI] [PubMed] [Google Scholar]
- 17.Braun, J. S., R. Novak, K. H. Herzog, S. M. Bodner, J. L. Cleveland, and E. I. Tuomanen. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5:298-302. [DOI] [PubMed] [Google Scholar]
- 18.Braun, J. S., R. Novak, P. J. Murray, C. M. Eischen, S. A. Susin, G. Kroemer, A. Halle, J. R. Weber, E. I. Tuomanen, and J. L. Cleveland. 2001. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J. Infect. Dis. 184:1300-1309. [DOI] [PubMed] [Google Scholar]
- 19.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun, J. S., E. I. Tuomanen, and J. L. Cleveland. 1999. Neuroprotection by caspase inhibitors. Expert Opin. Investig. Drugs 8:1599-1610. [DOI] [PubMed] [Google Scholar]
- 21.Burroughs, M. H., L. Tsenova-Berkova, K. Sokol, J. Ossig, E. Tuomanen, and G. Kaplan. 1995. Effect of thalidomide on the inflammatory response in cerebrospinal fluid in experimental bacterial meningitis. Microb. Pathog. 19:245-255. [DOI] [PubMed] [Google Scholar]
- 22.Caliezi, C., S. Zeerleder, M. Redondo, B. Regli, H. U. Rothen, R. Zurcher-Zenklusen, R. Rieben, J. Devay, C. E. Hack, B. Lammle, and W. A. Wuillemin. 2002. C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit. Care Med. 30:1722-1728. [DOI] [PubMed] [Google Scholar]
- 23.Cavazzana-Calvo, M., P. Bordigoni, G. Michel, H. Esperou, G. Souillet, T. Leblanc, J. L. Stephan, J. P. Vannier, F. Mechinaud, J. Reiffers, E. Vilmer, J. Landman-Parker, M. Benkerrou, A. Baruchel, J. Pico, F. Bernaudin, C. Bergeron, E. Plouvier, C. Thomas, J. Wijdenes, B. Lacour, S. Blanche, and A. Fischer. 1996. A phase II trial of partially incompatible bone marrow transplantation for high-risk acute lymphoblastic leukaemia in children: prevention of graft rejection with anti-LFA-1 and anti-CD2 antibodies. Société Française de Greffe de Moelle Osseuse. Br. J. Haematol. 93:131-138. [DOI] [PubMed] [Google Scholar]
- 24.De Beek, D. D., B. Schmand, J. De Gans, M. Weisfelt, H. Vaessen, J. Dankert, and M. Vermeulen. 2002. Cognitive impairment in adults with good recovery after bacterial meningitis. J. Infect. Dis. 186:1047-1052. [DOI] [PubMed] [Google Scholar]
- 25.De Gans, J., and B. D. van de Beek for the European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549-1556. [DOI] [PubMed] [Google Scholar]
- 26.Del Maschio, A., A. De Luigi, I. Martin-Padura, M. Brockhaus, T. Bartfai, P. Fruscella, L. Adorini, G. Martino, R. Furlan, M. G. De Simoni, and E. Dejana. 1999. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (junctional adhesion molecule). J. Exp. Med. 190:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diab, A., H. Abdalla, H. L. Li, F. D. Shi, J. Zhu, B. Hojberg, L. Lindquist, B. Wretlind, M. Bakhiet, and H. Link. 1999. Neutralization of macrophage inflammatory protein-2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect. Immun. 67:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont, R. A., B. D. Car, N. N. Voitenok, U. Junker, B. Moser, O. Zak, and T. O'Reilly. 2000. Systemic neutralization of interleukin-8 markedly reduces neutrophilic pleocytosis during experimental lipopolysaccharide-induced meningitis in rabbits. Infect. Immun. 68:5756-5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrenreich, H., T. R. Nau, C. Dembowski, M. Hasselblatt, M. Barth, A. Hahn, L. Schilling, A.-L. Siren, and W. Bruck. 2000. Endothelin b receptor deficiency is associated with an increased rate of neuronal apoptosis in the dentate gyrus. Neuroscience 95:993-1001. [DOI] [PubMed] [Google Scholar]
- 30.Enting, R. 1997. Dexamethasone for bacterial meningitis: we need the answer. Dutch Bacterial Meningitis Study Group. Lancet 349:1179-1180. [DOI] [PubMed] [Google Scholar]
- 31.Erickson, L., and P. De Wals. 1998. Complications and sequelae of meningococcal disease in Quebec, Canada, 1990-1994. Clin. Infect. Dis. 26:1159-1164. [DOI] [PubMed] [Google Scholar]
- 32.Ernst, J. D., J. M. Decazes, and M. Sande. 1983. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect. Immun. 41:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feigin, V. L., G. J. Rinkel, A. Algra, M. Vermeulen, and J. van Gijn. 1998. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 50:876-883. [DOI] [PubMed] [Google Scholar]
- 34.Feldman, W. E. 1977. Relation of concentrations of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patinets with bacterial meningitis. N. Engl. J Med. 296:433-435. [DOI] [PubMed] [Google Scholar]
- 35.Feng, D., J. A. Nagy, K. Pyne, H. F. Dvorak, and A. M. Dvorak. 1998. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187:903-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fishman, R. A., K. Sligar, and R. B. Hake. 1977. Effects of leukocytes on brain metabolism in granulocytic brain edema. Ann. Neurol. 2:89-94. [Google Scholar]
- 37.Free, S. L., L. M. Li, D. R. Fish, S. D. Shorvon, and J. M. Stevens. 1996. Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 37:400-405. [DOI] [PubMed] [Google Scholar]
- 38.Friedland, I. R., H. Jafari, S. Ehrett, S. Rinderknecht, M. Paris, M. Coulthard, H. Saxen, K. Olsen, and G. H. McCracken. 1993. Comparison of endotoxin release by different antimicrobial agents and the effect on inflammation in experimental Escherichia coli meningitis. J. Infect. Dis. 168:657-662. [DOI] [PubMed] [Google Scholar]
- 39.Garber, K. 2000. Protein C may be sepsis solution. Nat. Biotechnol. 18:917-918. [DOI] [PubMed] [Google Scholar]
- 40.Giampaolo, C., M. Scheld, J. Boyd, J. Savory, M. Sande, and M. Wills. 1981. Leukocyte and bacterial interrelationships in experimental meningitis. Ann. Neurol. 9:328-333. [DOI] [PubMed] [Google Scholar]
- 41.Granert, C., J. Raud, and L. Lindquist. 1998. The polysaccharide fucoidin inhibits the antibiotic-induced inflammatory cascade in experimental pneumococcal meningitis. Clin. Diagn. Lab. Immunol. 5:322-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granert, C., J. Raud, X. Xie, L. Lindquist, and L. Lindbom. 1994. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J. Clin. Investig. 93:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenman, R. L., R. M. Schein, M. A. Martin, R. P. Wenzel, N. R. MacIntyre, G. Emmanuel, H. Chmel, R. B. Kohler, M. McCarthy, and J. Plouffe. 1991. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA 266:1097-1102. [PubMed] [Google Scholar]
- 44.Grimwood, K., P. Anderson, V. Anderson, L. Tan, and T. Nolan. 2000. Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Arch. Dis. Child. 83:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harlan, J. M., and R. K. Winn. 2002. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit. Care Med. 30:S214-S219. [DOI] [PubMed] [Google Scholar]
- 46.Hartwell, R. C., and L. N. Sutton. 1993. Mannitol, intracranial pressure, and vasogenic edema. Neurosurgery 32:444-450. [PubMed] [Google Scholar]
- 47.Horn, J., and M. Limburg. 2001. Calcium antagonists for ischemic stroke: a systematic review. Stroke 32:570-576. [DOI] [PubMed] [Google Scholar]
- 48.Huber, J. D., R. D. Egleton, and T. P. Davis. 2001. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24:719-725. [DOI] [PubMed] [Google Scholar]
- 49.Ikonomidou, C., V. Stefovska, and L. Turski. 2000. Neuronal death enhanced by N-methyl-d-aspartate antagonists. Proc. Natl. Acad. Sci. USA 97:12885-12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jander, S., M. Schroeter, and G. Stoll. 2000. Role of NMDA receptor signaling in the regulation of inflammatory gene expression after focal brain ischemia. J. Neuroimmunol. 109:181-187. [DOI] [PubMed] [Google Scholar]
- 51.Jin, K. L., X. O. Mao, and D. A. Greenberg. 2000. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA 97:10242-10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jodar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 53.Kastenbauer, S., U. Koedel, B. F. Becker, and H. W. Pfister. 2002. Oxidative stress in bacterial meningitis in humans. Neurology 58:186-191. [DOI] [PubMed] [Google Scholar]
- 54.Kastenbauer, S., U. Koedel, and H. W. Pfister. 1999. Role of peroxynitrite as a mediator of pathophysiological alterations in experimental pneumococcal meningitis. J. Infect. Dis. 180:1164-1170. [DOI] [PubMed] [Google Scholar]
- 55.Kaufmann, A. M., and E. R. Cardoso. 1992. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J. Neurosurg. 77:584-589. [DOI] [PubMed] [Google Scholar]
- 56.Kempermann, G., D. Gast, and F. H. Gage. 2002. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52:135-143. [DOI] [PubMed] [Google Scholar]
- 57.Kirschfink, M., and T. E. Mollnes. 2001. C1-inhibitor: an anti-inflammatory reagent with therapeutic potential. Expert Opin. Pharmacother. 2:1073-1083. [DOI] [PubMed] [Google Scholar]
- 58.Koedel, U., I. Bayerlein, R. Paul, B. Sporer, and H. W. Pfister. 2000. Pharmacologic interference with NF-κB activation attenuates central nervous system complications in experimental pneumococcal meningitis. J. Infect. Dis. 182:1437-1445. [DOI] [PubMed] [Google Scholar]
- 59.Koedel, U., A. Bernatowicz, K. Frei, A. Fontana, and H. W. Pfister. 1996. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J. Immunol. 157:5185-5191. [PubMed] [Google Scholar]
- 60.Koedel, U., C. Gorriz, S. Lorenzl, and H. W. Pfister. 1997. Increased endothelin levels in cerebrospinal fluid samples from adults with bacterial meningitis. Clin. Infect. Dis. 25:329-330. [DOI] [PubMed] [Google Scholar]
- 61.Koedel, U., and H. W. Pfister. 1999. Models of experimental bacterial meningitis. Role and limitations. Infect. Dis. Clin. North. Am. 13:549-577, vi. [DOI] [PubMed]
- 62.Koedel, U., and H. W. Pfister. 1999. Oxidative stress in bacterial meningitis. Brain Pathol. 9:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 64.Koedel, U., F. Winkler, B. Angele, A. Fontana, R. A. Flavell, and H. W. Pfister. 2002. Role of caspase-1 in experimental pneumococcal meningitis: evidence from pharmacologic caspase inhibition and caspase-1-deficient mice. Ann. Neurol. 51:319-329. [DOI] [PubMed] [Google Scholar]
- 65.Koedel, U., F. Winkler, B. Angele, A. Fontana, and H. W. Pfister. 2002. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 22:39-49. [DOI] [PubMed] [Google Scholar]
- 66.Kornelisse, R. F., C. M. Westerbeek, A. B. Spoor, B. van der Heijde, L. Spanjaard, H. J. Neijens, and R. de Groot. 1995. Pneumococcal meningitis in children: prognostic indicators and outcome. Clin. Infect. Dis. 21:1390-1397. [DOI] [PubMed] [Google Scholar]
- 67.Krysan, D. J., and A. R. Kemper. 2002. Claims of equivalence in randomized controlled trials of the treatment of bacterial meningitis in children. Pediatr. Infect. Dis. J. 21:753-758. [DOI] [PubMed] [Google Scholar]
- 68.Lai, K., B. K. Kaspar, F. H. Gage, and D. V. Schaffer. 2003. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6:21-27. [DOI] [PubMed] [Google Scholar]
- 69.Lechner, F., U. Sahrbacher, T. Suter, K. Frei, M. Brockhaus, U. Koedel, and A. Fontana. 2000. Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J. Infect. Dis. 182:978-982. [DOI] [PubMed] [Google Scholar]
- 70.Lees, K. R., K. Asplund, A. Carolei, S. M. Davis, H. C. Diener, M. Kaste, J. M. Orgogozo, and J. Whitehead. 2000. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet 355:1949-1954. [DOI] [PubMed] [Google Scholar]
- 71.Leib, S. L., J. M. Clements, R. L. Lindberg, C. Heimgartner, J. M. Loeffler, L. A. Pfister, M. G. Tauber, and D. Leppert. 2001. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734-1742. [DOI] [PubMed] [Google Scholar]
- 72.Leib, S. L., Y. S. Kim, S. M. Black, J. H. Tureen, and M. G. Tauber. 1998. Inducible nitric oxide synthase and the effect of aminoguanidine in experimental neonatal meningitis. J. Infect. Dis. 177:692-700. [DOI] [PubMed] [Google Scholar]
- 73.Leib, S. L., Y. S. Kim, L. L. Chow, R. A. Sheldon, and M. G. Tauber. 1996. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J. Clin. Investig. 98:2632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leib, S. L., Y. S. Kim, D. M. Ferriero, and M. G. Tauber. 1996. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J. Infect. Dis. 173:166-171. [DOI] [PubMed] [Google Scholar]
- 75.Leib, S. L., D. Leppert, J. Clements, and M. G. Tauber. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leib, S. L., and M. G. Tauber. 1999. Pathogenesis of bacterial meningitis. Infect. Dis. Clin. North Am. 13:527-548. [DOI] [PubMed] [Google Scholar]
- 77.Leib, S. L., and M. G. Tauber. 2000. In search of strategies for preventing brain damage as a sequela of bacterial meningitis. Schweiz. Med. Wochenschr. 130:928-935. (In German.) [PubMed]
- 78.Leppert, D., S. L. Leib, C. Grygar, K. M. Miller, U. B. Schaad, and G. A. Hollander. 2000. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 31:80-84. [DOI] [PubMed] [Google Scholar]
- 79.Levin, M., P. A. Quint, B. Goldstein, P. Barton, J. S. Bradley, S. D. Shemie, T. Yeh, S. S. Kim, D. P. Cafaro, P. J. Scannon, and B. P. Giroir. 2000. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet 356:961-967. [DOI] [PubMed] [Google Scholar]
- 80.Lipovsky, M. M., L. Tsenova, F. E. Coenjaerts, G. Kaplan, R. Cherniak, and A. I. Hoepelman. 2000. Cryptococcal glucuronoxylomannan delays translocation of leukocytes across the blood-brain barrier in an animal model of acute bacterial meningitis. J. Neuroimmunol. 111:10-14. [DOI] [PubMed] [Google Scholar]
- 81.Loeffler, J. M., R. Ringer, M. Hablutzel, M. G. Tauber, and S. L. Leib. 2001. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J. Infect. Dis. 183:247-252. [DOI] [PubMed] [Google Scholar]
- 82.Lorenzl, S., U. Kodel, K. Frei, and H. W. Pfister. 1996. Effect of the bradykinin B2 receptor antagonist Hoe140 in experimental pneumococcal meningitis in the rat. Eur. J Pharmacol. 308:335-341. [DOI] [PubMed] [Google Scholar]
- 83.Lorenzl, S., U. Koedel, K. Frei, A. Bernatowicz, A. Fontana, and H. W. Pfister. 1995. Protective effect of a 21-aminosteroid during experimental pneumococcal meningitis. J. Infect. Dis. 172:113-118. (Erratum, J. Infect. Dis. 172:1174.) [DOI] [PubMed]
- 84.Lorenzl, S., U. Koedel, and H. W. Pfister. 1996. Mannitol, but not allopurinol, modulates changes in cerebral blood flow, intracranial pressure, and brain water content during pneumococcal meningitis in the rat. Crit. Care Med. 24:1874-1880. [DOI] [PubMed] [Google Scholar]
- 85.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 86.Luttun, A., G. Carmeliet, and P. Carmeliet. 2002. Vascular progenitors: from biology to treatment. Trends Cardiovasc. Med. 12:88-96. [DOI] [PubMed] [Google Scholar]
- 87.Maiden, M. C., and B. G. Spratt. 1999. Meningococcal conjugate vaccines: new opportunities and new challenges. Lancet 354:615-616. [DOI] [PubMed] [Google Scholar]
- 88.McAllister, C. K., and J. M. O'Donoghue. 1975. Experimental pneumococcal meningitis. II. Characterization and quantificationof the inflammatory process. J. Infect. Dis. 132:355-360. [DOI] [PubMed] [Google Scholar]
- 89.McIntyre, P. B., C. S. Berkey, S. M. King, U. B. Schaad, T. Kilpi, G. Y. Kanra, and C. M. Perez. 1997. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA 278:925-931. [DOI] [PubMed] [Google Scholar]
- 90.Mertsola, J., W. A. Kennedy, D. Waagner, X. Saez-Llorens, K. Olsen, E. J. Hansen, and G. H. McCracken, Jr. 1991. Endotoxin concentrations in cerebrospinal fluid correlate with clinical severity and neurologic outcome of Haemophilus influenzae type B meningitis. Am. J. Dis. Child 145:1099-1103. [DOI] [PubMed] [Google Scholar]
- 91.Michie, H. R. 1998. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J. Antimicrob. Chemother. 41(Suppl. A):47-49. [DOI] [PubMed] [Google Scholar]
- 92.Middleton, J., S. Neil, J. Wintle, I. Clark-Lewis, H. Moore, C. Lam, M. Auer, E. Hub, and A. Rot. 1997. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91:385-395. [DOI] [PubMed] [Google Scholar]
- 93.Minns, R. A., H. M. Engleman, and H. Stirling. 1989. Cerebrospinal fluid pressure in pyogenic meningitis. Arch. Dis. Child. 64:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moller, K., P. Hogh, F. S. Larsen, G. I. Strauss, P. Skinhoj, B. K. Sperling, and G. M. Knudsen. 2000. Regional cerebral blood flow during hyperventilation in patients with acute bacterial meningitis. Clin. Physiol. 20:399-410. [DOI] [PubMed] [Google Scholar]
- 95.Moller, K., F. S. Larsen, J. Qvist, J. H. Wandall, G. M. Knudsen, I. E. Gjorup, and P. Skinhoj. 2000. Dependency of cerebral blood flow on mean arterial pressure in patients with acute bacterial meningitis. Crit. Care Med. 28:1027-1032. [DOI] [PubMed] [Google Scholar]
- 96.Molyneux, E. M., A. L. Walsh, H. Forsyth, M. Tembo, J. Mwenechanya, K. Kayira, L. Bwanaisa, A. Njobvu, S. Rogerson, and G. Malenga. 2002. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 360:211-218. [DOI] [PubMed] [Google Scholar]
- 97.Mustafa, M. M., O. Ramilo, G. A. Syrogiannopoulos, K. D. Olsen, G. H. McCracken, Jr., and E. J. Hansen. 1989. Induction of meningeal inflammation by outer membrane vesicles of Haemophilus influenzae type b. J. Infect. Dis. 159:917-922. [DOI] [PubMed] [Google Scholar]
- 98.Nakatomi, H., T. Kuriu, S. Okabe, S. Yamamoto, O. Hatano, N. Kawahara, A. Tamura, T. Kirino, and M. Nakafuku. 2002. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429-441. [DOI] [PubMed] [Google Scholar]
- 99.Nau, R., and W. Bruck. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 100.Nau, R., and H. Eiffert. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin. Microbiol. Rev. 15:95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nau, R., A. soto, and W. Bruck. 1999. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 58:265-274. [DOI] [PubMed] [Google Scholar]
- 102.Ostergaard, C., R. V. Yieng-Kow, C. G. Larsen, N. Mukaida, K. Matsushima, T. Benfield, N. Frimodt-Moller, F. Espersen, A. Kharazmi, and J. D. Lundgren. 2000. Treatment with a monocolonal antibody to IL-8 attenuates the pleocytosis in experimental pneumococcal meningitis in rabbits when given intravenously, but not intracisternally. Clin. Exp. Immunol. 122:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paoletti, L. C., and L. C. Madoff. 2002. Vaccines to prevent neonatal GBS infection. Semin. Neonatol. 7:315-323. [DOI] [PubMed] [Google Scholar]
- 104.Paris, M. M., I. R. Friedland, S. Ehrett, S. M. Hickey, K. D. Olsen, E. Hansen, E. J. Thonar, and G. H. McCracken, Jr. 1995. Effect of interleukin-1 receptor antagonist and soluble tumor necrosis factor receptor in animal models of infection. J. Infect. Dis. 171:161-169. [DOI] [PubMed] [Google Scholar]
- 105.Paris, M. M., S. M. Hickey, M. Trujillo, A. Ahmed, K. Olsen, and G. H. McCracken, Jr. 1997. The effect of interleukin-10 on meningeal inflammation in experimental bacterial meningitis. J. Infect. Dis. 176:1239-1246. [DOI] [PubMed] [Google Scholar]
- 106.Park, W. S., Y. S. Chang, and M. Lee. 2000. The efficacy of pentoxifylline as an anti-inflammatory agent in experimental Escherichia coli meningitis in the newborn piglet. Biol. Neonate 77:236-242. [DOI] [PubMed] [Google Scholar]
- 107.Paul, R., U. Koedel, and H. W. Pfister. 1997. 7-Nitroindazole inhibits pial arteriolar vasodilation in a rat model of pneumococcal meningitis. J. Cereb. Blood Flow Metab. 17:985-991. [DOI] [PubMed] [Google Scholar]
- 108.Paul, R., U. Koedel, and H. W. Pfister. 2000. Reduction of intracranial pressure by nimodipine in experimental pneumococcal meningitis. Crit. Care Med. 28:2552-2556. [DOI] [PubMed] [Google Scholar]
- 109.Paul, R., S. Lorenzl, U. Koedel, B. Sporer, U. Vogel, M. Frosch, and H. W. Pfister. 1998. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann. Neurol. 44:592-600. [DOI] [PubMed] [Google Scholar]
- 110.Perry, V. H., D. C. Anthony, S. J. Bolton, and H. C. Brown. 1997. The blood-brain barrier and the inflammatory response. Mol. Med. Today 3:335-341. [DOI] [PubMed] [Google Scholar]
- 111.Petersdorf, R. G., and C. N. Luttrell. 1962. Studies on the pathogenesis of meningitis: intrathecal infection. J. Clin. Investig. 41:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pfister, H. W., G. D. Borasio, U. Dirnagl, M. Bauer, and K. M. Einhaupl. 1992. Cerebrovascular complications of bacterial meningitis in adults. Neurology 42:1497-1504. [DOI] [PubMed] [Google Scholar]
- 113.Pfister, H. W., W. Feiden, and K. M. Einhaupl. 1993. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch. Neurol. 50:575-581. [DOI] [PubMed] [Google Scholar]
- 114.Pfister, H. W., A. Fontana, M. G. Tauber, A. Tomasz, and W. M. Scheld. 1994. Mechanisms of brain injury in bacterial meningitis: workshop summary. Clin. Infect. Dis. 19:463-479. [DOI] [PubMed] [Google Scholar]
- 115.Pfister, H. W., K. Frei, B. Ottnad, U. Koedel, A. Tomasz, and A. Fontana. 1992. Transforming growth factor beta 2 inhibits cerebrovascular changes and brain edema formation in the tumor necrosis factor alpha-independent early phase of experimental pneumococcal meningitis. J. Exp. Med. 176:265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfister, H. W., U. Kodel, U. Dirnagl, R. L. Haberl, G. Ruckdeschel, and K. M. Einhaupl. 1992. Effect of catalase on regional cerebral blood flow and brain edema during the early phase of experimental pneumococcal meningitis. J. Infect. Dis. 166:1442-1445. [DOI] [PubMed] [Google Scholar]
- 117.Pfister, H. W., U. Koedel, U. Dirnagl, R. L. Haberl, W. Feiden, and K. M. Einhaupl. 1990. Superoxide dismutase inhibits brain oedema formation in experimental pneumococcal meningitis. Acta Neurochir. Suppl. (Vienna) 51:378-380. [DOI] [PubMed] [Google Scholar]
- 118.Pfister, H. W., U. Koedel, R. L. Haberl, U. Dirnagl, W. Feiden, G. Ruckdeschel, and K. M. Einhaupl. 1990. Microvascular changes during the early phase of experimental bacterial meningitis. J. Cereb. Blood Flow Metab. 10:914-922. [DOI] [PubMed] [Google Scholar]
- 119.Pfister, H. W., U. Koedel, S. Lorenzl, and A. Tomasz. 1992. Antioxidants attenuate microvascular changes in the early phase of experimental pneumococcal meningitis in rats. Stroke 23:1798-1804. [DOI] [PubMed] [Google Scholar]
- 120.Pfister, H. W., T. Kumpfel, and U. Koedel. 1995. Involvement of substance P in pial arteriolar vasodilatation during pneumococcal meningitis in the rat. Neuroreport 6:1301-1305. [DOI] [PubMed] [Google Scholar]
- 121.Pfister, L. A., J. H. Tureen, S. Shaw, S. Christen, D. M. Ferriero, M. G. Tauber, and S. L. Leib. 2000. Endothelin inhibition improves cerebral blood flow and is neuroprotective in pneumococcal meningitis. Ann. Neurol. 47:329-335. [PubMed] [Google Scholar]
- 122.Powell, K. R., L. I. Sugarman, A. E. Eskenazi, K. A. Woodin, M. A. Kays, K. L. McCormick, M. E. Miller, and C. D. Sladek. 1990. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J. Pediatr. 117:515-522. [DOI] [PubMed] [Google Scholar]
- 123.Qazi, S. A., M. A. Khan, N. Mughal, M. Ahmad, B. Joomro, Y. Sakata, N. Kuriya, T. Matsuishi, K. A. Abbas, and F. Yamashita. 1996. Dexamethasone and bacterial meningitis in Pakistan. Arch. Dis. Child. 75:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiu, F. H., K. Wada, G. L. Stahl, and C. N. Serhan. 2000. IMP and AMP deaminase in reperfusion injury down-regulates neutrophil recruitment. Proc. Natl. Acad. Sci. USA 97:4267-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quagliarello, V., and W. M. Scheld. 1992. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N. Engl. J Med. 327:864-872. [DOI] [PubMed] [Google Scholar]
- 126.Quagliarello, V. J., and W. M. Scheld. 1997. Treatment of bacterial meningitis. N. Engl. J. Med. 336:708-716. [DOI] [PubMed] [Google Scholar]
- 127.Radetsky, M. 1992. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr. Infect. Dis. J. 11:694-698. [DOI] [PubMed] [Google Scholar]
- 128.Ramilo, O., X. Saez-Llorens, J. Mertsola, H. Jafari, K. D. Olsen, E. J. Hansen, M. Yoshinaga, S. Ohkawara, H. Nariuchi, and G. H. McCracken, Jr. 1990. Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J. Exp. Med. 172:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ring, A., J. S. Braun, J. Pohl, V. Nizet, W. Stremmel, and J. L. Shenep. 2002. Group B streptococcal beta-hemolysin induces mortality and liver injury in experimental sepsis. J. Infect. Dis. 185:1745-1753. [DOI] [PubMed] [Google Scholar]
- 130.Rozdzinski, E., J. Sandros, M. van der Flier, A. Young, B. Spellerberg, C. Bhattacharyya, J. Straub, G. Musso, S. Putney, R. Starzyk, and E. Tuomanen. 1995. Inhibition of leukocyte-endothelial cell interactions and inflammation by peptides from a bacterial adhesin which mimic coagulation factor X. J. Clin. Investig. 95:1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rozdzinski, E., B. Spellerberg, M. van der Flier, C. Bhattacharyya, A. I. Hoepelman, M. A. Moran, A. Jarpe, S. D. Putney, R. M. Starzyk, and E. Tuomanen. 1995. Peptide from a prokaryotic adhesin blocks leukocyte migration in vitro and in vivo. J. Infect. Dis. 172:785-793. [DOI] [PubMed] [Google Scholar]
- 132.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci 22:11-28. [DOI] [PubMed] [Google Scholar]
- 133.Saez-Llorens, X., O. Ramilo, M. M. Mustafa, J. Mertsola, C. de Alba, E. Hansen, and G. H. McCracken, Jr. 1990. Pentoxifylline modulates meningeal inflammation in experimental bacterial meningitis. Antimicrob. Agents Chemother. 34:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sandercock, P., and I. Roberts. 2002. Systematic reviews of animal experiments. Lancet 360:586.. [DOI] [PubMed] [Google Scholar]
- 135.Saukkonen, K., S. Sande, C. Cioffe, S. Wolpe, B. Sherry, A. Cerami, and E. Tuomanen. 1990. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J. Exp. Med. 171:439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schneider, A., A. Martin-Villalba, F. Weih, J. Vogel, T. Wirth, and M. Schwaninger. 1999. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 5:554-559. [DOI] [PubMed] [Google Scholar]
- 137.Schneider, O., U. Michel, G. Zysk, O. Dubuis, and R. Nau. 1999. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53:1584-1587. [DOI] [PubMed] [Google Scholar]
- 138.Schoeman, J. F. 2000. Thalidomide therapy in childhood tuberculous menigitis. J. Child. Neurol. 15:838.. [DOI] [PubMed] [Google Scholar]
- 139.Schoeman, J. F., P. Springer, A. Ravenscroft, P. R. Donald, L. G. Bekker, A. J. van Rensburg, W. A. Hanekom, P. A. Haslett, and G. Kaplan. 2000. Adjunctive thalidomide therapy of childhood tuberculous meningitis: possible anti-inflammatory role. J. Child. Neurol. 15:497-503. [DOI] [PubMed] [Google Scholar]
- 140.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins. 1997. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 141.Seaberg, R. M., and D. van der Kooij. 2002. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 22:1784-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shenep, J. L., and E. Tuomanen. 1998. Perspective: targeting nitric oxide in the adjuvant therapy of sepsis and meningitis. J. Infect. Dis. 177:766-769. [DOI] [PubMed] [Google Scholar]
- 143.Singhi, S. C., P. D. Singhi, B. Srinivas, H. P. Narakesri, N. K. Ganguli, R. Sialy, and B. N. Walia. 1995. Fluid restriction does not improve the outcome of acute meningitis. Pediatr. Infect. Dis. J. 14:495-503. [DOI] [PubMed] [Google Scholar]
- 144.Spanaus, K. S., D. Nadal, H. W. Pfister, J. Seebach, U. Widmer, K. Frei, S. Gloor, and A. Fontana. 1997. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J. Immunol. 158:1956-1964. [PubMed] [Google Scholar]
- 145.Spratt, B. G., and B. M. Greenwood. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 356:1210-1211. [DOI] [PubMed] [Google Scholar]
- 146.Sullivan, G. W., J. Linden, B. L. Buster, and W. M. Scheld. 1999. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J. Infect. Dis. 180:1550-1560. [DOI] [PubMed] [Google Scholar]
- 147.Syrogiannopoulos, G. A., K. D. Olsen, and G. H. McCracken, Jr. 1987. Mannitol treatment in experimental Haemophilus influenzae type b meningitis. Pediatr Res. 22:118-122. [DOI] [PubMed] [Google Scholar]
- 148.Tang, T., P. S. Frenette, R. O. Hynes, D. D. Wagner, and T. N. Mayadas. 1996. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J. Clin. Investig. 97:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tauber, M. G., and B. Moser. 1999. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin. Infect. Dis. 28:1-11. [DOI] [PubMed] [Google Scholar]
- 150.Tauber, M. G., A. M. Shibl, C. J. Hackbarth, J. W. Larrick, and M. Sande. 1987. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J. Infect. Dis. 156:456-462. [DOI] [PubMed] [Google Scholar]
- 151.Townsend, G. C., and W. M. Scheld. 1993. Adjunctive therapy for bacterial meningitis: rationale for use, current status, and prospects for the future. Clin. Infect. Dis. 17(Suppl. 2):S537-S549. [DOI] [PubMed] [Google Scholar]
- 152.Tsenova, L., K. Sokol, V. H. Freedman, and G. Kaplan. 1998. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J. Infect. Dis. 177:1563-1572. [DOI] [PubMed] [Google Scholar]
- 153.Tuomanen, E. 1988. Partner drugs: a new outlook for bacterial meningitis. Ann. Intern. Med. 109:690-692. [DOI] [PubMed] [Google Scholar]
- 154.Tuomanen, E. 1993. Breaching the blood-brain barrier. Sci. Am. 268:80-84. [DOI] [PubMed] [Google Scholar]
- 155.Tuomanen, E., B. Hengstler, R. Rich, M. A. Bray, O. Zak, and A. Tomasz. 1987. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J. Infect. Dis. 155:985-990. [DOI] [PubMed] [Google Scholar]
- 156.Tuomanen, E., B. Hengstler, O. Zak, and A. Tomasz. 1986. The role of complement in inflammation during experimental pneumococcal meningitis. Microb. Pathog. 1:15-32. [DOI] [PubMed] [Google Scholar]
- 157.Tuomanen, E. I., K. Saukkonen, S. Sande, C. Cioffe, and S. D. Wright. 1989. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tureen, J., Q. Liu, and L. Chow. 1996. Near-infrared spectroscopy in experimental pneumococcal meningitis in the rabbit: cerebral hemodynamics and metabolism. Pediatr. Res. 40:759-763. [DOI] [PubMed] [Google Scholar]
- 159.Tureen, J. H., M. G. Tauber, and M. Sande. 1991. Effect of indomethacin on the pathophysiology of experimental meningitis in rabbits. J. Infect. Dis. 163:647-649. [DOI] [PubMed] [Google Scholar]
- 160.Tureen, J. H., M. G. Tauber, and M. Sande. 1992. Effect of hydration status on cerebral blood flow and cerebrospinal fluid lactic acidosis in rabbits with experimental meningitis. J. Clin. Investig. 89:947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Alphen, L., L. Spanjaard, E. A. van der, I. Schuurman, and J. Dankert. 1997. Effect of nationwide vaccination of 3-month-old infants in The Netherlands with conjugate Haemophilus influenzae type b vaccine: high efficacy and lack of herd immunity. J. Pediatr. 131:869-873. [DOI] [PubMed] [Google Scholar]
- 162.van Bruggen, N., H. Thibodeaux, J. T. Palmer, W. P. Lee, L. Fu, B. Cairns, D. Tumas, R. Gerlai, S. P. Williams, C. M. van Lookeren, and N. Ferrara. 1999. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J. Clin. Investig. 104:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van der Flier, M., F. Coenjaerts, J. L. Kimpen, A. M. Hoepelman, and S. P. Geelen. 2000. Streptococcus pneumoniae induces secretion of vascular endothelial growth factor by human neutrophils. Infect. Immun. 68:4792-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van der Flier, M., G. Stockhammer, G. J. Vonk, P. G. Nikkels, R. A. Diemen-Steenvoorde, G. J. Der Vlist, S. W. Rupert, E. Schmutzhard, E. Gunsilius, G. Gastl, A. I. Hoepelman, J. L. Kimpen, and S. P. Geelen. 2001. Vascular endothelial growth factor in bacterial meningitis: detection in cerebrospinal fluid and localization in postmortem brain. J. Infect. Dis. 183:149-153. [DOI] [PubMed] [Google Scholar]
- 165.van Furth, A. M., J. J. Roord, and R. van Furth. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect. Immun. 64:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang, V. J., R. Malley, G. R. Fleisher, S. H. Inkelis, and N. Kuppermann. 2000. Antibiotic treatment of children with unsuspected meningococcal disease. Arch. Pediatr. Adolesc. Med. 154:556-560. [DOI] [PubMed] [Google Scholar]
- 167.Wang, W., W. L. Dentler, and R. T. Borchardt. 2001. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am. J. Physiol. Heart Circ. Physiol. 280:H434-H440. [DOI] [PubMed] [Google Scholar]
- 168.Warren, B. L., A. Eid, P. Singer, S. S. Pillay, P. Carl, I. Novak, P. Chalupa, A. Atherstone, I. Penzes, A. Kubler, S. Knaub, H. O. Keinecke, H. Heinrichs, F. Schindel, M. Juers, R. C. Bone, and S. M. Opal. 2001. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286:1869-1878. [DOI] [PubMed] [Google Scholar]
- 169.Weber, J. R., K. Angstwurm, W. Burger, K. M. Einhaupl, and U. Dirnagl. 1995. Anti ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J. Neuroimmunol. 63:63-68. [DOI] [PubMed] [Google Scholar]
- 170.Weber, J. R., K. Angstwurm, T. Rosenkranz, U. Lindauer, W. Burger, K. M. Einhaupl, and U. Dirnagl. 1997. Histamine (H1) receptor antagonist inhibits leukocyte rolling in pial vessels in the early phase of bacterial meningitis in rats. Neurosci. Lett. 226:17-20. [DOI] [PubMed] [Google Scholar]
- 171.Weber, J. R., K. Angstwurm, T. Rosenkranz, U. Lindauer, D. Freyer, W. Burger, C. Busch, K. M. Einhaupl, and U. Dirnagl. 1997. Heparin inhibits leukocyte rolling in pial vessels and attenuates inflammatory changes in a rat model of experimental bacterial meningitis. J. Cereb. Blood Flow Metab. 17:1221-1229. [DOI] [PubMed] [Google Scholar]
- 172.Wellmer, A., J. Gerber, J. Ragheb, G. Zysk, T. Kunst, A. Smirnov, W. Bruck, and R. Nau. 2001. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 69:6881-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]
- 174.Ziegler, E. J., C. J. Fisher, Jr., C. L. Sprung, R. C. Straube, J. C. Sadoff, G. E. Foulke, C. H. Wortel, M. P. Fink, R. P. Dellinger, and N. N. Teng. 1991. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N. Engl. J. Med. 324:429-436. [DOI] [PubMed] [Google Scholar]
- 175.Zysk, G., W. Bruck, F. R. Fischer, M. Mader, P. Rieckmann, and R. Nau. 1997. Limited efficacy of pentoxifylline as anti-inflammatory agent in experimental pneumococcal meningitis. Clin. Exp. Immunol. 107:458-461. [DOI] [PubMed] [Google Scholar]
- 176.Zysk, G., W. Bruck, J. Gerber, Y. Bruck, H. W. Prange, and R. Nau. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 55:722-728. [DOI] [PubMed] [Google Scholar]