Abstract
Hepatitis C virus (HCV) is a major human pathogen associated with life-threatening liver disease. Entry into hepatocytes requires CD81 and a putative second receptor. In this study, we elucidated the postreceptor attachment stages of HCV entry using HCV pseudoparticles (HCVpp) as a model system. By means of dominant-negative mutants and short interfering RNAs of various cellular proteins, we showed that HCVpp enter via clathrin-coated vesicles and require delivery to early but not to late endosomes. However, the kinetics of HCV envelope glycoprotein-mediated fusion are delayed compared to those of other viruses that enter in early endosomes. Entry of HCVpp can be efficiently blocked by bafilomycin A1, which neutralizes the pH in early endosomes and impairs progression of endocytosis beyond this stage. However, low-pH exposure of bafilomycin A1-treated target cells does not induce entry of HCVpp at the plasma membrane or in the early stages of endocytosis. These observations indicate that, subsequent to internalization, HCVpp entry necessitates additional, low-pH-dependent interactions, modifications, or trafficking, and that these events are irreversibly disrupted by bafilomycin A1 treatment.
An estimated 2% of the world's population is infected with the hepatitis C virus (HCV) and is at risk of developing life-threatening liver disease (58). Little is known about the mechanism of entry into target cells of this important human pathogen. HCV tropism is restricted to hepatocytes by the E1 and E2 envelope glycoproteins (E1E2) (10, 15, 22, 24, 30, 32, 47, 53). The CD81 tetraspanin was identified as an E2 binding receptor and more recently was shown to be necessary for HCV entry into hepatocytes and certain hepatoma cells (51). However, this widely expressed protein is insufficient for HCV entry, and our recent findings indicate that E2 also interacts with a putative, hepatocyte-specific receptor (5, 8, 19, 41). Candidate second receptors include scavenger receptor class B type 1 (52), low-density lipoprotein receptor (1), and glycosaminoglycans (28). Finally, HCV entry into target cells is low-pH dependent, indicating a requirement for receptor-mediated endocytosis and fusion with intracellular membranes (26, 28, 59).
Cellular uptake pathways include clathrin-mediated endocytosis, caveolae, macropinocytosis, and novel nonclathrin/noncaveolae pathways (48). Viruses that require internalization for entry mostly take advantage of clathrin-mediated endocytosis, which is the major ubiquitous route of receptor internalization into cells (12). Clathrin and associated proteins assemble on the intracellular face of the plasma membrane to form invaginations that vesiculate through the action of dynamin (17, 39, 43). As they mature into early endosomes, clathrin-coated vesicles (CCV) shed their protein coat and become acidified. Early endosomes are major sorting stations for internalized cargo, which can be recycled to the plasma membrane or progress to late endosomes and subsequently to lysosomes (27, 40). Regulation of sorting and trafficking is determined by inbuilt signals on internalized receptors, signaling events within the cell, and regulatory proteins (11, 56). Viruses entering through this pathway generally follow the course of their attachment receptors until the low pH in early or late endosomes triggers fusion and delivery of the viral capsids to the cytoplasmic site of replication. They are therefore sensitive to lysosomotropic agents that inhibit endosomal acidification, including bafilomycin A1.
Molecular inhibitors in the form of small interfering RNAs (siRNAs) and dominant-negative cellular proteins have surpassed the use of chemical inhibitors in specifically blocking distinct stages of endocytosis. Moreover, these tools have been successfully used to study internalization and trafficking of intracellular pathogens, including viruses (55). For example, dominant-negative mutants of Eps15 and dynamin block formation of clathrin-coated pits and vesicle budding, respectively (7, 20). Overexpression of Eps15(Δ95/295) or DynK44A mutants was used to demonstrate the critical role of clathrin-mediated endocytosis in entry of Semliki Forest virus (SFV), vesicular stomatitis virus (VSV), and several flaviviruses (14, 23, 29, 33, 57). Mutants of Rab GTPases interfere with the regulation of vesicle budding, mobility, and fusion through the endosomal pathway. In particular, the Rab5 mutant Rab5(S34N) blocks CCV transport to the early endosome and homotypic early endosome fusion, whereas the Rab7 mutant Rab7(T22N) blocks delivery of cargo to late endosomes (3, 25, 34, 60). Rab5 and Rab7 dominant-negative mutants were used to demonstrate different trafficking requirements for SFV, VSV, and influenza virus (54). Here, we have successfully applied these novel and powerful techniques to study HCV endocytosis.
Retroviral particles pseudotyped with E1E2 (HCVpp) authentically recapitulate many aspects of HCV entry into hepatocytes and represent a convenient model system for the study of HCV entry (4, 5, 19, 26, 31, 36, 45, 59). In this study, HCVpp were used to investigate viral internalization and trafficking. Note that HCVpp were comprised of a human immunodeficiency virus type 1 core bearing E1E2 of the H77 (1a) isolate (8, 19) and express the luciferase reporter gene (8, 19). Alternatively, envelope glycoproteins of other viruses were incorporated onto the retroviral core, providing essential controls and relative points of comparison. Using dominant-negative mutants and siRNAs of various cellular proteins, we showed that HCVpp enter target cells via CCV and require delivery to early but not to late endosomes. However, fusion was significantly delayed compared to that of other viruses that enter early endosomes. Also, HCVpp entry could not be induced at the cell surface or after internalization by extracellular low-pH exposure of bafilomycin A1-treated cells. Together, these observations indicate that HCV E1E2 envelope glycoproteins require additional interactions or trafficking in order to achieve fusion and entry.
MATERIALS AND METHODS
Cell culture and pseudoparticle production.
Human embryo kidney cells (293T) and human hepatoma cells (Huh7) were grown at 37°C in 5% CO2 in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, minimal essential medium nonessential amino acids, and sodium pyruvate.
293T cells (1.2 × 106) were lipofected as previously described by us (8) with a 1:2 ratio of NL luc+ env mutant reporter vector and vectors expressing the envelope glycoproteins of human T-cell leukemia virus type 1 (HTLV-1), VSV, SFV, Ebola virus, or HCV (18). Supernatants were collected 24 h postlipofection and filtered (0.45 μm). Huh7 target cells (2 × 104) were infected with different pseudoparticles, and luciferase activity (in relative light units [RLU]/milliliter) was measured in cell lysates 48 h postinfection using the Promega Luciferase kit according to the manufacturer's instructions.
RNA interference assay.
Huh7 (104) cells were transfected with siRNA (5 mM; Santa Cruz) directed against the clathrin heavy chain (HC; Sigma) or a nonspecific control siRNA (5 mM; QIAGEN) using lipofectamine 2000 (Invitrogen). For immunoblotting, transfected Huh7 cells were lysed (50 mM Tris-HCl, pH 7.4, 120 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 0.2 mM Na3VO4, 1 mM dithiothreitol) in the presence of Complete protease inhibitors (Roche Applied Sciences). Samples were adjusted for protein content (determined by the Bradford method) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by transfer onto nitrocellulose membranes (Bio-Rad). Membranes were probed by standard protocols using mouse monoclonal antibody (TD.1) against clathrin HC (1:1,000; Santa Cruz Biotechnology). Staining was revealed with an anti-mouse immunoglobulin G horseradish peroxidase (HRP)-conjugated antibody (Amersham Biosciences) and developed using the Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer). To control for the amount of protein loaded per well, membranes were stripped with Re-Blot Western Blot (Chemicon International) and reprobed with anti-β-tubulin HRP-conjugated antibody (H-235) (1:1,000; Santa Cruz Biotechnology). In parallel, cells were infected 72 h posttransfection with HCVpp or HTLV-1 pseudoparticles (HTLV-1pp) at dilutions that would yield similar RLU, and luciferase activity was measured in cell lysates 48 h postinfection.
Generation and expression of wild-type and dominant-negative cellular proteins.
Coding sequences of wild-type Rab5 and Rab5(S34N) as well as wild-type Rab7 and Rab7(T22N) (provided by C. R. Roy, Yale University School of Medicine) were PCR amplified and spliced to the 3′ end of the enhanced green fluorescent protein (EGFP) coding sequence. The EGFP-Rabs as well as EGFP-Eps15(D3Δ2) and EGFP-Eps15(Δ95/295) (7) (provided by A. Dautry-Varsat, Pasteur Institut, France) fusion constructs were subcloned into the pQCXIP retroviral vector (Clontech). In order to generate pseudoparticles, lipofectamine 2000 (Invitrogen) was used to transfect 293T cells with 3 μg of pQCXIP expression vectors, 3 μg of murine leukemia virus Gag-Pol packaging vector, and 8 μg of the VSV envelope glycoprotein expression vector. Supernatants were collected 48 h posttransfection, filtered (0.45 μm), and used immediately to infect Huh7 cells. Expression of wild-type and dominant-negative EGFP fusion proteins was ascertained by flow cytometry analyses 48 h posttransduction. Transduced cells were infected with different pseudoparticles at dilutions that would yield similar RLU, and luciferase activity was measured in cell lysates 48 h postinfection. For all experiments, only values within the linear range of the assay (104 to 106 RLU) were taken into account.
Time course of entry inhibition.
HCVpp were added to cells and spinoculated for 1 h at 2,000 rpm at 4°C. Cells were washed twice with cold phosphate-buffered saline (PBS) to remove unbound particles. Postadsorptive events required for virus penetration were initiated by shifting the cells to 37°C. Medium at 37°C containing bafilomycin A1 (25 nM) was added at different time points, from 0 to 4 h. The drug was removed 3 h later by a change of medium. Alternatively, Huh7 cells were washed in cold PBS and incubated with proteinase K (50 μg/ml) for 1 h at 4°C under gentle agitation. Detached cells were pelleted, washed in cold PBS, and replated in fresh medium at 37°C. Luciferase activity was measured in cell lysates 48 h postinfection. Nonlinear regression was used to fit curves to the experimental data: one-phase exponential association was fitted to SFV pseudoparticle (SFVpp) and VSV pseudoparticle (VSVpp) entry, whereas sigmoidal dose-response was fitted to HCVpp entry. This is because HCVpp entry undergoes an initial, slow phase, associated with CD81 binding.
Fusion at the cell surface.
Huh7 cells were pretreated for 30 min with bafilomycin A1 (25 nM) or the solvent dimethyl sulfoxide (DMSO) (1:1,000). HCVpp supplemented with drug or solvent were added to the cells and spinoculated for 1 h at 2,000 rpm at 4°C. Cells were washed twice with cold PBS to remove unbound particles. Cells were washed with PBS (at 4°C for time = 0 and at 37°C for subsequent time points), and DMEM (pH 7.2 or pH 5.3) at 37°C was added for 5 min. Subsequently, cells were washed with PBS and incubated in culture medium supplemented with bafilomycin A1 (25 nM) or DMSO (1:1,000) for an additional 3 h, at which time point the medium was changed. Fresh medium was added to cells, and luciferase activity was measured 48 h postinfection.
RESULTS
HCVpp enter target cells via clathrin-mediated endocytosis.
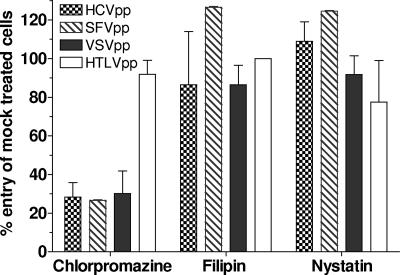
The most common internalization pathway exploited by viruses is clathrin-mediated endocytosis. However, other pathways, including caveolae, macropinocytosis, and noncaveolin/nonclathrin endocytosis, can be used (48). We initially observed that chlorpromazine (25 μM; ∼9 μg/ml), a drug that prevents clathrin polymerization and blocks internalization by CCV (14), effectively inhibited HCVpp entry into hepatoma cells (Fig. 1). Entry mediated by SFV and VSV envelope glycoproteins also was inhibited. HTLV-1 pseudoparticle (HTLV-1pp) entry, however, was not affected, showing that chlorpromazine specifically blocked entry of pH-dependent viruses and was not cytotoxic at the concentration used. In contrast, nystatin (12.5 μg/ml) or filipin (5 μg/ml), which complex with plasma membrane cholesterol, did not affect entry of HCVpp or control pseudoviruses. Together, our observations suggested that HCVpp entry occurs through CCV and does not depend on cholesterol. Because of the pleiotropic effects of these drugs on cell function, we established the role of clathrin-mediated endocytosis in HCV entry using more specific inhibitors of CCV formation.
FIG. 1.
Inhibition of viral entry by drugs that target different internalization pathways. Huh7 cells were treated with chlorpromazine (25 μM), filipin (5 μg/ml), or nystatin (12.5 μg/ml) for 1 h at 37°C. The mock treatments were water for chlorpromazine and nystatin as well as methanol for filipin. Cells were infected with HCVpp, SFVpp, VSVpp, or HTLV-1pp in the continued presence of drugs or corresponding mock treatment. Media were changed after 2 h, and luciferase activity in cellular extracts was measured 48 h postinfection. The percent entry was calculated relative to mock-treated cells, and values are means of at least three independent experiments ± standard deviations.
HCVpp-permissive hepatoma cells (Huh7) were transfected with an siRNA designed to block expression of the clathrin heavy chain (HC), an important component of the clathrin triskelion (43). Seventy-two hours posttransfection, clathrin HC was undetectable by Western blotting compared to control siRNA-transfected cells (Fig. 2A). At this time point, cells were infected with pseudoparticles comprising the envelope glycoproteins of HCV or HTLV-1, the latter being a virus that fuses at the plasma membrane. Note that here and throughout this study different pseudoparticles were used at dilutions that yielded similar luciferase signals in an attempt to measure inhibition of comparable levels of entry. HCVpp entry into clathrin-depleted cells was inhibited 65% compared to control cells (P < 0.001; Fig. 2B). In contrast, neither the anti-clathrin HC nor the control siRNA affected HTLV-1pp entry to any significant degree (P = 0.9737).
FIG. 2.
Inhibition of pseudoparticle entry by agents that target clathrin-mediated endocytosis. (A) Huh7 cells were lipofected with a nonspecific siRNA (control) or an siRNA targeting the clathrin HC (α-CHC). Seventy-two hours posttransfection, cellular extracts were analyzed for clathrin HC and β-tubulin expression by Western blotting. (B) Huh7 cells were infected with HCVpp or HTLV-1pp 72 h after transfection with siRNAs. Luciferase activity in cell lysates was measured 48 h postinfection. The percent entry was calculated relative to the nonspecific siRNA-transfected cells. Results are from at least three independent experiments ± standard deviations. (C) Huh7 cells were transduced with pQCXIP backbone vector, pQCXIP-EGFP-Eps15(D3Δ2), or pQCXIP-EGFP-Eps15(Δ95/295). Cells were infected with HCVpp, SFVpp, or HTLV-1pp 48 h posttransduction. Luciferase activity in cell lysates was measured 48 h postinfection. The percent entry was calculated relative to the backbone vector-transduced cells. Results are from at least three independent experiments ± standard deviations.
We also blocked clathrin-mediated endocytosis by overexpression of a transdominant mutant of Eps15, a regulatory protein that is requisite for the formation of clathrin-coated pits. Huh7 hepatoma cells were transduced by a retroviral vector expressing transdominant Eps15Δ95/295 or a mutant with no activity, Eps15D3Δ2 (7). Alternatively, cells were transduced with the backbone vector. The Eps15 proteins are fused to EGFP, and their expression was determined by flow cytometry to be maximal in >90% of cells at 48 h posttransduction (data not shown). At this time point, cells were infected with retroviral pseudoparticles bearing HCV, SFV, or HTLV-1 envelope glycoproteins. Expression of the transdominant mutant inhibited HCVpp entry by 60% (P = 0.0034; Fig. 2C). A similar decrease was also observed for SFV pseudoparticles (SFVpp) (P = 0.0312), which enter cells via CCV (23). As expected, HTLV-1pp entry was not affected (P = 0.85). There was no effect of the inactive mutant Eps15D3Δ2 on any of the viruses. Together, these observations demonstrate that HCV internalization is mediated by clathrin-dependent endocytosis.
HCVpp entry requires delivery to early but not to late endosomes.
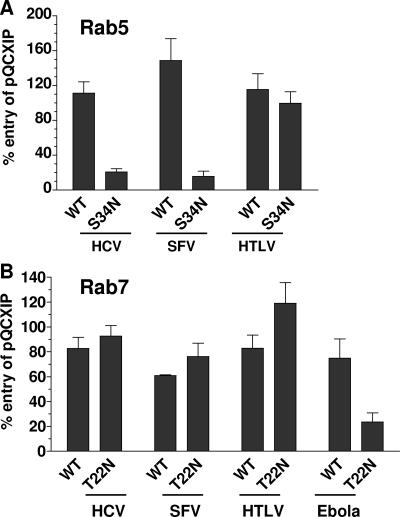
Viruses that undergo low-pH-dependent entry preferentially fuse either in early or in late endosomes (48). To determine which of these compartments are necessary for HCVpp entry, Huh7 hepatoma cells were transduced with retroviral vectors expressing Rab5 or Rab7 wild type or dominant-negative mutants, all of which are fused to EGFP. Alternatively, cells were transduced with the empty vector. Expression of the EGFP-Rab fusion proteins was determined by flow cytometry to be maximal in >90% of cells at 48 h posttransduction (data not shown). At this time point, cells were infected with pseudoparticles bearing different envelope glycoproteins.
Expression of the Rab5 dominant-negative mutant Rab5(S34N), which interferes with formation of early/sorting endosomes, reduced HCVpp entry by 70% compared to entry into cells transduced by vector alone (P < 0.001; Fig. 3A). A similar reduction in entry was observed for pseudoparticles comprising SFV (P = 0.0073) envelope glycoproteins, which are known to induce fusion in early endosomes (54). HTLV-1pp entry was not significantly reduced by Rab5(S34N) expression. In contrast, expression of the Rab7(T22N) mutant, which blocks delivery of cargo to the late endosome, did not inhibit HCVpp entry to any significant degree (Fig. 3B). As expected, SFVpp and HTLV-1pp entry also were not affected, because these viruses fuse in early endosomes and at the plasma membrane, respectively. In fact, Rab7(T22N) expression only inhibited entry of Ebola virus pseudoparticles, which fuse in late endosomes (P = 0.045; Fig. 3B) (13). Based on these observations, we concluded that HCVpp entry requires delivery to early but not to late endosomes.
FIG. 3.
Inhibition of pseudoparticle entry by Rab5 and Rab7 transdominant-negative mutants. (A) Huh7 cells were transduced with pQCXIP backbone vector, pQCXIP-EGFP-Rab5(WT), or pQCXIP-EGFP-Rab5(S34N). (B) Alternatively, Huh7 cells were transduced with pQCXIP backbone vector, pQCXIP-EGFP-Rab7(WT), or pQCXIP-EGFP-Rab7(T22N) and infected with viral pseudotypes. Forty-eight hours posttransduction, cells were infected with HCVpp, SFVpp, or HTLV-1pp. Luciferase activity in cell lysates was measured 48 h postinfection. The percent entry was calculated relative to the backbone vector-transduced cells. Results are from at least three independent experiments ± standard deviations. WT, wild type.
Evidence for a significant delay between HCVpp internalization and fusion.
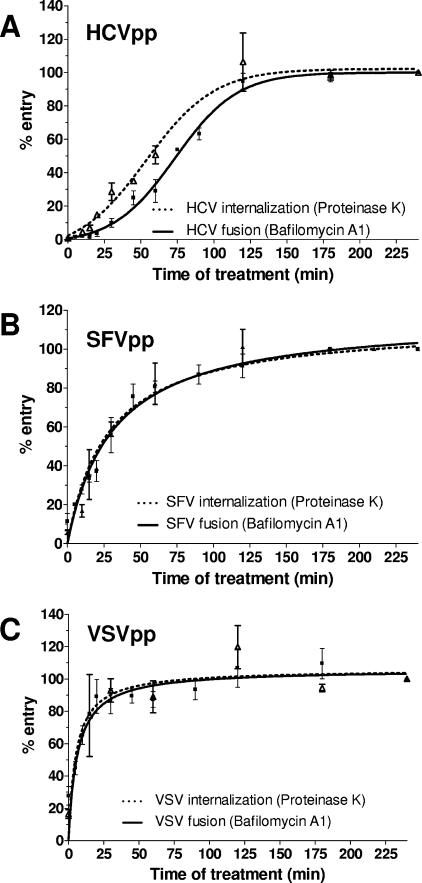
In order to ascertain that HCVpp enter early endosomes, we compared the kinetics of internalization and fusion mediated by HCV E1E2. SFV and VSV pseudoparticles were chosen as controls because, like HCV, they require CCV and delivery to early endosomes. Moreover, SFV and VSV fusion in early endosomes is triggered by the drop in pH (37). Pseudoparticles were adsorbed to Huh7 cells at 4°C, and entry was initiated by shifting cells to 37°C. At different time points thereafter, pseudoparticles remaining on the cell surface were inactivated by proteinase K treatment. Internalized particles, however, were resistant to proteolysis and could proceed with subsequent stages of entry. At 3 h, no further effect of proteinase K treatment on entry was evident, indicating that maximal (100%) internalization was achieved. The percentage of internalization at any given time point therefore was calculated relative to the measurement at 3 h. The fraction of pseudoparticles having undergone fusion was quantified similarly by measuring bafilomycin A1 sensitivity at different time points after initiation of entry. The percentage of entry was calculated relative to the signal at 3 h, when no further effect of bafilomycin A1 was observed. Note that the two assays are comparable, because conditions are identical up to addition of inhibitors and are irrelevant thereafter.
We previously showed that half-maximal CD81 binding was attained in 17 min (8), and the plateau at the beginning of the HCVpp time course curves probably reflects this event (Fig. 4A). Half-maximal (50%) HCVpp internalization occurred 53 min after initiation of entry, as determined by proteinase K sensitivity (Fig. 4A). Half-maximal HCVpp fusion required 73 min, as determined by bafilomycin A1 sensitivity. Hence, there is a 20-min time lag between HCVpp internalization and fusion. In contrast, half-maximal SFVpp internalization occurred in 27 min, whereas 31 min were required for fusion (Fig. 4B). Similar experiments were also performed with VSVpp; half-maximal internalization and fusion occurred at 5 and 6 min, respectively (Fig. 4C). In our assay, therefore, SFVpp and VSVpp internalization and fusion were near-simultaneous events. The delay between internalization and fusion mediated by HCV envelope glycoproteins suggests the necessity for additional events in early endosomes or for further trafficking to other intracellular compartments. Note that the times required for half-maximal internalization and fusion were calculated by nonlinear regression analyses using GraphPad Prizm 4.0 software. Differences were statistically significant, as determined by an unpaired t test to calculate two-tailed P values.
FIG. 4.
Time course of proteinase K and bafilomycin A1 sensitivity of viral entry. (A) HCVpp, (B) SFVpp, or (C) VSVpp were prebound to Huh7 cells at 4°C for 1 h. Entry was initiated by shifting cells to 37°C. At the indicated time points, cells were treated with proteinase K (50 μg/ml) at 4°C for 1 h (dotted line). Cells were washed, resuspended in fresh medium, and reseeded. Alternatively, bafilomycin A1 (25 nM) was added at the indicated time points (solid line). After 3 h, the drug was removed by a change of medium. Luciferase activity was measured in cellular extracts 48 h postinfection. The percent entry was calculated relative to the 3-h time point, and values are means of at least three independent experiments ± standard deviations.
HCVpp fusion cannot be rescued by low-pH treatment.
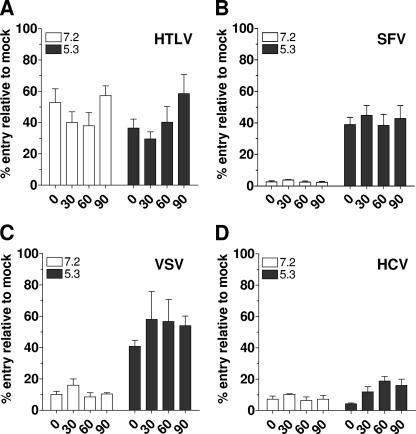
We next asked whether receptor binding and low pH (normally encountered by the virus in early endosomes) were sufficient to trigger HCVpp fusion and entry. For this, endosomal acidification was inhibited by addition of bafilomycin A1, thereby precluding pH-dependent envelope glycoprotein-mediated fusion in endosomes (29). Pseudoparticles were allowed to attach to Huh7 target cells at 4°C and then shifted to 37°C in order to initiate early entry events. To induce fusion, extracellular treatment with neutral or low-pH solutions was applied at different time points posttemperature shift. The percentage of pseudoparticle entry was calculated relative to cells that were DMSO (solvent) treated only. Note that the background levels of entry at time zero or at neutral pH (for SFV and VSV) are due to incomplete inhibition of entry by bafilomycin A1 treatment.
HTLV-1 envelope glycoprotein pseudoparticles entered cells regardless of extracellular pH, since this virus normally fuses at the plasma membrane (Fig. 5A). Importantly, this control demonstrated that there was no significant loss of infectivity of bound viral particles over the time course of our assay, nor was there inactivation by exposure to low pH. After treatment with acidic but not neutral pH, retroviral particles pseudotyped with SFV envelope glycoproteins readily entered cells at similar levels, regardless of the preincubation time (Fig. 5B). Note that bafilomycin A1 does not inhibit internalization but blocks cargo trafficking beyond early endosomes (2, 6, 35, 46). Even after 90 min, therefore, when >80% of SFVpp are internalized, entry could be rescued by extracellular low-pH treatment (Fig. 5B). We observed similar results for VSVpp at time points when nearly 90% of VSVpp are internalized (60 and 90 min; Fig. 5C). These observations show that low-pH treatment can overcome the inhibitory effect of bafilomycin on fusion after virus internalization. In contrast, no significant entry of HCVpp was observed after low-pH treatment at 30 min postattachment (P = 0.6650) (Fig. 5D). The slight increase in HCVpp entry observed after low-pH treatment at 60 and 90 min was only marginally higher than entry observed at the same time points after neutral-pH treatment (P = 0.0174 and P = 0.1138, respectively) (Fig. 5D). Exposure to low pH, even at a time point when most HCVpp should have attained early endosomes, therefore cannot overcome the inhibitory effect of bafilomycin A1. Similar results were observed at pH 3, 4, and 6 (data not shown). This suggests that endosomal acidification is required for events other than triggering E1E2-mediated membrane fusion.
FIG. 5.
Rescue of pseudoparticle entry by extracellular low-pH treatment. (A) HTLV-1pp, (B) SFVpp, (C) VSVpp, or (D) HCVpp were prebound to Huh7 cells in the presence of bafilomycin A1 (25 nM) or DMSO (1:1,000) at 4°C for 1 h. Unbound particles were washed off, and internalization was initiated by shifting cells to 37°C. At the indicated time points, cells were incubated for 5 min with DMEM (pH 7.2 or pH 5.3) and then returned to fresh medium supplemented with bafilomycin A1. After 2 h, the drug was removed by a change of medium. Luciferase activity was measured in cellular extracts 48 h postinfection. The percent entry was calculated relative to cells treated with DMSO, and values are means of at least three independent experiments ± standard deviations.
DISCUSSION
The fusogenic activity of viral envelope glycoproteins can be triggered by interactions with cellular receptors, in which case entry occurs at the plasma membrane (38, 44). Alternatively, viruses first undergo internalization by receptor-mediated endocytosis, followed by fusion with intracellular membranes. The most commonly used internalization pathway is clathrin dependent, and envelope-mediated membrane fusion is triggered by the low pH in endosomes (38, 48). Hence, VSV and SFV fuse within early endosomes, whereas influenza virus fuses in late endosomes. Certain viruses exhibit more complex entry pathways. For example, simian virus 40 is internalized through caveolae and enters in the endoplasmic reticulum (49). Receptor interactions are needed to prime the envelope glycoproteins for pH-dependent fusion of avian leukosis virus (42). Ebola virus fusion not only requires receptor interactions and low pH but the envelope glycoprotein also needs to be processed by acid-dependent cathepsins in late endosomes (13). Viruses therefore use an array of interactions, modifications, and internalization pathways to deliver their genomes into target cells.
Here, we elucidated the postreceptor attachment stages of HCV entry. HCV attachment to target cells is mediated by a putative hepatocyte-specific receptor and the CD81 tetraspanin (5, 8, 19, 41). Other plasma membrane components may be recruited by the virion-receptor complex, and additional conformational or enzymatic modifications of E1E2 may prime the envelope for downstream events (8, 19, 28). Krey et al. have recently suggested a role for disulfide bonds in the entry of another flaviviridae, bovine viral diarrhea virus (29). However, we were not able to inhibit HCVpp entry with the specific protein disulfide isomerase inhibitor bacitracin and the nonspecific thiol blocker 2,4-dinitrothiocyanatebenzene (L. Meertens, unpublished results). Disulfide bond isomerization therefore does not appear to play a role in HCV entry. Cholesterol depletion from the plasma membrane by filipin and nystatin also did not affect HCVpp entry. Note that these agents also disrupt caveolin-dependent internalization. However, Huh7 cells express very small amounts of caveolin-1 (21), and it is unlikely that this pathway contributes significantly to virus internalization in these cells.
Inhibitory drugs, anti-clathrin HC siRNAs, and transdominant mutants of Eps15 were used to unambiguously demonstrate that HCVpp are predominantly internalized via clathrin-mediated endocytosis, confirming recently published observations (9, 16, 26, 31, 59). The partial inhibition of entry, also observed for SFVpp and VSVpp, was due to working under conditions wherein inhibitors did not cause cytotoxicity but also did not block 100% of clathrin-mediated endocytosis. We also determined that half-maximal HCVpp internalization requires approximately 1 h. However, CD81 does not possess any intracellular internalization motifs and undergoes extremely slow endocytosis (30% in 12 h) (50). This suggests that HCVpp internalization is mediated by other cell surface molecules, such as the putative hepatocyte-specific receptor. Alternatively, hepatocyte-specific proteins associated with CD81 via the tetraspanin web may promote endocytosis of the virus-CD81 complex. A final possibility is that CD81 oligomerization through multiple interactions with the viral envelope may lead to more rapid internalization via CCV. Recruitment of other cell surface components or CD81 molecules into a viral entry complex may account for the 30-min delay between CD81 binding and internalization.
Transdominant mutants of the GTPases Rab5 and Rab7 were used to show that HCVpp entry requires delivery of the viruses to early but not to late endosomes, suggesting that early endosomes are the site of fusion. However, differences between internalization and fusion kinetics suggested a more complex mechanism of entry. Indeed, we observed a 20-min lag between HCVpp internalization and fusion, whereas the two events were closely associated during entry of SFVpp and VSVpp, two viruses that fuse in early endosomes (37, 61). Moreover, low-pH exposure after bafilomycin A1 treatment of target cells could not induce HCVpp entry at the cell surface, even after the virus had interacted with the receptor and CD81. At a time point when approximately 80% of HCVpp are internalized, there still was very little rescue of entry by low-pH treatment. Extracellular low-pH exposure readily rescued SFVpp and VSVpp entry. Tscherne et al. performed similar experiments using replicating virus (HCVcc) but interpreted the modest level of entry (approximately 15%) after low-pH treatment to indicate rescue of fusion (59). It is difficult to assess the significance of their observations, because no other viruses were used as positive controls.
We interpreted these observations to mean that receptor binding and low pH are not sufficient to induce HCV E1E2-mediated membrane fusion. We cannot completely exclude the possibility that conformational changes and protein dissociation kinetics leading up to fusion are exceptionally slow for HCV E1E2 and require the continuous presence of low pH. However, we favor the interpretation that HCV entry necessitates additional postinternalization events which are irreversibly inhibited by bafilomycin A1 treatment and cannot be reactivated by low-pH exposure. These events could be interactions with specific protein factors or enzymatic modifications within early endosomes (or other intracellular compartments). One possibility we considered was that HCV entry requires the action of a pH-dependent protease. However, treatment of Huh7 cells with a wide array of protease inhibitors did not significantly or specifically affect HCVpp entry (L. Meertens, unpublished). Tscherne et al. also did not observe any effect on HCVcc entry of cathepsin B and L inhibitors (59). An alternative possibility is that trafficking to other intracellular compartments may be necessary because the physicochemical properties of their membranes are amenable to HCV E1E2-induced fusion. A requirement for additional interactions with intracellular factors or trafficking of HCV particles constitutes a novel and unique mechanism of viral entry. Detailed understanding of these postreceptor binding events may reveal new targets for antivirals. Like all entry inhibitors, these would have the advantage of preventing viral replication within host cells.
Acknowledgments
We thank Craig Roy (Yale University School of Medicine) for generously providing us with the Rab GTPase transdominant mutants and Dautry-Varsat (Pasteur Institute) for providing us with Eps15 mutants.
This work was supported by NIH grant AI066198 and The Burroughs Wellcome Fund Investigators in Pathogenesis of Infectious Diseases. This work was also supported in part by the NIAID Centers for AIDS Research grant AI051519 to the Albert Einstein College of Medicine.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baravalle, G., D. Schober, M. Huber, N. Bayer, R. F. Murphy, and R. Fuchs. 2005. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 320:99-113. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri, M. A., G. Li, M. I. Colombo, and P. D. Stahl. 1994. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269:18720-18722. [PubMed] [Google Scholar]
- 4.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F. L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. [DOI] [PubMed] [Google Scholar]
- 6.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 8.Bertaux, C., and T. Dragic. 2006. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J. Virol. 80:4940-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard, E., S. Belouzard, L. Goueslain, T. Wakita, J. Dubuisson, C. Wychowski, and Y. Rouille. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 80:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boisvert, J., X. S. He, R. Cheung, E. B. Keeffe, T. Wright, and H. B. Greenberg. 2001. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J. Infect. Dis. 184:827-835. [DOI] [PubMed] [Google Scholar]
- 11.Brennwald, P. 2000. Reversal of fortune. Do Rab GTPases act on the target membrane? J. Cell Biol. 149:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. [DOI] [PubMed] [Google Scholar]
- 13.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu, J. J., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocquerel, L., J. C. Meunier, A. Op de Beeck, D. Bonte, C. Wychowski, and J. Dubuisson. 2001. Coexpression of hepatitis C virus envelope proteins E1 and E2 in cis improves the stability of membrane insertion of E2. J. Gen. Virol. 82:1629-1635. [DOI] [PubMed] [Google Scholar]
- 16.Codran, A., C. Royer, D. Jaeck, M. Bastien-Valle, T. F. Baumert, M. P. Kieny, C. A. Pereira, and J. P. Martin. 2006. Entry of hepatitis C virus pseudotypes into primary human hepatocytes by clathrin-dependent endocytosis. J. Gen. Virol. 87:2583-2593. [DOI] [PubMed] [Google Scholar]
- 17.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 18.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 19.Cormier, E. G., F. Tsamis, F. Kajumo, R. J. Durso, J. P. Gardner, and T. Dragic. 2004. CD81 is an entry coreceptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA 101:7270-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deleersnyder, V., A. Pillez, C. Wychowski, K. Blight, J. Xu, Y. S. Hahn, C. M. Rice, and J. Dubuisson. 1997. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 71:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 131:1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornfeld, S., and I. Mellman. 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5:483-525. [DOI] [PubMed] [Google Scholar]
- 28.Koutsoudakis, G., A. Kaul, E. Steinmann, S. Kallis, V. Lohmann, T. Pietschmann, and R. Bartenschlager. 2006. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 80:5308-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krey, T., H. J. Thiel, and T. Rumenapf. 2005. Acid-resistant bovine pestivirus requires activation for pH-triggered fusion during entry. J. Virol. 79:4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanford, R. E., D. Chavez, F. V. Chisari, and C. Sureau. 1995. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J. Virol. 69:8079-8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavillette, D., B. Bartosch, D. Nourrisson, G. Verney, F. L. Cosset, F. Penin, and E. I. Pecheur. 2006. Hepatitis C virus glycoproteins mediate low pH-dependent membrane fusion with liposomes. J. Biol. Chem. 281:3909-3917. [DOI] [PubMed] [Google Scholar]
- 32.Lavillette, D., A. W. Tarr, C. Voisset, P. Donot, B. Bartosch, C. Bain, A. H. Patel, J. Dubuisson, J. K. Ball, and F. L. Cosset. 2005. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41:265-274. [DOI] [PubMed] [Google Scholar]
- 33.Lecot, S., S. Belouzard, J. Dubuisson, and Y. Rouille. 2005. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 79:10826-10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, G., and P. D. Stahl. 1993. Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 268:24475-24480. [PubMed] [Google Scholar]
- 35.Liebl, D., F. Difato, L. Hornikova, P. Mannova, J. Stokrova, and J. Forstova. 2006. Mouse polyomavirus enters early endosomes, requires their acidic pH for productive infection, and meets transferrin cargo in Rab11-positive endosomes. J. Virol. 80:4610-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logvinoff, C., M. E. Major, D. Oldach, S. Heyward, A. Talal, P. Balfe, S. M. Feinstone, H. Alter, C. M. Rice, and J. A. McKeating. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 101:10149-10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh, M., E. Bolzau, and A. Helenius. 1983. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 32:931-940. [DOI] [PubMed] [Google Scholar]
- 38.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science 285:215-220. [DOI] [PubMed] [Google Scholar]
- 40.Maxfield, F. R., and T. E. McGraw. 2004. Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5:121-132. [DOI] [PubMed] [Google Scholar]
- 41.McKeating, J. A., L. Q. Zhang, C. Logvinoff, M. Flint, J. Zhang, J. Yu, D. Butera, D. D. Ho, L. B. Dustin, C. M. Rice, and P. Balfe. 2004. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J. Virol. 78:8496-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 43.Mousavi, S. A., L. Malerod, T. Berg, and R. Kjeken. 2004. Clathrin-dependent endocytosis. Biochem. J. 377:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nisole, S., and A. Saib. 2004. Early steps of retrovirus replicative cycle. Retrovirology 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Op de Beeck, A., Y. Rouille, M. Caron, S. Duvet, and J. Dubuisson. 2004. The transmembrane domains of the prM and E proteins of yellow fever virus are endoplasmic reticulum localization signals. J. Virol. 78:12591-12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel, J., A. H. Patel, and J. McLauchlan. 1999. Covalent interactions are not required to permit or stabilize the non-covalent association of hepatitis C virus glycoproteins E1 and E2. J. Gen. Virol. 80:1681-1690. [DOI] [PubMed] [Google Scholar]
- 48.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 49.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 50.Petracca, R., F. Falugi, G. Galli, N. Norais, D. Rosa, S. Campagnoli, V. Burgio, E. Di Stasio, B. Giardina, M. Houghton, S. Abrignani, and G. Grandi. 2000. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J. Virol. 74:4824-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 52.Scarselli, E., H. Ansuini, R. Cerino, R. M. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21:5017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selby, M. J., E. Glazer, F. Masiarz, and M. Houghton. 1994. Complex processing and protein:protein interactions in the E2:NS2 region of HCV. Virology 204:114-122. [DOI] [PubMed] [Google Scholar]
- 54.Sieczkarski, S. B., and G. R. Whittaker. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4:333-343. [DOI] [PubMed] [Google Scholar]
- 55.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 56.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113:183-192. [DOI] [PubMed] [Google Scholar]
- 57.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 338:53-60. [DOI] [PubMed] [Google Scholar]
- 58.Thomson, B. J., and R. G. Finch. 2005. Hepatitis C virus infection. Clin. Microbiol. Infect. 11:86-94. [DOI] [PubMed] [Google Scholar]
- 59.Tscherne, D. M., C. T. Jones, M. J. Evans, B. D. Lindenbach, J. A. McKeating, and C. M. Rice. 2006. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 80:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitelli, R., M. Santillo, D. Lattero, M. Chiariello, M. Bifulco, C. B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272:4391-4397. [DOI] [PubMed] [Google Scholar]
- 61.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]