Abstract
The patterns of cellular immune responses induced by live attenuated influenza vaccine (LAIV) versus those of the trivalent inactivated influenza vaccine (TIV) have not been studied extensively, especially in children. The goals of this study were to evaluate the effects of TIV and LAIV immunization on cellular immunity to live influenza A virus in children and adults and to explore factors associated with variations in responses to influenza vaccines among individuals. A gamma interferon (IFN-γ) flow cytometry assay was used to measure IFN-γ-producing (IFN-γ+) NK and T cells in peripheral blood mononuclear cell cultures stimulated with a live influenza A virus strain before and after LAIV or TIV immunization of children and adults. The mean percentages of influenza A virus-specific IFN-γ+ CD4 and CD8 T cells increased significantly after LAIV, but not TIV, immunization in children aged 5 to 9 years. No increases in the mean levels of influenza A virus-reactive IFN-γ+ T cells and NK cells were observed in adults given LAIV or TIV. TIV induced a significant increase in influenza A virus-reactive T cells in 6-month- to 4-year-old children; LAIV was not evaluated in this age group. The postvaccination changes (n-fold) in the percentages of influenza A virus-reactive IFN-γ+ T and NK cells in adults were highly variable and correlated inversely with the prevaccination percentages, in particular with that of the CD56dim NK cell subset. In conclusion, our findings identify age, type of vaccine, and prevaccination levels of immune reactivity to influenza A virus as factors significantly associated with the magnitude of cellular immune responses to influenza vaccines.
Human influenza A and B viruses are major infectious pathogens of the respiratory tract that affect people of all ages, with the highest rates of mortality and severe morbidity in the elderly and very young (30). The annual worldwide influenza epidemics and the threat of a new pandemic represent major public-health concerns. The risks of influenza infection and the associated morbidity and mortality are significantly reduced by vaccination (3, 28). The two types of influenza vaccines currently licensed in the United States are the inactivated trivalent influenza vaccine (TIV), given by intramuscular injection (28), and the live attenuated influenza vaccine (LAIV), administered intranasally (3). TIV consists of the viral surface proteins hemagglutinin (HA) and neuraminidase (NA), which are partially purified from detergent-extracted, inactivated virions of the seasonal circulating influenza strains. TIV is approved for use in adults and children aged 6 months or older. The newly licensed LAIV is manufactured using cold-adapted influenza strains as the “genetic backbone” into which HA and NA genes from circulating strains are inserted by gene reassortment. The attenuated phenotypes of the LAIV vaccine result from several changes in the internal genes that reduce the ability of the virus to replicate in the respiratory tract (24, 27). LAIV is currently approved for use in children and adults aged 5 to 49 years. Both TIV and LAIV are licensed as safe and effective vaccines in the approved age groups, although the relative levels of efficacy between the two commercial vaccines have not been compared directly. Notably, while the two vaccines represent different formulations and routes of administration, they contain similar or identical HA and NA antigens.
Antibodies to HA and NA have been associated with protection from disease or viral replication after natural influenza infection or vaccination in adults and children (4, 5, 11, 19, 20, 26, 40). Cellular immune responses, including innate NK responses and adaptive T-cell responses, are thought to play a pivotal role in clearing influenza virus infection; however, these conjectures are based primarily on studies in animal models (8, 12, 13, 18, 33, 35, 48). Few systematic studies of cellular immunity against influenza virus have been done in people, especially in young children (21, 34). The relationship between innate and adaptive immune responses to influenza vaccination has not been investigated extensively in humans. In particular, the effects of the prevaccination baseline immunity to influenza A virus on the cellular immune responses to influenza vaccinations are poorly understood.
We developed an IFN-γ flow cytometric assay that allows simultaneous detection of influenza A virus-specific T cells and influenza A virus-responsive NK cells in peripheral blood mononuclear cells (PBMCs) (22, 23). By staining cell surface phenotype markers and intracellular gamma interferon (IFN-γ), this assay measures the percentages of IFN-γ-producing (IFN-γ+) cells in T- and NK cell subsets after a 17-h exposure of PBMCs to influenza A virus. Production of IFN-γ by NK cells in this assay depends on exposure of influenza A virus-specific memory T cells to the virus (22). In the current study, we evaluated the T-cell and NK cell responses to a live influenza A virus strain in children and adults before and after immunization with TIV or LAIV during the 2004-2005 influenza season. The goals of this study were (i) to use IFN-γ production to examine patterns of T-cell and NK cell immunity to live influenza A virus in children and adults before vaccination, (ii) to evaluate the effects of TIV and LAIV immunization on cellular immunity to live influenza A virus in children and adults, and (iii) to explore the factors associated with variations in responses to influenza vaccines among individuals.
MATERIALS AND METHODS
Human subjects and vaccination protocols.
Prior to the 2004-2005 influenza season, 26 children 6 months to 4 years of age, 39 children 5 to 9 years of age, and 44 adults 22 to 49 years of age were enrolled in a multiproject influenza vaccine study during the period from 23 September to 1 December 2004. Table 1 summarizes the demographic information of the study subjects in the three age groups, as well as the vaccination and blood-sampling protocols for each age group. After obtaining informed consent from the subjects or their parents and assent from children 7 years old and older, the subjects were immunized with either TIV (Fluzone; Aventis-Pasteur) or LAIV (FluMist; MedImmune) following current guidelines for influenza vaccination. All of the adult subjects were participants in a study in the previous 2003-2004 flu season, in which they were randomized in a 1:1 ratio to receive either TIV or LAIV. For the current study, these subjects were immunized with one dose of the same vaccine that they had received in the previous year. Subjects who were 5 to 9 years of age were randomized in a 1:1 ratio to receive either TIV or LAIV. For those children who had not received influenza vaccine previously, a second dose of the same vaccine was given at approximately 28 days (for TIV) or 42 days (for LAIV) after the first dose based on current vaccination guidelines. Children in the youngest age group (6 months to 4 years) were all influenza vaccine naïve and therefore received two doses of TIV by intramuscular injection approximately 28 days apart. The 2004-2005 TIV contained influenza A/New Caledonia (H1N1), A/Wyoming (H3N2), and B/Jiangsu virus strains. Each dose of TIV contained 45 μg of HA in the recommended ratio of 15 μg from each of the three vaccine strains. The 2004-2005 LAIV contained influenza A/New Caledonia (H1N1), A/Wyoming (H3N2), and B/Jilin virus strains. Each dose of LAIV contained 106.5 to 107.5 median tissue culture infectious doses of live attenuated influenza virus reassortants of each of the three vaccine strains.
TABLE 1.
Demographic information on the study population
| Age group | Vaccine type | Sampling time (day) | n | No. of females/males | Agea (mean yr. ± SD) |
|---|---|---|---|---|---|
| Adult (22-49 yr) | LAIV | 0, 10, 28 | 20 | 12/8 | 29.9 ± 5.7 |
| TIV | 0, 10, 28 | 24 | 12/12 | 33.5 ± 8.7 | |
| Child (5-9 yr) | LAIV | 0, 10, 28 | 9 | 4/5 | 6.0 ± 1.1 |
| 0, 10, 42 | 9 | 5/4 | 7.6 ± 1.3 | ||
| TIV | 0, 10, 28 | 21 | 4/17 | 7.1 ± 1.4 | |
| Child (6 mo-4 yr) | TIV | 0 | 24 | 12/12 | 2.5 ± 1.3 |
| 10 after dose 1 | 7 | 4/3 | 3.3 ± 0.8 | ||
| 10 after dose 2 | 10 | 5/5 | 2.3 ± 1.3 |
There was no significant difference in age between different vaccine and sampling time groups within each age group.
The subjects were free of major medical illnesses before enrollment, as documented by a review of their medical histories and a clinical examination at their first visit. Subjects were excluded if they had an allergy to eggs or a history of immunodeficiency or were taking immunosuppressive medications. Three blood samples were collected from the adults and children aged 5 to 9 years. The first two samples were collected on day 0 just prior to vaccination (baseline) and on approximately day 10 (9.8 ± 1.1; mean ± standard deviation) after the first vaccine dose. For the adults receiving TIV or LAIV and children receiving TIV, the third blood samples were collected approximately 28 (29.3 ± 2.1) days after the first vaccine dose (immediately prior to the second dose of TIV for the vaccine-naïve children). For the vaccine-naïve LAIV recipients aged 5 to 9 years (n = 9), the third blood sample was collected approximately 42 (44.5 ± 6.0) days after vaccination, immediately prior to administration of the second dose of LAIV. For the previously vaccinated LAIV recipients aged 5 to 9 years (n = 9), the third blood sample was collected on approximately day 28 (28.8 ± 0.8). Two blood samples were scheduled to be collected from each of the 6-month- to 4-year-old subjects, who were randomized in a 1:1 ratio to two different sampling schedules for the second blood sample. The first blood sample was collected from all of the subjects at baseline, while the second was collected on approximately day 10 (9.2 ± 0.6) after either the first or second dose, depending upon random assignment. There were 24 blood samples drawn on day 0, 7 samples drawn on day 10 after the first dose, and 10 samples on day 10 after the second dose available for this study because of missed appointments or insufficient volumes of blood samples collected. No children aged 2 years or younger returned for testing after vaccination.
Preparation of influenza virus.
Purified wild-type influenza virus A/Wyoming/03/2003 (H3N2) was prepared as previously described (23). In brief, virus was grown in 11-day old embryonated specific-pathogen-free hen's eggs (Charles River Laboratories). Allantoic fluid was harvested 48 h after infection and assayed for virus by measuring the concentration of influenza HA. Virus-containing allantoic fluid was pooled and centrifuged to pellet influenza A virus particles. The virus pellet was resuspended in phosphate-buffered saline and further purified by continuous 15% to 60% sucrose gradient centrifugation. The purified virus was reconstituted in phosphate-buffered saline, stabilized with sucrose-phosphate-glutamate buffer (BioWhittaker), dispensed into single-use aliquots, and stored at −70°C. The virus titer was determined with Madin-Darby canine kidney cells by standard procedures (31).
Cytokine flow cytometry assay.
PBMCs were prepared with standard Ficoll gradient centrifugation from heparinized whole blood and incubated with or without influenza A virus as previously described (22), with minor modifications. In brief, 1.5 × 106 to 2.0 × 106 cells were resuspended in 0.1 ml of RPMI 1640 medium without serum. Purified influenza A virus/Wyoming was added to the cells at a multiplicity of infection of 3 and incubated at 37°C in 5% CO2 for 1 h. The same volume of sucrose-phosphate-glutamate buffer was used as the negative control. RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics was then added to a final volume of 0.7 ml. The cells were incubated for another 16 h, with brefeldin A (Sigma-Aldrich) added to a final concentration of 10 μg/ml for the last 4 h of incubation. Staining and flow cytometric analysis were done as described previously (22), with modifications. In brief, the cells were first stained with allophycocyanin-Cy7-labeled anti-CD8 and phycoerythrin-Cy7-labeled anti-CD56 (BD Pharmingen) and then treated with FACS Lysing Solution and FACS Permeabilizing Solution (BD Biosciences). The permeabilized cells were subsequently stained with a mixture of the following antibodies: phycoerythrin-labeled anti-IFN-γ, fluorescein isothiocyanate-labeled anti-perforin, and PerCP-labeled anti-CD3 (BD Biosciences). The stained cells were analyzed with an LSR II flow cytometer (BD Biosciences). The percentages of influenza A virus-specific IFN-γ+ cells were determined for the following cell subsets after subtraction of the corresponding percentage in the negative control or the background: CD4 T cells (gated on the CD3+ CD8− lymphocyte population), CD8 T cells (gated on the CD3+ CD8+ lymphocyte population), CD56bright NK cells (gated on the CD3− CD56bright perforin− lymphocyte population), and CD56dim NK cells (gated on the CD3− CD56dim perforin+ lymphocyte population) (22). The limit of detection for the assay was 0.01%. The average background level was 0.03% for CD4 and CD8 T cells, 1.21% for CD56bright NK cells, and 0.37% for CD57dim NK cells.
Statistical analysis.
Data below the detection threshold (0.01%) were imputed to 0.005%, which is one-half of the detection threshold. In order to compare the change (n-fold) in percentages of IFN-γ+ cells, which is likely a more appropriate measurement for the size of change in immune cell activity than the difference in percentages, all data were log10 transformed for analysis. Therefore, the reported means are geometric mean percentages (GMP). Regression models were fitted to the log10-tranformed repeated-measures data using generalized estimating equations (GEE) (32), with generalized score tests used to test hypotheses (7). Spearman's coefficient was computed for correlations and partial correlations (9, 47) to estimate the degree of monotonic association. Attained significance levels with a P value of <0.05 were considered to be statistically significant. The type I error rate was controlled at 5% across multiple comparisons within each set of analyses by using sequential Bonferroni adjustment (25, 42).
RESULTS
Percentages of influenza A virus-reactive IFN-γ+ T cells and NK cells in adults and 5- to 9-year-old children before and after immunization with TIV or LAIV.
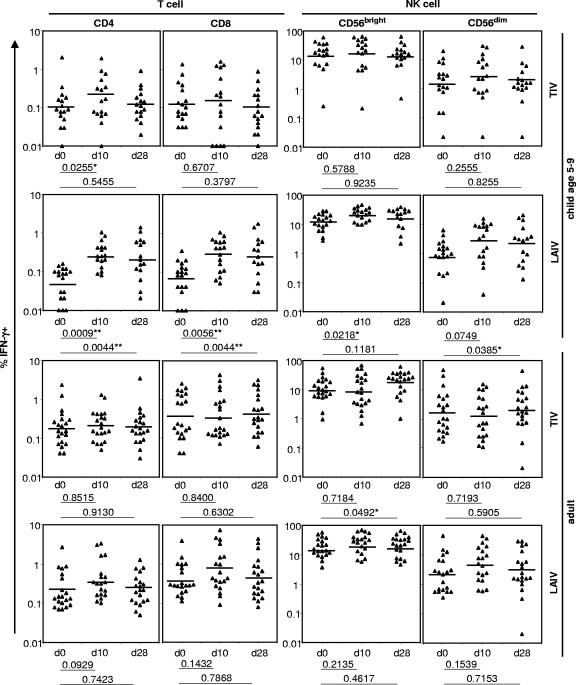
Adults aged 22 to 49 years and children aged 5 to 9 years received TIV or LAIV before the 2004 to 2005 influenza season. Blood samples were collected at baseline (day 0), on approximately day 10 after vaccination, and either on day 28 (for children and adults receiving TIV and 9/18 children requiring only one dose of LAIV) or on day 42 (for 9/18 children requiring two doses of LAIV) after vaccination (Table 1) (see Materials and Methods). The PBMCs were incubated for 17 h with live wild-type influenza virus A/Wyoming, which was a component H3N2 virus strain in TIV and LAIV, and the percentages of IFN-γ+ cells in the CD4 T-cell, CD8 T-cell, CD56bright NK cell, and CD56dim NK cell subsets were determined (Fig. 1). Since no significant differences were detected in the percentages of IFN-γ+ cells in any T- or NK cell subsets between the children given LAIV and tested on day 28 or day 42 (P > 0.5; Student's t test), the results were pooled and identified as day 28 samples in the data analyses.
FIG. 1.
Percentages of IFN-γ+ cells in CD4 and CD8 T cells and CD56bright and CD56dim NK cells at day 0 (d0; baseline), day 10 (d10), and day 28 (d28) after immunization with TIV or LAIV in children aged 5 to 9 years and adults. The bars denote the GMP of IFN-γ+ cells as estimated by GEE regression analysis. The criteria for statistical significance were adjusted across all four cell subsets within each vaccine and age group to control the type I error rate at 5% across these multiple comparisons. **, result that remained statistically significant after the adjustment; *, result with a P value of <0.05 in an individual test but that became nonsignificant after the adjustment.
The GMP of influenza A virus-reactive IFN-γ+ CD4 T cells and CD8 T cells were significantly higher than baseline (day 0) on both day 10 and day 28 for 5- to 9-year-old LAIV recipients, even after adjustment for multiple comparisons (Fig. 1). The estimated GMP of IFN-γ+ CD56bright NK cells and CD56dim NK cells were also higher after vaccination, although the differences were not statistically significant after adjustment for multiple comparisons (Fig. 1). In contrast, children in this age group given TIV had no significant changes in the GMP of any cell subsets after vaccination, when adjusted for multiple comparisons. In adult subjects, statistically significant changes were not observed in the GMP of influenza A virus-reactive IFN-γ+ cells after TIV or LAIV immunization, after adjustment for multiple comparisons (Fig. 1).
Comparison of changes in the percentages of influenza A virus-reactive IFN-γ+ cells after immunization with TIV versus LAIV.
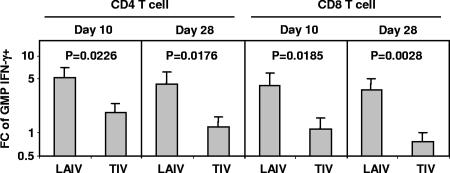
Cellular immune responses, measured as the change (n-fold) in the GMP of influenza A virus-reactive IFN-γ+ cells from baseline to day 10 and from baseline to day 28, were compared between the TIV and LAIV recipients in 5- to 9-year-old children for each lymphocyte subpopulation. The change (n-fold) in GMP was significantly greater in the LAIV recipients than the TIV recipients for CD4 and for CD8 T cells at both time points (Fig. 2). These results suggest that LAIV induced more vigorous T-cell responses than TIV in children aged 5 to 9 years. The changes (n-fold) in GMP for CD56bright and CD56dim NK cell subsets were also higher after LAIV versus TIV vaccination, but the differences were not statistically significant (P > 0.19; GEE regression). Significant differences were not observed in the change (n-fold) in IFN-γ+ cells in any of the lymphocyte subsets between adults given TIV or LAIV (P > 0.18; GEE regression). Notably, no significant difference was detected between the baseline (day 0) levels of IFN-γ+ cells in the CD4 T-cell, CD8 T-cell, and CD56dim NK cell subsets between adult TIV and LAIV recipients (P > 0.5; Student's t test). The baseline level of IFN-γ+ cells in the CD56bright subset was higher in the LAIV group than in the TIV group (P = 0.05; Student's t test), but the difference was not statistically significant after adjustment for multiple comparisons.
FIG. 2.
Differences in LAIV- versus TIV-induced changes (n-fold) in IFN-γ+ CD4 and CD8 T cells in children aged 5 to 9 years. The height of each bar denotes the estimated change (n-fold; days 0 to 10 or days 0 to 28, as indicated) in the GMP (plus 1 standard error). The estimates and P values were from GEE regression analysis. FC, change (n-fold).
Comparison of the vaccine-induced changes in percentages of influenza A virus-reactive IFN-γ+ cells in children versus adults.
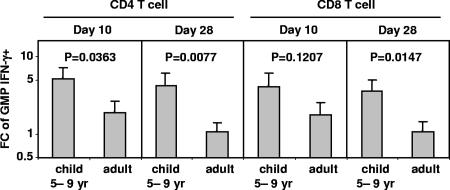
When the changes (n-fold) in the GMP of influenza A virus-reactive IFN-γ+ cells in lymphocyte subpopulations were compared between children 5 to 9 years old and adults given LAIV, the average change (n-fold) in the GMP for CD4 T cells was significantly greater in children both on day 10 and on day 28 (Fig. 3). The change (n-fold) in the GMP was also higher for the CD8 T-cell subset in children versus adults, although the difference was significant only on day 28. These results suggest that T-cell responses to LAIV are more vigorous in children than in adults. The estimated changes (n-fold) in the GMP of IFN-γ+ CD56bright and CD56dim NK cells were also greater in children, but these differences were not statistically significant (P > 0.24; GEE regression). Significant change (n-fold) differences between children and adults given TIV were not detected in any T- or NK cell subsets (P > 0.15; GEE regression).
FIG. 3.
Differences in the change (n-fold) in GMP for IFN-γ+ CD4 and CD8 T cells after immunization with one dose of LAIV in children aged 5 to 9 years versus adults. The height of each bar denotes the estimated change (n-fold; days 0 to 10 or days 0 to 28, as indicated) in the GMP (plus 1 standard error). The estimates and P values were from GEE regression analysis. FC, change (n-fold).
Percentage of influenza A virus-reactive IFN-γ+ cells in children 6 months to 4 years old after immunization with TIV.
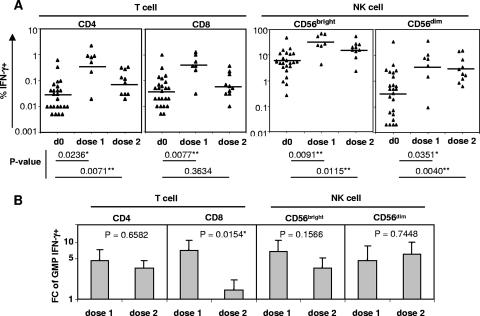
Children aged 6 months to 4 years who had not previously been immunized received two doses of TIV at a 28-day interval and were randomized for testing at baseline (day zero) and 10 days after either the first or the second TIV dose. At day 10 after the first dose, the GMP of influenza A virus-reactive IFN-γ+ cells increased from baseline in all four lymphocyte subsets (Fig. 4A). These estimated increases were significant in all individual tests but were nonsignificant for CD4 T cells and CD56dim NK cells after adjustment for multiple comparisons. Ten days after the second dose, the GMP of IFN-γ+ cells were significantly higher than baseline for CD4 T cells and both NK cell subsets, but not for CD8 T cells, after adjustment for multiple comparisons. In contrast, older children and adults did not have a significant increase in influenza A virus-reactive IFN-γ+ cells in any lymphocyte subset after TIV immunization (Fig. 1).
FIG. 4.
NK cell and T-cell responses to TIV in children aged 6 months to 4 years. (A) Percentages of IFN-γ+ cells in the NK and T-cell subsets measured at baseline (d0; n = 24) before the first dose of TIV was given and at day 10 (range, 9 to 11) after dose 1 (n = 7) or dose 2 (n = 10) of TIV, which was given approximately 28 days after the first dose. The GMP of IFN-γ+ cells before and after each dose of TIV were compared via GEE regression analysis. The criteria for statistical significance were adjusted across all four cell subsets to control the type I error rate at 5% across these multiple comparisons. **, result that remained statistically significant after the adjustment; *, result with a P value of <0.05 in an individual test but that became nonsignificant after the adjustment. (B) Comparison of T-cell or NK cell responses at day 10 after dose 1 versus day 10 after dose 2 of TIV. The estimated changes (n-fold) in the GMP of IFN-γ+ cells (plus 1 standard error) were compared via GEE regression analysis. *, result with a P value of <0.05 in an individual test but that became nonsignificant after adjustment for multiple comparisons. FC, change (n-fold).
Among children aged 6 months to 4 years, changes (n-fold) from baseline were estimated to be greater in children tested 10 days after the first dose than in those tested 10 days after the second dose in three of the four lymphocyte subsets, including CD8 T cells (Fig. 4B). For CD8 T cells, the change (n-fold) was significantly greater after the first dose, although the difference was nonsignificant after adjustment for multiple comparisons. The change (n-fold) for the CD56dim NK cell subset was estimated to be greater after the second dose, but this difference was not statistically significant in the individual test. Thus, the second dose of TIV in children aged 6 months to 4 years may not boost the percentages of influenza A virus-reactive IFN-γ+ T cells and NK cells above the levels after the first dose of TIV.
Effects of age on the change in influenza A virus-reactive IFN-γ+ cells after TIV vaccination.
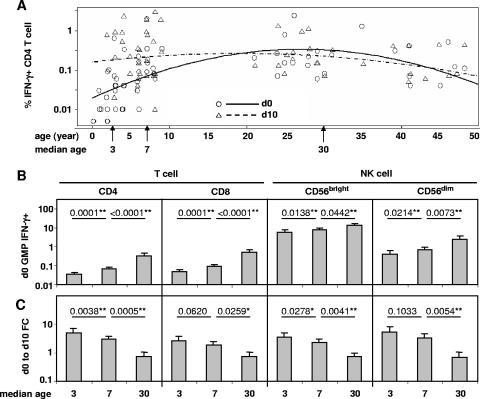
To explore further the age effects on immune responses in TIV recipients in all three age groups, log-transformed percentages of IFN-γ+ cells at baseline (day zero) and 10 days after the first dose were regressed on age for each lymphocyte subset. Figure 5A is a plot of the regression results for the CD4 T-cell subset as an example. Since the regression is on age as a continuous variable, hypotheses were tested at the median age for each age group (ages 3, 7, and 30 years). Baseline levels of the GMP of influenza A virus-reactive IFN-γ+ cells in each of the cell subsets increased significantly from age 3 to age 7 to age 30 years (Fig. 5B). In contrast, the estimated change (n-fold) in the GMP in each cell subset decreased from age 3 to age 7 to age 30 years, and the differences between the adjacent median ages were significant before and after adjustment for multiple comparisons in six of eight comparisons, except those for CD8 T-cell and CD56dim NK cell subsets between the median ages of 3 and 7 years (Fig. 5C). Four children younger than age 2 had only a baseline blood sample tested. The trend of reduced change (n-fold) with increased age remained when these four baseline samples were excluded, although the differences between adjacent median ages became nonsignificant (P ≥ 0.05), except for CD4 T cells (P < 0.04) (data not shown). These results suggest that while the average baseline levels of influenza A virus-reactive IFN-γ+ cells were higher in children of intermediate ages and even higher in adults compared to the youngest children, the change (n-fold) in the GMP of IFN-γ+ cells after the first dose of TIV tended to be greatest in the youngest children with the lowest baseline levels and least in the adults with the highest baseline levels.
FIG. 5.
Association between age and T- and NK cell responses to TIV. Child and adult subjects in all three age groups receiving TIV were pooled for this analysis, consisting of children aged 6 months to 4 years (n = 24 at day 0; n = 7 at day 10), children aged 5 to 9 years (n = 17 at day 0; n = 20 at day 10), and adults (n = 21 at day 0; n = 23 at day 10). The base 10 logarithms of the percentages of IFN-γ+ cells in each of the cell subsets at day 0 (d0; baseline) and day 10 (d10) after the first dose of TIV were regressed on age using GEE. The GMP of IFN-γ+ cells was estimated from the regression fit at the median age for each of the three groups: 3, 7, and 30 years. (A) Fitted GEE regression model (lines) and raw data (symbols) for CD4 T cells. (B) Estimated baseline GMP (plus 1 standard error) of IFN-γ+ cells in each lymphocyte subset for the three median ages. (C) Estimated change (n-fold; day 0 to day 10) in the GMP of IFN-γ+ cells in each lymphocyte subset for the three median ages. P values are provided for comparisons of baseline GMP (panel B) or change (n-fold) in the GMP (panel C) between adjacent median ages. The criteria for statistical significance were adjusted across all four cell subsets to control the type I error rate at 5% across these multiple comparisons. **, result that remained statistically significant after the adjustment; *, result with a P value of <0.05 in an individual test but that became nonsignificant after the adjustment. FC, change (n-fold).
Relationship between the baseline levels of influenza A virus-reactive IFN-γ+ cells and changes after vaccination.
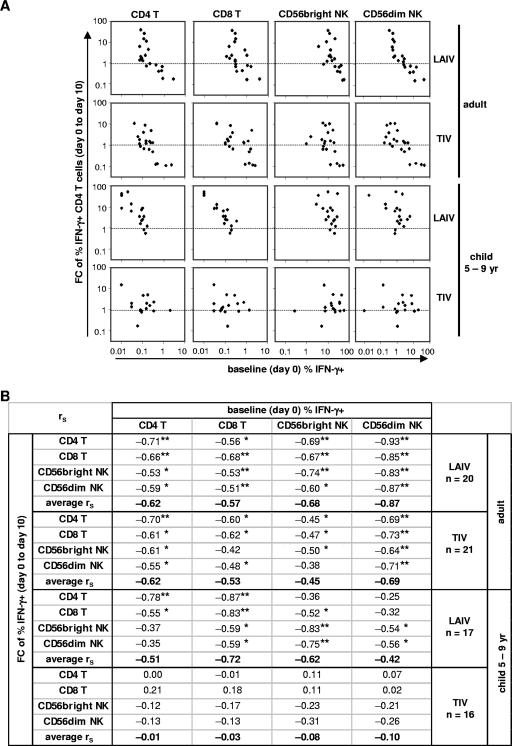
Although adults as a group had no significant increases in the GMP of IFN-γ+ cells after vaccination, subsets of individuals demonstrated distinct patterns of responses. For example, in the 19 adults given LAIV, the percentages of influenza A virus-reactive IFN-γ+ CD4 and CD8 T cells at day 10 increased in 11 (58%) subjects and decreased in 8 (42%) subjects compared to baseline. Baseline percentages of IFN-γ+ CD4 and CD8 T cells were significantly lower in the first subgroup than in the second subgroup (P < 0.05; Student's t test). In addition, children had lower baseline levels of IFN-γ+ cells than adults and were more likely to have increases in the GMP of IFN-γ+ CD4 and CD8 T cells after LAIV immunization (Fig. 1 and 3). In order to assess the relationships between the baseline levels of IFN-γ+ T cells and NK cells, which reflect cellular immunity to influenza A virus at the time of vaccination, and changes (n-fold) in IFN-γ+ cells after vaccination, which reflect cellular immune responses to the vaccine, we computed Spearman's correlation coefficients (rS) between baseline percentages of influenza A virus-reactive IFN-γ+ cells and the changes (n-fold) in the percentage of IFN-γ+ cells of each cell subset in individual subjects after vaccination by age and vaccine group (Fig. 6). Significant inverse correlations were detected between the change (n-fold; day 0 to day 10) in IFN-γ+ cells and the baseline levels of IFN-γ+ cells in the four lymphocyte subsets examined in adults given LAIV or TIV. Figure 6A shows the inverse correlations for the change (n-fold) in the CD4 T-cell subset as an example. The inverse correlation was, in general, stronger among the LAIV recipients than the TIV recipients, as indicated by the size of rS and the number of rS coefficients remaining statistically significant after adjustment for multiple comparisons (Fig. 6B). In both vaccine groups, the strongest inverse correlation was observed between the change (n-fold) in IFN-γ+ cells for any of the cell subsets following vaccination and the baseline percentage of IFN-γ+ cells in the CD56dim NK cell subset, as indicated by the size and statistical significance of rS (Fig. 6B).
FIG. 6.
Associations between baseline percentages of IFN-γ+ cells and changes (n-fold) in the percentages after influenza vaccination. All subjects with data available for day 0 (baseline) and day 10 were included. (A) Scatter plots for the change (n-fold) in IFN-γ+ CD4 T cells versus baseline percentages of IFN-γ+ cells in CD4 and CD8 T-cell subsets and CD56bright and CD56dim NK cell subsets in adult and child (5- to 9-year-old) groups receiving LAIV or TIV. For reference, the dotted lines indicate a change (n-fold) of 1. (B) Complete list of estimated Spearman correlation coefficients, rS, for change (n-fold) versus the baseline level of IFN-γ+ cells in all lymphocyte subsets. The criteria for statistical significance were adjusted across four cell subsets within each vaccine/age group to control the type I error rate at 5% across the 16 multiple comparisons. **, result that remained statistically significant after the adjustment; *, result with a P value of <0.05 in an individual test but that became nonsignificant after the adjustment. The average rS was calculated across the change for four lymphocyte subsets versus the baseline percentage of each cell type in each age/vaccine group. FC, change (n-fold).
Significant inverse correlations were also detected between the change (n-fold) in IFN-γ+ cells in 5- to 9-year-old children given LAIV and their baseline percentages of IFN-γ+ cells for some lymphocyte subsets, although fewer correlations were statistically significant than for the adult LAIV recipients. In contrast to adults, in children, no statistically significant correlation was detected with the baseline level of IFN-γ+ cells in the CD56dim NK cell subset after adjustment for multiple comparisons (Fig. 6B). Significant correlation was not detected between the change (n-fold) in IFN-γ+ cells in any cell subset after TIV immunization and the baseline levels of IFN-γ+ cells in any cell subset in this age group (rS < 0.31; P > 0.15), even without adjustment for multiple comparisons. The results did not change when children aged 6 months to 4 years with data available for calculating day 0 to day 10 changes (n-fold; n = 6) were included in the analysis (average rS = −0.17 and P > 0.08 in all 16 individual tests) (data not shown).
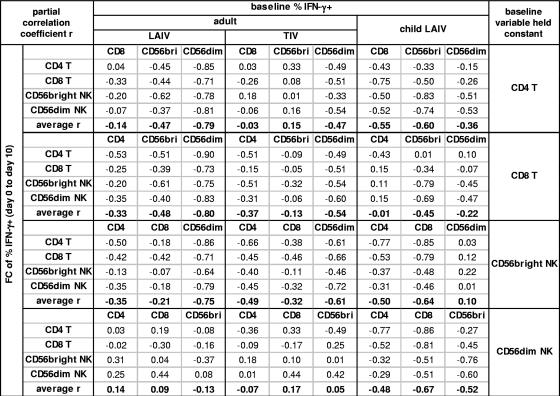
To explore further which lymphocyte subset at the time of vaccination had the most proximal association with the change (n-fold) in influenza A virus-reactive IFN-γ+ cells on day 10 after vaccination, partial correlation coefficients between the day 0 to day 10 change (n-fold) and the baseline percentage of IFN-γ+ cells were computed. This analysis held constant the baseline level of IFN-γ+ cells in each of the T- and NK cell subsets one at a time (47). Figure 7 summarizes these results for the groups of adults given LAIV or TIV and children given LAIV, in which inverse correlations were observed (Fig. 6). In the adults, when the baseline percentages in the CD4 T-, CD8 T-, or CD56bright NK cell subsets were held constant, the strong inverse correlation remained between the baseline percentages of IFN-γ+ CD56dim NK cells and the day 0 to day 10 change (n-fold). In contrast, the inverse correlation declined toward zero when the baseline percentage of IFN-γ+ cells in the CD56dim NK cell subset was held constant. The same pattern was observed in both adult LAIV and adult TIV groups but was more pronounced in the LAIV group, as indicated by the larger sizes of partial correlation coefficients for baseline CD56dim NK cells in this group. These results suggest that the baseline level of IFN-γ+ cells in the CD56dim NK cells has the most direct association with T- and NK cell responses after vaccination of adults. In contrast to the adults, when the baseline level of IFN-γ+ CD56dim NK cells was held constant in children given LAIV, substantial inverse correlations persisted between the change (n-fold) and baseline levels of influenza A virus-reactive IFN-γ+ cells (Fig. 7). The different patterns of negative correlation between baseline and postvaccination responses in children versus adults and in LAIV recipients versus TIV recipients further suggests that both age and type of vaccine influence cellular immune responses to influenza vaccines.
FIG. 7.
Estimated partial correlation coefficients for change (n-fold) versus baseline levels of IFN-γ+ cells, with the baseline level of each other lymphocyte subset held constant. The partial correlation coefficients (47) were calculated using the rS between the change (n-fold) in IFN-γ+ cells and the baseline percentage of IFN-γ+ cells (Fig. 6B) and the rS between the baseline percentage of IFN-γ+ cells in each cell subset. FC, change (n-fold).
DISCUSSION
In this study, cellular immune responses to a live influenza A virus strain were evaluated in children aged 6 months to 9 years and adults aged 22 to 48 years before and after immunization with TIV or LAIV. After one dose of LAIV, the average percentage of influenza A virus-reactive IFN-γ+ cells in T-cell and NK cell subsets, detected after stimulation with live influenza A virus/Wyoming (H3N2), increased significantly in children aged 5 to 9 years but not in adults. In children aged 5 to 9 years, LAIV was more likely to induce an increase in the percentage of influenza A virus-specific IFN-γ+ CD4 and CD8 T cells than TIV. Age also significantly influenced immune responses to LAIV and TIV. The change (n-fold) in IFN-γ+ T cells induced by LAIV was smaller among the adults than among 5- to 9-year-old children. Significant inverse correlations between the postvaccination change (n-fold) in influenza A virus-reactive IFN-γ cells in various lymphocyte subsets and their prevaccination levels were observed in all age and vaccine groups, except for children given TIV. The strongest correlation was observed in adults given LAIV. Among adults, the baseline levels of IFN-γ+ cells in the CD56dim NK cell subset were associated most directly with change (n-fold) in IFN-γ+ cells after either LAIV or TIV vaccination. Although differences in the pattern of the inverse correlation were observed, the vaccine-induced change (n-fold) in influenza A virus-reactive IFN-γ+ cells in all four lymphocyte subsets, including CD4 and CD8 T cells and CD56bright and CD56dim NK cells, correlated positively with each other in all age and vaccine groups (data not shown). Notably, the cellular immune responses to TIV and LAIV showed no correlation with the day 0 to day 10 change (n-fold) in neutralizing antibody titers against influenza A virus/Wyoming (unpublished data).
In a limited number of influenza vaccine-naïve children younger than age 5, the average percentage of influenza A virus-reactive IFN-γ+ cells increased significantly in the CD8 T-cell and CD56bright NK cell subsets after one dose of TIV, while significant changes were not observed in any T- or NK cell subsets in older children or adults after TIV vaccination. The reason for the different responses in different age groups remains unclear but may be related to the lack of preexisting influenza virus immunity in the youngest age group. Interestingly, the percentages of IFN-γ+ cells detected in the subjects tested at day 10 after a second dose of TIV given 28 days after the first dose were not greater than the levels observed in the subjects tested at day 10 after the first dose. Possible explanations for this unexpected result include a difference in the kinetics of the secondary responses or in the functional status of immune cells that are reexposed to the HA and NA antigens after a recent primary exposure. These issues should be addressed in future studies with larger sample sizes in order to optimize the use of TIV in this youngest age group. However, it is interesting that a similar lack of boosting effect was observed in the same subjects in their antibody-secreting-cell responses to the second dose of TIV (unpublished data).
Presentation of exogenous protein antigen to CD8 T cells, such as the influenza virus proteins in the TIV formulation, requires cross-presentation of the antigen through the major histocompatibility complex class I pathway (44). Dendritic cells (DCs) are the major antigen-presenting cells that have the capability to cross-present exogenous protein antigens to CD8 T cells (1, 43, 46). The detection of influenza A virus-specific CD8 T cells following immunization with TIV in children younger than 5 years old suggests that the cross-presentation pathway is functional in this age group. It is unclear why TIV failed to induce a significant T- or NK cell response in older children, although one might speculate that the preexisting influenza virus immunity diminished the ability of DCs to present viral antigen efficiently. Interestingly, LAIV appears to be more likely to induce influenza A virus-specific IFN-γ+ T-cell responses than does TIV, as demonstrated in the 5- to 9-year-old vaccinees. This seems likely to be due to the provision of additional antigens in the form of endogenously synthesized viral proteins in LAIV-immunized subjects. It is also possible that the replication of LAIV in the respiratory tract provides a more favorable environment for DCs and other antigen-presenting cells to present viral antigens to T cells. These findings may have implications for the relative protective effects of LAIV and TIV in children. It will be important to directly compare responses to TIV and LAIV in children younger than 5 years old to determine whether these two vaccines also differ in the capacity to induce T-cell responses in the youngest age group and to examine the efficiency of cross-priming versus conventional endogenous priming for the stimulation of primary major histocompatibility complex class I-restricted responses in influenza-naïve children. It will also be of interest to investigate the shedding of replicating vaccine virus in LAIV recipients and to investigate if vaccine replication is associated with T-cell responses in recipients.
The innate immune response, which is rapid but not considered antigen specific, provides a first line of defense against viral infection and influences the subsequent adaptive T-cell responses (36, 37, 49). It was reported that in human volunteers experimentally challenged with wild-type influenza virus, infection caused a significant increase in NK cell activity (13). Despite the importance of innate immunity in host defense against viral infection, most previous immunological studies of influenza vaccines have focused on adaptive immunity, i.e., B-cell and T-cell responses, and rarely on innate immunity. In limited studies of the NK cell response to inactivated influenza vaccine, an enhanced NK cell cytotoxicity has been related to vaccination in some studies (38, 45), but not others (29).
IFN-γ is known for its direct antiviral activity, as well as its immune-regulatory activity (14). Rapid production of IFN-γ and other inflammatory cytokines by NK cells, including the CD56bright and CD56dim NK cell subsets, is an important component of the innate immune response (6). In the current study, we demonstrated that the IFN-γ response of NK cells to influenza A virus antigens can be enhanced by vaccination, especially with LAIV, in the majority of children, as well as in some adults. Based on our previous finding that the IFN-γ response of NK cells to influenza A virus is dependent on the preexisting memory T cells specific for influenza A virus (22), we propose that the enhanced NK cell reactivity to influenza A virus is secondary to the enhanced T-cell response after vaccination. In agreement with this hypothesis, we found that the change (n-fold) in the percentage of IFN-γ+ NK cells correlated positively with the change (n-fold) in IFN-γ+ T cells in both adults and children receiving either LAIV or TIV (data not shown).
Although the adults as a group did not respond to TIV or LAIV as assessed by a change in the average levels of influenza A virus-reactive IFN-γ+ cells, the responses were highly variable among individual subjects, who showed either increases or decreases in the percentage of IFN-γ+ T cells and NK cells after immunization. Significant positive and negative correlations were observed between measured parameters, indicating that such person-to-person variation reflects the intrinsic interaction between the vaccine and the host immune system rather than the measurement error of the assay. One of the most intriguing findings of this study was that in adults, who are likely to differ in their previous exposures to influenza A virus, CD4 and CD8 T-cell responses to vaccination best correlated inversely with the levels of CD56dim NK cell reactivity at the time of vaccination. To our knowledge, this is the first report of the relationship between the baseline levels of an innate immune response against a virus and the cellular immune responses after vaccination. The findings in this study, together with our previous findings on interactions between influenza A virus-reactive T cells and NK cells, suggest a new model of relationships between innate immunity and adaptive immunity, as well as between preexisting memory T cells and antigen recall responses, in the contexts of viral infection and vaccination.
Previously, we showed that IFN-γ production by NK cells in response to influenza A virus depends on preexisting influenza A virus-specific memory T cells and that interleukin-2 produced by the activated influenza A virus-specific memory T cells determines the IFN-γ response of NK cells to influenza A virus (22). Based on these observations and the inverse correlation between cellular responses to influenza vaccines and the baseline percentage of IFN-γ+ CD56dim NK cells, we speculate that the interaction between NK cells and DCs is critical in determining the immunologic outcome of exposure to influenza vaccination or infection.
DCs play a central role in both innate and adaptive immunity. Influenza A virus-infected DCs are the major producers of type I IFN and interleukin-12, which activate NK cells to produce cytokines and mediate cytotoxic activity (6, 10, 15). On the other hand, DCs process viral antigens and present them to specific T cells, resulting in activation and amplification of virus-specific T cells, which constitute the primary or secondary T-cell response to viral infection or vaccination. Depending on their maturity and functional status, DCs can also render T cells anergic to cognate antigens (2). Studies using in vitro-cultured NK cells and DCs have shown that NK cell-DC interaction may result in their reciprocal activation, as well as inhibition of DCs by NK cells, in different circumstances (16, 17, 37, 41, 49). At low NK cell/DC ratios, DC responses were amplified dramatically, while at high NK cell/DC ratios, DC responses were inhibited completely. The inhibition of DC functions by NK cells was mediated by the potent DC-killing activity of the autologous NK cells (41), which is most likely mediated by the CD56dim NK cell subset, which expressed high levels of perforin (39). Therefore, depending on the magnitude of CD56dim NK cell activity during the early stage of infection in vivo, the NK cell response could either enhance or suppress subsequent adaptive T-cell responses by activating or inhibiting DC responses.
Based on our observations, the levels of influenza A virus-specific memory T cells are low in some adults, consistent with less previous exposure to influenza A virus infection or vaccination and resulting in low levels of baseline NK cell reactivity to influenza A virus. During influenza A virus infection or LAIV vaccination, the lower levels of baseline T-cell and NK cell reactivity are likely to result in higher levels of viral replication, as well as higher levels of DC activity, which are likely to be associated with a more vigorous influenza A virus-specific T-cell response. In contrast, higher baseline levels of influenza A virus-specific T-cell and NK cell reactivities should limit virus replication upon reexposure to natural infection or vaccination and inhibit DC functions. The outcome would be to limit the further activation of influenza A virus-specific T cells.
The variable patterns in the immune responses that we observed in influenza vaccinees also raise important questions regarding the roles of innate and adaptive cellular immunity during natural infection with pathogenic influenza viruses. The current population may have limited cross-reactive neutralizing antibody activity but significant T-cell cross-reactivity against newly emerged epidemic or pandemic influenza strains, as neutralizing antibodies primarily target the highly variable HA and NA proteins while T-cell immunity may target more conserved structural and nonstructural proteins of the virus. Do modulation of DC functions and subsequent T-cell responses by high levels of NK reactivity, which is due to high levels of preexisting memory T cells, accelerate or delay the recovery of infected individuals? Since innate immune responses to infecting virus may occur well before the onset of clinical symptoms, it is difficult to study the interaction between DC, NK cell, and T-cell immunities at the earliest stages of natural infection, as opposed to vaccination. Our study suggests that LAIV immunization may serve as a valuable model to address these types of critical questions concerning host responses to influenza virus and other viral infections, since early events can be studied directly in relation to the time of exposure.
Acknowledgments
We thank L. Liu, K. Dionis, P. Stepick-Biek, S. Stamatis, L. Rott, and C. Crumpton for technical assistance, J. Oehlert for database management, and the children, their parents, and adult subjects for participating in these studies.
This work was supported by NIH grants AI057229, DK56339, and 5M01RR000070.
A. M. Arvin and H. B. Greenberg are paid consultants of Medimmune Vaccines, the manufacturer of the licensed live attenuated influenza vaccine used in this study.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Belshe, R. B. 2004. Influenza vaccines—live, p. 371. In S. A. Plotkin and W. Ornstein (ed.), Vaccines. Saunders, Philadelphia, Pa.
- 4.Belshe, R. B., and W. C. Gruber. 2001. Safety, efficacy and effectiveness of cold-adapted, live, attenuated, trivalent, intranasal influenza vaccine in adults and children. Phil. Trans. R. Soc. Lond. B 356:1947-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe, R. B., K. L. Nichol, S. B. Black, H. Shinefield, J. Cordova, R. Walker, C. Hessel, I. Cho, and P. M. Mendelman. 2004. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5-49 years. Clin. Infect. Dis. 39:920-927. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 7.Boos, D. 1992. On generalized score tests. Am. Stat. 46:327-333. [Google Scholar]
- 8.Bot, A., A. Reichlin, H. Isobe, S. Bot, J. Schulman, W. M. Yokoyama, and C. A. Bona. 1996. Cellular mechanisms involved in protection and recovery from influenza virus infection in immunodeficient mice. J. Virol. 70:5668-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conover, W. J., and R. L. Iman. 1981. Rank transformation as a bridge between parametric and nonparametric statistics. Am. Stat. 35:124-129. [Google Scholar]
- 10.Cooper, M. A., T. A. Fehniger, S. C. Turner, K. S. Chen, B. A. Ghaheri, T. Ghayur, W. E. Carson, and M. A. Caligiuri. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97:3146-3151. [DOI] [PubMed] [Google Scholar]
- 11.Couch, R. B. 2003. An overview of serum antibody responses to influenza virus antigens. Dev. Biol 115:25-30. [PubMed] [Google Scholar]
- 12.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 13.Ennis, F. A., A. Meager, A. S. Beare, Y. H. Qi, D. Riley, G. Schwarz, G. C. Schild, and A. H. Rook. 1981. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet ii:891-893. [DOI] [PubMed] [Google Scholar]
- 14.Farrar, M. A., and R. D. Schreiber. 1993. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 11:571-611. [DOI] [PubMed] [Google Scholar]
- 15.Fehniger, T. A., M. A. Cooper, G. J. Nuovo, M. Cella, F. Facchetti, M. Colonna, and M. A. Caligiuri. 2003. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101:3052-3057. [DOI] [PubMed] [Google Scholar]
- 16.Ferlazzo, G., M. L. Tsang, L. Moretta, G. Melioli, R. M. Steinman, and C. Munz. 2002. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerosa, F., A. Gobbi, P. Zorzi, S. Burg, F. Briere, G. Carra, and G. Trinchieri. 2005. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 174:727-734. [DOI] [PubMed] [Google Scholar]
- 18.Graham, M. B., and T. J. Braciale. 1997. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 186:2063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber, W. C., R. B. Belshe, J. C. King, J. J. Treanor, P. A. Piedra, P. F. Wright, G. W. Reed, E. Anderson, F. Newman, et al. 1996. Evaluation of live attenuated influenza vaccines in children 6-18 months of age: safety, immunogenicity, and efficacy. J. Infect. Dis. 173:1313-1319. [DOI] [PubMed] [Google Scholar]
- 20.Gruber, W. C., L. H. Taber, W. P. Glezen, R. D. Clover, T. D. Abell, R. W. Demmler, and R. B. Couch. 1990. Live attenuated and inactivated influenza vaccine in school-age children. Am. J. Dis. Child 144:595-600. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie, T., C. G. Hobbs, V. Davenport, R. E. Horton, R. S. Heyderman, and N. A. Williams. 2004. Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adults. J. Infect. Dis. 190:1927-1935. [DOI] [PubMed] [Google Scholar]
- 22.He, X. S., M. Draghi, K. Mahmood, T. H. Holmes, G. W. Kemble, C. L. Dekker, A. M. Arvin, P. Parham, and H. B. Greenberg. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, X. S., K. Mahmood, H. T. Maecker, T. H. Holmes, G. W. Kemble, A. M. Arvin, and H. B. Greenberg. 2003. Analysis of the frequencies and of the memory T cell phenotypes of human CD8+ T cells specific for influenza A viruses. J. Infect. Dis. 187:1075-1084. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, E., K. Mahmood, Z. Chen, C. F. Yang, J. Spaete, H. B. Greenberg, M. L. Herlocher, H. Jin, and G. Kemble. 2005. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J. Virol. 79:11014-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 43:223-225. [Google Scholar]
- 26.Hurwitz, E. S., M. Haber, A. Chang, T. Shope, S. Teo, M. Ginsberg, N. Waecker, and N. J. Cox. 2000. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA 284:1677-1682. [DOI] [PubMed] [Google Scholar]
- 27.Jin, H., B. Lu, H. Zhou, C. Ma, J. Zhao, C. F. Yang, G. Kemble, and H. Greenberg. 2003. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306:18-24. [DOI] [PubMed] [Google Scholar]
- 28.Kilbourne, E. D. 1999. Inactivated influenza vaccines, p. 531-52. In S. A. Plotkin and E. A. Mortimer (ed.), Vaccines, 3rd ed. Saunders, Philadelphia, Pa.
- 29.Kutza, J., P. Gross, D. Kaye, and D. M. Murasko. 1996. Natural killer cell cytotoxicity in elderly humans after influenza immunization. Clin. Diagn. Lab. Immunol. 3:105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1533-79. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 31.Lennette, D. A. 1995. General principles for laboratory diagnosis of viral, rickettsial, and chlamydial infections, p. 3-25. In E. H. Lennette, D. A. Lennette, and E. T. Lennette (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th ed. American Public Health Association, Washington, D.C.
- 32.Liang, K., and S. L. Zeger. 1986. Longitudinal data analysis using generalized linear models. Biometrika 73:13-22. [Google Scholar]
- 33.Liu, B., I. Mori, M. J. Hossain, L. Dong, K. Takeda, and Y. Kimura. 2004. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J. Gen. Virol. 85:423-428. [DOI] [PubMed] [Google Scholar]
- 34.Mbawuike, I. N., P. A. Piedra, T. R. Cate, and R. B. Couch. 1996. Cytotoxic T lymphocyte responses of infants after natural infection or immunization with live cold-recombinant or inactivated influenza A virus vaccine. J. Med. Virol. 50:105-111. [DOI] [PubMed] [Google Scholar]
- 35.McMichael, A. J., F. M. Gotch, G. R. Noble, and P. A. Beare. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309:13-17. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 37.Moretta, A. 2002. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2:957-964. [DOI] [PubMed] [Google Scholar]
- 38.Mysliwska, J., P. Trzonkowski, E. Szmit, L. B. Brydak, M. Machala, and A. Mysliwski. 2004. Immunomodulating effect of influenza vaccination in the elderly differing in health status. Exp. Gerontol. 39:1447-1458. [DOI] [PubMed] [Google Scholar]
- 39.Nagler, A., L. L. Lanier, S. Cwirla, and J. H. Phillips. 1989. Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol. 143:3183-3191. [PubMed] [Google Scholar]
- 40.Nichol, K. L., P. M. Mendelman, K. P. Mallon, L. A. Jackson, G. J. Gorse, R. B. Belshe, W. P. Glezen, and J. Wittes. 1999. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137-144. [DOI] [PubMed] [Google Scholar]
- 41.Piccioli, D., S. Sbrana, E. Melandri, and N. M. Valiante. 2002. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice, W. R. 1989. Analyzing tables of statistical tests. Evolution 43:223-225. [DOI] [PubMed] [Google Scholar]
- 43.Rock, K. L., L. Rothstein, S. Gamble, and C. Fleischacker. 1993. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J. Immunol. 150:438-446. [PubMed] [Google Scholar]
- 44.Rock, K. L., and L. Shen. 2005. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 207:166-183. [DOI] [PubMed] [Google Scholar]
- 45.Schapiro, J. M., Y. Segev, L. Rannon, M. Alkan, and B. Rager-Zisman. 1990. Natural killer (NK) cell response after vaccination of volunteers with killed influenza vaccine. J. Med. Virol. 30:196-200. [DOI] [PubMed] [Google Scholar]
- 46.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 47.Sokal, R. R., and F. J. Rohlf. 1981. Biometry, 2nd ed. W. H. Freeman and Company, New York, N.Y.
- 48.Yap, K. L., G. L. Ada, and I. F. McKenzie. 1978. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273:238-239. [DOI] [PubMed] [Google Scholar]
- 49.Zitvogel, L. 2002. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 195:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]