NATURE OF THE PROBLEM
BW agents.
The release of a biological weapon (BW) agent by a terrorist group or military force would likely be silent and undetectable or nearly so. As shown by anthrax attack during the fall of 2001 in the eastern United States, patients would begin appearing at hospitals and clinics within several days of exposure, most presenting with nonspecific flu-like symptoms. The first days of the outbreak might not even cause undue concern. However, depending on the type of agent and the method of dispersal, the public healthcare system would rapidly be stretched to capacity and beyond.
The qualities that make a good BW agent are its relationship between aerosolization, infectivity, or toxicity and the amount of agent required to produce an effect (48). In addition, criteria such as environmental stability, ease of production, disease severity, and communicability determine which agents are the most likely to be utilized. For maximum effect, an optimal agent should be highly lethal and easily produced in large quantities and have limited options for preventive or prophylactic treatment. Given that the respiratory route is the most effective for most BW agents, stability in an aerosol form and the capability to be readily dispersed also in an aerosol (1- to 10-μm particle size) are necessary. When potential agents are reviewed for these characteristics, Bacillus anthracis (anthrax) and variola major virus (smallpox) are considered to have the greatest potential for mass casualties and civil disruption. Also high on a prospective list of agents are botulinum neurotoxins, Yersinia pestis, and Francisella tularensis (48, 91, 92). Lower on the prospective list are Burkholderia pseudomallei and Burkholderia mallei, Rickettsia sp., Coxiella burnetii, Venezuelan equine encephalitis virus, Marburg and Ebola viruses, and influenza viruses (48, 63, 91, 92).
Emerging infectious disease agents.
In addition to diseases caused by intentional epidemics, there are several emerging infectious diseases (ID) with the potential for significant public health consequences, including dengue fever, West Nile fever, and Rift Valley fever as well as the recent reemergence of malaria in the eastern United States (48, 63, 91, 92). As with BW agents, emerging ID agents may be directly transmissible or vector borne (63). A complex interplay of factors can influence disease emergence, including genetic variation, environmental changes, and population pressures. Further compounding this already complicated situation, are the estimated 600 million international tourists annually, many with the potential to the spread disease globally in a matter of hours (63). Clearly, the challenges facing modern clinical microbiologists and immunologists are daunting enough without the added difficulties posed by the intentional release of BW/ID agents!
Because of the threat posed by both BW and emerging or reemerging ID agents, there is a need to rapidly identify such agents in the clinical setting in order to treat the individuals at risk and to improve public health surveillance and epidemiology. To meet this challenge, a vast array of assay strategies has been developed for use in clinical diagnostics and environmental detection. Over the past 20 years, technologies have been developed or adapted to the challenges posed by these agents, permitting detection and identification in several minutes to hours. In particular, the development of improved reagents and detection equipment has led to dramatic improvements in the sensitivity and specificity of immunoassay systems, allowing an ever-increasing range of analytes to be identified and quantitated. Recent developments in molecular biology techniques have made possible the production of fusion antibody conjugates, which may lead to further improvements in the sensitivity and cost of reagents, as well as possibly revolutionizing the production of monoclonal antibodies. At the same time, simple-to-use, inexpensive assay systems have been developed with the necessary reliability, accuracy, and sensitivity to bring immunoassay technology to much more diverse areas such as outpatient monitoring, large screening programs in developing countries, and remote environmental surveillance. As a result of these continual improvements, immunoassays are now the most widely used analytical technique in laboratory medicine, embracing a vast repertoire of analytes that are analyzed by an increasingly diverse range of devices. This brief review will detail the current state of the art in rapid immunological assays for the detection and identification of BW/ID agents and also offer a look into the future of the technology and its application.
ANTIBODY GENERATION
Antibodies—the most critical reagent.
The single most critical reagent in immunological assays remains the antibody. Extensively used as diagnostic tools, they are key components in the vast array of tests used by clinical laboratories. The phenomenal growth in the range and scope of immunoassays has largely arisen through the exquisite specificity that can be achieved by different types of antibodies. Even the nature of the antibody reagents critical to such assays is evolving in technological complexity. Over the last quarter century, polyclonal antibodies have been largely replaced in clinical assays by monoclonal antibodies while recombinant antibodies may be poised as the next-generation reagent. While each type of antibodies has its own advantages and disadvantages in terms of generation, cost, and overall utility, monoclonal and recombinant antibodies have a key advantage over their polyclonal brethren: the potential of an unlimited supply of uniform reagents, enabling broad standardization of methodologies.
As might be expected, all currently fielded rapid immunological assays for the detection and identification of BW/ID agents share one common trait: they require the use of antibodies. In most cases, antibody affinity and specificity are the limiting factors of these assays. As a result, the antibodies selected for use in an assay must be chosen with great care. Criteria for selection include, but are not limited to, the ability to bind to a desired target with high affinity while at the same time retaining high specificity. These attributes are determined empirically based on extensive testing with a robust panel of target agents, molecular and environmental mimics, and potential interferents. Such a specificity panel usually includes (or should include) genetic near neighbors as well as material or agents from environmental or biological sources likely to be contaminants of samples.
Antibody development.
Suitable polyclonal and monoclonal antibodies can be developed in a number of different ways. The most straightforward is to inject host animals, such as mice for monoclonal development or rabbits or goats for polyclonal development, with live or inactivated material and then fuse the spleens or collect the blood of those animals that have high titers to the target agent (31). If done correctly, this method tends to produce high-affinity antibodies with neutralizing titers (31). However, antibodies produced in this manner do not tend to be overly specific for a particular organism but rather broadly cross-reactive to many related species. An effective screening procedure is thus critical for obtaining specific antibodies that are not cross-reactive. As a result, this approach works the best with purified toxins or other large proteins that have unique antigenic sites readily accessible for antibody binding and maturation. A second approach involves the immunization of animals with selected fractions of viral and bacterial lysates (31). For example, to detect an intact live agent from clinical or environmental specimens, the best antibodies are usually generated by using membrane-bound or -associated protein fractions as immunogens. Suitable antigens can be solubilized with detergent or left as an insoluble matrix (31) and then used to immunize an appropriate host. A third approach involves the immunization of animals with highly purified proteins (31). Such proteins are selected on the basis of being unique to the target organism as well as based on immunogenic potential. These antigens can be either biochemically purified or genetically engineered and expressed by standard methods. A final approach to antibody development is genetic immunization (5, 38, 83). Genetic immunization can be used to generate specific antibodies by delivering the gene, which encodes the target protein in a eukaryotic expression vector, rather than the protein itself (5, 38, 83). This approach is well suited to cases in which the protein is difficult to purify or when a gene, but not the protein, has been obtained.
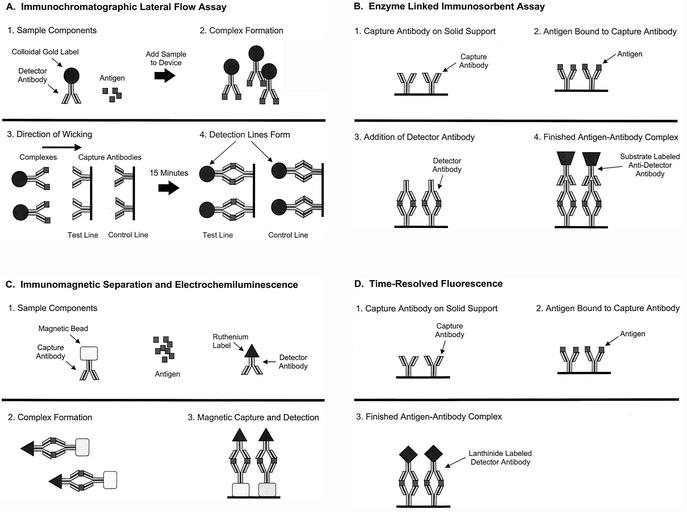
Once antibodies of the desired specificity and affinity have been developed, they can be utilized in a broad range of immunoassays (Table 1). We will discuss four immunoassays that are the most widely used for the detection and identification of potential BW/ID agents: immunochromatographic lateral flow assays, enzyme-linked immunosorbent assays (ELISA), time-resolved fluorescence (TRF) assays, and immunomagnetic separation-electrochemiluminescence (IMS-ECL) assays.
TABLE 1.
Examples of BW/ID agents, accepted detection and identification methods, and available rapid immunological assaysa
| Agent | Standard method(s) | Immunological assay
|
|||
|---|---|---|---|---|---|
| HHA | ELISA | ECL | TRF | ||
| Bacteria | |||||
| Bacillus anthracis | Culture, γ phage, FA, | X | X | X | X |
| Brucella sp. | Culture, FA | X | X | ||
| Burkholderia sp. | Culture | X | X | ||
| Coxiella bumetii | Culture, serology, FA, | X | X | ||
| Escherichia coli O157:H7 | Culture, serology | X | X | X | |
| Francisella tula- rensis | Culture, FA | X | X | X | |
| Salmonella sp. | Culture, serology | X | X | ||
| Shigella sp. | Culture | X | X | ||
| Vibrio cholerae | Culture, serology | X | X | ||
| Yersinia pestis | Culture, FA | X | X | ||
| Parasites, Plasmo- dium sp. | Microscopy | X | X | ||
| Toxins | |||||
| Aflatoxins | HPLC, MS | X | |||
| C. botulinum neurotoxins | Bioassay | X | X | X | X |
| Ricin | ELISA | X | X | X | |
| Saxitoxin | Bioassay | X | |||
| Shigatoxin | Bioassay | X | X | ||
| Staphylococcal enterotoxins | ELISA | X | X | X | X |
| Viruses | |||||
| Dengue | Culture, CF, FA | X | X | ||
| Ebola | Culture, EM, FA | X | |||
| Rift Valley fever | Culture, FA | X | |||
| Variola major (smallpox) | Culture, EM | X | X | ||
| West Nile fever | Culture, serology | X | |||
| Yellow fever | Culture, CF, FA | X | |||
Abbreviations: CF, complement fixation; EM, electron microscopy; FA, fluorescent antibody; HHA, hand held assay; HPLC, high-performance liquid chromatography; MS, mass spectroscopy; X, assay available.
IMMUNOCHROMATOGRAPHIC LATERAL FLOW ASSAYS
Current assay technology and limitations.
Immunochromatographic assays were first described in the late 1960s and were originally developed to assess the presence of serum proteins (47). Other early assays that used an immunochromatographic technique include those for the quantification of drugs in biological fluids (100), theophylline in whole blood (57), and mouse immunoglobulin (36). Over the past decade many immunochromatographic assays have been reported for the detection of infectious diseases (2-4, 8, 9, 13, 15-17, 21, 22, 27, 29, 37, 42, 59-61, 69, 74-76, 84, 94), cancer (35, 72), cardiovascular problems (54, 65, 66, 73), pancreatitis (43), and illicit drugs (7, 95). Other promising areas for the use of such assays are drug monitoring (93), food safety (10), and veterinary medicine (45, 50).
Assays using this format are rapid, taking approximately 15 min to run, and are also simple to use, requiring only the dilution of the test agent in a sample buffer and applying several drops (∼200 μl) to the test strip (Fig. 1A). Typical handheld assay devices contain a colloidal gold (or other)-labeled antibody dried onto a filter pad affixed to a nitrocellulose strip. A capture antibody is applied in a line on the strip and dried. To perform the test, a specimen is suspended in buffer and added to the pad containing the colloidal gold-labeled antibody. The antibody specifically binds to antigen present in the specimen, and the resulting complex wicks down the membrane where it binds to the capture antibody. A positive reaction is visualized as a red line created by the bound colloidal gold. Similar assays using different detection systems have been described in the literature, including those based on latex particles and upconverting phosphatases (30, 64).
FIG. 1.
Principles of the four primary immunological assays. (A) Lateral flow immunochromatographic assay (handheld assay); (B) ELISA; (C) ECL; (D) TRF.
The present generation of handheld assays have several limitations. First, only one agent can be detected per assay strip. Thus, if an unknown sample needs to be characterized, several handheld assays must usually be run to obtain a presumptive identification. The second limitation is that each of the assays have varying sensitivity levels to their respective target agents. Assays for bacterial agents tend to be the most sensitive, able to detect from 2 × 105 to 2 × 106 CFU/ml while those for toxins have sensitivities ranging from 50 pg/ml to 50 ng/ml. Assays specific for viruses usually have the lowest sensitivities, ranging from 2 × 105 to 5 × 107 PFU/ml. Third, since these assays are visualized as a red line created by the bound colloidal gold, the sensitivity is limited to what can be seen by the unaided (and uncalibrated) human eye. Typically, an arbitrary quantitation of the detection sensitivity of these assays is done by assigning a number between 0 and 5, with the increasing intensity of the red line assigned a higher value. Besides the somewhat arbitrary nature of this process, numeric values can vary based on the skill of the technician responsible for validating a given lot of assays.
Enhanced labeling and detection approaches.
Recent advances in detection and labeling technologies that would in some instances improve the sensitivities of assays by at least an order of magnitude and make detection quantitative, not merely subjective, may offset the disadvantages inherent to present handheld assays. To detect very low levels of antigen, which may be present at low concentrations in vivo or in environmental samples, the sensitivity of conventional gold-labeled lateral flow assays can be enhanced up to one order of magnitude by using a silver enhancement step. Lateral flow assays are run, as described above, washed in phosphate-buffered saline and a Tween 20 solution, and then immersed in a silver enhancer reagent for 5 min. Horton and coworkers reported sensitivities of 100 ng/ml before enhancement and 100 pg/ml after enhancement (36). This system has the advantage of greater sensitivity but does not need a specialized mechanism to read the assay, which is advantageous for use in a field setting.
Alternative approaches to antibody labeling coupled with specialized quantitative readers can also lead to significant improvements in the sensitivity of lateral flow immunoassays. For example, superparamagnetic nanoparticles comprising either iron oxide (Fe3O4) or iron oxide and a polysaccharide matrix can be used to label antibodies in place of gold. Their broad potential for laboratory applications has been demonstrated by their use in detection of typhoid-specific antibodies, cell separation, and antibody sorting (53, 88-90). More recently, systems using paramagnetic particle labels have been described that make it possible to use iron oxide particles as labels in place of gold (6, 49, 70, 71). The labeled antibody-antigen mixture wicks up the membrane, as described above, and is deposited at the site of the solid-phase antibody, and the magnetic flux is measured in the antigen capture zone. This technology has three advantages. First, the signal is permanent and can be read more than one time. Second, the signal is quantitative and can be assigned a value in millivolts. Third, the signal generated is comparable to the detection limits seen with radionucleotide labels or nephelometric techniques (70, 71).
ELISAS
In the early 1970s, the search for simple but sensitive methods for the quantitative detection of antigen and antibody that did not rely upon particle agglutination or radiolabeled reagents led to the development of solid-phase enzyme-coupled reagent assays (23). In principal, the labeling by chemical conjugation of an enzyme bound to either antigen or antibody allows detection of immune complexes formed on a solid phase as the fixed enzyme, once washed free of excess reagents, and on subsequent substrate interaction, yields a colored product that is directly visualized and/or quantitatively measured by optical density. The resulting assays, ELISAs, are economical, versatile, robust, and simple assays that achieve separation of bound and free moieties by use of a solid-phase support. Because of their combined simplicity and sensitivity, ELISAs can be used reliably for screening large numbers of small-volume test samples in the simplest of laboratory environments. This technical advance has had its greatest impact in epidemiology and in the diagnosis of ID (41, 67, 96).
In general, two-site antigen-capture assays are used for the detection of BW/ID agents. This assay format is simple, specific, sensitive, and readily converted to the lateral flow assay format previously described above. As illustrated in Fig. 1B, a capture antibody (often a monoclonal of high affinity) affixed to the solid phase is exposed to a test sample (as well as to positive- and negative-control samples) and, after washing, the complex is exposed further to diluted detector antibody specific to the same antigen. Finally, a conjugate antibody is added and the reaction is visualized. In this system, the antigen must have multiple epitopes for antibody binding, or a repeating, spatially distant, single epitope. Such an assay can be very sensitive and specific. The use of a monoclonal antibody as the capture antibody usually results in a finished assay of high specificity and low background. However, the addition of a polyclonal antibody can greatly increase the breadth of the assay to detect multiple isolates of the same species of bacteria, virus, or fungus. With minor modifications, this assay can detect serum immune complexes. The antibody isotypes of the immune complexes can be determined and quantified by using a panel of isotype-specific antiglobulin conjugates on the same test samples in repeated assays.
TRF ASSAYS
Assays based on TRF use lanthanide chelate labels with unique fluorescence properties (24, 25, 33, 34, 55, 56, 58, 78, 79). Key among these properties is a very long fluorescence decay time and an exceptionally large Stokes' shift. The long fluorescence decay time allows the user to measure fluorescence after the background has fully subsided. Additionally, the label, a lanthanide chelate, is dissociated from the antibody or reporter molecule into a new, highly fluorescent chelate within a protective micelle. Most importantly, these lanthanide chelates can be used in place of other labels typically used in ELISA-based procedures. Cumulatively, these factors contribute to the high sensitivity and low backgrounds characteristic of immunologic assays based on TRF.
TRF assays are set up in a traditional 96-well two-site antigen-capture ELISA format. As illustrated in Fig. 1C, a capture antibody (most often a monoclonal of high affinity) affixed to the solid phase is exposed to a test sample (as well as to positive- and negative-control samples) and, after washing, the complex is exposed further to diluted detector antibody specific to the same antigen which is labeled with a lanthanide chelate (europium, samarium, terbium, or dysprosium). Europium, Eu3+, is the label that is mainly used. A low-pH enhancement solution is then added, which causes the lanthanide to dissociate from the labeled compound. This form of the lanthanide is highly fluorescent. As in an ELISA, the antigen must have multiple epitopes for antibody binding, or a repeating, spatially distant, single epitope. TRF assays have many of the same limitations as ELISAs; primarily, these are a function of the antibodies used. Additionally, special care must be taken with these assays to avoid lanthanide contamination, including the use of dedicated measuring devices and rigorous washing techniques.
A key advantage of TRF assays is that they can be up to one order of magnitude more sensitive because of the highly fluorescent nature of the lanthanide label (68). Recently several papers have been published which report the use of this reporter technology in clinical specimens for the detection of low-level proteins that are below the sensitivity limit of traditional ELISAs as well as BW agents in both clinical and environmental specimens (18-20, 26, 39, 40, 68, 85, 97). Also, as with ELISAs, these assays can be modified to detect serum immune complexes (1). The antibody isotypes of the immune complexes can be determined and quantified by using a panel of isotype-specific antiglobulin conjugates on the same test samples in repeated assays.
IMS-ECL ASSAYS
Recent advances in IMS and ECL have led to the development of several related technologies and systems including the ORIGEN immunoassay system (Igen, Inc., Rockville, Md.), QPCR 5000 (Applied Biosystems, Foster City, Calif.), and magnetic ECL detection systems (11, 98, 99).
IMS has been used for soluble and particulate antigen capture, separation, purification, and concentration efficiently with high-affinity antibodies for several decades (32, 51, 86). A key feature of this technology is its ability to capture and concentrate antigens from a variety of complex biological matrices. One of the major advantages of IMS is the increased reaction kinetics as a result of the potentially greater surface area on the magnetic beads compared to conventional ELISA and immunological reaction within a turbulent bead suspension (62, 80). Additionally, beads can be mixed rapidly or slowly to encourage rapid capture of soluble antigens or gentle docking with particulate antigens. A further advantage of the magnetic beads is the rapid separation of antibody-captured materials from the surrounding milieu when placed in a magnetic field. These beads contain paramagnetic magnetite (FE3O4) that is magnetizable in the presence of an external field but not in its absence. The beads have a large number of different sizes, ranging from a few nanometers to several micrometers. They are usually spherical, but the shape is dependent on the manufacturing process and the needs of the end user.
The format and principle of an ECL assay is similar to that of assays based on ELISA and TRF technologies (Fig. 1D). Detection is accomplished by the heavy metal chelate ruthenium (II) tris-bipyridal Ru(bpy)32+ conjugated to a detector antibody. Initially Ru(bpy)32+ and tripropylamine (TPA) are oxidized at the surface of an anode. TPA immediately loses a proton, becoming a powerful reducer. This causes Ru(bpy)33+ to enter a high-energy state by a high-energy electron transfer from the electron carrier, TPA. Relaxation to the ground state results in light emission detectable at 620 nm. Ru(bpy)32+ is not consumable during the reaction and may be oxidized and excited again due to excess TPA used in the buffer (98). The ECL assay format has been used by several groups to detect BW agents, including B. anthracis and staphylococcal enterotoxin B (28, 44, 99).
As with TRF assays, assays based on IMS and ECL technology have limitations similar to those of ELISA, and again, these are primarily a function of the antibodies used—the better the avidity and affinity of the antibody, the more sensitive and specific the assay. As with other highly sensitive assays, signal-to-noise ratios need to be closely studied and the limit of detection needs to be carefully analyzed (68, 77).
FUTURE TRENDS
The advances in immunological reagents and assay formats are being matched by improvements in complementary laboratory technology, ranging from automated analyzers and microarrays that facilitate the analysis of large numbers of samples, to self-contained miniaturized devices that enable an immunoassay to be performed at the point of care or in a field setting. Together, these novel reagents and new technologies are likely to transform diagnostic medicine over the next decade as much as our recognition of the civilian public health threat posed by BW/ID agents has.
On the horizon are even further advances in sensitivity and throughput. Most are based a combination of existing immunological systems coupled with electronic sensing modules (12). An automated system has been described that utilizes solid-phase ELISA coupled with a multichannel optical flow cell with a sensor composed of a light-emitting diode and a photodetector (46). Simultaneous detection of staphylococcal enterotoxin B, bacteriophage M13, and Escherichia coli has been accomplished with this immunosensor. Another prototype system that uses a light-addressable potentiometric sensor and a flowthrough immunofiltration enzyme assay can detect eight agents simultaneously within 15 min (87). While the limit of specific detection is considerably higher than that of more mature assays, the speed and multiplexing offered by this approach are promising. Other approaches, based on microfluidic arrays and photosensors, have also been reported and show promising improvements in sensitivity with no loss in specificity (81, 82). The main advantages offered by these experimental systems is that in addition to automation, multiplexing, and throughput, they offer a quantitative assessment of the agent present, detecting in some instances fewer than 50 copies of target agent.
While the systems described above are all fairly elaborate, laboratory-based systems, similar improvements in handheld immunoassays are also on the horizon. Recently, a self-contained handheld biosensor has been described that uses immunoaffinity for specificity and fluorescence for quantitation (14). The prototype is automated and requires no special storage. It is also multiuse: approximately 100 measurements can be made before refurbishment is required. Sensitivity is also promising. Using aflatoxins, a detection limit has been demonstrated at concentrations from 0.1 to 50 ppb in less than 2 min. The design flexibility suggests that it could be readily adapted for the detection of other biological analytes.
Besides these advances in automation and throughput, another technique has been demonstrated that may significantly improve the sensitivity of current solid-phase immunoassays. This approach, based on force differentiation, subjects a labeled antigen-antibody complex to a magnetic field of defined magnitude and orientation, displacing weakly bound nonspecific particles while leaving the specific immunochemical complex intact (52). The number of antigen-antibody complexes bound to the surface after applying the differentiation force was related to the analyte concentration, permitting the development of an optical detection scheme to count the number of such complexes. In this prototype, the sensitivity of the force differentiation assay was one to two orders of magnitude higher than conventional solid-phase immunoassay techniques, while retaining 99% specificity.
Although new and improved assays and sensor formats will continue to be developed, the most critical component of any immunological test will likely remain the antibody itself. Ultimately, improvements in the affinity, specificity, and mass production of antibodies will dictate the success or failure of a given immunoassay technology. Sensitive and specific detection of BW and ID agents by immunoassays has improved by several orders of magnitude over the past 30 years. If recent scientific progress is a fair indicator, the future promises continued improvements in immunoassays with an ever-increasing array of applications.
REFERENCES
- 1.Aggerbeck, H., B. Norgaard-Pedersen, and I. Heron. 1996. Simultaneous quantitation of diphtheria and tetanus antibodies by double antigen, time-resolved fluorescence immunoassay. J. Immunol. Methods 190:171-183. [DOI] [PubMed] [Google Scholar]
- 2.Aidoo, S., W. K. Ampofo, J. A. Brandful, S. V. Nuvor, J. K. Ansah, N. Nii-Trebi, J. S. Barnor, F. Apeagyei, T. Sata, D. Ofori-Adjei, and K. Ishikawa. 2001. Suitability of a rapid immunochromatographic test for detection of antiboies to human immunodeficiency virus in Ghana, West Africa. J. Clin. Microbiol. 39:2572-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allwinn, R., C. Schieferstein, S. Clauke, and H. W. Doerr. 1999. Rapid diagnosis of primary dengue fever by the immunochromatographic test and by electron microscopy-a case report. Infection 27:365-367. [DOI] [PubMed] [Google Scholar]
- 4.Araz, E., M. Tanyuksel, N. Ardic, and C. Tabuk. 2000. Performance of a commercial immunochromatographic test for the diagnosis of vivaz malaria in Turkey. Trans. R. Soc. Trop. Med. Hyg. 94:55-56. [DOI] [PubMed] [Google Scholar]
- 5.Barry, M. A., M. E. Barry, and S. A. Johnston. 1994. Production of monoclonal antibodies by genetic immunization. BioTechniques 16:616-618, 620. [PubMed]
- 6.Baselt, D. R., G. U. Lee, M. Natesan, S. W. Metzger, P. E. Sheehan, and R. J. Colton. 1998. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 13:731-739. [DOI] [PubMed] [Google Scholar]
- 7.Beck, O., M. Kraft, M. R. Moeller, B. L. Smith, S. Schneider, and R. Wennig. 2000. Frontline immunochromatographic device for on-site urine testing of amphetamines: laboratory validation using authentic specimens. Ann. Clin. Biochem. 37:199-204. [DOI] [PubMed] [Google Scholar]
- 8.Berdal, B. P., R. Mehl, H. Haaheim, M. Loksa, R. Grunow, J. Burans, C. Morgan, and H. Meyer. 2000. Field detection of Francisella tularensis. Scand. J. Infect. Dis. 32:287-291. [DOI] [PubMed] [Google Scholar]
- 9.Bhaskar, S., S. Singh, and M. Sharma. 1996. A single-step immunochromatographic test for the detection of Entamoeba histolytica in stool samples. J. Immunol. Methods 196:193-198. [DOI] [PubMed] [Google Scholar]
- 10.Bird, C. B., R. L. Miller, and B. M. Miller. 1999. Reveal for Salmonella test system. J. AOAC Int. 82:625-633. [PubMed] [Google Scholar]
- 11.Blackburn, G. F., H. P. Shah, J. H. Kenten, J. Leland, R. A. Kamin, J. Link, J. Peterman, M. J. Powell, A. Shah, D. B. Talley, S. K. Tyagi, E. Wilkens, T. Wu, and R. J. Massey. 1991. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin. Chem. 37:1534-1539. [PubMed] [Google Scholar]
- 12.Bossi, A., S. A. Piletsky, P. G. Righetti, and A. P. Turner. 2000. Capillary electrophoresis coupled to biosensor detection. J. Chromatogr. A 892:143-153. [DOI] [PubMed] [Google Scholar]
- 13.Buser, J., L. Risch, T. Rutz, S. Mannang, and J. Munzinger. 2001. Comparison of a rotavirus latex agglutination test with two rapid immunochromatographic test devices for detection of rotavirus in human feces. Eur. J. Clin. Microbiol. Infect. Dis. 20:295-296. [DOI] [PubMed] [Google Scholar]
- 14.Carlson, M. A., C. B. Bargeron, R. C. Benson, A. B. Fraser, T. E. Phillips, J. T. Velky, J. D. Groopman, P. T. Strickland, and H. W. Ko. 2000. An automated, handheld biosensor for aflatoxin. Biosens. Bioelectron. 14:841-848. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarti, A., R. Gur, N. Berry, and M. D. Mathur. 2000. Evaluation of three commercially available kits for serological diagnosis of dengue haemorrhagic fever. Diagn. Microbiol. Infect. Dis. 36:273-274. [DOI] [PubMed] [Google Scholar]
- 16.Chanteau, S., L. Rahalison, M. Ratsitorahina, Mahafaly, M. Rasolomaharo, P. Biosier, T. O'Brien, J. Aldrich, A. Keleher, C. Morgan, and J. Burans. 2000. Early diagnosis of bubonic plague using F1 antigen capture ELISA assay and rapid immunogold dipstick. Int. J. Med. Microbiol. 290:279-283. [DOI] [PubMed] [Google Scholar]
- 17.Ching, W. M., D. Rowland, Z. Ahang, A. L. Bourgeois, D. Kelly, G. A. Dasch, and P. L. Devine. 2001. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin. Diagn. Lab. Immunol. 8:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daijo, J. E., and J. R. Sportsman. 1999. A time-resolved fluorescence immunoassay for insulin in rodent plasma. J. Pharm. Biomed. Anal. 19:335-342. [DOI] [PubMed] [Google Scholar]
- 19.Diamandis, E. P. 1991. Multiple labeling and time-resolvable fluorophores. Clin. Chem. 37:1486-1491. [PubMed] [Google Scholar]
- 20.Diamandis, E. P., and T. K. Christopoulos. 1991. Time-resolved immunofluorometric detection of antigens separated by high-performance liquid chromatography and coated to polystyrene. BioTechniques 10:646-648. [PubMed] [Google Scholar]
- 21.Dominguez, J., N. Gali, S. Blanco, P. Pedroso, C. Prat, L. Matas, and V. Ausina. 2001. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest 119:243-249. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez, J., N. Gali, L. Matas, P. Pedroso, A. Hernandez, E. Padilla, and V. Ausina. 1999. Evaluation of a rapid immunochromatographic assay for the detection of Legionella antigen in urine samples. Eur. J. Clin. Microbiol. Infect. Dis. 18:896-898. [DOI] [PubMed] [Google Scholar]
- 23.Engvall, E., and P. Perlmann. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871-874. [DOI] [PubMed] [Google Scholar]
- 24.Evangelista, R. A., A. Pollak, B. Allore, E. F. Templeton, R. C. Morton, and E. P. Diamandis. 1988. A new europium chelate for protein labelling and time-resolved fluorometric applications. Clin. Biochem. 21:173-178. [DOI] [PubMed] [Google Scholar]
- 25.Evangelista, R. A., A. Pollak, and E. F. Templeton. 1991. Enzyme-amplified lanthanide luminescence for enzyme detection in bioanalytical assays. Anal. Biochem. 197:213-224. [DOI] [PubMed] [Google Scholar]
- 26.Fiet, J., F. Giton, P. Boudou, J. M. Villette, H. Soliman, G. Morineau, A. Boudi, and H. Galons. 2001. A new specific and sensitive time resolved-fluoroimmunoassay of 11-deoxycortisol in serum. J. Steroid Biochem. Mol. Biol. 77:143-150. [DOI] [PubMed] [Google Scholar]
- 27.Garcia, L. S., and R. Y. Shimizu. 2000. Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J. Clin. Microbiol. 38:1267-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatto-Menking, D. L., H. Yu, J. G. Bruno, M. T. Goode, M. Miller, and A. W. Zulich. 1995. Sensitive detection of biotoxoids and bacterial spores using an immunomagnetic electrochemiluminescence sensor. Biosens. Bioelectron. 10:501-507. [DOI] [PubMed] [Google Scholar]
- 29.Grunow, R., W. Splettstoesser, S. McDonald, C. Otterbein, T. O'Brien, C. Morgan, J. Aldrich, E. Hofer, E. J. Finke, and H. Meyer. 2000. Detection of Francisella tularensis in biological specimens using a capture enzyme-linked immunosorbent assay, an immunochromatographic handheld assay, and a PCR. Clin. Diagn. Lab. Immunol. 7:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hampl, J., M. Hall, N. A. Mufti, Y. M. Yao, D. B. MacQueen, W. H. Wright, and D. E. Cooper. 2001. Upconverting phosphor reporters in immunochromatographic assays. Anal. Biochem. 288:176-187. [DOI] [PubMed] [Google Scholar]
- 31.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Haukanes, B. L., and C. Kyam. 1993. Application of magnetic beads in bioassays. Bio/Technology 11:60-63. [DOI] [PubMed] [Google Scholar]
- 33.Hemmila, I. 1988. Lanthanides as probes for time-resolved fluorometric immunoassays. Scand. J. Clin. Lab. Investig. 48:389-399. [DOI] [PubMed] [Google Scholar]
- 34.Hemmila, I., S. Dakuba, V. M. Mukkala, H. Siitari, and T. Lovgren. 1984. Europium as a label in time-resolved immunofluorometric assays. Anal. Biochem. 137:335-343. [DOI] [PubMed] [Google Scholar]
- 35.Hochmeister, M. N., B. Budowle, O. Rudin, C. Gehrig, U. Borer, M. Thali, and R. Dirnhofer. 1999. Evaluation of prostate-specific antigen (PSA) membrane test assays for the forensic identification of seminal fluid. J. Forensic Sci. 44:1057-1060. [PubMed] [Google Scholar]
- 36.Horton, J. K., S. Swinburne, and M. J. O'Sullivan. 1991. A novel, rapid, single-step immunochromatographic procedure for the detection of mouse immunoglobulin. J. Immunol. Methods 140:131-134. [DOI] [PubMed] [Google Scholar]
- 37.Jelinek, T., S. Eichenlaub, and T. Loscher. 1999. Sensitivity and specificity of a rapid immunochromatographic test for diagnosis of visceral leishmaniasis. Eur. J. Clin. Microbiol. Infect. Dis. 18:669-670. [DOI] [PubMed] [Google Scholar]
- 38.Johnston, S. A., and D. C. Tang. 1994. Gene gun transfection of animal cells and genetic immunization. Methods Cell Biol. 43(Pt A):353-365. [DOI] [PubMed] [Google Scholar]
- 39.Kahan, I., A. Papanastasiou-Diamandi, G. Ellis, S. K. Makela, J. McLaurin, M. D'Costa, and E. P. Diamandis. 1990. Sensitive time-resolved fluorescence immunoassay of somatotropin in serum. Clin. Chem. 36:503-508. [PubMed] [Google Scholar]
- 40.Kakabakos, S. E., T. K. Christopoulos, and E. P. Diamandis. 1992. Multianalyte immunoassay based on spatially distinct fluorescent areas quantified by laser-excited solid-phase time-resolved fluorometry. Clin. Chem. 38:338-342. [PubMed] [Google Scholar]
- 41.Katti, M. K. 2001. Are enzyme-linked immunosorbent assay and immunoblot assay independent in immunodiagnosis of infectious diseases? Clin. Infect. Dis. 32:1114. [DOI] [PubMed] [Google Scholar]
- 42.Kaur, H., and A. Mani. 2000. Evaluation and usefulness of a immunochromatographic test for rapid detection of Plasmodium falciparum infection. Ind. J. Med. Sci. 54:421-424. [PubMed] [Google Scholar]
- 43.Kemppainen, E. A., J. I. Hedstrom, P. A. Puolakkainen, V. S. Sainio, R. K. Haapianen, V. Perhoniemi, S. Osman, E. O. Kivilaakso, and U. H. Stenman. 1997. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N. Engl. J. Med. 336:1788-1793. [DOI] [PubMed] [Google Scholar]
- 44.Kijek, T. M., C. A. Rossi, D. Moss, R. W. Parke, and E. A. Henchal. 2000. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of staphylococcal enterotoxin B. J. Immunol. Methods 236:9-17. [DOI] [PubMed] [Google Scholar]
- 45.Klingenberg, K. D. V., and J. Esfandiari. 1996. Evaluation of a one-step test for rapid, in practice detection of rotavirus in farm animals. Vet. Rec. 138:393-395. [DOI] [PubMed] [Google Scholar]
- 46.Koch, S., H. Wolf, C. Danapel, and K. A. Feller. 2000. Optical flow-cell multichannel immunosensor for the detection of biological warfare agents. Biosens. Bioelectron. 14:779-784. [DOI] [PubMed] [Google Scholar]
- 47.Kohn, J. 1968. An immunochromatographic technique. Immunology 15:863-865. [PMC free article] [PubMed] [Google Scholar]
- 48.Kortepeter, M. G., and G. W. Parker. 1999. Potential biological weapons threats. Emerg. Infect. Dis. 5:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriz, K., J. Gehrke, and D. Kriz. 1998. Advancements toward magneto immunoassays. Biosens. Bioelectron. 13:817-823. [DOI] [PubMed] [Google Scholar]
- 50.Laitinen, M. P. A., and M. Vuento. 1996. Immunochromatographic assay for quantitation of milk progesterone. Acta Chem. Scand. 50:141-145. [DOI] [PubMed] [Google Scholar]
- 51.Lea, T., F. Vartdal, K. Nustad, S. Funderud, A. Berge, T. Ellingsen, R. Schmid, P. Stenstad, and J. Ugelstad. 1988. Monosized, magnetic polymer particles: their use in separation of cells and subcellular components, and in the study of lymphocyte function in vitro. J. Mol. Recognit. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 52.Lee, G. U., S. Metzger, M. Natesan, C. Yanavich, and Y. F. Dufrene. 2000. Implementation of force differentiation in the immunoassay. Anal. Biochem. 287:261-271. [DOI] [PubMed] [Google Scholar]
- 53.Lim, P. L., F. C. Tam, Y. M. Cheong, and M. Jegathesan. 1998. One-step 2-minute test to detect typhoid-specific antibodies based on particle separation in tubes. J. Clin. Microbiol. 36:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lou, S. C., C. Patel, S. Ching, and J. Gordon. 1993. One-step competitive immunochromatographic assay for semiquantitative determination of lipoprotein(a) in plasma. Clin. Chem. 39:619-624. [PubMed] [Google Scholar]
- 55.Lovgren, T., I. Hemmilia, K. Pettersson, and P. Halonen. 1985. Time-resolved fluorometry in immunassay, p. 203-217. In T. Collins and W. Hoh (ed.), Alternative immunoassays. Wiley and Sons Ltd., Chichester, United Kingdom.
- 56.Lovgren, T., L. Merio, K. Mitrunen, M. Makinen, K. Blomberg, T. Palenius, and K. Pettersson. 1996. One-step all-in-one dry reagent immunoassay with fluorescent europium chelate label and time-resolved fluorometry. Clin. Chem. 42:1196-1201. [PubMed] [Google Scholar]
- 57.McKenzie, S. 1988. Whole blood assay of theophylline concentrations using immunochromatographic stick. Arch. Dis. Child. 63:571-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikola, H., T. Takalo, and I. Hemmilia. 1995. Synthesis and properties of luminescent lanthanide chelate labels and labeled haptenic antigens for homogeneous immunoassays. Bioconjug. Chem. 6:235-241. [DOI] [PubMed] [Google Scholar]
- 59.Mills, C. D., D. C. H. Burgess, H. J. Taylor, and K. C. Kain. 1999. Evaluation of a rapid and inexpensive dipstick assay for the diagnosis of Plasmodium falciparum malaria. Bull. W. H. O. 77:553-559. [PMC free article] [PubMed] [Google Scholar]
- 60.Miwa, H., S. Akamatsu, T. Tachikawa, T. Sogabe, K. Ohtaka, A. Nagahara, Y. Sugiyama, and N. Sato. 2001. On-site diagnosis of H. pylori infection by urine. Diagn. Microbiol. Infect. Dis. 39:95-97. [DOI] [PubMed] [Google Scholar]
- 61.Mohanty, S., S. K. Mishra, A. Mohanty, and B. S. Das. 1999. Immunochromatographic test for the diagnosis of Falciparum malaria. J. Assoc. Physicians India 47:201-202. [PubMed] [Google Scholar]
- 62.Nguyen, V., N. Leclerc, C. Wolff, P. Kennel, P. Fonteneau, R. Deyes, J. Warter, and P. Poindron. 1999. Protection of immunoreactivity of dry immobilized proteins on microtitration plates in ELISA application for detection of autoantibodies in myasthenia gravis. J. Biotechnol. 72:115-125. [DOI] [PubMed] [Google Scholar]
- 63.Nichol, S. T., J. Arikawa, and Y. Kawaoka. 2000. Emerging viral diseases. Proc. Natl. Acad. Sci. USA 97:12411-12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ortega-Vinuesa, J. L., and D. Bastos-Gonzalez. 2001. A review of factors affecting the performance of latex agglutination tests. J. Biomater. Sci. Polym. Ed. 12:379-408. [DOI] [PubMed] [Google Scholar]
- 65.Pagani, F., C. Serena, C. Bosio, C. Cuccia, and M. Panteghini. 2001. Evaluation of a rapid bedside immunochromatographic assay for detection of cardiac troponin I in whole blood. Clin. Chem. Lab. Med. 39:458-459. [DOI] [PubMed] [Google Scholar]
- 66.Panteghini, M., and F. Pagani. 1996. Characterization of a rapid immunochromatographic assay for simultaneous detection of high concentrations of myoglobin and CK-MB in whole blood. Clin. Chem. 42:1292-1293. [PubMed] [Google Scholar]
- 67.Payne, J. W. J., D. L. Marshall, R. K. Shockley, and W. J. Martin. 1988. Clinical laboratory applications of monoclonal antibodies. Clin. Microbiol. Rev. 1:313-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peruski, A. H., L. H. Johnson, III, and L. F. Peruski, Jr. 2002. Rapid and sensitive detection of biological warfare agents using time-resolved fluorescence assays. J. Immunol. Methods 263:35-41. [DOI] [PubMed] [Google Scholar]
- 69.Pillai, D. R., and K. C. Kain. 1999. Immunochromatographic strip-based detection of Entamoeba histolytica-E. dispar and Giardia lamblia coproantigen. J. Clin. Microbiol. 37:3017-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson, J., P. Hawkins, and R. Luxton. 2001. The use of coated paramagnetic particles as a physical label in a magneto-immunoassay. Biosens. Bioelectron. 16:989-993. [DOI] [PubMed] [Google Scholar]
- 71.Richardson, J., A. Hill, R. Luxton, and P. Hawkins. 2001. A novel measuring system for the determination of paramagnetic particle labels for use in magneto-immunoassays. Biosens. Bioelectron. 16:1127-1132. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Carbayo, M., E. Herrero, J. Megias, A. Mira, and F. Soria. 1999. Initial evaluation of the new urinary bladder cancer rapid test in the detection of transitional cell carcinoma of the bladder. Urology 54:656-661. [DOI] [PubMed] [Google Scholar]
- 73.Schouten, Y., R. J. D. Winter, J. P. Gorgels, R. W. Koster, R. Adams, and G. T. Sanders. 1998. Clinical evaluation of the CARDIAC STATus, a rapid immunochromatographic assay for simultaneous detection of elevated concentrations of CK-MB and myoglobin in whole blood. Clin. Chem. Lab. Med. 36:469-473. [DOI] [PubMed] [Google Scholar]
- 74.Schramm, W., S. E. Wade, B. B. Angulo, P. C. Torres, and A. Burgess-Cassler. 1998. A simple whole-blood test for detecting antibodies to human immunodeficiency virus. Clin. Diagn. Lab. Immunol. 5:263-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schrier, W. H., R. J. Schoengold, J. T. Baker, J. L. Norell, C. L. Jaseph, Y. Okin, J. Y. Doe, and H. Chandler. 1998. Development of FlexSure HP-an immunochromatographic method to detect antibodies against Helicobacter pylori. Clin. Chem. 44:293-298. [PubMed] [Google Scholar]
- 76.Shin, H. S., C. K. Kim, K. S. Shin, H. K. Chung, and T. R. Heo. 2001. Pretreatment of whole blood for use in immunochromatographic assays for hepatitis B virus surface antigen. Clin. Diagn. Lab. Immunol. 8:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith, D. R., C. A. Rossi, T. M. Kijek, E. A. Henchal, and G. V. Ludwig. 2001. Comparison of dissociation-enhanced lanthanide fluorescent immunoassays to enzyme-linked immunosorbent assays for detection of staphylococcal enterotoxin B, Yersinia pestis-specific F1 antigen, and Venezuelan equine encephalitis virus. Clin. Diagn. Lab. Immunol. 8:1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soini, E., and I. Hemmila. 1979. Fluoroimmunoassay: present status and key problems. Clin. Chem. 25:353-361. [PubMed] [Google Scholar]
- 79.Soini, E., and H. Kojola. 1983. Time-resolved fluorometer for lanthanide chelates-a new generation of nonisotopic immunoassays. Clin. Chem. 29:65-68. [PubMed] [Google Scholar]
- 80.Stenberg, M., and H. Nygren. 1988. Kinetics of antigen-antibody reactions at solid-liquid interfaces. J. Immunol. Methods 113:3-15. [DOI] [PubMed] [Google Scholar]
- 81.Stokes, D. L., M. J. Sepaniak, and T. Vo-Dinh. 1997. Development of a new capillary electrophoresis-based fibre optic sensor. Biomed. Chromatogr. 11:187-192. [DOI] [PubMed] [Google Scholar]
- 82.Szurdoki, F., K. L. Michael, and D. R. Walt. 2001. A duplexed microsphere-based fluorescent immunoassay. Anal. Biochem. 291:219-228. [DOI] [PubMed] [Google Scholar]
- 83.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 84.Tjitra, E., S. Suprianto, M. Dyer, B. J. Currie, and N. M. Anstey. 1999. Field evaluation of the ICT malaria P.f/P.v immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia. J. Clin. Microbiol. 37:2412-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tschop, M., H. M. Behre, E. Nieschlag, R. A. Dressendorfer, and C. J. Strasburger. 1998. A time-resolved fluorescence immunoassay for the measurement of testosterone in saliva: monitoring of testosterone replacement therapy with testosterone buciclate. Clin. Chem. Lab. Med. 36:223-230. [DOI] [PubMed] [Google Scholar]
- 86.Ugelstad, J., P. Stenstad, L. Kilaas, W. S. Prestvik, R. Herie, A. Berge, and E. Hornes. 1993. Monodisperse magnetic polymer particles. New biochemical and biomedical applications. Blood Purif. 11:349-369. [DOI] [PubMed] [Google Scholar]
- 87.Uithoven, K. A., J. C. Schmidt, and M. E. Ballman. 2000. Rapid identification of biological warfare agents using an instrument employing a light addressable potentiometric sensor and a flow-through immunofiltration-enzyme assay system. Biosens. Bioelectron. 14:761-770. [DOI] [PubMed] [Google Scholar]
- 88.Vaccaro, D. E., and J. E. Markinac. 1995. Use of monoclonal antibodies with magnetic particles to separate cell subpopulations by negative selection. Methods Mol. Biol. 45:245-252. [DOI] [PubMed] [Google Scholar]
- 89.Vaccaro, D. E., and J. E. Markinac. 1995. Use of monoclonal antibodies with magnetic particles to separate cell subpopulations by positive selection. Methods Mol. Biol. 45:253-259. [DOI] [PubMed] [Google Scholar]
- 90.Valenti, S., A. Sarkissian, G. Giordano, and K. D. Dahl. 1995. A technique for sorting rat gonadotropes using anti-LH or anti-FSH antibodies covalently attached to magnetic beads. J. Neuroendocrinol. 7:673-679. [DOI] [PubMed] [Google Scholar]
- 91.Vorobjev, A. A., B. L. Cherkassey, A. V. Stepanow, A. A. Kyuregyan, and Y. M. Fjedorov. 1994. “Criterion rating” as a measure of portable use of bioagents as biological weapons. Report to the Working Group on Biological Weapons Control of the Committee on International Security and Arms Control (CISAC). National Academy of Sciences, Washington, D.C.
- 92.Vorobjev, A. A., B. L. Cherkassey, A. V. Stepanow, A. A. Kyuregyan, and Y. M. Fjedorov. 1997. Key problems of controlling especially dangerous infections, p. 71-76. In Proceedings of the International Symposium of Severe Infectious Disease: epidemiology, express-diagnostics and prevention. State Scientific Institution, Volgo-Vyatsky Center of Applied Biotechnology, Kirov, Russia.
- 93.Wannamaker, B., L. Denio, W. E. Dodson, F. Dreifuss, C. Crosby, N. Santilli, P. Duffner, P. Ryan-Dudeck, C. Conboy, and E. Ellis. 1989. Immunochromatographic measurement of phenobarbital in whole blood with a non-instrumented assay. Neurology 39:1215-1218. [DOI] [PubMed] [Google Scholar]
- 94.Weaver, P. C., E. P. Yzerman, K. J. Kuijper, P. Speelman, and J. Dankert. 2000. Rapid diagnosis of Legionnaires disease using an immunochromatographic assay for Legionella pneumophila serogroup 1 antigen in urine during an outbreak in The Netherlands. J. Clin. Microbiol. 38:2738-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wennig, R., M. R. Moeller, J. M. Haguenoer, A. Marocchi, F. Aoppi, B. L. Smith, R. D. L. Torre, C. A. C. Goerlach-Graw, J. Schaeffler, and R. Leinberger. 1998. Development and evaluation of immunochromatographic rapid tests for screening of cannabinoids, cocaine, and opiates in urine. J. Anal. Toxicol. 22:148-155. [DOI] [PubMed] [Google Scholar]
- 96.Wright, P. F., E. Nilsson, E. M. V. Rooij, M. Lelenta, and M. H. Jeggo. 1993. Standardisation and validation of enzyme-linked immunosorbent assay techniques for the detection of antibody in infectious disease diagnosis. Rev. Sci. Tech. 12:435-450. [DOI] [PubMed] [Google Scholar]
- 97.Wu, F. B., Y. F. He, and S. Q. Han. 2001. Matrix interference in serum totalthyroxin (T4) time-resolved fluorescence immunoassay (TRFIA) and its elimination with the use of streptavidin-biotin separation technique. Clin. Chim. Acta 308:117-126. [DOI] [PubMed] [Google Scholar]
- 98.Yang, H., J. K. Leland, D. Yost, and R. J. Massey. 1994. Electrochemiluminescence: a new diagnostic and research tool. ECL detection technology promises scientists new “yardsticks” for quantification. Bio/Technology 12:193-194. [DOI] [PubMed] [Google Scholar]
- 99.Yu, H. 1998. Comparative studies of magnetic particle-based solid phase fluorogenic and electrochemiluminescent immunoassay. J. Immunol. Methods 218:1-8. [DOI] [PubMed] [Google Scholar]
- 100.Zuk, R. F., V. K. Ginsberg, T. Gouts, J. Rabbie, H. Merrick, E. F. Ullman, M. M. Fischer, C. C. Sizto, S. N. Stiso, and D. J. Litman. 1985. Enzyme immunochromatography-a quantitative immunoassay requiring no instrumentation. Clin. Chem. 31:1144-1150. [PubMed] [Google Scholar]