Abstract
To understand the role of immune mechanisms in protecting chickens from Salmonella infections, we examined the immune responses of Salmonella enterica serovar Enteritidis-infected chickens and the effect of chicken anemia virus (CAV), a T-cell-targeted virus, on S. enterica serovar Enteritidis-induced immune responses. One-day-old chicks were orally inoculated with S. enterica serovar Enteritidis with or without intramuscular injection of CAV. The bacterial infection, pathology, and immune responses of chickens were evaluated at 14, 28, and 56 days postinoculation. The infection increased the levels of S. enterica serovar Enteritidis-specific mucosal immunoglobulin A (IgA), the number of gut-associated T cells, and the titer of serum IgG specific for S. enterica serovar Enteritidis surface antigens. CAV infection depressed these immune responses, especially the mucosal immune responses, but did not increase the number of S. enterica serovar Enteritidis-infected cells in the intestine. The severity of pathological lesions appeared to be reciprocal to the level of immune responses, but the S. enterica serovar Enteritidis infection persisted. These results suggest that oral infection of S. enterica serovar Enteritidis in chickens induces both mucosal and systemic immune responses, which have a limited effect on the S. enterica serovar Enteritidis infection under conditions designed to mimic the field situation.
Salmonella is one of the primary causes of human food poisoning throughout the world (19). Salmonella enterica serovar enteritidis carried by chickens and poultry products is the major source of human intestinal infections (12, 14, 16, 20, 26, 37). S. enterica serovar Enteritidis outbreaks have been found to be associated with the consumption of contaminated and undercooked poultry products, such as eggs and egg-containing products (15), and have become a serious economic and public health problem.
The common sources of S. enterica serovar Enteritidis infection for chickens are contaminated feed and feces. S. enterica serovar Enteritidis infection in chickens is initiated by extensive colonization of S. enterica serovar Enteritidis in the intestine (11, 28, 29). S. enterica serovar Enteritidis colonization in the gastrointestinal tract can persist for as long as 18 weeks postinoculation in hens (22). S. enterica serovar Enteritidis can then be rapidly spread among chickens through shedding S. enterica serovar Enteritidis-contaminated feces (21). After the initial colonization at the intestinal epithelial surface, S. enterica serovar Enteritidis invades and spreads among a wide range of tissues. After oral inoculation, S. enterica serovar Enteritidis is detected in the liver, spleen, ovary, and oviduct (4, 6, 21, 43). In addition to S. enterica serovar Enteritidis infection of ovaries, persistent colonization of S. enterica serovar Enteritidis in the gastrointestinal tract of the laying hens is also a major factor for S. enterica serovar Enteritidis contamination of shell eggs. Therefore, measures that effectively protect chickens from S. enterica serovar Enteritidis colonization are essential for the reduction of S. enterica serovar Enteritidis contamination of poultry products.
The immune system is a naturally existing protective system for pathogen infection. Vaccines stimulate specific immune responses to pathogens that provide animals with protection. The development of effective vaccines against S. enterica serovar Enteritidis for chickens has been hindered by a lack of knowledge concerning the immune responses against Salmonella in chickens. In general, the mucosal immune system of the intestine, including mucosal immunoglobulin A (IgA) and mucosa-associated lymphocytes and leukocytes, forms the first line of defense against S. enterica serovar Enteritidis infection. Systemic immune responses, including humoral and cell-mediated responses, play important roles in the resistance and clearance of S. enterica serovar Enteritidis infection. The humoral immune responses of chickens after infection with S. enterica serovar Enteritidis have been extensively studied for diagnostic purposes (5). The fundamental mechanism of mucosal resistance to infection and clearance of S. enterica serovar Enteritidis from the gut of chickens has received scant attention (5). The effects of immune suppression by chemicals (2, 13, 16) or infectious bursal disease virus (38) on immune responses to S. enterica serovar Enteritidis have been reported earlier. These studies show that the intestinal shedding rate of S. enterica serovar Enteritidis increases after cyclophosphamide-testosterone propionate treatments that preferentially deplete B cell precursors and B cells (2) or bursal disease virus infection that preferentially destroys precursor B cells in the bursa of Fabricius (38). This suggests an important role for humoral immune responses in the control of S. enterica serovar Enteritidis infection in chickens. Protection and clearance of S. enterica serovar Enteritidis infection by humoral mechanisms alone is unlikely, as S. enterica serovar Enteritidis is a facultative intracellular bacterium. There is sufficient evidence from various animal models that cell-mediated immunity plays a major role in controlling Salmonella infection (23, 30). CD3+, CD4+, and CD8+ T cells were observed to proliferate in the reproductive tract of S. enterica serovar Enteritidis-infected chickens (45, 46). However, T-cell immunosuppression with cyclosporine A showed no significant effect on S. enterica serovar Enteritidis infection in chickens (2). It is therefore unclear whether T cells play a role in immune responses against S. enterica serovar Enteritidis in chickens.
Chicken anemia virus (CAV), a small, nonenveloped icosahedral virus, has been shown to cause severe anemia and atrophy of lymphoid organs in young chickens (23, 24, 41, 48). CAV is commonly found in commercially produced chickens. Erythroid progenitors in the bone marrow, precursor T cells in the thymus, but not B cells, are the targets of CAV. Early reports showed that destruction of these cells in young chickens less than 3 weeks of age resulted in anemia and suppression of T-cell-mediated immune responses (31, 42), which enhances the pathogenicity of secondary infectious agents (1, 25, 44). The selective depletion of the precursor T cells makes CAV-infected chickens a good model system for the study of T cell function in the chickens' immune response against S. enterica serovar Enteritidis infection.
To understand the immunobiology of S. enterica serovar Enteritidis in the chicken, the humoral and cellular immune responses at the mucosal surface of the intestine and in the peripheral blood and spleens of chickens infected with S. enterica serovar Enteritidis were analyzed. The immune responses to S. enterica serovar Enteritidis in healthy chickens and chickens infected with CAV were compared.
MATERIALS AND METHODS
Animals and experimental design.
One-day-old specific-pathogen-free White Leghorn chickens were purchased from SPAFAS (Storrs, Conn.). They were housed in isolation units at the College of Veterinary Medicine, University of Maryland (College Park) in a biosafety-level-2 research facility. The chicks were provided with water and commercial antibiotic-free feed ad libitum. The experimental procedures and protocols were undertaken in accordance with the animal care and management guidelines of the University of Maryland. All of the cloacae of the chickens were sampled prior to inoculation and were found to be negative for Salmonella by culture.
To follow the S. enterica serovar Enteritidis infection in young birds, 1-day-old specific-pathogen-free chickens were divided into 4 groups of 15 birds each. The first group of chickens was kept as uninfected controls. Birds in the second group were inoculated intramuscularly with 0.5 ml of 105 50% tissue culture infective doses of CAV CL-1 strain/ml. Birds in the fourth group were individually given 1 × 107 CFU of S. enterica serovar Enteritidis by crop gavage. Birds in the third group were inoculated with both CAV and S. enterica serovar Enteritidis. The four groups of chickens were housed in four different isolators to prevent cross-contamination and observed twice daily for signs of illness and mortality. At 14, 28, and 56 days postinoculation (DPI), 5 chicks were randomly taken from each group and euthanized. The cecal contents were cultured individually for S. enterica serovar Enteritidis. The intestinal mucus, intestine, and spleen tissue samples were collected and processed for further analyses. Viable S. enterica serovar Enteritidis was found in the cecal content of all inoculated chickens at 14, 28, and 56 DPI.
Bacteria and virus.
S. enterica serovar Enteritidis phage type 4 (PT4) strain 338 was originally isolated by the Food and Drug Administration (Laurel, Md.) from egg-associated field outbreaks. This strain was transformed with a green fluorescent protein (GFP)-containing plasmid that permits constitutive production of GFP under the control of a lac promoter. Bacteria were cultured in 10 ml of tryptic soy broth (Difco, Detroit, Mich.) at 37°C for 18 h and diluted to 1 × 107 CFU/ml in phosphate-buffered saline (PBS) before inoculation.
CAV strain CL-1 (32) was used. The infective tissue culture fluid of this strain was prepared and its titer was determined in a chicken B lymphoblastoid cell line, MDCC-MSB1. An inoculum of 1 × 105 mean 50% tissue culture infective doses per ml was used to infect the chickens.
Antibodies.
Goat polyclonal antibodies specific to chicken IgA and IgM were purchased from Bethyl Laboratories, Inc. (Montgomery, Tex.), and mouse anti-chicken CD3, CD4, and CD8 and goat anti-chicken IgG were from Southern Biotechnology Associates (Birmingham, Ala.). Goat anti-Salmonella common structural antigens and rabbit anti-goat IgG conjugated to horseradish peroxidase (HRP) were from KPL Laboratories, Inc. (Gaithersburg, Md.).
Bacteriological assay.
Cecal contents of birds were enriched in buffered peptone water for 18 h and in Hajna tetrathionate broth (Difco) for 24 h and then plated on XLT4 Salmonella-selective agar medium (Difco) by employing standard procedures.
PCR detection of CAV.
DNA was extracted from a pooled suspension of thymus and spleen collected at 14, 28, and 56 DPI by using DNAZOL reagent (Life Technologies, Inc., Rockville, Md.) according to the manufacturer's protocol. The purified DNA was stored at −20°C for further analysis. PCR amplification of the highly conserved VP2 and VP3 overlapping coding region of CAV was carried out by using the method described by Yamaguchi et al. (47). The primers used for PCR were P3, AATGAACGCTCTCCAAGAAG (locations 464 to 483), and P4, GGAGGCTTGGGTTGATCGGTC (locations 715 to 695). This set of primer pairs was expected to be applicable for most of the isolates of CAV based on the nucleotide sequences of CAV in the DNA databases (47).
Histology and immunohistochemistry.
For histology, tissues were fixed in 10% neutral buffered formalin and processed per standard procedures. Paraffin-embedded sections were cut at 5- to 8-μm thickness and stained with hematoxylin and eosin.
For immunohistochemistry, tissues from different parts of the intestine and spleen were fixed in 4% paraformaldehyde for 4 to 6 h, perfused with different grades of sucrose, embedded in TissueTek OCT compound (Sakura Finetek USA, Torrance, Calif.), snap-frozen in liquid nitrogen, and stored at −70°C. Sections were cut at 5- to 7-μm thickness with a Leitz HM-500 cryostat, air dried at room temperature, and fixed in acetone for 20 min. Endogenous peroxidase activity was blocked with 0.3 to 0.6% H2O2 in methanol for 30 min. Nonspecific binding was minimized by treating the sections with 0.1% bovine serum albumin (BSA) or 1% normal chicken serum in PBS for 30 min. The sections were then blotted with anti-chicken IgA and CD3 and anti-Salmonella antibodies and their corresponding HRP-conjugated secondary antibodies. For negative controls, 1% BSA was used instead of the primary antibody. Sections were analyzed under a light microscope, and positively stained cells from at least five randomly selected fields (at ×100 magnification), representing an area of 0.07 mm2 (46), were counted. The mean cell numbers for each experimental group were calculated.
Intestinal mucosal IgA.
The duodenum, jejunum, and ileum of the intestine were collected, and the intestinal lumen was exposed. The mucus was collected by scraping the mucosal surface of the intestine, and the mucus from each chicken was suspended in 10 ml of PBS and centrifuged at 5,000 × g for 30 min at 4°C. The supernatant was mixed with equal quantities of PBS containing 0.01 M phenylmethylsulfonyl fluoride, 0.1% sodium azide, and 1% BSA. The resulting mixture was stored at −20°C for further analysis.
Salmonella-specific IgA in intestinal mucus was measured by enzyme-linked immunosorbent assay (ELISA) as described by Desmidt et al. (16). Ninety-six-well-microtiter plates (Nalgene Nunc, Rochester, N.Y.) were coated either with affinity-purified goat anti-chicken IgA antibody (Bethyl Laboratories, Inc.) or with the outer membrane proteins of S. enterica serovar Enteritidis (1 μg/ml) in coating buffer (50 mM sodium carbonate-bicarbonate buffer, pH 9.6) overnight at 4°C. After washing, the mucus samples were added to triplicate wells, and chicken Ig reference serum (Bethyl laboratories, Inc.) was added to wells coated with goat anti-chicken IgA antibody, serving as standards. After incubation for 1 h, the plates were incubated with HRP-conjugated goat anti-chicken IgA in PBS containing 0.05% Tween 20 and 1% BSA and washed, and orthophenylenediamine (Sigma, St. Louis, Mo.) and 0.22% H2O2 in 0.04 M citric acid buffer (pH 5.0) were added. The reaction was stopped after 10 min by adding 50 μl of 0.5 M sulfuric acid. The absorbance was read at 490 nm on a 96-well plate reader (Bio-Rad, Hercules, Calif.). The concentration of Salmonella-specific IgA was calculated by using the standard curve generated from the reference serum.
Cell proliferation.
Mononuclear cells (MNCs) were isolated from chicken spleen and peripheral blood as described previously (3). Briefly, the spleen was washed and minced in Hanks' balanced salt solution, and the residual tissue was removed with a cell strainer. The cell suspension from the spleen and the blood was layered under with Histopaque (1.077 g/ml) and centrifuged at 400 × g for 30 min at room temperature. MNCs were obtained from the interface and washed twice with Hanks' balanced salt solution. The final pellet was suspended in RPMI 1640 medium containing 5% heat-inactivated fetal calf serum. MNCs (4 × 105) were incubated with either RPMI 1640 medium alone or medium containing concanavalin A (1.6 μg/well) or flagella isolated from S. enterica serovar Enteritidis in 96-well plates at 40°C for 48 h. The cells were incubated with 1 μCi of [3H]thymidine for an additional 24 h and harvested. Radioactivity was counted with a microbeta counter.
Flow cytometry.
Flow cytometric analysis of MNCs was carried out as previously described (39). MNCs were incubated with R-phycoerythrin (R-PE)-conjugated anti-chicken CD3 and fluorescein isothiocyanate (FITC)-conjugated anti-chicken IgG (Southern Biotechnology, Inc.). Duplicate samples of MNCs were stained with R-PE-conjugated anti-chicken CD4 and FITC-conjugated anti-chicken CD8 (Southern Biotechnology, Inc.). R-PE- and FITC-conjugated isotype control samples were also included. Cells were analyzed with an EPICS Elite flow cytometer (Beckman Coulter). For gating purposes, the viable MNC population was determined by forward light scatter versus side light scatter analysis. Five thousand cells were analyzed for each sample.
Statistical analysis.
Statistical analyses were carried out with Windows-based statistical software (Statistix, version 7.0). The differences between the groups were examined by one-way analysis of variance. The means were compared by Tukey's test. Significant differences were estimated at P values of <0.05.
RESULTS
S. enterica serovar Enteritidis and CAV infection.
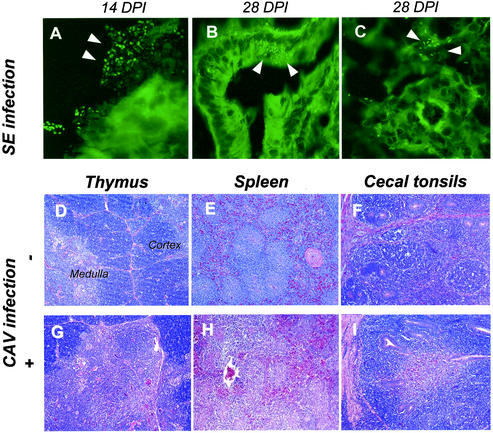
The distribution of S. enterica serovar Enteritidis in tissues of infected chickens was examined by immunohistochemistry and fluorescence microscopy. Clusters of fluorescent rod-shaped bacteria were seen in the lumen of the intestine adhering to the villous epithelium (Fig. 1A) at 14 DPI. By 28 DPI, they moved into the mucosal epithelial cells, lamina propria, crypts, and core of villi (Fig. 1B) and within cells of the spleen (Fig. 1C). Immunohistochemistry analysis gave similar results (data not shown).
FIG. 1.
Tissue distribution of orally inoculated S. enterica serovar Enteritidis and pathological changes in CAV-infected chickens. (A to C) The distribution of S. enterica serovar Enteritidis (SE) that constitutively expressed GFP was analyzed under a fluorescence microscope with a 100× objective. Fluorescent rod-shaped Salmonella cells were observed on the epithelium of the villus (A), in the host cells of intestinal crypt (B), and around the artery of spleen (C). (D to I) Paraffin-embedded sections from the thymus (D and G), spleen (E and H), and cecal tonsil (F and I) of mock-infected control chickens (D to F) and CAV-infected chickens (G to I) were stained with hematoxylin and eosin. Images were acquired by using 10× objectives.
Even though Salmonella-infected chickens generally looked healthy with normal weight gain, the pathology of the infection was readily detectable by histology, including necrosis of the villous epithelium, MNC infiltration in the villi and lamina propria of the intestine, and occasionally hemorrhagic enteritis. These lesions were prominent at 14 DPI, moderate at 28 DPI, and less noticeable at 56 DPI.
CAV infection was followed by histology and PCR. The thymus of CAV-infected chickens showed lymphocyte depletion in the cortex (Fig. 1G). The lymphocyte depletion appeared mild to moderate at 14 DPI, progressed to severe lymphoid depletion and necrosis at 28 DPI, and returned to almost normal at 56 DPI. Similar lesions were detected in the spleens (Fig. 1H) and cecal tonsils (Fig. 1I) of CAV-infected birds. PCR analysis showed that all CAV-infected chickens carried the virus for up to 56 DPI.
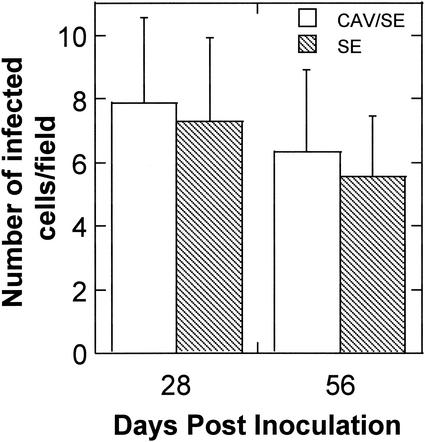
Chickens inoculated with both CAV and S. enterica serovar Enteritidis showed pathological lesions similar to those seen in chickens inoculated with CAV or S. enterica serovar Enteritidis alone, except that the lesions appeared more pronounced in the dually infected chickens. S. enterica serovar Enteritidis infection in chickens inoculated with S. enterica serovar Enteritidis alone or both CAV and S. enterica serovar Enteritidis was evaluated by enriched culture of feces and immunohistochemistry. Feces from both singly and dually infected groups remained S. enterica serovar Enteritidis positive for at least 56 DPI. The number of intestinal cells that were stained by antibodies specific for Salmonella common structural antigens did not significantly differ between the singly and dually infected groups (Fig. 2). This suggests that coinfection of CAV has no significant effect on the level of S. enterica serovar Enteritidis infection in the intestine.
FIG. 2.
Salmonella-positive host cells in the intestine of chickens infected with S. enterica serovar Enteritidis (SE) alone and dually infected with S. enterica serovar Enteritidis and CAV. Cryosections of the intestine were incubated with antibody specific for Salmonella and an HRP-conjugated secondary antibody. The sections were further stained with hematoxylin. Images were acquired by using a 100× objective. The number of host cells showing positive staining on five randomly selected microscopic areas of each chicken was counted. Shown are the averages (± standard deviations) of the results from five chickens of each group.
Mucosal immune responses.
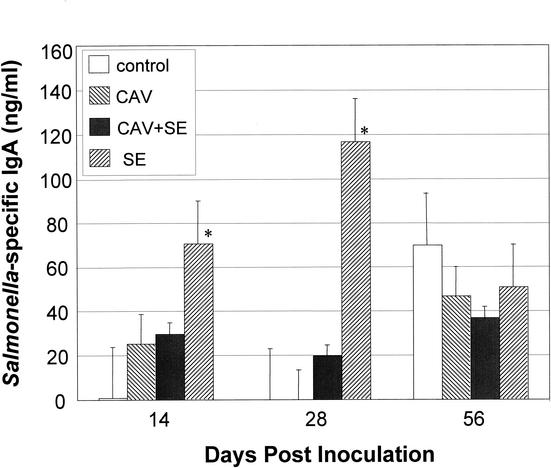
IgA is one of the major isotypes of Igs secreted on the mucosal surface. Salmonella-specific IgA in the intestinal mucus of different groups of birds was quantified by ELISA. As shown in Fig. 3, at 14 and 28 DPI, the Salmonella-specific IgA was dramatically increased in birds inoculated with S. enterica serovar Enteritidis alone. Coinfection of birds with CAV and S. enterica serovar Enteritidis significantly reduced the IgA level in comparison to those of birds infected with S. enterica serovar Enteritidis alone. By 56 DPI, the IgA level in S. enterica serovar Enteritidis-infected birds decreased, and the basal level of IgA that bound to the outer membrane molecules of S. enterica serovar Enteritidis in control birds increased. No significant differences in the level of Salmonella-specific IgA between different infection groups were detected (Fig. 3).
FIG. 3.
S. enterica serovar Enteritidis (SE)-specific IgA responses in the intestinal mucosa. The concentration of S. enterica serovar Enteritidis-specific IgA in chicken intestinal mucus was determined by ELISA with anti-chicken IgA antibody. S. enterica serovar Enteritidis envelope proteins were used as antigens. The concentration of IgA was calculated by using the standard curve generated from the reference IgA with a known concentration. Shown are the averages (± standard deviations) from triplicate samples for each group of chickens. Asterisks represent the significant (P < 0.05) difference from the control group.
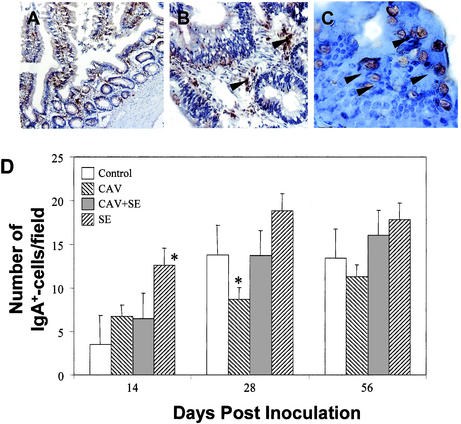
Intestinal cells secreting or carrying IgA were detected with chicken IgA-specific antibodies by immunohistochemistry. IgA+ cells were often found in the lamina propria (Fig. 4B), submucosa, and core of the villi (Fig. 4A) of the different parts of the intestine, such as the duodenum, ileum, and cecum. Some goblet cells (Fig. 4C) were also positively stained for IgA, suggesting the presence of secreted IgA. The IgA+ cells were detected in small numbers throughout the experimental period in all of the birds, and the number of IgA+ cells increased with the age of the chickens (Fig. 4D). Importantly, birds infected with S. enterica serovar Enteritidis alone had significantly higher numbers of IgA+ cells in the intestines than did chickens in all of the other groups, especially at 14 DPI. Coinfecting birds with both Salmonella and CAV reduced this increase (Fig. 4D). By 56 DPI, the difference in the number of IgA+ cells between different infection groups was much less pronounced than those at 14 DPI (Fig. 4D).
FIG. 4.
IgA-positive cells in the intestine. Cryosections of the intestine were incubated with anti-chicken IgA and HRP-conjugated secondary antibodies. The sections were further stained with hematoxylin. Images were acquired by using 10× (A), 40× (B), and 100× (C) objectives. The number of cells showing IgA-specific staining on five randomly selected microscopic areas of each chicken was counted. Shown are the averages (± standard deviations) of the results from five chickens of each group (D). Asterisks represent the significant (P < 0.05) difference from the control group. IgA-positive cells were found in the lamina propria of the intestine (B), and goblet cells lining the intestinal epithelium also carry IgA (C). SE, serovar Enteritidis.
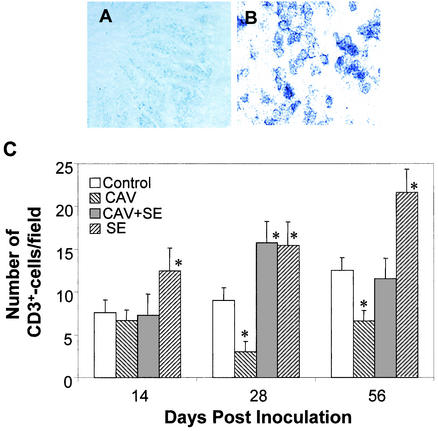
Gut-associated T cells were detected with a chicken CD3-specific antibody by immunohistochemistry. CD3+ T cells were primarily detected in the epithelium and core of the villi (Fig. 5A and B). In the control group, the number of gut-associated T cells slightly increased with the age of the chickens (Fig. 5C). Compared to the uninfected group, the number of T cells in the intestines of birds inoculated with S.enterica serovar Enteritidis alone increased significantly (Fig. 5C). In contrast, the number of T cells in the intestines of birds infected with CAV alone decreased, especially at 28 and 56 DPI (Fig. 5C). Moreover, the number of gut-associated CD3+ T cells in dually infected chickens was significantly lower than that in birds infected with S. enterica serovar Enteritidis alone, especially at 56 DPI (Fig. 5C).
FIG. 5.
Gut-associated T cells. Cryosections of the chicken intestine were stained with anti-chicken CD3 antibody and an HRP-conjugated secondary antibody. Images were acquired by using 10× (A) and 100× (B) objectives. CD3+ T cells were found in the epithelium (A) and the core of the intestinal villi (B). The number of CD3+ T cells from five randomly selected microscopic areas of each chicken was counted. Shown are the averages (± standard deviations) of the results from five chickens of each group (C). Asterisks represent the significant (P < 0.05) difference from the control group. SE, serovar Enteritidis.
These data indicate that the mucosal immunity gradually matures with the age of chickens. Oral inoculation of young chicks with S. enterica serovar Enteritidis stimulated mucosal immune responses, including increases in the level of Salmonella-specific IgA and the number of gut-associated T cells. In contrast, CAV infection reduced these responses.
Systemic immune responses.
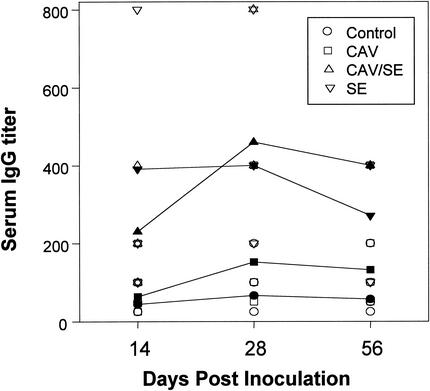
The titers of S. enterica serovar Enteritidis-specific serum IgG of different infection groups were compared by ELISA (Fig. 6). Serum titers of the birds of the control and CAV-infected groups remained low over the course of the study. S. enterica serovar Enteritidis inoculation increased the serum titer. The serum titers of chickens dually infected with CAV and S. enterica serovar Enteritidis were comparable to those of chickens infected with S. enterica serovar Enteritidis alone, indicating that CAV infection has no effect on the humoral IgG response against S. enterica serovar Enteritidis.
FIG. 6.
Titer of Salmonella-specific IgG in serum. The titers of S. enterica serovar Enteritidis (SE)-specific IgG in chicken sera were determined by ELISA with an anti-chicken IgG antibody. S. enterica serovar Enteritidis envelope proteins were used as antigens. Shown are the geometric mean titers of triplicate samples from five chickens in each group. Open symbols, data from each individual chicken of a group; filled symbols, averages of the data from five chickens of each group.
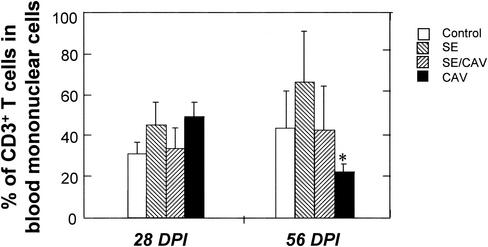
Subsets of lymphocytes in the peripheral blood and spleens of different groups of chickens were analyzed by flow cytometry. T cells were stained with anti-chicken CD3, and B cells were stained with anti-chicken IgG antibody. The percentage of the IgG+-B-cell population in MNCs of the peripheral blood and spleen was not significantly affected by CAV and Salmonella infections (data not shown). S. enterica serovar Enteritidis infection had no significant effect on the subpopulation of T cells in the peripheral blood and spleen at 28 DPI (data not shown). A dramatic decrease in the number of CD3+ T cells in the peripheral blood, including CD4+ CD8+, CD4+, and CD8+ T cells, was observed in CAV-infected chickens at 56 DPI (Fig. 7).
FIG. 7.
Percentages of T lymphocytes in the peripheral blood of chickens. MNCs were isolated from the peripheral blood of each chicken by density gradient centrifugation and labeled with anti-chicken CD3 and its corresponding secondary antibody conjugated to a fluorochrome. Cells were analyzed with a flow cytometer. Shown are the average results (±standard deviations) from five chickens of each group.
Lymphocyte activity was further evaluated by the ability to proliferate after antigenic and mitogenic stimulations. MNCs from the peripheral blood and spleen of Salmonella-infected birds only proliferated in the presence of mitogen concanavalin A but did not proliferate when stimulated by purified flagella from S. enterica serovar Enteritidis (data not shown). This suggests that specific lymphocytes in the peripheral blood and spleen are not activated by oral infection with S. enterica serovar Enteritidis.
Thus, oral infection with S. enterica serovar Enteritidis increased the serum titer of antibodies specific for Salmonella but did not stimulate an S. enterica serovar Enteritidis-specific lymphocyte activation. CAV infection reduced the number of T cells in the peripheral blood but had no effect on the serum level of S. enterica serovar Enteritidis-specific antibodies.
DISCUSSION
To understand the immune mechanisms involved in the defense against Salmonella infections in chickens, we examined the pathology and immune responses of S. enterica serovar Enteritidis-infected chickens and the effect of CAV on S. enterica serovar Enteritidis-induced immune responses.
Mucosal immunity provides the first line of protection following oral exposure to pathogens. Here, we found that oral inoculation of young chicks with S. enterica serovar Enteritidis increased the concentration of S. enterica serovar Enteritidis-specific IgA in the intestinal mucus, the number of IgA+ cells, and the number of gut-associated T cells in the intestine. This demonstrates that oral infection of chickens with S. enterica serovar Enteritidis can induce both humoral and cell-mediated immune responses at the mucosal surface. As in mammalian species, it has been well documented that the chicken mucosal immune response is characterized by an IgA-dominated antibody profile. In particular, the involvement of mucosal IgA in protection against salmonellosis has been reported (27). Secretory IgA limits the mucosal colonization of S. enterica serovar Enteritidis by preventing adherence and thereby subsequent invasion of the bacteria (40). Our study showed that S. enterica serovar Enteritidis-specific mucosal IgA peaked at 28 DPI. This peak of IgA in the intestinal mucus was accompanied by the reduction of pathological lesions in chickens, suggesting that the IgA response may have a role in controlling S. enterica serovar Enteritidis infection in the intestine. Similarly, reduced shedding has been reported in bursectomized versus normal chickens after infection with Salmonella enterica serovar Typhimurium (10), S. enterica serovar Enteritidis PT13 (2), or S. enterica serovar Enteritidis PT4 (22). As noted in this and other studies (7, 35), the number of IgA+ cells and secretory IgA in the intestine increased rather slowly over time, suggesting that the mucosal humoral immunity is not fully developed in newly hatched chicks and requires a certain period of time to mature. The oral inoculation of S. enterica serovar Enteritidis induced a significant increase in S. enterica serovar Enteritidis-specific IgA in the intestine. However, the IgA response was rather transient, and by 56 DPI, the IgA level returned to the baseline even though S. enterica serovar Enteritidis infection persisted. Furthermore, lymphocytes in the peripheral blood and spleen were not activated in orally infected chicks. These combined data suggest that the mucosal IgA response induced by oral S. enterica serovar Enteritidis infection is probably a primary humoral response (18). Interestingly, the basal level of S. enterica serovar Enteritidis-specific IgA levels in uninfected chickens increased as chicks matured. This may reflect an increase in the level of natural or spontaneous antibodies in the intestine as chicks mature. Natural antibodies specific for bacteria, viruses, and toxins are found in healthy, nonimmunized humans and mice. The B1 type of B cells is the source of these antibodies. Such spontaneously generated antibodies have been shown to be essential for the first line of defense against pathogen infection (8, 36). However, the presence and generation of natural antibodies in the chicken intestine has not yet been studied.
S. enterica serovar Enteritidis is a facultative intracellular bacterium. When S. enterica serovar Enteritidis resides inside host cells, humoral immunity alone is unlikely to control the infection. In mammalian models, cell-mediated immunity has been shown to play a major role (23, 30, 33) in limiting Salmonella infections. In chickens, it has been reported that local cell-mediated immunity plays a major role in controlling Salmonella infections in the oviduct (46). Here, we showed that oral S. enterica serovar Enteritidis infection increased the number of CD3+ T cells in the intestine, suggesting that S. enterica serovar Enteritidis infection either stimulates gut-associated T cells to expand or recruits more T cells to the mucosal tissues.
Oral infection of chickens with S. enterica serovar Enteritidis also stimulates systemic immunity. This study showed that S. enterica serovar Enteritidis infection increased S. enterica serovar Enteritidis-specific IgG titers in the serum but did not induce the significant proliferation of MNCs from the blood and spleen, suggesting that oral infection is not capable of activating peripheral or circulating lymphocytes that are specific for S. enterica serovar Enteritidis. This is probably one of the main reasons why the disseminated bacteria can persist in chickens for their lifetime without encountering elimination by the immune system (17). How S. enterica serovar Enteritidis escapes the specific immunity of chickens is unclear.
To better understand the roles of the immune system in protecting chickens from Salmonella infection, we used a T-cell immunosuppression model generated by infecting chickens with CAV. It has been previously shown that infection of chickens less than 3 weeks of age with CAV can result in permanent immunosuppression (24, 42, 48), mediated by selective destruction of CD4+ CD8+ T cells (31, 32). Here, we found that CAV infection induced lymphocyte depletion in the thymus, spleen, and cecal tonsil and decreased the number of T cells in the peripheral blood and at the intestinal mucosal surfaces. However, CAV had no effect on the number of B cells in the peripheral blood and spleen. This is consistent with previous findings that CAV specifically targets T cells. Different from the previous reports (24, 42, 48), the effect of CAV infection on the chicken immune system appeared to be transient. Even though infected chickens remained CAV positive, lymphocyte depletion appeared to improve with time. This difference may be a result of the CAV strain used for the infection. It has been shown previously that the CAV CL-1 strain induces transient lymphocyte depletion (9). We chose this mild CAV strain to insure that all infected young chicks will survive through the infection and to assess the effects of T-cell immunity during the early stages of colonization by S. enterica serovar Enteritidis. However, the mild strain of CAV may not be very effective in causing long-term T-cell suppression. Because the effects of different strains of CAV on the immune system of young chicks have not been well studied, it is not known whether transient or severe T-cell depletion is the typical consequence of natural infections of CAV. Importantly, our data showed the decrease in the number of circulating and gut-associated T cells was concurrent with decreases in the number of IgA+ cells and the level of Salmonella-specific IgA in the intestines of chickens infected with both S. enterica serovar Enteritidis and CAV. This suggests that T cells may play a role in activating antibody responses against Salmonella infection on the mucosal surface. Therefore, CAV induces deficiencies in both T cells and B-cell-mediated responses on the intestinal mucosal surface. However, no significant increase in the number of S. enterica serovar Enteritidis-positive host cells was observed in chickens dually infected with CAV and S. enterica serovar Enteritidis compared to those of chickens infected with S. enterica serovar Enteritidis alone. This is consistent with previous reports of T-cell immunosuppression by cyclosporine A not increasing the intestinal shedding of S. enterica serovar Enteritidis in chickens (2, 13). The results from this and other studies indicate that T cells have a very limited role in the chicken immune response against Salmonella. The ineffectiveness of T cells in oral S. enterica serovar Enteritidis infection is probably due to S. enterica serovar Enteritidis effectively avoiding or suppressing the activation of T cells.
In summary, oral inoculation of chickens with S. enterica serovar Enteritidis stimulates the mucosal immune responses, including humoral and cell-mediated responses. For the systemic immune response, the S. enterica serovar Enteritidis infection increased the serum level of S. enterica serovar Enteritidis IgG but failed to stimulate lymphocyte proliferation. CAV infection weakened the immune responses, especially the mucosal immune responses, against S. enterica serovar Enteritidis, but this did not result in a detectable increase in S. enterica serovar Enteritidis colonization of the intestine. The increases in the immune responses were concurrent with a decrease in S. enterica serovar Enteritidis-induced pathological lesions in the intestine but failed to eradicate S. enterica serovar Enteritidis infection. These results suggest that oral infection of chickens with Salmonella cannot induce immune responses that are effective in controlling Salmonella infection. Further studies on the immune mechanisms of chickens are required to identify new strategies that increase the efficacy of chicken immune responses and therefore oral Salmonella vaccines for chickens.
Acknowledgments
This work was supported by grants from the Joint Institute for Food Safety and Applied Nutrition to W. Song and R. A. Heckert.
REFERENCES
- 1.Adair, B. M. 2000. Immunopathogenesis of chicken anemia virus infection. Dev. Comp. Immunol. 24:247-255. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. W., and P. S. Holt. 1995. Response to Salmonella enteritidis infection by the immunocompromised avian host. Poult. Sci. 74:656-665. [DOI] [PubMed] [Google Scholar]
- 3.Babu, U., and M. L. Failla. 1989. Superoxide dismutase activity and blastogenic response of lymphocytes from copper-deficient rats fed diets containing fructose and cornstarch. Nutr. Res. 9:273-282. [Google Scholar]
- 4.Barrow, P. A. 1991. Experimental infection of chickens with Salmonella enteritidis. Avian Pathol. 20:145-153. [DOI] [PubMed] [Google Scholar]
- 5.Barrow, P. A. 1992. ELISAs and the serological analysis of Salmonella infections in poultry: a review. Epidemiol. Infect. 109:361-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow, P. A., M. B. Huggins, M. A. Lovell, and J. M. Simpson. 1987. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res. Vet. Sci. 42:194-199. [PubMed] [Google Scholar]
- 7.Bar-Shira, E., D. Sklan, and A. Friedman. 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27:147-157. [DOI] [PubMed] [Google Scholar]
- 8.Bos, N. A., H. Kimura, C. G. Meeuwsen, H. De Visser, M. P. Hazenberg, B. S. Wostmann, J. R. Pleasants, R. Benner, and D. M. Marcus. 1989. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur. J. Immunol. 19:2335-2339. [DOI] [PubMed] [Google Scholar]
- 9.Bounous, D. I., M. A. Goodwin, R. L. Brooks, Jr., C. M. Lamichhane, R. P. Campagnoli, J. Brown, and D. B. Snyder. 1995. Immunosuppression and intracellular calcium signaling in splenocytes from chicks infected with chicken anemia virus, CL-1 isolate. Avian Dis. 39:135-140. [PubMed] [Google Scholar]
- 10.Brownell, J. R., W. W. Sadler, and M. J. Fanelli. 1970. Role of bursa of Fabricius in chicken resistance to Salmonella typhimurium. Avian Dis. 14:142-152. [PubMed] [Google Scholar]
- 11.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2001. Preliminary food net data on the incidence of foodborne illnesses-selected sites, United States. Morb. Mortal. Wkly. Rep. 51:325-329. [PubMed] [Google Scholar]
- 13.Corrier, D. E., M. H. Elissalde, R. L. Ziprin, and J. R. DeLoach. 1991. Effect of immunosuppression with cyclophosphamide, cyclosporin, or dexamethasone on Salmonella colonization of broiler chicks. Avian Dis. 35:40-45. [PubMed] [Google Scholar]
- 14.Cowden, J. M., D. Chisholm, M. O'Mahony, D. Lynch, S. L. Mawer, G. E. Spain, L. Ward, and B. Rowe. 1989. Two outbreaks of Salmonella enteritidis phage type 4 infections associated with the consumption of fresh shell-egg products. Epidemiol. Infect. 103:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle, E. F., S. R. Palmer, and C. D. Riberio. 1988. Salmonella phage type 4 infection associated with hen's eggs. Lancet ii:1295-1297. [DOI] [PubMed] [Google Scholar]
- 16.Desmidt, M., R. Ducatelle, J. Mast, B. M. Goddeeris, B. Kaspers, and F. Haesebrouck. 1998. Role of the humoral immune system in Salmonella enteritidis phage type four infection in chickens. Vet. Immunol. Immunopathol. 63:355-367. [DOI] [PubMed] [Google Scholar]
- 17.Dunlap, N. E., W. H. Benjamin, R. D. McCall, A. B. Tilden, and D. E. Briles. 1991. A safe-site for Salmonella typhimurium is within splenic cells during the early phase of infection in mice. Microb. Pathol. 10:297-310. [DOI] [PubMed] [Google Scholar]
- 18.Fagarasan, S., and T. Honjo. 2003. Intestinal IgA synthesis: regulation of front-line body defenses. Nat. Rev. Immunol. 3:63-72. [DOI] [PubMed] [Google Scholar]
- 19.Fantasia, M., and E. Filetici. 1994. Salmonella enteritidis in Italy. Int. J. Food Microbiol. 21:7-13. [DOI] [PubMed] [Google Scholar]
- 20.Fantasia, M., E. Filetici, M. P. Anastasio, M. D. Marcozzi, M. P. Gramenzi, and P. Aureli. 1991. Italian experience in Salmonella enteritidis 1978-1988: characterization of isolates from food and man. Int. J. Food Microbiol. 12:353-362. [DOI] [PubMed] [Google Scholar]
- 21.Gast, R. K., and C. W. Beard. 1990. Production of Salmonella enteritidis contaminated eggs by experimentally infected hens. Avian Dis. 34:438-446. [PubMed] [Google Scholar]
- 22.Gast, R. K., and S. T. Benson. 1995. The comparative virulence for chicks of Salmonella enteritidis phage type 4 isolates and isolates of phage types commonly found in poultry in the United States. Avian Dis. 39:567-574. [PubMed] [Google Scholar]
- 23.George, A., R. Nair, S. Rath, S. N. Ghosh, and R. S. Kamat. 1987. Regulation of cell-mediated immunity in mice immunized with Salmonella enteritidis. J. Med. Microbiol. l23:239-246. [DOI] [PubMed] [Google Scholar]
- 24.Goryo, M., H. Sugimura, S. Matsumoto, T. Umemura, and C. Itakura. 1985. Isolation of an agent inducing chicken anemia. Avian Pathol. 14:483-496. [DOI] [PubMed] [Google Scholar]
- 25.Goryo, M., Y. Shibata, T. Suwa, T. Umemura, and C. Itakura. 1987. Outbreak of anemia associated with chicken anemia agent in young chicks. Jpn. J. Vet. Sci. 49:867-873. [DOI] [PubMed] [Google Scholar]
- 26.Hasenson, L. B., L. Kaftyreva, V. G. Laszlo, E. Woitenkova, and M. Nesterova. 1992. Epidemiological and microbiological data on Salmonella enteritidis. Acta Microbiol. Hung. 39:31-39. [PubMed] [Google Scholar]
- 27.Hassan, J. O., S. B. Porter, and R. Curtis III. 1993. Effect of infective dose on humoral immune responses and colonization in chickens experimentally infected with Salmonella typhimurium. Avian Dis. 37:19-26. [PubMed] [Google Scholar]
- 28.Henderson, S. C., D. I. Bounous, and M. D. Lee. 1999. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 67:3580-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinton, M., G. R. Pearson, E. J. Threlfall, B. Rowe, M. Woodward, and C. Wray. 1989. Experimental Salmonella enteritidis infection in chicks. Vet. Rec. 124:223. [DOI] [PubMed] [Google Scholar]
- 30.Hsu, H. S. 1989. Pathogenesis and immunity in murine salmonellosis. Microbiol. Rev. 53:390-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu, L. B., B. Lucio, and K. A. Sachet. 1993. Depletion of CD4+ and CD8+ T lymphocyte subpopulations by CIA-1, a chicken infectious anemia virus. Avian Dis. 37:492-500. [PubMed] [Google Scholar]
- 32.Lamichhane, C. M., D. B. Snyder, M. A. Goodwin, S. A. Mengel, J. Brown, and T. G. Dickson. 1991. Pathogenicity of CL-1 chicken anemia agent. Avian Dis. 35:515-522. [PubMed] [Google Scholar]
- 33.Liebler, E. M., C. M. Press, and T. Landsverk. 1994. Lymphocyte subpopulations in jejunal and ileal Peyer's patches of calves with experimental Salmonella dublin infection. Zentralbl. Veterinarmed. B 41:113-125. [DOI] [PubMed] [Google Scholar]
- 34.Lillehoj, H. S., and K. S. Cheung. 1992. Postnatal development of T cell subpopulations in the intestinal intra epithelium and lamina propria in chickens. Vet. Immunol. Immunopathol. 31:347-360. [DOI] [PubMed] [Google Scholar]
- 35.Mast, J., and B. M. Goddeeris. 1999. Development of immunocompetence of broiler chickens. Vet. Immunol. Immunopathol. 70:245-256. [DOI] [PubMed] [Google Scholar]
- 36.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624-630. [DOI] [PubMed] [Google Scholar]
- 37.Perales, I., and A. Audicana. 1988. Salmonella enteritidis and eggs. Lancet ii:1133. [DOI] [PubMed] [Google Scholar]
- 38.Phillips, R. A., and H. M. Opitz. 1995. Pathogenicity and persistence of Salmonella enteritidis and egg contamination in normal and infectious bursal disease virus-infected leghorn chicks. Avian Dis. 39:778-787. [PubMed] [Google Scholar]
- 39.Sasai, K., K. Yoshimura, H. S. Lillehoj, G. S. Withanage, T. Fukata, E. Baba, and A. Arakawa. 1997. Analysis of splenic and thymic lymphocyte subpopulations in chickens infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 59:359-367. [DOI] [PubMed] [Google Scholar]
- 40.Shroff, K. E., K. Meslin, and J. J. Cebra. 1995. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 63:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth, J. A., D. A. Moffett, M. S. McNulty, D. Todd, and D. P. Mackie. 1993. A sequential histopathologic and immunocytochemical study of chicken anemia virus infection at one day of age. Avian Dis. 37:324-338. [PubMed] [Google Scholar]
- 42.Taniguchi, T., N. Yuasa, M. Maeda, and T. Horiuchi. 1982. Hematopathological changes in dead and moribund chicks induced by chicken anemia agent. Natl. Inst. Anim. Health Q. (Tokyo) 22:61-69. [PubMed] [Google Scholar]
- 43.Turnbull, P. C., and G. H. Snoeyenbos. 1974. Experimental salmonellosis in the chicken. 1. Fate and host response in alimentary canal, liver, and spleen. Avian Dis. 18:153-177. [PubMed] [Google Scholar]
- 44.Von Bulow, V., and K. A. Schat. 1997. Infectious anemia, p. 739-756. In B. W. Calnek, H. J. Barnes, C. W. Beard, I. R. McDougald, and M. Saif Ames (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames.
- 45.Wigley, P., A. Berchieri, K. L. Page, A. L. Smith, and B. A. Barrow. 2001. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect. Immun. 69:7873-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Withanage, G. S., K. Sasai, T. Fukata, T. Miyamoto, E. Baba, and H. S. Lillehoj. 1998. T lymphocytes, B lymphocytes, and macrophages in the ovaries and oviducts of laying hens experimentally infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 66:173-184. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi, S., N. Kaji, H. M. Munangandu, C. Kojima, M. Mase, and K. Tsukamoto. 2000. Quantification of chicken anemia virus by competitive polymerase chain reaction. Avian Pathol. 29:305-310. [DOI] [PubMed] [Google Scholar]
- 48.Yuasa, N., T. Taniguchi, and I. Yoshida. 1979. Isolation and some characteristics of an agent inducing anemia in chicks. Avian Dis. 23:366-385. [Google Scholar]