Abstract
Macrophage inflammatory protein-1α (MIP-1α) and MIP-1β play an important role in modulating immune responses. To understand their importance in immunity to placental malaria (PM) and in human immunodeficiency virus (HIV)-PM coinfection, we investigated levels of these chemokines in the placental intervillous blood plasma (IVB plasma) and cord blood plasma of HIV-negative PM-negative, HIV-negative PM-positive, HIV-positive PM-negative, and HIV-positive PM-positive women. Compared to HIV-negative PM-negative women, the MIP-1β concentration in IVB plasma was significantly elevated in HIV-negative PM-positive women and HIV-positive PM-positive women, but it was unaltered in HIV-positive PM-negative women. Also, PM-infected women, irrespective of their HIV status, had significantly higher levels of MIP-1β than HIV-positive PM-negative women. The MIP-1α level was not altered in association with either infection. The IVB plasma levels of MIP-1α and MIP-1β positively correlated with the cord blood plasma levels of these chemokines. As with IVB plasma, only cord plasma from PM-infected mothers had significantly elevated levels of MIP-1β compared to PM-negative mothers, irrespective of their HIV infection status. MIP-1β and MIP-1α levels in PM-positive women were positively associated with parasite density and malaria pigment levels. Regardless of HIV serostatus, the IVB MIP-1β level was significantly lower in women with PM-associated anemia. In summary, an elevated level of MIP-1β was associated with PM. HIV infection did not significantly alter these two chemokine levels in IVB plasma.
In areas where malaria is endemic, pregnant women are more susceptible to Plasmodium falciparum infection and experience a greater severity of malaria than their nonpregnant counterparts (3, 14). In holoendemic areas of malaria, placental malaria (PM) has been found to be associated with the low birthweight of the offspring, which increases infant morbidity and mortality (20). Furthermore, in tropical regions of Africa, where malaria is endemic, the rate of human immunodeficiency virus (HIV) infection has rapidly increased. Importantly, pregnant women with HIV infection have a much higher risk of acquiring P. falciparum infection than those with no HIV infection (19). However, it is not known why HIV-infected pregnant women are more susceptible to P. falciparum infection than HIV-negative women.
In a previous study, our investigators demonstrated that production of gamma interferon (IFN-γ) by maternal placental blood (intervillous blood [IVB]) mononuclear cells is associated with protective immunity to PM (16). Subsequently, our investigators also reported that the impaired IFN-γ and interleukin-12 (IL-12) responses by IVB mononuclear cells are associated with the increased susceptibility to PM in HIV-positive women (5, 15). These findings suggested that alterations in the cytokine balance in HIV-positive pregnant women may be one of the contributing factors for the enhanced susceptibility to P. falciparum infection.
In addition to cytokines, chemokines also play an important role in the regulation of immune responses and in controlling HIV infection and progression. Macrophage inflammatory protein-1α (MIP-1α) and MIP-1β are from the CC (cysteine-cysteine) chemokine subfamily. These soluble factors are chemotactic for specific types of leukocyte populations and are involved in the regulation of cell-mediated immunity (13). MIP-1α and MIP-1β are produced by monocytes, macrophages, lymphocytes, and other cell types (13). CCR1, CCR4, and CCR5 are the known receptors that bind to MIP-1α (12, 17). CCR1, CCR5, and CCR8 are the known receptors for MIP-1β (12, 17). In addition, it has been demonstrated that these CC chemokines can enhance IFN-γ production (10, 23) through the engagement of the CCR5 receptor.
Despite the significant roles that these CC chemokines play in immune and inflammatory responses, their role in protection against PM is not well understood. Tkachuk et al. have shown that macrophages and Hofbauer cells from PM-infected but not PM-uninfected placentas express CCR5 (24). Since CCR5 is a coreceptor for macrophage-tropic HIV type 1 (HIV-1) (8), this finding suggests that upregulation of this receptor may increase the potential reservoir for HIV in the placenta by increasing the number of HIV target cells. However, it was not known from this study whether any of the chemokines that bind to this receptor, such as RANTES, MIP-1α, or MIP-1β, were upregulated following PM infection.
Thus, to understand further the role of these chemokines in immune responses to PM and HIV-PM coinfection, we addressed the following questions in this study: (i) does PM upregulate MIP-1α or MIP-1β in the IVB? (ii) Is there a correlation between these chemokine levels in the IVB plasma versus cord plasma? (iii) Does HIV infection alter the levels of these chemokines in the IVB with any potential implications for the transient loss of immunity to malaria during pregnancy in HIV-infected mothers? (iv) Is there any correlation between severe outcomes associated with PM, such as anemia, and these CC chemokine levels? These questions were addressed using IVB and cord blood plasma obtained from a cohort of western Kenyan women with (i) HIV-negative PM-negative, (ii) HIV-negative PM-positive, (iii) HIV-positive PM-negative, or (iv) HIV-positive PM-positive infection status.
MATERIALS AND METHODS
Study site, participants, and samples.
This study was conducted retrospectively using IVB plasma samples obtained from women who participated in a cohort study to assess the impact of PM on mother-to-child HIV-1 transmission (2). Pregnant women attending the antenatal clinic and delivery ward in the New Nyanza Provincial General Hospital in Kisumu, western Kenya, participated in this study. Kisumu experiences intense perennial transmission of P. falciparum malaria, with two peak transmission periods occurring from November to December and May to July.
Informed consent was obtained from all patients associated with this study, and human subjects guidelines of the Centers for Disease Control and Prevention (CDC) institutional review board, the University of Georgia, and the Kenya Medical Research Institute (KEMRI) ethical review committee were strictly followed. Only healthy women (in both HIV-positive and HIV-negative groups) with uncomplicated labor and singleton, vaginal deliveries were included for this study. Women who were likely to have AIDS were excluded. A total of 98 women were included in this study, and their distribution in different groups was as follows: 32 HIV negative, PM negative; 22 HIV negative, PM positive; 32 HIV positive, PM negative; and 12 HIV positive, PM positive. The geometric mean (interquartile range [IQR]) of parasite density was 0.6 × 103 (0.1 × 103 to 3.2 × 103) and 2 × 103 (1.9 × 103 to 30 × 103) per μl of whole blood for HIV-negative, PM-positive and HIV-positive, PM-positive women, respectively. There was no significant difference in the parasite density between these two groups. The HIV status was determined by simultaneously testing with two HIV rapid test kits as reported previously (16). Women with discordant HIV test results were excluded. The mean age and gravidity were not significantly different between the four infection status groups.
Collection of plasma samples.
A sample of IVB was collected by making an incision on the maternal side (basal plate) of the placenta. The IVB and cord blood samples were collected in EDTA-containing tubes. Plasma from the IVB and cord blood samples was separated within a few hours by centrifugation and stored in liquid nitrogen until used.
Determination of parasite density.
Thick blood films were used to assess P. falciparum parasitemia in the IVB (16). Placental thick blood smears were prepared from IVB, stained with 10% Giemsa, and examined under oil immersion for malaria parasites. The parasite density was calculated by assuming that each microliter of blood contains 8,000 leukocytes; this represents only an approximation, since the actual leukocyte count will vary among different people. In this study, high-density P. falciparum malaria infection was indicated by counts of ≥10,000 parasitized erythrocytes per μl of blood, whereas low-density P. falciparum malaria infection was indicated by counts of <10,000 parasitized erythrocytes per μl of blood.
Scoring of hemozoin pigment.
Hemozoin pigments in leukocytes were examined on IVB thick blood smears by light microscopy. The percentage of leukocytes containing malaria pigments was scored as 0 = none/300 leukocytes, 1 = <10%, 2 = 10 to 25%, 3 = 26 to 50%, and 4 = >50%.
Measurement of Hb.
At the time of delivery, maternal peripheral blood from either a fingerprick or venipuncture was collected for hemoglobin (Hb) determination. Hb levels were measured using a Hemocue machine (HemoCue AB, Angelholm, Sweden). Of 34 PM-positive women, the Hb data were missing for 2 individuals. In this study, any PM-positive woman with an Hb level of <8 g/dl was considered to have a malaria-associated anemia.
Measurement of cytokine and chemokine levels in supernatants by ELISA.
A double-sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure chemokine levels, as described elsewhere (16). The MIP-1α level was detected using a capture mouse anti-human MIP-1α monoclonal antibody (clone 93321) and a biotinylated goat anti-human MIP-1α antibody from R&D Systems Inc. (Minneapolis, Minn.). The MIP-1β level was measured using a capture mouse monoclonal anti-human MIP-1β monoclonal antibody (clone 24006.111) and a biotinylated goat anti-human MIP-1β antibody from R&D Systems Inc. Cytokine concentrations were determined using a standard curve obtained from the known concentration of cytokine standards included in each assay plate.
Statistical analyses.
Unless otherwise indicated, women were grouped according to infection status: (i) HIV negative, PM negative; (ii) HIV negative, PM positive; (iii) HIV positive, PM negative; and (iv) HIV positive, PM positive. The SAS statistical software package (version 8.02; SAS Institute, Inc., Cary, N.C.) was used for data analysis. Since the distribution of data from this study was assumed to lack normality and due to the small sample size, nonparametric tests were used for analysis of the ranked data. The nonparametric Wilcoxon rank-sum (WRS) test was used for comparisons of two independent sample groups. To compare the averages of the ranked data in more than two groups, the nonparametric Kruskal-Wallis (KW) test was performed. If the result from a comparison of multiple groups by KW test yielded statistical significance, the permutation method from the MULTTEST procedure (SAS proc multtest) was used to obtain the adjusted P value for each pair of groups in multiple comparisons. To quantify correlations between chemokine level and clinical parameter, the Spearman ranked correlation test was used. The strength of correlation between two variables was determined by the Spearman's rank correlation coefficient (rho). Two-sided P values of ≤0.05 were considered statistically significant.
RESULTS
IVB MIP-1α and MIP-1β in relation to PM and HIV infection status.
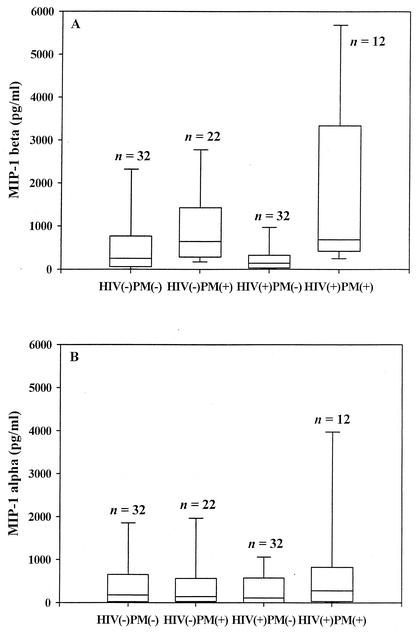
We compared the levels of MIP-1α and MIP-1β in the IVB plasma of HIV-positive and HIV-negative mothers with and without PM (Fig. 1). Overall, there was a significant difference in the MIP-1β levels in IVB plasma among groups of mothers with different HIV and PM status (P = 0.0003; KW). IVB plasma MIP-1β levels were significantly elevated in PM-positive mothers, irrespective of their HIV status, in comparison to PM-negative mothers (P < 0.0001; WRS). In contrast, HIV infection alone did not significantly impact MIP-1β levels, since there was no significant difference in this chemokine level between HIV-positive women and HIV-negative women, irrespective of their PM status (P = 0.2; WRS).
FIG. 1.
The placental IVB plasma levels of MIP-1α and MIP-1β in HIV-infected and HIV-uninfected women with or without PM. The box plot encloses the range from the 25th to the 75th percentiles of chemokine levels and is transected by a line at the median. The lowermost and uppermost horizontal lines indicate the 10th and 90th percentiles. The sample size is indicated on top of each box plot. (A) MIP-1β levels in IVB plasma. Overall, 95% (93 of 98) of the participants had a positive MIP-1β response. HIV negative PM negative versus HIV negative PM positive, P = 0.05; HIV negative PM negative versus HIV positive PM negative, not significant (P > 0.05); HIV negative PM negative versus HIV positive PM positive, P = 0.03; HIV positive PM negative versus HIV positive PM positive, P = 0.002; HIV negative PM positive versus HIV positive PM negative, P = 0.002. (B) MIP-1α levels in IVB plasma. Overall, 81% (79 of 98) of the participants had a positive MIP-1α response. There were no significant differences between any of the groups of mothers with HIV and PM infection status (P > 0.05; KW).
To assess the impact of these infections as single or coinfections on MIP-1β levels, the four groups were examined in multiple comparisons. These analyses revealed that the MIP-1β levels of PM-positive women, regardless of HIV serostatus, were significantly higher than those of HIV-negative, PM-negative mothers (HIV-negative, P = 0.05; HIV-positive, P = 0.03; proc multtest). In addition, HIV-positive, PM-positive mothers had a significantly higher level of MIP-1β than HIV-positive, PM-negative mothers (P = 0.002; proc multtest). In contrast, HIV-positive, PM-negative mothers did not have a significant difference in the MIP-1β level compared to HIV-negative, PM-negative mothers (P > 0.05).
There were no significant differences in the IVB plasma levels of MIP-1α between the four different groups of mothers (P = 0.6; KW), suggesting that neither PM infection nor HIV infection significantly modulated the placental IVB plasma response of this chemokine (PM positive versus PM negative, P = 0.3; HIV negative versus HIV positive, P = 0.5).
Cord MIP-1α and MIP-1β in relation to PM and HIV infection status.
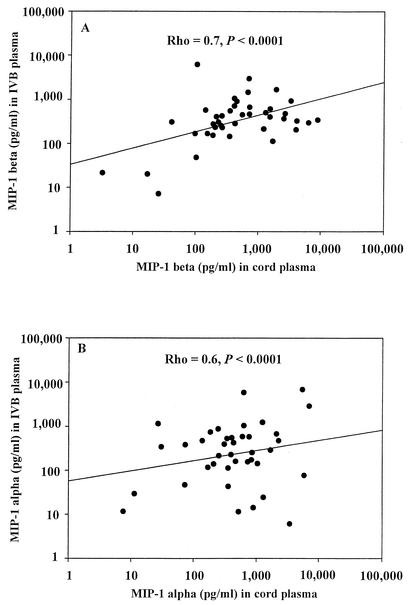
We determined the levels of MIP-1α and MIP-1β in the cord plasma. There was a strong positive correlation between cord MIP-1β and IVB MIP-1β levels (rho = 0.7; P < 0.0001) (Fig. 2A). Similarly, MIP-1α levels in the cord and IVB plasma showed significant positive correlation (rho = 0.6; P < 0.0001) (Fig. 2B). The level of MIP-1β in the cord plasma of mothers with PM infection (median = 407 pg/ml; IQR = 284 to 1,452; n = 10) was significantly elevated compared to the level in mothers with PM-negative status (median = 223 pg/ml; IQR = 0 to 444; n = 44), irrespective of their HIV status (P = 0.01; WRS). However, there was no significant difference in the MIP-1β levels in the cord plasma among four different groups of mothers when stratified based on both HIV and PM infection status (P = 0.08; KW).
FIG. 2.
Correlation between IVB and cord plasma levels of MIP-1β and MIP-1α. The data from both HIV-positive and HIV-negative women are included. A total of 54 matched-pair plasma samples were included; their distributions in each of the groups were 22 HIV negative PM negative, 5 HIV negative PM positive, 5 HIV positive PM negative, and 22 HIV positive PM positive. Other details are as described in the legend for Fig. 1. Common log scale types were set for both the y axis and x axis. Each data point represents the chemokine concentration for the individual participant. (A) Scatter plot of IVB MIP-1β levels and cord MIP-1β levels. Spearman's rank correlation coefficient (rho) = 0.7; P < 0.0001. (B) Scatter plot of IVB MIP-1α levels and cord MIP-1α levels. Spearman's rank correlation coefficient (rho) = 0.6; P < 0.0001.
The levels of cord MIP-1 α were not significantly different among groups of mothers with different PM and HIV status (P = 0.6; KW).
Gravidity-based differences.
There were no statistically significant differences observed in the levels of MIP-1α (P = 0.5; KW) or MIP-1β (P = 0.4; KW) when women were compared on the basis of gravidity (data not shown).
Association between parasite density and chemokine levels.
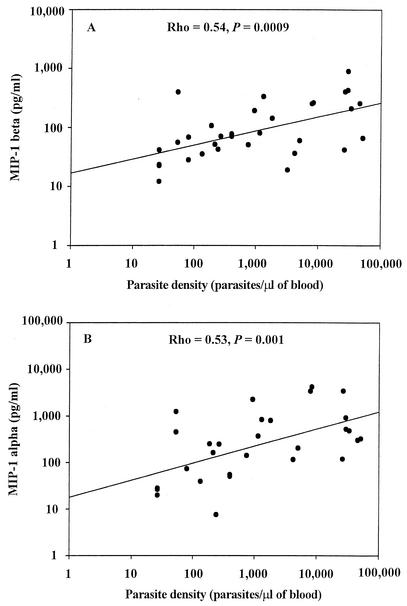
The parasite density positively correlated with IVB MIP-1β levels (Fig. 3A) (rho = 0.54; P = 0.0009) and MIP-1α levels (Fig. 3B) (rho = 0.53; P = 0.001). Women with high-density P. falciparum malaria infection (n = 7) in the placenta had significantly elevated levels of MIP-1β (median [IQR] level, 2,563 [662 to 4,274] pg/ml; P < 0.02; WRS) and MIP-1α (median [IQR] level, 484 [304 to 921] pg/ml; P < 0.05; WRS) compared to mothers with low-density PM (n = 27; MIP-1β median [IQR] level, 559 [283 to 1,069] pg/ml; MIP-1α median [IQR] level, 115 [26 to 453] pg/ml).
FIG. 3.
Association between parasite density and IVB MIP-1α and IVB MIP-1β levels. The data from both HIV-positive (n = 12) and HIV-negative (n = 22) women with PM were included. Other details are as described in the legend for Fig. 1. Common log scale types were set for both the y axis and x axis. Each data point represents the chemokine concentration for the individual participant. (A) Scatter plot of MIP-1β levels in IVB plasma and parasite density. Spearman's rank correlation coefficient (rho) = 0.54; P = 0.0009. (B) Scatter plot of MIP-1α levels in IVB plasma and parasite density. Spearman's rank correlation coefficient (rho) = 0.53; P = 0.001.
Association between malaria pigment levels and CC chemokine levels.
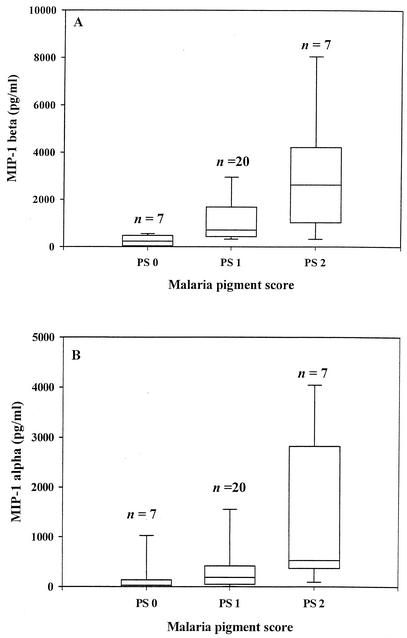
Since a previous study had shown that malaria pigments can stimulate MIP-1α and MIP-1β production (18), we investigated whether there is an association between these chemokine levels and malaria pigment load (as determined by pigment scores) (Fig. 4). Both MIP-1β (P < 0.001 [proc multtest]) and MIP-1α (P < 0.05 [proc multtest]) levels were significantly elevated in groups with the highest pigment score (PS 2) compared to their respective no-pigment groups (Fig. 4A). On the other hand, only MIP-1β (P < 0.01 [proc multtest]), and not MIP-1α, was significantly elevated in the low-pigment group (PS 1) in comparison to the respective no-pigment groups (Fig. 4B).
FIG. 4.
MIP-1α and MIP-1β levels in relation to malaria pigment scores. The chemokine levels (median with IQR) in three different groups based on the relative malaria pigment loads are shown. Pigment scoring was as follows: PS 0 (no detectable pigment), PS 1 (low pigment score), and PS 2 (intermediate and high pigment scores combined). The box encloses the range from the 25th to the 75th percentiles and is transected by a line at the median. The lowermost and uppermost horizontal lines indicate the 10th and 90th percentiles. Values on top of each box plot indicate sample size. The data from both HIV-positive and HIV-negative women are included. Other details are as described in the legend for Fig. 1. (A) MIP-1β levels in IVB plasma. PS 0 versus PS 1, P < 0.01; PS 0 versus PS 2, P < 0.001 (proc multtest). (B) MIP-1α levels in IVB plasma. PS 0 versus PS 1, P > 0.05; PS 0 versus PS 2, P < 0.05 (proc multtest).
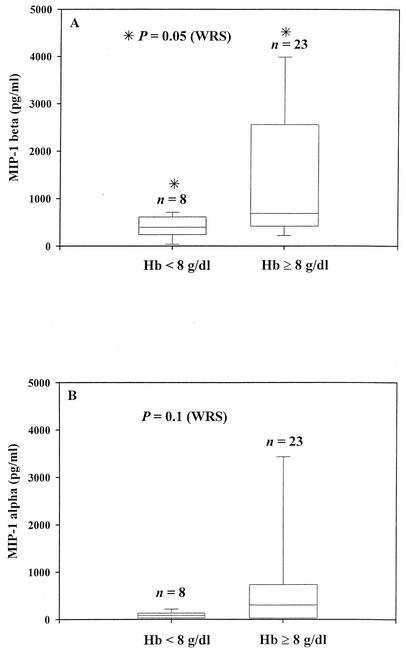
Association between Hb levels and CC chemokine levels.
We assessed if there is an association between the PM-associated anemia status and the CC chemokine levels measured in this study (Fig. 5). Since there was no significant difference in the CC chemokine levels between HIV-negative, PM-positive and HIV-positive, PM-positive mothers (P > 0.5; WRS), we grouped all PM-positive mothers (n = 32) together, irrespective of their HIV status. Among PM-positive mothers, MIP-1β levels (Fig. 5A) were significantly reduced in mothers with anemia (Hb < 8 g/dl; P = 0.05; WRS). On the other hand, although the MIP-1α median level was lower in the anemic mothers than in nonanemic mothers, the difference was not significant (Fig. 5B). These CC chemokine levels were not associated with poor birth outcomes such as low birth weight and preterm delivery (data not shown).
FIG. 5.
MIP-1β (A) and MIP-1α (B) levels in relation to Hb level. The chemokine data (median with IQR) from PM-positive women (including both HIV-positive and HIV-negative women) with anemia (Hb < 8g/dl) were compared to those for subjects without anemia (Hb ≥ 8g/dl). The box encloses the range from the 25th to the 75th percentiles and is transected by a line at the median. The lowermost and uppermost horizontal lines indicate the 10th and 90th percentiles. Values on top of each box plot are sample size. Other details are as described in the legend for Fig. 1. A significant difference is indicated by an asterisk.
DISCUSSION
MIP-1α and MIP-1β play important roles in recruiting macrophages, dendritic cells, and T cells to sites of infection and lymphoid organs (6). In this study, we found that PM infection significantly upregulated the IVB plasma level of MIP-1β, irrespective of the maternal HIV status. MIP-1α levels were elevated only in women with high-density PM infection. However, HIV infection itself did not significantly alter the levels of these two chemokines in the IVB plasma. Since MIP-1α and MIP-1β levels were not significantly altered due to HIV infection, it is unlikely that these factors directly contribute to the increased susceptibility to malaria in HIV-infected women during pregnancy. However, their impact on mother-to-child transmission of HIV, especially in HIV-PM-coinfected women, remains to be investigated.
A characteristic feature of PM is the accumulation of large numbers of monocytes/macrophages in the infected placenta. However, the role of chemokines, such as MIP-1α and MIP-1β, in recruiting the macrophage/monocyte populations to the P. falciparum-infected placenta has not been well understood. The results of the present study show that in the malaria-infected IVB, there is a significant increase in the production of MIP-1β, with MIP-1α being upregulated in the presence of high-density P. falciparum infection. Our findings are consistent with a recent report (1) which showed that MIP-1α and to a lesser extent MIP-1β are elevated in placental IVB plasma and correlate with mononuclear cell density in the placenta. Together, these findings suggest that these CC chemokines may play an important role in attracting T-cell and monocyte/macrophage populations to the malaria-infected placenta.
MIP-1α and MIP-1β levels in the cord blood are positively correlated with the IVB plasma levels of these two chemokines. It is unclear whether these chemokines are to some extent transported from the maternal circulation to that of the fetus or that the fetal blood cells themselves produce these chemokines. Previous studies have reported the presence of these chemokines in cord blood (9, 22). One of these studies showed that cord blood mononuclear cells can produce these chemokines in response to lipopolysaccharide in vitro, which suggested that cord blood cells are competent to produce these chemokines. It is not known whether the presence of higher concentrations of MIP-1β in the cord plasma of neonates from PM-infected mothers is due to transfer from the maternal side, or due to activation of cord blood cells by the passively acquired malarial antigens from the maternal side, or both.
In some previous studies, MIP-1α has been reported to inhibit erythropoiesis (4, 7, 21). Therefore, we determined if there is any correlation between the levels of these chemokines in the IVB plasma and malaria-associated anemia. Surprisingly, we found a significant negative correlation between malaria-associated anemia and MIP-1 β, but not MIP-1α, levels. It is unlikely that MIP-1β is directly involved in the pathogenesis of malarial anemia, since a recent study has clearly demonstrated that this chemokine does not affect erythropoiesis (11). Therefore, we speculate that the most likely explanation for the present finding is that low levels of MIP-1β may be indirectly associated with malaria-associated anemia.
A previous study showed that malaria pigments can induce MIP-1α and MIP-1β production in human peripheral blood mononuclear cells (18). Our results are consistent with this finding, in that MIP-1α and MIP-1β levels in the IVB plasma were related to malaria pigment loads. Thus, it appears that the presence of large numbers of malaria pigment-loaded macrophages in the placenta may serve to activate production of MIP-1α and MIP-1β. Alternatively, it is also possible that increased production of these chemokines in the high-pigment group may be simply due to higher parasite load, since high-density parasitemia will ultimately result in a higher pigment load in the macrophages. Further investigation in in vitro studies will be necessary to determine whether pigments are more potent than malaria antigens and/or parasites in activating IVB mononuclear cells to produce MIP-1α and MIP-1β.
Unlike MIP-1β, MIP-1α levels were significantly elevated only in women with high-density PM infection. This observation suggests that activation of MIP-1α by malarial antigens or malaria pigments may require a higher threshold of activation than MIP-1β. Further studies will be required to determine how malarial antigens and malaria pigments activate production of these chemokines and any consequent effects on immunity to malaria.
In conclusion, we have shown that PM infection is associated with the significant upregulation of MIP-1β, but not MIP-1α. We speculate that MIP-1β may be involved in regulating macrophages and mobilization of protective Th1 lymphocyte populations to the malaria-infected placenta. This study has also shown that increased susceptibility to PM in HIV-infected women is not associated with a defective production of MIP-1α and MIP-1β. Furthermore, low levels of MIP-1β in the IVB were associated with malaria-associated anemia in pregnant women.
Acknowledgments
This investigation received financial support from the Medical Scholars Program of Mahidol University (to S.C.), United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (grant 960568 to V.U., Principle Investigator [PI]), and U.S. Agency for International Development (grants A0T0483-PH1-2171 and HRN-A-00-04-00010-02 to B.L.N. [PI]). J.M.M. was supported by the National Institutes of Health (grant AI-50240).
We thank all of the study participants for donating their placentas for our research efforts. We thank KEMRI and the Director of KEMRI, Davy Koech, for his approval with regard to publication of this paper. We also acknowledge the cooperation of the NNGPH Labour Ward staff and the diligent efforts of the CDC/KEMRI staff in conducting this study. We thank John Vulule, Director of the Centre for Vector Biology Control and Research, KEMRI, and Richard Steketee, Chief, Malaria Epidemiology Branch, DPD, CDC, for their continued support. We appreciate Charles Todd for his valuable comments.
REFERENCES
- 1.Abrams, E. T., H. Brown, S. W. Chensue, G. D. Turner, E. Tadesse, V. M. Lema, M. E. Molyneux, R. Rochford, S. R. Meshnick, and S. J. Rogerson. 2003. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J. Immunol. 170:2759-2764. [DOI] [PubMed] [Google Scholar]
- 2.Ayisi, J. G., A. M. van Eijk, F. O. ter Kuile, M. S. Kolczak, J. A. Otieno, A. O. Misore, P. A. Kager, R. W. Steketee, and B. L. Nahlen. 2000. Risk factors for HIV infection among asymptomatic pregnant women attending an antenatal clinic in western Kenya. Int. J. STD AIDS 11:393-401. [DOI] [PubMed] [Google Scholar]
- 3.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer, H. E., B. Sherry, S. Cooper, L. Lu, R. Maze, M. P. Beckmann, A. Cerami, and P. Ralph. 1993. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J. Immunol. 150:3448-3458. [PubMed] [Google Scholar]
- 5.Chaisavaneeyakorn, S., J. M. Moore, J. Otieno, S. C. Chaiyaroj, D. J. Perkins, Y. P. Shi, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Immunity to placental malaria. III. Impairment of interleukin (IL)-12, not IL-18, and interferon-inducible protein-10 responses in the placental intervillous blood of human immunodeficiency virus/malaria-coinfected women. J. Infect. Dis. 185:127-131. [DOI] [PubMed] [Google Scholar]
- 6.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 7.de Wynter, E. A., J. Durig, M. A. Cross, C. M. Heyworth, and N. G. Testa. 1998. Differential response of CD34+ cells isolated from cord blood and bone marrow to MIP-1 alpha and the expression of MIP-1 alpha receptors on these immature cells. Stem Cells 16:349-356. [DOI] [PubMed] [Google Scholar]
- 8.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 9.Hariharan, D., W. Ho, J. Cutilli, D. E. Campbell, and S. D. Douglas. 2000. C-C chemokine profile of cord blood mononuclear cells: selective defect in RANTES production. Blood 95:715-718. [PubMed] [Google Scholar]
- 10.Luther, S. A., and J. G. Cyster. 2001. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2:102-107. [DOI] [PubMed] [Google Scholar]
- 11.Majka, M., J. Ratajczak, B. Lee, M. Honczarenko, R. Douglas, M. A. Kowalska, L. Silberstein, A. M. Gewirtz, and M. Z. Ratajczak. 2000. The role of HIV-related chemokine receptors and chemokines in human erythropoiesis in vitro. Stem Cells 18:128-138. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Garcia, J., D. L. Kolson, and F. Gonzalez-Scarano. 2002. Chemokine receptors in the brain: their role in HIV infection and pathogenesis. AIDS 16:1709-1730. [DOI] [PubMed] [Google Scholar]
- 13.Matsukawa, A., C. M. Hogaboam, N. W. Lukacs, and S. L. Kunkel. 2000. Chemokines and innate immunity. Rev. Immunogenet. 2:339-358. [PubMed] [Google Scholar]
- 14.McGregor, I. A., M. E. Wilson, and W. Z. Billewicz. 1983. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans. R. Soc. Trop. Med. Hyg. 77:232-244. [DOI] [PubMed] [Google Scholar]
- 15.Moore, J. M., J. Ayisi, B. L. Nahlen, A. Misore, A. A. Lal, and V. Udhayakumar. 2000. Immunity to placental malaria. II. Placental antigen-specific cytokine responses are impaired in human immunodeficiency virus-infected women. J. Infect. Dis. 182:960-964. [DOI] [PubMed] [Google Scholar]
- 16.Moore, J. M., B. L. Nahlen, A. Misore, A. A. Lal, and V. Udhayakumar. 1999. Immunity to placental malaria. I. Elevated production of interferon-gamma by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. J. Infect. Dis. 179:1218-1225. [DOI] [PubMed] [Google Scholar]
- 17.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 18.Sherry, B. A., G. Alava, K. J. Tracey, J. Martiney, A. Cerami, and A. F. Slater. 1995. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 45:85-96. [PubMed] [Google Scholar]
- 19.Steketee, R. W., J. J. Wirima, P. B. Bloland, B. Chilima, J. H. Mermin, L. Chitsulo, and J. G. Breman. 1996. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am. J. Trop. Med. Hyg. 55:42-49. [DOI] [PubMed] [Google Scholar]
- 20.Steketee, R. W., J. J. Wirima, L. Slutsker, D. L. Heymann, and J. G. Breman. 1996. The problem of malaria and malaria control in pregnancy in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 55:2-7. [DOI] [PubMed] [Google Scholar]
- 21.Su, S., N. Mukaida, J. Wang, Y. Zhang, A. Takami, S. Nakao, and K. Matsushima. 1997. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1α by interacting mainly with a C-C chemokine receptor, CCR1. Blood 90:605-611. [PubMed] [Google Scholar]
- 22.Sullivian, S. E., S. L. Staba, J. A. Gersting, A. D. Hutson, D. Theriaque, R. D. Christensen, and D. A. Calhoun. 2002. Circulating concentrations of chemokines in cord blood, neonates, and adults. Pediatr. Res. 51:653-657. [DOI] [PubMed] [Google Scholar]
- 23.Taub, D. D., S. M. Turcovski-Corrales, M. L. Key, D. L. Longo, and W. J. Murphy. 1996. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 156:2095-2103. [PubMed] [Google Scholar]
- 24.Tkachuk, A. N., A. M. Moormann, J. A. Poore, R. A. Rochford, S. W. Chensue, V. Mwapasa, and S. R. Meshnick. 2001. Malaria enhances expression of CC chemokine receptor 5 on placental macrophages. J. Infect. Dis. 183:967-972. [DOI] [PubMed] [Google Scholar]