Abstract
We have found that NS1 serotype-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) can be used to differentiate primary and secondary dengue virus infections. This is due to the fact that the NS1-specific IgG antibody cannot be detected before day 9 of illness for primary infection, so the NS1-specific IgG antibodies measured in acute-phase sera must come from previous infection. Comparison of NS1 serotype-specific IgG ELISA with envelope- and membrane-specific capture IgM and IgG ELISA in the differentiation of primary and secondary dengue virus infections showed good correlation (95.90% agreement). Most important, we have found that the serotype of the dengue virus from the majority of patients with primary infection could be correctly identified when convalescent-phase or postinfection sera were analyzed by NS1 serotype-specific IgG ELISA. These findings suggested that NS1 serotype-specific IgG ELISA could be reliably applied for serodiagnosis and seroepidemiological study of dengue virus infection.
Dengue virus is a mosquito-borne flavivirus and the most prevalent arbovirus in tropical and subtropical regions of Asia, Africa, and Central and South America (8). It causes a spectrum of illness, ranging from unapparent infection to mild undifferentiated fever, classical dengue fever, and a more severe form, dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), with high morbidity and mortality. There are four distinct serotypes, DEN-1, DEN-2, DEN-3, and DEN-4. Infection induces a lifelong protective immunity to the homologous serotype, but there is no cross-reactive immunity to the heterologous serotypes. Instead, it has been generally accepted that secondary or multiple dengue virus infection is a major risk factor for DHF/DSS, in addition to other factors, such as viral virulence and host genetic background (2, 7, 9, 15). Therefore, differentiation of primary versus secondary or multiple dengue virus infection is critical in analyzing data for epidemiological, pathological, clinical, and immunological studies.
Until recently, the most commonly used serological techniques for the routine diagnosis of dengue virus infection have been the hemagglutination inhibition (HI) test (4) and capture immunoglobulin M (IgM) and/or IgG enzyme-linked immunosorbent assay (ELISA) (6, 10, 14, 19). Traditionally, the HI test was used to differentiate primary and secondary dengue virus infections due to its simplicity, sensitivity, and reproducibility. The patient sera were classified as showing secondary dengue virus infection when the HI titer was greater than or equal to 1:2,560 and as showing primary dengue virus infection when the titer was lower (19). However, many investigators have raised doubts concerning the general applicability of using the HI titer to classify primary versus secondary infections in regions where two or more flaviviruses are cocirculating, since the IgG antibodies measured are broadly flavivirus reactive (14, 19). Innis et al. (10) first proposed that primary or secondary infection be classified by determining the ratio of dengue virus IgM antibodies to IgG antibodies. They showed that acute-phase sera from patients with primary dengue virus infections had higher IgM/IgG ratios, whereas sera from patients with secondary infections had lower IgM/IgG ratios.
We have recently developed an NS1 isotype- and serotype-specific ELISA for the serodiagnosis and seroepidemiological studies of flavivirus infection (16, 17, 18). Our main goals are to set up an ELISA system that can be easily and reliably used to differentiate (i) Japanese encephalitis (JE) virus and dengue virus infections, (ii) JE vaccination and JE virus infection, (iii) primary and secondary dengue virus infections, and (iv) the serotype of dengue virus infection. Investigation of the NS1-specific antibody response to JE virus showed that NS1-specific IgM and IgA antibodies from JE patients did not cross-react with dengue virus NS1 glycoprotein, while IgG antibodies from ∼10% of these patients showed significant cross-reactivity. However, careful analysis suggested that the cross-reactive IgG antibodies to dengue virus NS1 antigen found in a few JE patients were largely related to previous infection with a heterologous flavivirus, most likely dengue virus (17). Preliminary study on 30 convalescent-phase sera from both dengue fever and DHF patients showed that the dengue virus serotypes could be correctly identified for 75 to 80% of primary versus 20 to 30% of secondary dengue virus infections when the HI titer was used to define primary and secondary infections (16). More recently, a retrospective seroepidemiological study on serum samples collected from Liuchiu Hsiang, Pingtung County, in southern Taiwan demonstrated that NS1 serotype-specific IgG ELISA could replace the plaque reduction neutralization test for seroepidemiological study to differentiate JE and dengue virus infections and for the dengue virus serotyping of primary infection (18).
In this study, we report the comparison of a modified capture IgM and IgG ELISA and NS1 serotype-specific IgG ELISA in the detection and differentiation of primary and secondary infections. A total of 244 acute- and convalescent-phase sera collected from 194 confirmed dengue patients between days 4 and 45 after the onset of symptoms, covering all four serotypes, were analyzed. Good correlation was found between these two assays. However, the NS1 serotype-specific IgG ELISA has an additional advantage, since the dengue virus serotypes from the majority of patients with primary infection could be correctly identified when convalescent-phase or postinfection sera were analyzed.
MATERIALS AND METHODS
Human serum samples.
The serum samples used in this study were collected from patients with confirmed cases of dengue reported to Center for Disease Control, Department of Health, during 1998 to 2001. Dengue virus infections were defined as febrile illness associated with the isolation of dengue virus, a positive reverse transcriptase PCR test (13), or the detection of dengue virus-specific IgM and IgG antibodies (capture IgM and IgG ELISA). Serum samples collected between days 3 and 30 after the onset of symptoms from DEN-2 outbreaks in Kaohsiung city and DEN-3 outbreaks in Tainan city in 1998 were used for study of the correlation between the HI test and capture IgM and IgG ELISA (IgM/IgG ratio). For comparison of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA, 244 acute- and convalescent-phase sera collected from 194 confirmed dengue patients (50 patients had paired serum samples) between days 4 and 45 after the onset of symptoms during dengue outbreaks, covering all four serotypes, 1998 to 2001 were analyzed. Sera collected during days 1 to 7 after the onset of symptoms are referred to as acute-phase samples. Early- and late-convalescent-phase sera refer to specimens collected during days 8 to 13 and days 14 to 45, respectively. Postinfection sera refer to specimens collected more than 45 days after the onset of symptoms.
Cell culture and antigen preparation.
Vero cells (8 × 106) were grown in 15 ml of RPMI 1640 medium (Gibco, Grand Island, N.Y.) containing 5% fetal calf serum in T-75 flasks. After 1 day of incubation in 5% CO2 at 37°C, a monolayer was formed and infected with one of four dengue virus serotypes of local Taiwan strains, i.e., DEN-1 (strain 8700828), DEN-2 (454009), DEN-3 (8700829), and DEN-4 (8700544), or with a JE virus vaccine strain (Beijing) at a multiplicity of infection of 1. Virus culture supernatants were harvested 2 to 4 days later, inactivated by UV irradiation, aliquoted, and stored at −80°C until use. The culture supernatants were used as the source of viral envelope and membrane (E/M) and NS1 antigens for ELISA. The control antigen was prepared by the same procedure from Vero cells cultured without viral infection.
MAb production and purification.
D2/8-1 is an NS1-specific monoclonal antibody (MAb) that was generated and characterized as previously described (3). D56-3 is an envelope-specific MAb with flavivirus group specificity. The ascitic fluids were generated from Pristane (2,6,10,14-tetramethylpentadecane)-primed BALB/c mice injected intraperitoneally with 5 × 106 cells and harvested after 7 to 14 days. MAbs were purified from ascitic fluid by protein A-Sepharose 4B Fast Flow affinity chromatography (Pharmacia Biotech) as described previously (5).
HI tests.
The HI test was performed as described by Clarke and Casals (4) and was modified for microtiter plates. DEN-1 (Hawaii strain) and JE (JaGAr strain) viral antigens were prepared from infected suckling mouse brains by sucrose acetone extraction and used for the measurement of HI antibodies to dengue and JE viruses, respectively. Results are expressed as the reciprocal of the dilution.
ELISA.
(i) NS1 serotype-specific IgG ELISA.
NS1 serotype-specific IgG ELISA was performed as previously described (16). Briefly, each microtiter eight-well strip (Nunc-Immuno; Nunc A/S, Roskilde, Denmark) was coated with MAb D2/8-1 (5 μg/ml; 100 μl/well) in 0.1 M carbonate buffer (Na2CO3-NaHCO3, pH 9.5). After washing with phosphate-buffered saline-0.05% Tween 20 (PBST), wells were blocked with 200 μl of phosphate-buffered saline-1% bovine serum albumin (BSA) for 1 h at 37°C. After washing, the wells were incubated with 1:3-diluted NS1-containing culture supernatants of DEN-1, DEN-2, DEN-3, DEN-4, or JE virus-infected Vero cells in PBST-1% BSA-5% normal rabbit serum for 1 h at 37°C. After washing, serum samples were added at a 1:50 dilution and incubated for 1 h at 37°C. Goat anti-human IgG conjugated to alkaline phosphatase (Jackson Immunochemicals, West Grove, Pa.) was then added and incubated for 1 h at 37°C. Finally, the enzyme activity was developed with the addition of the substrate p-nitrophenylphosphate (Chemicon, Temecula, Calif.), and the optical density (OD) was determined 30 min later at the dual wavelengths of 405 and 630 nm with a Dynatech MR700 microplate reader.
(ii) Capture IgM and IgG ELISA.
A modified capture IgM and IgG ELISA was performed to measure the IgM and IgG antibodies in patients infected with dengue virus as previously described by Innis et al. (10). Major modifications included using (i) mixed viral antigens of four dengue virus serotypes from culture supernatants harvested from cells infected with each of the four dengue virus serotypes, (ii) unlabeled flavivirus-specific MAb, (iii) mixed viral antigens and MAb in a single step, (iv) alkaline phosphatase-conjugated goat anti-mouse IgG (γ chain specific) as the tracer and p-nitrophenylphosphate as the substrate, and (v) OD readings directly at the dual wavelengths of 405 and 630 nm without stopping the color-developing reaction. Most importantly, primary or secondary dengue virus infection was defined by the ratio of the IgM to IgG readings (≧1.2 or <1.2, respectively) directly without calculating the antibody unit through a standard control. Briefly, each microtiter eight-well strip was coated overnight at 4°C with affinity-purified goat anti-human IgM (μ chain specific) or IgG (γ chain specific) antibodies (Jackson Immunochemicals) at 5 μg/ml (100 μl/well) in 0.1 M carbonate buffer (Na2CO3/NaHCO3, pH 9.5). After washing and blocking, the wells were incubated with 100 μl of 1:100-diluted serum in PBST-1% BSA-5% normal rabbit serum for 1 h at 37°C. After washing, the wells were incubated with 100 μl of a cocktail containing 1:3-diluted pooled virus antigens from culture supernatants of DEN-1-, DEN-2-, DEN-3-, or DEN-4-infected Vero cells and 1 μg of MAb D56.3 per ml for 1 h at 37°C. After washing, the wells were incubated with 1:1,000-diluted alkaline phosphatase-conjugated goat anti-mouse IgG (γ chain specific) (Zymed Laboratories, Inc., San Francisco, Calif.) and incubated for 1 h at 37°C. The enzyme activity was developed, and the OD was measured 30 min later.
For accuracy, we always run capture IgM and IgG ELISA on the same 96-well plate, using an 8-well strip format coated with affinity-purified goat anti-human IgM (μ-chain-specific) or IgG (γ-chain-specific) antibodies. In addition, each plate has appropriate control sera to verify the sensitivity and specificity of the test results. For example, a primary dengue control serum would give ODs of 2.5 for IgM and 1.5 for IgG, whereas a secondary dengue control serum would give ODs of 1.5 for IgM and 2.5 for IgG.
Data analysis.
The ODs read from culture supernatants of Vero cells with or without dengue virus infection were designated the test absorbance and negative control value for each sample in the ELISA. Positivity was determined by comparison to individual negative controls. A positive sample was defined as having a test absorbance/negative control ratio of ≧2.0, and a negative sample was defined as having a ratio of <2.0. For those sera with positive NS1-specific IgG antibody responses, NS1 serotyping was determined by the ratio of the highest OD value to the second highest OD value read from the four dengue virus serotypes. Positive serotype specificity is defined if the OD ratio is ≧1.2, and negative serotype specificity is defined if the OD ratio is <1.2. Based on the NS1 serotype-specific IgG ELISA, primary dengue virus infection was defined if (i) a negative NS1-specific IgG antibody response was found for sera collected between days 1 and 14 of illness or (ii) a positive serotype specificity was found for sera collected after ≧9 days of illness. Secondary dengue virus infection was defined if (i) a positive NS1-specific IgG antibody response was found for sera collected between days 1 and 8 of illness or (ii) a positive NS1-specific IgG antibody response and negative serotype specificity was found at any time after the onset of symptoms.
RESULTS
Comparison of modified capture IgM and IgG ELISA and HI in the differentiation of primary and secondary dengue virus infections.
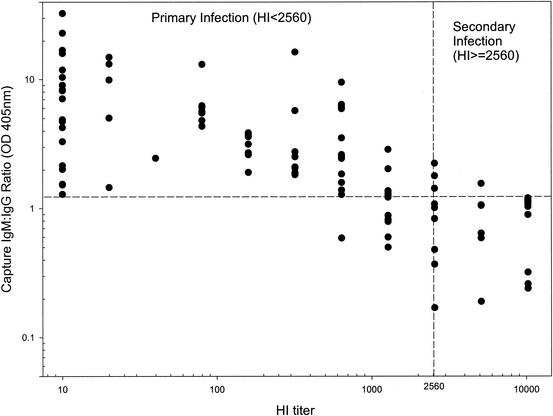
In an attempt to set up a simple and reliable assay to detect and differentiate primary and secondary dengue virus infections, we have modified the capture IgM and IgG ELISA originally reported by Innis et al. (10) in order to improve the sensitivity and specificity. One of the major modifications we have made is that primary or secondary dengue virus infection was defined by the ratio of IgM to IgG readings directly without calculating the antibody unit through a standard control. To ascertain its usefulness, a pilot study was initiated to evaluate the correlation between capture IgM/IgG ratio and HI and to determine the cutoff value suitable for the discrimination of primary and secondary infections. Figure 1 shows the correlation plot for 103 sera from dengue patients with primary or secondary infection collected between days 3 and 30 of illness. Based on the results, dengue virus infection was defined as primary if the capture IgM/IgG ratio was ≧1.2 or as secondary if the ratio was <1.2. The results showed good correlation between these two assays, with 89.4% agreement (92 sera) (κ = 0.727). Among the 11 discordant sera, 8 sera were found to have HI titers in the range of 1:1,280 to 1:2,560. When paired sera were analyzed in parallel, the results were in favor of capture IgM/IgG ratio. We have found that three variables, i.e., the virus strain (the DEN-1 serotype was used for the HI test), the time of serum collection, and the flavivirus cross-reactive IgG antibodies, influenced the HI titer much more than the capture IgM/IgG ratio (data not shown). Accordingly, we replaced the HI test with capture IgM and IgG ELISA in our later routine serodiagnosis of flavivirus infection.
FIG. 1.
Comparison of HI test and capture IgM/IgG ratio. A total of 103 serum samples collected between days 3 and 30 after the onset of symptoms were analyzed for HI and capture IgM/IgG ratio. Good correlation was found, with a result concordance of 89.4%.
Comparisons between capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA in serodiagnosis and differentiation of primary and secondary dengue virus infections.
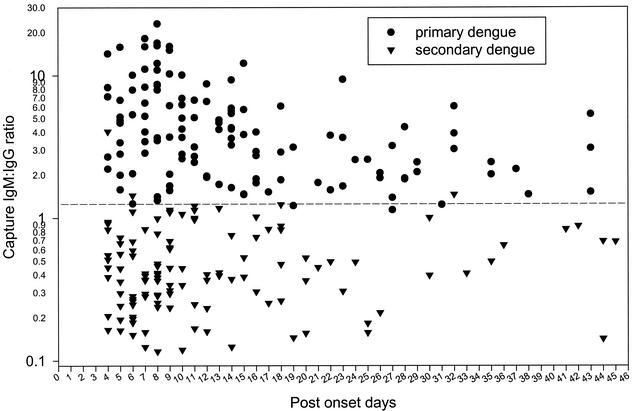
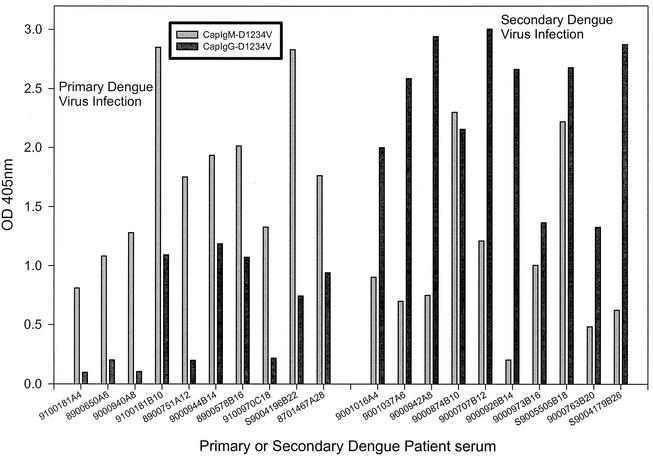
To further evaluate the usefulness of the capture IgM and IgG ELISA and compare it with NS1 serotype-specific IgG ELISA in serodiagnosis and differentiation of primary and secondary dengue virus infections, we analyzed 244 acute- and convalescent-phase sera collected from 194 confirmed dengue patients (50 patients had paired serum samples), between days 4 and 45 of illness, covering all four serotypes. Table 1 shows a summary of the correlation between capture IgM/IgG ratio and NS1-specific IgG ELISA from all 244 sera tested in this study. For NS1 serotype-specific IgG ELISA, primary dengue virus infection was defined if (i) a negative NS1-specific IgG antibody response was found for sera collected between days 1 and 14 of illness or (ii) a positive serotype specificity (the test absorbance ratio of the highest OD value to the second-highest OD value was ≧1.2) was found for sera collected ≧9 days of illness. Secondary dengue virus infection was defined if (i) a positive NS1-specific IgG antibody response was found for sera collected between days 1 and 8 of illness or (ii) a positive NS1-specific IgG antibody response and negative serotype specificity were found at any time after the onset. One hundred twenty-two (50%) of the serum samples were classified as showing primary infection by both methods, while 112 (45.90%) were classified as showing secondary infection by both methods, with a resultant concordance of 95.90%. Ten sera were found to have discordant results. Among these, five sera showed primary infection in the NS1 assay but were classified as secondary by capture IgM/IgG ratio, and the other five showed the opposite pattern. The results suggested that good correlation existed for these two assays (κ = 0.918). For those few sera with discordant results, paired acute- and convalescent- phase sera were analyzed for capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA to make the best judgment. Based on the dual analysis, 123 out of 244 sera tested were designated as showing primary infection, whereas the other 121 sera showed secondary infection. Figure 2 shows the range and dynamic plot of the capture IgM/IgG ratio from these 244 sera. The results clearly showed that the majority of sera tested could be correctly grouped into primary or secondary infection if a capture IgM/IgG ratio of 1.2 was used as the cutoff. Figure 3 shows representative data to illustrate the different patterns of primary and secondary infections determined by using capture IgM and IgG ELISA.
TABLE 1.
Comparison of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA in the differentiation of primary and secondary dengue virus infections
| NS1 serotype-specific IgG resulta | No. of samples (% of total sera tested) with capture IgM/ IgG ratio showingb:
|
Total no. of samples | |
|---|---|---|---|
| Primary infection | Secondary infection | ||
| Primary infection | 122 (50) | 5 (2.05) | 127 |
| Secondary infection | 5 (2.05) | 112 (45.90) | 117 |
| Total | 127 | 117 | 244 |
Primary dengue virus infection was defined if (i) a negative NS1-specific IgG antibody response was found for sera collected between days 1 and 14 of illness or (ii) there was positive serotype specificity for sera collected at ≥9 days of illness. Secondary dengue virus infection was defined if (i) a positive NS1-specific IgG antibody response was found for sera collected between days 1 and 8 of illness or (ii) a positive NS1-specific IgG antibody response and negative serotype specificity was found any time after the onset.
Infection was defined as primary if the capture IgM/IgG ratio was ≥1.2 or as secondary if the ratio was <1.2
FIG. 2.
Range and dynamic plot of capture IgM/IgG ratio from serum samples of patients with primary or secondary dengue virus infection. A total of 244 sera collected from 194 confirmed dengue patients (50 patients had paired serum samples) between days 4 and 45 after the onset of symptoms were analyzed by capture IgM and IgG ELISA. Among these, 123 sera were designated as showing primary infection, whereas the other 121 sera showed secondary infection, based on the dual analyses of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA. A capture IgM/IgG ratio of ≧1.2 or <1.2 is used to define primary or secondary dengue virus infection, respectively.
FIG. 3.
Different patterns of capture IgM and IgG ELISA for serum samples from patients with primary or secondary dengue virus infections. Representative data are shown to illustrate the different patterns of capture IgM and IgG ELISA from acute- and convalescent-phase sera with primary or secondary dengue virus infection. The last number(s) in the identification number of each dengue patient indicates the days after the onset of illness. D1234V indicates a mixture of culture supernatants harvested from DEN-1, DEN-2, DEN-3, and DEN-4 virus-infected Vero cells.
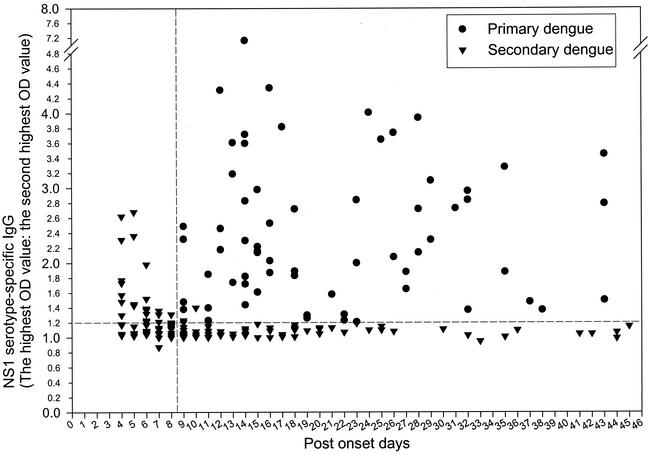
Range and dynamic plot of NS1 serotype-specific IgG ELISA from sera designated as showing primary or secondary infection.
Figure 4 shows the range and dynamic plot of the NS1 serotype-specific IgG ELISA from the same sera used for Fig. 2, designated as showing primary or secondary infection based on dual analyses of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA. The results clearly showed that the majority of sera tested could be correctly differentiated into primary or secondary infection by NS1 serotype-specific ELISA. Based on the results of NS1 serotype-specific ELISA, primary dengue virus infection was defined if (i) a negative NS1-specific IgG antibody response was found for sera collected between days 1 and 14 of illness or (ii) a positive serotype specificity was found for sera collected ≧ 9 days after the onset. Secondary dengue virus infection was defined if (i) a positive NS1-specific IgG antibody response was found for sera collected between days 1 and 8 of illness or (ii) a positive NS1-specific IgG antibody response and negative serotype specificity were found at any time after the onset. Accordingly, all of those serum samples collected between days 1 and 8 of illness shown in Fig. 4 were from secondary dengue virus infection, having a positive NS1-specific IgG antibody response regardless of positive (OD ratio of ≧1.2) or negative (OD ratio of <1.2) serotype specificity.
FIG. 4.
Range and dynamic plot of NS1 serotype-specific IgG ELISA from serum samples of patients with primary or secondary dengue virus infection. A total of 244 sera collected from 194 confirmed dengue patients (50 patients had paired serum samples) between days 4 and 45 after the onset of symptoms were analyzed. Among these, 123 sera were designated as showing primary infection, whereas the other 121 sera showed secondary infection, based on the dual analyses of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA. NS1 serotyping was done by calculating the ratio of the highest OD value to the second highest OD value read from the four dengue virus serotypes. For further details, see Materials and Methods.
Acute-phase sera from patients with secondary dengue virus infection can be used to identify the serotype of the first dengue virus infection.
The results from Fig. 4 (sera between days 4 and 8 of illness) also indicated that identification of the dengue virus serotype of the first dengue infection from patients with a second dengue infection was possible if acute-phase sera were available. This is due to the fact that the NS1-specific IgG antibody cannot be detected before day 9 of illness for primary infection, so the NS1-specific IgG antibody measured in the acute-phase sera must come from previous infection. Table 2 shows representative data from paired sera collected from Kaohsiung city in southern Taiwan during the 2000 DEN-2 outbreak. The results clearly showed that the acute-phase sera indicate a previous DEN-1 infection for these patients. This is in accordance with the largest DEN-1 outbreak during 1987 to 1988 in this area (11, 12). Further, we have found that it is possible to differentiate secondary and third or fourth dengue virus infections if very early acute-phase sera (days 1 to 3) are available for analysis, since serum samples collected at days 1 to 3 after onset of illness from secondary infection would show a primary NS1 serotype-specific IgG response, while serum samples from patients with a third or fourth infection would show a strong and complex NS1 serotype-specific IgG response (Table 2 and unpublished data).
TABLE 2.
Identification of the serotype of the first dengue virus infection from acute-phase sera from a second dengue virus infection
| Dengue patient serum | Day | NS1 serotype-specific IgG ELISA result (OD405)
|
NS1 serotypingb | |||||
|---|---|---|---|---|---|---|---|---|
| DEN-1a | DEN-2 | DEN-3 | DEN-4 | JE virus | Cell control | |||
| 9000899A | 2 | 1.435 | 0.608 | 0.356 | 0.155 | 0.184 | 0.092 | 2.36 |
| 9000899B | 8 | 2.206 | 2.041 | 2.598 | 2.055 | 0.079 | 0.091 | 1.18 |
| S9006049A | 2 | 1.564 | 0.32 | 0.509 | 0.236 | 0.167 | 0.150 | 3.07 |
| S9006049B | 22 | 3.722 | 3.745 | 3.999 | 2.958 | 0.144 | 0.144 | 1.07 |
| 9000936A | 3 | 0.925 | 0.181 | 0.239 | 0.118 | 0.138 | 0.203 | 3.87 |
| 9000936B | 5 | 3.657 | 3.573 | 3.530 | 2.313 | 0.170 | 0.133 | 1.02 |
| 9000913A | 4 | 0.820 | 0.169 | 0.258 | 0.184 | 0.182 | 0.174 | 3.18 |
| 9000913B | 15 | 2.193 | 1.834 | 1.851 | 0.601 | 0.095 | 0.075 | 1.18 |
| 9000923A | 4 | 0.682 | 0.120 | 0.260 | 0.131 | 0.215 | 0.175 | 2.62 |
| 9000923B | 21 | 2.185 | 2.103 | 2.375 | 1.297 | 0.137 | 0.090 | 1.13 |
| 9000763A | 5 | 0.618 | 0.151 | 0.231 | 0.150 | 0.174 | 0.150 | 2.68 |
| 9000763B | 20 | 1.551 | 1.301 | 1.483 | 0.869 | 0.097 | 0.089 | 1.05 |
| 9001090A | 6 | 0.574 | 0.180 | 0.340 | 0.397 | 0.199 | 0.155 | 1.45 |
| 9001090B | 20 | 2.641 | 2.371 | 2.311 | 1.738 | 0.112 | 0.080 | 1.11 |
| 9000924A | 6 | 1.620 | 0.817 | 0.553 | 0.489 | 0.115 | 0.099 | 1.98 |
| 9000924B | 25 | 2.481 | 2.114 | 2.259 | 1.708 | 0.108 | 0.084 | 1.10 |
Boldface indicates positive serotype specificity of DEN-1 from previous primary infection.
NS1 serotyping was done by calculating the ratio of the highest OD value to the second highest OD value. Positive serotype specificity (values in boldface) is defined if the ratio is ≥1.2
Serotyping of primary dengue virus infection by NS1 serotype-specific IgG ELISA.
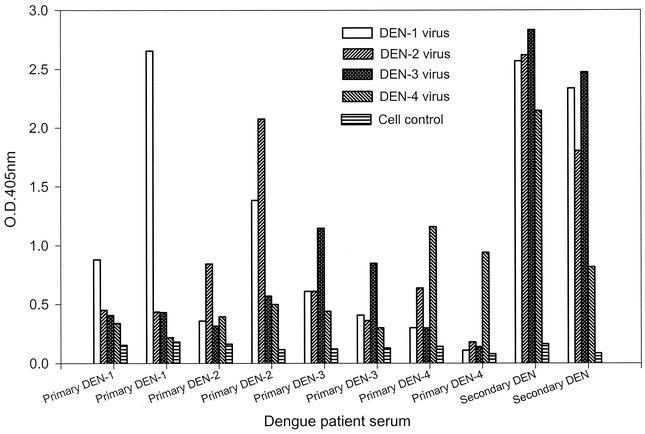
Representative data for NS1 serotype-specific IgG ELISA from sera with primary DEN-1, DEN-2, DEN-3, or DEN-4 or secondary dengue virus infection are shown in Fig. 5. The results demonstrated that the dengue virus serotype of the primary infection could be correctly identified when convalescent-phase sera were analyzed, whereas the strong and complex pattern induced in secondary infection would not allow correct serotyping.
FIG. 5.
Dengue virus serotyping by NS1 serotype-specific IgG ELISA. Convalescent-phase serum samples from patients with primary DEN-1, DEN-2, DEN-3, or DEN-4 or secondary dengue virus infection were analyzed, and representative data are shown to illustrate the NS1 serotype-specific IgG antibody responses.
Correlation plot of capture IgM and IgG ELISA and NS1 serotype-specific IgG ELISA from serum samples from patients with primary and secondary dengue virus infections.
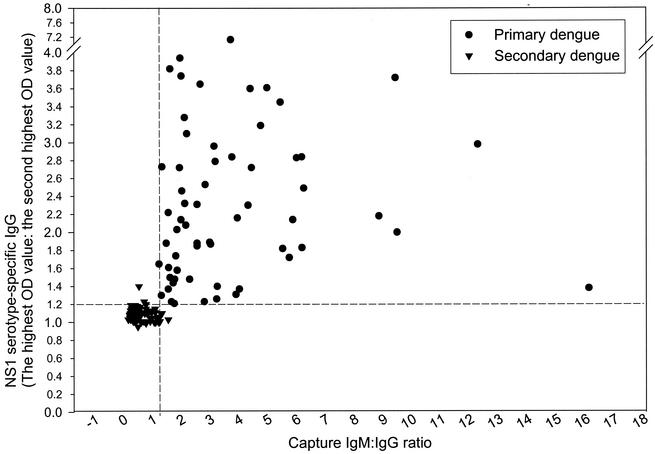
Figure 6 shows the correlation plot for the capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA with a subset of the sera in Table 1 designated as showing primary or secondary infection collected between days 9 and 45 of illness. A total of 132 serum samples were used, with 66 sera classified as showing primary infections and 66 sera as showing secondary infections. The results clearly showed a good correlation and that both NS1 serotype-specific IgG ELISA and the capture IgM/IgG ratio are valuable in the serodiagnosis of flavivirus infection.
FIG. 6.
Correlation plot of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA, showing a comparison of individual assay values from sera designated as showing primary or secondary infection, collected between days 9 and 45 of illness, from a subset of the 244 sera used for Table 1. A total of 132 serum samples were used, with 66 sera classified as showing primary infection and 66 sera classified as showing secondary infection.
DISCUSSION
Among available serological assays, capture IgM and/or IgG ELISA and the HI test are the most commonly used serological techniques for the diagnosis of dengue virus infections. Although the HI test had been traditionally used for the differentiation of primary and secondary dengue virus infections, it had become less popular recently and was gradually replaced by capture IgM and IgG ELISA. This is due to the inherent disadvantages of the HI test, including (i) the need for paired sera collected at appropriate time intervals, (ii) the inability to measure isotype-specific antibody response, and (iii) high cross-reactivity of flavivirus-specific IgG antibodies, which made definite diagnosis very difficult. In contrast, capture IgM and IgG ELISA has many advantages, including that (i) a correct diagnosis is often possible for single serum sample, (ii) the assay is able to measure IgM and IgG antibodies (IgM antibody is highly specific in the differentiation of flavivirus infection), (iii) the cross-reactivity of flavivirus-specific IgG antibodies measured by capture IgG ELISA is much less than that in the HI test, and (iv) full automation is possible. Accordingly, capture IgM and IgG ELISA has become the most powerful assay for serodiagnosis due to its high sensitivity, specificity, and simplicity. The results shown in this study of the comparison of the HI test with the capture IgM/IgG ratio support the current trend toward using capture IgM and IgG ELISA to differentiate primary and secondary infections (Fig. 1). Furthermore, we have recently modified the capture IgM and IgG ELISA originally developed by Innis et al. (10) for the routine diagnosis of JE, dengue, and yellow fever infections by using virus-infected culture supernatants as the source of viral antigens. The results showed that differential diagnosis of acute or recent flavivirus infection could be made by tests against a panel of viral antigens (data not shown).
In this study, we evaluated the usefulness of NS1 serotype-specific IgG ELISA in the serodiagnosis and compared it with capture IgM and IgG ELISA in the differentiation of primary and secondary dengue virus infections. A total of 244 acute- and convalescent-phase sera, collected between days 4 and 45 after the onset, covering all four dengue virus serotypes were analyzed. Among these, 123 sera were designated as showing primary infection, whereas the other 121 sera were secondary based on the dual analyses of capture IgM/IgG ratio and NS1 serotype-specific IgG ELISA. Good correlation was found between these two assays, with 95.90% agreement. Among the 10 discordant sera, several factors were found to contribute to the discrepancy. These included (i) a high titer of cross-reactive E/M-specific IgG antibodies from previous JE infection, leading to a lower IgM/IgG ratio (≦1.2) (one false secondary dengue infection); (ii) secondary dengue infection (strong and complex NS1 serotype-specific IgG response) with a higher IgM/IgG ratio, ranging from 1.2 to 1.5 (five false primary infections); (iii) secondary dengue infection (capture IgM/IgG ratio in the range between 0.45 and 0.69) with the NS1 serotype-specificity (the test absorbance ratio of the highest OD value to the second highest OD value) value in the range between 1.2 and 1.5 for the convalescent-phase sera (three false primary infections); and (iv) an older individual (62 years old) showing negative NS1-specific IgG antibodies for acute-phase sera but medium and complex NS1-specific IgG responses for convalescent-phase sera (IgM/IgG ratios of 0.39 and 0.5 for acute- and convalescent-phase sera) (one false primary infection). It is tempting to speculate that the NS1-specific IgG antibody from a few older individuals would persist for several decades after infection and then the titer would start to decline to an undetectable level.
Our data agreed well with those reported by Innis et al. (10) in that sera collected between days 4 and 45 of illness could be used for serodiagnosis and differentiation of primary and secondary dengue virus infections. However, the original ELISA was modified in that the capture IgM/IgG ratio (≧1.2 for primary infection) was directly used to differentiate primary and secondary infections without calculating the antibody unit. Sera collected between days 4 and 45 of illness could also be used for NS1 serotype-specific IgG ELISA. Indeed, we have found that this was an easy and reliable way to define an acute, primary dengue infection if NS1-specific IgG antibodies were not detected between days 1 and 14 after the onset. This is due to the fact that NS1-specific IgG antibodies would not be detected before day 9 of illness after the onset. Interestingly, Alcon et al. recently reported that the NS1 antigen was found circulating from the first day after the onset of fever up to day 9 (1). This is in contrast with data from Young et al., who reported that NS1 could not be detected in either acute- or convalescent-phase serum samples taken from patients with serologically confirmed primary infection (20). Our unpublished data from more than 100 acute-phase sera with either primary or secondary infection are in agreement with Alcon et al. Accordingly, our data suggest that NS1 antigen was detectable during days 1 to 8 of illness and that NS1-specific IgG antibodies started to appear after that.
Compared to capture IgM and IgG ELISA, an additional advantage of NS1 serotype-specific IgG ELISA in the serodiagnosis is that dengue virus serotyping is possible for patients with primary dengue infection if convalescent-phase or postinfection sera are available. Figure 5 and Table 2 show representative data from dengue virus serotyping of convalescent-phase sera from primary and secondary infections and acute- and convalescent-phase sera from secondary infections. The results demonstrate that the dengue virus serotypes could be correctly identified for convalescent-phase sera from primary infection and acute-phase sera from secondary infection but not for convalescent-phase sera from secondary infection. More recently, we have initiated a retrospective study to analyze postinfection sera covering all four serotypes from patients with confirmed primary or secondary infection in Tainan city in southern Taiwan. The results showed an excellent correlation between convalescent-phase sera and postinfection sera in the identification of the dengue virus serotype from patients with primary infection (data not shown).
In summary, we have reported the development of an easy, sensitive, and specific NS1 serotype-specific IgG ELISA that can be reliably used for serodiagnosis and seroepidemiological study of dengue virus infection.
Acknowledgments
We thank Hsiu-Ling Pan and Chih-Heng Chen for their expert technical assistance.
This work was supported in part by grants NSC 89-2318-B-043B-001-M51 from the National Science Council and DOH90-DC-2015 from the Center for Disease Control, Department of Health, Taiwan, Republic of China.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bravo, J. R., M. G. Guzman, and G. P. Kouri. 1987. Why dengue haemorrhagic fever in Cuba? I. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans. R. Soc. Trop. Med. Hyg. 81:816-820. [DOI] [PubMed] [Google Scholar]
- 3.Chen, L. K., C. L. Liao, C. G. Lin, S. C. Lai, C. I. Liu, S. H. Ma, Y. Y. Huang, and Y. L. Lin. 1996. Persistence of Japanese encephalitis virus is associated with abnormal expression of nonstructural protein NS1 in host cells. Virology 217:220-229. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne virus. Am. J. Trop. Med. Hyg. 7:561-573. [DOI] [PubMed] [Google Scholar]
- 5.Ey, P. L., S. J. Prowse, and C. R. Jenkin. 1978. Isolation of pure IgG1, IgG2a, and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 15:429-436. [DOI] [PubMed] [Google Scholar]
- 6.Groen, J., P. Koraka, J. Velzing, C. Copra, and A. D. M. E. Osterhaus. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubler, D. J., D. Reed, L. Rosen, and J. C. J. Hitchcock. 1978. Epidemiologic, clinical, and virologic observations on dengue in the kingdom of Tonga. Am. J. Trop. Med. Hyg. 27:581-589. [DOI] [PubMed] [Google Scholar]
- 8.Gubler, D. J. 1997. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem, p. 1-22. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, N.Y.
- 9.Halstead, S. B., H. Shotwell, and J. Casals. 1973. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J. Infect. Dis. 128:15-22. [DOI] [PubMed] [Google Scholar]
- 10.Innis, B. L., A. Nisalak, S. Nimmannitya, S. Kusalerdchariya, V. Chongswasdi, S. Suntayakorn, P. Puttisri, and C. H. Hoke. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418-427. [DOI] [PubMed] [Google Scholar]
- 11.Ko, Y. C. 1989. Epidemiology of dengue fever in Taiwan. Gaoxiong Yi Xue Ke Xue Za Zhi 5:1-11. [PubMed] [Google Scholar]
- 12.Ko, Y. C., M. J. Chen, and S. M. Yeh. 1992. The predisposing and protective factors against dengue virus transmission by mosquito vector. Am. J. Epidemiol. 136:214-220. [DOI] [PubMed] [Google Scholar]
- 13.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino, Y., M. Tadano, M. Saito, N. Maneekarn, N. Sittisombut, V. Sirisanthana, B. Poneprasert, and T. Fukunaga. 1994. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol. Immunol. 38:951-955. [DOI] [PubMed] [Google Scholar]
- 15.Rosen, L. 1977. The Emperor's New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 26:337-343. [DOI] [PubMed] [Google Scholar]
- 16.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2000. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J. Med. Virol. 62:224-232. [DOI] [PubMed] [Google Scholar]
- 17.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, T. H. Lin, and J. H. Huang. 2001. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine 19:1753-1763. [DOI] [PubMed] [Google Scholar]
- 18.Shu, P. Y., L. K. Chen, S. F. Chang, Y. Y. Yueh, L. Chow, L. J. Chien, C. Chin, H. H. Yang, T. H. Lin, and J. H. Huang. 2002. Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J. Clin. Microbiol. 40:1840-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 20.Young, P., R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]