Abstract
Laboratory diagnosis of Bartonella henselae infections can be accomplished by serology or PCR assay on biopsy samples. The purpose of our work was to assess immunofluorescence detection (IFD) in lymph node smears using a specific monoclonal antibody directed against B. henselae and a commercial serology assay (IFA) compared with PCR detection. Among 200 lymph nodes examined from immunocompetent patients, 54 were positive for B. henselae by PCR, of which 43 were also positive by IFD. Among the 146 PCR-negative lymph nodes, 11 were positive by IFD. Based on PCR results, the specificity of this new technique was 92.5%, the sensitivity was 79.6%, and the positive predictive value was 79.6%. At a cutoff titer of 64, the sensitivity of the IFA was 86.8% and the specificity was 74.1%. Diagnosis of cat scratch disease (CSD) may be improved, with a specificity of 100%, when the two tests (IFD and IFA) were negative; the sensitivity was 97.4% if one of the two tests was positive. Since PCR-based detection with biopsy samples is available only in reference laboratories, we suggest using IFD coupled with the commercial serology test for the diagnosis of CSD.
Sixteen species within the genus Bartonella are now characterized, including three that have been extensively characterized as human pathogens, i.e., B. bacilliformis, the agent of Carrion's disease (21), B. quintana (31), and B. henselae (2). B. henselae, a species first recognized in 1990 (45), is the main etiological agent of cat scratch disease (CSD) (35) and is also responsible for bacillary angiomatosis and peliosis hepatis in immunocompromised (mainly AIDS) patients (25), bacteremia, and endocarditis (2). Cats are the main reservoir of B. henselae, and humans may be contaminated by cat scratches or bites; additionally, the role of the cat flea (Ctenocephalides felis) as a vector for human transmission has been proposed (2).
Techniques for diagnosing Bartonella-related infections include culturing of the pathogen (7, 24, 27), molecular biology techniques, especially PCR amplification of Bartonella spp. genes (1, 19, 23, 37, 38, 48), and serology (32, 43). Isolation of B. henselae in CSD patients has rarely been achieved (16, 27). Amplification of Bartonella spp. DNA from skin, lymph nodes, granulomatous lesions, osteolytic lesions, or less frequently from other organ biopsies or in leukoclastic vasculitis has been often reported in patients suffering CSD (1, 4, 19, 38, 40, 48) or bacillary angiomatosis (17, 37). PCR-based detection of Bartonella species from human specimens by use of various target genes remains the best method for the diagnosis of CSD. Nevertheless, this technique is only available in reference laboratories and contamination may impair its specificity. Serology is the only noninvasive diagnostic technique and has been evaluated for the diagnosis of CSD (18, 32, 36, 39, 43) and other Bartonella-related infections, including bacteremia (8-10, 22) and endocarditis (12, 15, 30, 33). The sensitivity of serology varies from one laboratory to another, ranging from near 100% to less than 30% (43). Commercially prepared antigen slides are now available for B. henselae and B. quintana serology (18, 20, 34, 39, 44, 46). Accurate diagnosis of CSD is necessary because the presentation and course of the disease may resemble more severe diseases such as malignant tumors or mycobacterial infections.
The purpose of our work was to assess an immunofluorescence assay on lymph node smears using a specific monoclonal antibody (MAb) directed against B. henselae and a commercial serology test in comparison to PCR detection using two different target genes as a reference technique to determine the best strategy for the diagnosis of CSD.
MATERIALS AND METHODS
Clinical specimens from patients suspected to have CSD between October 2001 and October 2002 were included in the study. A case of definite diagnosis of B. henselae infection (CSD group) was defined as a case in which a patient had regional lymphadenitis and a history of recent cat contact and where direct identification of a Bartonella sp. was made by PCR from lymph node tissue by using two different target genes. A standardized questionnaire (which gathered information about contact with cats or cat fleas, fever, cat scratches or bites, cutaneous lesion at the inoculation site, and treatment) was completed for each patient. When available, a serum sample from each patient was tested for Bartonella antibodies (32).
All available sera were examined for the presence of anti-B. henselae antibodies by using a commercial immunofluorescence assay (IFA; Focus Technologies, Cypress, Calif.; distributed in France by Eurobio, Paris, France) (32). The slides were prepared with the B. henselae Houston-1 and B. quintana Oklahoma strains grown in Vero cells for detection of immunoglobulin G (IgG). IgG titers of ≥64 were used as cutoffs for B. henselae antigen.
DNA extraction and PCR amplification from lymph nodes.
Total genomic DNA was extracted from samples with a QIAamp tissue kit (Qiagen, Hilden, Germany) as previously described (48). Samples were handled under sterile conditions to avoid the risk of cross-contamination. Genomic DNA was stored at 4°C until used as the template in PCR assays. The primers used for amplification and sequencing (its and pap31 genes) have been evaluated previously in our laboratory (Table 1) (48). For amplification of the Bartonella pap31 gene, a seminested PCR using three primers was applied. In each case, the PCR was carried out with PTC-200 automated thermocyclers and by using a Taq polymerase kit (Gibco-BRL, Cergy Pontoise, France). PCR amplification was performed under the following conditions: initial 3 min of denaturation at 94°C, followed by 44 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C for both genes, and extension for 45 s at 72°C. The PCR products were separated by electrophoresis on 1.5% agarose gels, visualized by staining with ethidium bromide, and then purified with a QIAquick PCR purification kit (Qiagen). PCR products were sequenced with a d-Rhodamine terminator cycle sequencing reading kit (Perkin-Elmer, Coignieres, France). Sequencing products were resolved with an ABI377 or ABI310 automated sequencer (Perkin-Elmer). Sequences obtained were compared with those in the GenBank DNA database by using the BLAST program (version 2.0; National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov])
TABLE 1.
Oligonucleotide primers used for PCR amplification and sequencing
| Primer (reference gene) | Nucleotide sequence | Detected organism | Reference(s) |
|---|---|---|---|
| URBarto1 (its) | CTT CGT TTC TCT TTC TTC A | Bartonella species | 42 |
| URBarto2 (its) | CTT CTC TTC ACA ATT TCA AT | Bartonella species | 42 |
| PAPn1 (pap31) | TTC TAG GAG TTG AAA CCG AT | Bartonella species | 47, 48 |
| PAPn2 (pap31) | GAA ACA CCA CCA GCA ACA TA | Bartonella species | 47, 48 |
| PAPnS2 (pap31) | GCA CCA GAC CAT TTT TCC TT |
For immunofluorescence detection (IFD), thin smears of all lymph nodes were made before PCR sampling. The IFD test was performed on fresh lymph node specimens. The slides were air dried, fixed with methanol for 10 min at room temperature, and stained for 30 min at 37°C with a mouse MAb specific for B. henselae (H2A10; titer of 1/3,200 diluted at 1/800 in phosphate-buffered saline [PBS]) (41). Slides were washed first in PBS (pH 7.2) with Tween and then in PBS (10 min each) and then rinsed with distilled water (5 min). After being air dried, slides were incubated with a fluorescein-isothiocyanate anti-mouse conjugate (Immunotech, Marseille, France) diluted 1:100 in PBS containing 0.2% Evans blue (Biomerieux, Marcy l'Etoile, France) for 30 min. The slides were washed as described above, air dried, mounted with Fluoprep (Biomerieux), and then examined with an epifluorescence microscope (Axioskop 20; Carl Zeiss, Göttingen, Germany) at a ×400 magnification. For each lymph node, a negative control was performed with a mouse MAb directed against Tropheryma whipplei (28). A positive control (smear from a B. henselae PCR-positive lymph node) was used in each experiment. A positive smear was defined as the presence of specific bacterial fluorescence on the slide stained with the MAb directed against B. henselae and an absence of fluorescence on the smear from the same lymph node stained with the control MAb. All smears were examined in two different experiments to confirm the results and to determine interoperator variability. Slides were viewed carefully since bacteria could be seen only in separate foci, and thus the entire smear was read.
Microbiologic cultures of lymph nodes for Bartonella isolation were performed either on blood agar plates that were incubated at 37°C with 5% CO2, examined weekly, and held for 2 months or by using a shell vial assay with human erythroid leukemia cells at 37°C with 5% CO2 as previously described (27). When other bacteria or mycobacteria were isolated, they were identified by Gram stain and biochemical reactions using standard bacteriologic methods. Histologic examination of lymph nodes was performed for all samples. Formalin-fixed paraffin-embedded tissue samples were cut and stained using routine methods, including Gram staining, hematoxylin and eosin, and periodic acid Schiff and Warthin-Starry.
Statistics.
For data comparison, the Student t test was performed using Epi Info software.
RESULTS
Overall, we used PCR to test 200 lymph node samples (from 189 patients) sent to our laboratory with a suspicion of CSD. The majority of the patients in this study were immunocompetent. Among these, amplicons were obtained for the two target genes in 54 lymph nodes of 52 patients (27.5%) with clinical and epidemiological evidence of B. henselae infection (CSD group). The sequences derived from these PCR products were 100% identical to those of B. henselae for both genes. In our study, one of these patients was coinfected with an atypical mycobacterium. Histologic examination of lymph nodes showed a granulomatous and necrotizing lymphadenitis with characteristic stellate microabscesses surrounded by palisading histiocytes. Of the remaining 137 patients (accounting for 146 lymph node samples), 55 (29%) had lymphadenopathy due to other infectious agents, 17 had malignant tumors (9%), 3 had sarcoidosis, and 62 had lymphadenopathy of unknown cause (Table 2). The mean age ± standard deviation of the 52 patients with proven B. henselae lymphadenopathy was 23.4 ± 17.8 years (range, 1 to 56 years) versus 37.8 ± 22.9 years (range, 1 to 78 years) for those in the non-CSD group (n = 137). This difference was statistically significant (P < 0.05, the Student t test). The sex ratio (male to female) was 1.28 for the CSD group versus 1.64 for the non-CSD group. For the 52 patients with CSD, all noted contact with cats, 34 (65.4%) reported scratches or bites, and 30 (57.7%) had a temperature of >38.5°C. One patient was bitten by a mouse. The localization of the lymph node was noted in 37 patients, with the following results: 16 (40.5%) axillary, 12 (32.4%) inguinal, 2 groin, 4 arm, and 3 cervical or jugulocarotid. For the non-CSD group, the localization of the lymph node was noted in 102 patients, with the following results: 21 (20.6%) axillary, 26 (25.5%) cervical, 15 (14.7%) inguinal, 8 (7.8%) mediastinal, and 32 of various sites. No lymph nodes grew Bartonella henselae after 3 months of culture.
TABLE 2.
Diagnoses for 189 patients with lymphadenopathy
| Diagnosis | No. (%) of patients |
|---|---|
| CSD | 52 (28) |
| Bacterial infection | |
| Coagulase-negative Staphylococcus | 13 |
| Staphylococcus aureus | 8 |
| Propionibacterium acnes | 3 |
| Streptococcus agalactiae | 2 |
| Pseudomonas aeruginosa | 1 |
| Enterococcus faecium | 1 |
| Methylobacterium sp. | 1 |
| Micrococcus sp. | 1 |
| Pasteurella sp. | 1 |
| Tuberculosis | 31 (16) |
| Lymphoma | 18 (10) |
| Cancer | 12 (6) |
| Sarcoidosis | 5 (3) |
| Q fever | 2 (1) |
| Tularemia | 1 (1) |
| B. quintana bacteremia | 1 (1) |
| Bacillary angiomatosis | 1 (1) |
| Candidiasis | 1 (1) |
| Unknown | 62 (33) |
| Total | 189 (100) |
Serum samples were available for 38 of 52 (73.1%) patients in the CSD group and for 58 of 137 (42.3%) patients in the non-CSD group. The sensitivity of the IFA test using a cutoff titer of ≥64 for the detection of anti-B. henselae IgG antibodies was 86.8%, and the specificity was 74.1%. For a cutoff titer of ≥128, the sensitivity was 60.5% and the specificity reached 86.2%. A final diagnosis for the 15 non-CSD patients who had positive CSD serology (titer of ≥1:64) was established in seven cases, including two cases of mycobacterial infection, one of lymphoma, one of sarcoidosis, one of Kaposi's sarcoma, and two of Staphylococcus aureus infection. In one case, Propionibacterium acnes was obtained from the lymph node and could be considered as a contaminant.
The 200 lymph nodes were tested on smears by IFD test. For the IFD assay, smears were viewed totally at a ×400 magnification and each smear was viewed for 10 min by two different operators. Among the 54 B. henselae PCR-positive lymph nodes, 43 were also positive by IFD (Fig. 1) and 11 were negative. For the 43 lymph nodes positive by IFD test, 13 were treated with antibiotics and 30 were not, whereas 3 of the 11 samples negative by IFD test were treated and 8 were not treated (P > 0.05, the Student t test). Among the 146 PCR-negative lymph nodes, 135 were IFD negative and 11 were IFD positive. Overall, the sensitivity was 79.6% and the specificity was 92.5%, with a positive predictive value of 79.6% and a negative predictive value of 92.5%.
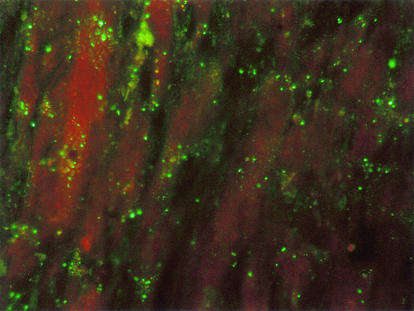
FIG. 1.
Detection of B. henselae by IFD test in the lymph node of a patient with CSD by using a MAb directed against B. henselae as viewed by fluorescence microscopy (magnification, ×400).
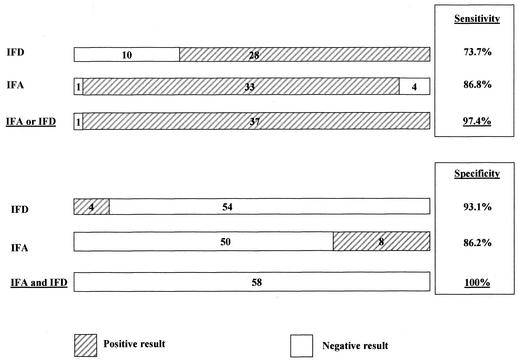
A final diagnosis for the 11 patients in the non-CSD group with IFD-positive smears was identified in seven cases, including two cases of mycobacterial infection, one of lymphoma, one of cancer, and three of Staphylococcus aureus infection. These false-positive results were obtained with samples different from those that produced false-positive results with the IFA serological test. Moreover, for the 11 patients with a negative immunofluorescence smear but who were positive by PCR, serology was available for 10 and was positive for 9 patients when the IFA cutoff titer was ≥64. Sensitivity and specificity could be improved with the coupling of tests as follows: sensitivity was 97.4% with a positive IFA (cutoff titer, ≥64) or a positive IFD, whereas specificity was 100% with a negative IFA (cutoff titer, ≥128) and negative IFD (Fig. 2).
FIG. 2.
Sensitivities and specificities obtained with IFA and/or IFD tests. For sensitivity, the cutoff titer for IFA was set at ≥64, whereas for specificity, it was set at ≥128.
DISCUSSION
CSD is usually suspected clinically, and it is confirmed by the detection of Bartonella DNA in lymph nodes. The infection is usually a self-limited disease, with frequent development of extensive regional lymph node enlargement lasting typically 2 to 3 months and occasionally longer. Surgical extirpation or lymph node aspiration is rarely needed. However, in cases of atypical presentation, lymph node biopsy is often performed to rule out more severe diseases such as malignant disease or mycobacterial infection. The isolation of B. henselae from lymph nodes of patients suffering from CSD has rarely been reported, since the bacteria are very fastidious (3, 6, 27). Thus, there is a need for suitable alternative tests or combinations of different tests to improve the diagnosis of B. henselae infections. Serological analysis by immunofluorescence or enzyme-linked immunosorbent assay is a useful noninvasive diagnostic method for the diagnosis of CSD, but specificities and sensitivities may vary according to the antigens used and disease case definition (5, 11, 32). To avoid this problem in the present study, all B. henselae cases were unambiguously diagnosed by PCR amplification of B. henselae from a lymph node biopsy sample by using two different target genes as previously reported (48). This study evaluated a new direct method for diagnosing CSD, i.e., an IFD assay using a MAb directed against B. henselae. This MAb (H2A10) was of the IgG2a subclass, reacted with a 43-kDa epitope present only in B. henselae strains, and did not cross-react with other Bartonella species (41). This MAb has been successfully used to specifically demonstrate the presence of B. henselae in erythrocytes from bacteremic cats (41). Moreover, we also evaluated a commercial IFA test to compare the two methods and to propose a strategy for the diagnosis of CSD.
We found in this study that about 28% of patients suspected of having CSD were found to have proven CSD (clinical and PCR-based detection). This proportion is very close to the result of a previous report which found that 39% of 274 patients with lymphadenopathy had CSD confirmed by PCR (48). B. henselae has been described as the main agent of CSD, but B. quintana and B. clarridgeiae have also been proposed as possible agents of the disease (13, 26, 29; D. Raoult, M. Drancourt, A. Carta, and J. A. Gastaut, Letter, Lancet 343:977, 1994). B. clarridgeaie and B. quintana were not been amplified from the lymph nodes in this study or in a previous study by our group (48). The only differences found in the patients' characteristics between the two groups (CSD and non-CSD) were in the mean age and sex ratio, which were both lower in the CSD group. These results are in accordance with the fact that the disease usually occurs in young patients (48). In the non-CSD group, patients were older and some of the chronic cases were due to more serious diseases such as tuberculosis or lymphoma.
The sensitivity and specificity of the serological test used in this study were similar to those previously reported (32) and confirm the results of studies showing the high sensitivity of the IFA test for the diagnosis of CSD (44, 46). We report a sensitivity of 86.8% at a cutoff titer of ≥64. However, specificity was low, since we found malignant processes and mycobacterial infections in the non-CSD group. Giladi et al. and Ridder et al. have reported four cases of patients with malignant tumors and high antibody titers against B. henselae (18, 39). The study of Sander et al. excluded patients with malignancy (43). Moreover, the seroprevalence in a particular geographic area as well as the antigen preparation of the respective IFA tests used should be determined before interpretation of the results (43). Even when two different serological tests were coupled, the specificity and the sensitivity were not sufficient for the diagnosis of CSD (32). False-negative PCR results could be due to a previous infection, with disappearance of the bacteria in the lymph nodes while antibodies against B. henselae remained present, suggesting that the results of PCR strongly depend on the duration of illness (39). Since CSD lymphadenopathy may last for months, it is indeed possible that patients with positive serology and negative PCR results had a previous infection due to B. henselae which was not acute at the time of sampling. This may also explain why isolation of the bacteria from lymph nodes is so difficult (27), because bacteria have disappeared from the node and positive serology results reflect a previous infection. If so, then the sensitivity and specificity of PCR and IFD would be less if we consider a retrospective diagnosis. However, for an acute diagnosis at the time of sampling, PCR and IFD were more predictive of the disease. For these reasons, we believe that diagnosis of B. henselae infections should not be made only by serology. Exclusion of other diagnoses, especially of more severe diseases such as lymphoma and mycobacterial infections, should be performed in atypical cases by histological analysis of lymph nodes.
Our new IFD assay was very easy to perform since we only needed lymph node smears. Interpretation of clinical samples, compared to negative and positive controls, was reproducible between operators. This method can be used in all laboratories with an epifluorescence microscope. Moreover, this method can be easily coupled with histological analysis and conventional culture to exclude more severe diseases, especially malignant processes and other bacterial infections. One of the problems with the IFD test was that fluorescence was not homogeneously distributed in the smears, as the bacteria were only found focally. Thus, all experiments were carried out twice to confirm the results. One may explain the 11 false-negative IFD tests by the focal presence of bacteria in the lymph node. Quantitative detection of Bartonella DNA would be of great interest to support this hypothesis but was not performed in this study. For the non-CSD group, this assay is 92.5% specific, but this is not enough since we have also found severe diseases such as mycobacterial infections and malignant processes in the non-CSD group. This type of result can lead to lack of treatment and serious consequences. Nevertheless, misdiagnosis for these cases was avoided by histological analysis of the lymph nodes. The three cases of S. aureus infection were also easily identified since culture of the bacteria was rapid and since this bacterium is known to cause false-positive results in immunofluorescence assays due to nonspecific reactions with protein A (14).
Interestingly, false-positive results with the IFA test and the IFD test were obtained with different samples and thus the two tests appear to be complementary. From our study, it is possible to propose a strategy for the diagnosis of CSD. To obtain a specificity and a positive predictive value of 100%, both IFA and IFD should be positive (using a cutoff titer for the serology of ≥128), whereas a sensitivity higher than 95% can be achieved when only one of the two tests is positive. These results for sensitivity and specificity should be interpreted cautiously since they are based on a small number of patients.
In conclusion, the present study shows the high specificity of the IFD using a MAb directed against B. henselae and performed with lymph node smears from CSD patients, especially when associated with histological analysis and conventional bacterial culture. The test described in this study could play an important role in the diagnosis of CSD since it requires only an epifluorescence microscope and can reduce costs and delays for diagnosis. Since PCR-based detection with biopsy samples is available only in reference laboratories, we suggest using this test coupled with the commercial serology test for the diagnosis of CSD.
Acknowledgments
We thank S. Dumler for reviewing the manuscript.
REFERENCES
- 1.Anderson, B., K. Sims, R. Regnery, L. Robinson, J. Schmidt, C. Goral, C. Hager, and K. Edwards. 1994. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J. Clin. Microbiol. 32:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avidor, B., Y. Kletter, S. Abulafia, Y. Golan, M. Ephros, and M. Giladi. 1997. Molecular diagnosis of cat scratch disease: a two-step approach. J. Clin. Microbiol. 35:1924-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoub, E. M., J. McBride, M. Schmiederer, and B. Anderson. 2002. Role of Bartonella henselae in the etiology of Henoch-Schonlein purpura. Pediatr. Infect. Dis. J. 21:28-31. [DOI] [PubMed] [Google Scholar]
- 5.Barka, N. E., T. Hadfield, M. Patnaik, W. A. Schartzman, and J. B. Peter. 1993. EIA for detection of Rochalimaea henselae-reactive IgG, IgM and IgA antibodies in patients with suspected cat-scratch disease. J. Infect. Dis. 167:1503-1504. [DOI] [PubMed] [Google Scholar]
- 6.Bergmans, A. M. C., M. F. Peeters, J. F. P. Schellekens, M. C. Vos, L. J. M. Sabbe, J. M. Ossewaarde, H. Verbakel, H. J. Hooft, and L. M. Schouls. 1997. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J. Clin. Microbiol. 35:1931-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, S. A., J. A. Rooney, P. Manzewitsch, and R. L. Regnery. 1997. Isolation of Bartonella (Rochalimaea) henselae: effects of methods of blood collection and handling. J. Clin. Microbiol. 35:544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouqui, P., B. Lascola, V. Roux, and D. Raoult. 1999. Chronic Bartonella quintana bacteremia in homeless patients. N. Engl. J. Med. 340:184-189. [DOI] [PubMed] [Google Scholar]
- 9.Comer, J. A., C. Flynn, R. L. Regnery, D. Vlahov, and J. E. Childs. 1997. Antibodies to Bartonella species in inner-city intravenous drug users in Baltimore, Md. Arch. Intern. Med. 156:2491-2495. [PubMed] [Google Scholar]
- 10.Cooper, M., M. Hollingdale, J. Vinson, and J. Costa. 1976. A passive hemagglutination test for diagnosis of trench fever due to Rochalimaea quintana. J. Infect. Dis. 134:605-609. [DOI] [PubMed] [Google Scholar]
- 11.Dalton, M. J., L. E. Robinson, J. Cooper, R. L. Regnery, J. G. Olson, and J. E. Childs. 1995. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a National Referral Center. Arch. Intern. Med. 155:1670-1676. [PubMed] [Google Scholar]
- 12.Drancourt, M., J. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt, M., V. Moal, P. Brunet, B. Dussol, Y. Berland, and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infection in a seronegative hemodialyzed patient. J. Clin. Microbiol. 34:1158-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsgren, A., and J. Sjoquist. 1966. “Protein A” from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J. Immunol. 97:822-827. [PubMed] [Google Scholar]
- 15.Fournier, P. E., J. L. Mainardi, and D. Raoult. 2002. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 9:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier, P. E., J. Robson, Z. Zeaiter, R. McDougall, S. Byrne, and D. Raoult. 2002. Improved culture from lymph nodes of patients with cat scratch disease and genotypic characterization of Bartonella henselae isolates in Australia. J. Clin. Microbiol. 40:3620-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasquet, S., M. Maurin, P. Brouqui, H. Lepidi, and D. Raoult. 1998. Bacillary angiomatosis in immunocompromised patients. AIDS 12:1793-1803. [DOI] [PubMed] [Google Scholar]
- 18.Giladi, M., Y. Kletter, B. Avidor, E. Metzkor-Cotter, M. Varon, Y. Golan, M. Weinberg, I. Riklis, M. Ephros, and L. Slater. 2001. Enzyme immunoassay for the diagnosis of cat-scratch disease defined by polymerase chain reaction. Clin. Infect. Dis. 33:1852-1858. [DOI] [PubMed] [Google Scholar]
- 19.Goral, S., B. Anderson, C. Hager, and K. M. Edwards. 1994. Detection of Rochalimaea henselae DNA by polymerase chain reaction from suppurative nodes of children with cat-scratch disease. Pediatr. Infect. Dis. J. 13:994-997. [DOI] [PubMed] [Google Scholar]
- 20.Harrison, T. G., and N. Doshi. 1999. Serological evidence of Bartonella spp. infection in the UK. Epidemiol. Infect. 123:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihler, G. M. 1996. Bartonella bacilliformis: dangerous pathogen slowly emerging from deep background. FEMS Microbiol. Lett. 144:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, L. A., D. H. Spach, D. A. Kippen, N. K. Sugg, R. L. Regnery, M. H. Sayers, and W. E. Stamm. 1996. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J. Infect. Dis. 173:1023-1026. [DOI] [PubMed] [Google Scholar]
- 23.Jalava, J., P. Kotilainen, S. Nikkari, M. Skurnik, E. Vanttinen, O. P. Lehtonen, E. Eerola, and P. Toivanen. 1995. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin. Infect. Dis. 21:891-896. [DOI] [PubMed] [Google Scholar]
- 24.Koehler, J. E., F. D. Quinn, T. G. Berger, P. E. Leboit, and J. W. Tappero. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 327:1625-1631. [DOI] [PubMed] [Google Scholar]
- 25.Koehler, J. E., M. A. Sanchez, C. S. Garrido, M. J. Whitfeld, F. M. Chen, T. G. Berger, M. C. Rodriguez-Barradas, P. E. Leboit, and J. W. Tappero. 1997. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis-peliosis. N. Engl. J. Med. 337:1876-1883. [DOI] [PubMed] [Google Scholar]
- 26.Kordick, D. L., E. J. Hilyard, T. L. Hadfield, K. H. Wilson, A. G. Steigerwalt, D. J. Brenner, and E. B. Breitschwerdt. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J. Clin. Microbiol. 35:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Scola, B., and D. Raoult. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, Z., B. La Scola, and D. Raoult. 2002. Monoclonal antibodies to immunodominant epitope of Tropheryma whipplei. Clin. Diagn. Lab. Immunol. 9:156-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margileth, A. M., and D. F. Baehren. 1998. Chest-wall abscess due to cat-scratch disease (CSD) in an adult with antibodies to Bartonella clarridgeiae: case report and review of the thoracopulmonary manifestations of CSD. Clin. Infect. Dis. 27:353-357. [DOI] [PubMed] [Google Scholar]
- 30.Maurin, M., F. Eb, J. Etienne, and D. Raoult. 1997. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J. Clin. Microbiol. 35:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurin, M., and D. Raoult. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurin, M., J. M. Rolain, and D. Raoult. 2002. Comparison of in-house and commercial slides for detection of immunoglobulins G and M by immunofluorescence against Bartonella henselae and Bartonella quintana. Clin. Diagn. Lab. Immunol. 9:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 34.Rath, P. M., G. von Recklinghausen, and R. Ansorg. 1997. Seroprevalence of immunoglobulin G antibodies to Bartonella henselae in cat owners. Eur. J. Clin. Microbiol. Infect. Dis. 16:326-327. [DOI] [PubMed] [Google Scholar]
- 35.Regnery, R. L., B. E. Anderson, J. E. Clarridge, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regnery, R. L., T. G. Olson, B. A. Perkins, and W. Bibb. 1992. Serological response to Rochalimaea henselae antigen in suspected cat-scratch disease. Lancet 339:1443-1445. [DOI] [PubMed] [Google Scholar]
- 37.Relman, D. A., J. S. Loutit, T. M. Schmith, S. Falkow, and L. S. Tompkins. 1990. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573-1580. [DOI] [PubMed] [Google Scholar]
- 38.Renesto, P., J. Gouvernet, M. Drancourt, V. Roux, and D. Raoult. 2001. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 39:430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridder, G. J., C. C. Boedeker, K. Technau-Ihling, R. Grunow, and A. Sander. 2002. Role of cat-scratch disease in lymphadenopathy in the head and neck. Clin. Infect. Dis. 35:643-649. [DOI] [PubMed] [Google Scholar]
- 40.Rolain, J. M., V. Chanet, H. Laurichesse, J. Beytout, and D. Raoult. Cat scratch disease with vertebral osteomyelitis and spleen abscesses. Ann. N. Y. Acad. Sci., in press. [DOI] [PubMed]
- 41.Rolain, J. M., B. La Scola, Z. Liang, B. Davoust, and D. Raoult. 2001. Immunofluorescent detection of intraerythrocytic Bartonella henselae in naturally infected cats. J. Clin. Microbiol. 39:2978-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roux, V., and D. Raoult. 1995. The 16S-23S rRNA intergenic spacer region of Bartonella (Rochalimaea) species is longer than usually described in other bacteria. Gene 156:107-111. [DOI] [PubMed] [Google Scholar]
- 43.Sander, A., R. Berner, and M. Ruess. 2001. Serodiagnosis of cat scratch disease: response to Bartonella henselae in children and a review of diagnostic methods. Eur. J. Clin. Microbiol. Infect. Dis. 20:392-401. [DOI] [PubMed] [Google Scholar]
- 44.Sander, A., M. Posselt, K. Oberle, and W. Bredt. 1998. Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: evaluation and comparison of two commercial serological tests. Clin. Diagn. Lab. Immunol. 5:486-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slater, L., D. F. Welch, D. Hensel, and D. W. Coody. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N. Engl. J. Med. 323:1587-1593. [DOI] [PubMed] [Google Scholar]
- 46.Zbinden, R., N. Michael, M. Sekulovski, A. von Graevenitz, and D. Nadal. 1997. Evaluation of commercial slides for detection of immunoglobulin G against Bartonella henselae by indirect immunofluorescence. Eur. J. Clin. Microbiol. Infect. Dis. 16:648-652. [DOI] [PubMed] [Google Scholar]
- 47.Zeaiter, Z., P. E. Fournier, H. Ogata, and D. Raoult. 2002. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int. J. Syst. Evol. Microbiol. 52:165-171. [DOI] [PubMed] [Google Scholar]
- 48.Zeaiter, Z., P. E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]