Abstract
Recent clinical and experimental observations showed that specific probiotic microorganisms may provide therapeutic benefits in inflammatory bowel disease. However, a rigorous screening for new candidate probiotic strains with optimized therapeutic properties necessitates also determining possible adverse interactions with the host, particularly in individuals who are not healthy. We have evaluated the persistence of strains of lactic acid bacteria (LAB) in the digestive tracts of mice, their immunomodulation capacity, and their safety in healthy animals and in a colitis model. Following daily administration of 109 CFU of viable LAB orally, intragastrically, or intrarectally, the animals' feces were examined for bacterial excretion and cytokines were quantified in intestinal samples by quantitative reverse transcription-PCR. The level of bacterial translocation was assessed in healthy mice and in mice suffering from colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). Irrespective of the route of administration, the potential probiotic strain Lactobacillus plantarum NCIMB8826 was found to persist for up to 10 days in the digestive tracts of mice. This strain did not induce detrimental effects in healthy or in TNBS-treated animals, as was reflected by the absence of weight loss, intestinal inflammation, modification of cytokine levels in the ileum and colon (healthy mice), and bacterial dissemination (healthy and colitic animals). Moreover, the translocation of endogenous microflora to the mesenteric lymph nodes and spleen was greatly reduced in the TNBS-treated mice after administration of LAB. This property, together with the strain's persistence capacity and innocuousness renders L. plantarum NCIMB8826 an attractive candidate as a probiotic to be used in the prevention or treatment of chronic inflammation.
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is a significant health care problem of unknown etiology which affects 0.5% of the population in northern Europe. It is a chronic immune-mediated disease in which endogenous bacteria are thought to play an important role, as suggested by numerous clinical observations and experimental studies, summarized in recent reviews (21, 41). Notably, most of the models in which animals develop spontaneous or chemically induced inflammatory colitis are influenced by the bacterial flora present in the intestinal lumen. Despite the fact that our current understanding of the complexity of the intestinal flora is very limited, many studies have shown that not all bacterial species have equal activities in promoting or reducing intestinal inflammation (15, 35-38, 46). This evidence has led to a new concept in the therapy of IBD based on probiotic preparations which usually contain lactobacilli, bifidobacteria, or Escherichia coli strains (6, 12, 18, 20, 25, 39, 45). However, many of these therapeutic trials make limited allowances for (i) optimal frequency and route of probiotic administration, (ii) rational selection of bacterial strains, (iii) possible adverse effects, and (iv) in vivo immune effects of probiotics on the intestinal mucosa.
Hence, the selection of new probiotic strains should take into account the above-mentioned points. To optimize the dose and frequency of administration, the persistence of the bacteria in the gastrointestinal tract (GIT) should be investigated. At present, these data are available only for a very few strains used in oral therapeutic trials. In animal models most data are obtained from intragastric gavages. Other modes of administration, such as the oral and rectal routes, also deserve to be evaluated in mice, as they may be more relevant to the treatment of certain pathologies.
The influence of probiotic strains on the immune system is often evaluated through in vitro or ex vivo measurements of cytokine or immunoglobulin production, T- or B-cell proliferation, or nitric oxide induction (14). The physiological relevance of these observations remains unclear because they show very little correlation with in vivo and clinical studies. Moreover, the techniques used for cytokine quantification (reverse transcriptase PCR, immunohistochemical analysis, or enzyme-linked immunosorbent assay) differ greatly between studies and may be the cause of sometimes contradictory conclusions.
Among the possible adverse events of regular probiotic feeding, the risk of bacterial translocation (BT; i.e., the passage of viable indigenous bacteria from the GIT to the mesenteric lymph nodes [MLN] and other extraintestinal sites) (5) should be evaluated carefully. In a healthy host, BT is a highly regulated event which occurs continuously at a low rate. When the integrity of the intestinal barrier is disturbed or when the immune system is not able to confine an infection, pathogenic bacteria can reach the bloodstream and cause septicemia (4). Consequently, it appears necessary to verify the safety of probiotic strains by assessing their potential to translocate, not only in healthy individuals but also in individuals with injured intestinal mucosae.
Our laboratory selected the human isolate Lactobacillus plantarum NCIMB8826 as a promising probiotic strain based, on the one hand, on its technological properties and, on the other hand, on its ability to survive passage through the human stomach (47) and to induce secretion of anti-inflammatory cytokines by peripheral blood mononuclear cells (32). In this paper, we evaluated the persistence of strain NCIMB8826 after oral, intragastric, or intrarectal administration to mice and compared its persistence to that of other lactic acid bacteria (LAB), including the reference probiotic strain Lactobacillus salivarius UCC118. This strain is indeed well documented for its survival and persistence in mouse and human GITs, its influence on cytokine expression, and its therapeutic effects in animal models of colitis and in patients with IBD (12, 13, 34). Furthermore, we compared L. plantarum NCIMB8826 and Lactococcus lactis MG1363 (a dairy starter derivative) to determine their influence on cytokine levels in the intestinal mucosa. Finally, we evaluated the potential risk of BT in a model that uses 2,4,6-trinitrobenzene sulfonic acid (TNBS) to induce colitis (22, 33) by using TNBS doses that very severely damaged (histological score, 6) the intestinal barrier.
MATERIALS AND METHODS
Bacteria and growth conditions.
Three Lactobacillus strains and one Lactococcus strain were used in this study. L. plantarum NCIMB8826 (National Collection of Industrial and Marine Bacteria, Aberdeen, United Kingdom) was isolated from human saliva. L. plantarum 256 (provided by P. Pouwels, TNO, Leiden, The Netherlands) is a silage isolate (S. Ahrné, personal communication), and L. salivarius spp. salivarius UCC118 (provided by J. K. Collins, University College Cork, Cork, Ireland) was isolated from the human intestine. Lactococcus lactis MG1363 (17) is a nonpathogenic strain derived from a cheese starter. In order to monitor the bacteria after their administration to the mice, the strains were electrotransformed with a plasmid conferring resistance to chloramphenicol or erythromycin. For chloramphenicol resistance, pTG2247 (23) was used with the L. plantarum strains and pNZ8020 (7) was used with the L. salivarius strain, and for erythromycin resistance, pTREX (50) was used with the Lactococcus lactis strain. Lactobacilli were grown at 37°C in MRS medium (Difco, Detroit, Mich.) supplemented with 10 μg of chloramphenicol (Sigma, St. Louis, Mo.) per ml under limited aeration. Lactococcus lactis was grown at 30°C in M17 medium (Difco) supplemented with 0.5% glucose (GM17) and 5 μg of erythromycin (Sigma) per ml under limited aerobic conditions. It was verified that culturing the strains in vitro without antibiotic selection pressure did not lead to plasmid loss for at least a few generations.
Preparation of the bacterial strains and administration to mice.
The strains were grown to an A600 of 1 to 2 (exponential growth phase), harvested by centrifugation at 3,000 × g for 10 min, washed with phosphate-buffered saline (PBS), and resuspended at the appropriate cell concentration either in 0.2 M NaHCO3 buffer containing 2% glucose or in PBS. Bacterial suspensions were prepared daily. For oral administration, mice received 109 CFU resuspended in bicarbonate buffer by mouth (25 μl) or intragastrically (100 μl). Intrarectal administration of 109 CFU resuspended in PBS (100 μl) was performed with mice anaesthetized with 3 mg of ketamine (Imalgene 1000; Mérial, Lyon, France), 46.7 μg of diazepam (Valium; Roche Diagnostics), and 15 μg of atropine (Aguettant Laboratory, Lyon, France), using a 3.5 F catheter (EO 3416-1; Biotrol, Chelles, France) inserted 4 cm proximal to the anus.
Study design.
Animal experiments were performed in an accredited establishment (number A59107; animal facility of the Institut Pasteur de Lille) according to French governmental guidelines (number 86/609/CEE). Adult male BALB/c mice were purchased from Iffa Credo (Saint Germain sur l'Arbresle, France) and kept under filter top hoods. The mice had free access to tap water and standard mouse chow.
Persistence of LAB in the GITs of mice.
Groups of four animals received a single dose per day of lactobacilli or lactococci for three consecutive days by the oral, intragastric, or intrarectal route. Fecal samples were collected daily, resuspended at 100 mg of feces/ml in one-fourth-strength Ringer's diluent, and mechanically homogenized. Dilutions were plated on the selected media described above and incubated at 37 or 30°C for 2 days before enumeration. The experiment was repeated three times for each strain and for each mode of administration tested.
Effects of LAB on the immune response.
Groups of eight to nine mice were given L. plantarum NCIMB8826, Lactococcus lactis MG1363, or carbonate buffer once a day for 4 days and were sacrificed on day 5 by cervical dislocation. Total RNA was extracted from ileal and colonic samples kept in TriReagent (Euromedex, Souffelweyersheim, France), and reverse transcribed into cDNA as described previously (9). Quantification of β-actin, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-10 cDNA was performed by PCR on 1 μg of cDNA using the appropriate competitors and primers (11). Quantification of the amplified cDNA was performed by electrophoresis in a 3% agarose gel by means of an image analyzer (Gel Analyst; Clara Vision, Orsa, France). Results of the cytokine measurements were expressed as cytokine cDNA per β-actin cDNA in picograms per milliliter.
Safety assessment of LAB in mice.
The risk of BT was determined for healthy mice and mice with TNBS-induced colitis. This colitis model was used, as it results in severe colonic inflammation similar to that described for patients with IBD (31). For both experiments, groups of five to nine mice received buffer (negative control), L. plantarum NCIMB8826, or Lactococcus lactis MG1363 orally once a day for 4 days. Colitis was induced in the experimental group of mice on day 5 by intrarectal administration of TNBS (150 mg/kg of body weight; Fluka, Saint Quentin Fallavier, France) mixed with an equal volume of ethanol, as previously described (9). Control mice received an intrarectal administration of 50% ethanol. Healthy mice were sacrificed on day 5, and mice with TNBS-induced colitis were sacrificed on day 7. The MLN and spleen were aseptically removed and immediately placed in one-fourth-strength Ringer's diluent. The samples were mechanically homogenized, and cultures for both aerobic and anaerobic bacteria were performed on Columbia blood agar supplemented with 0.05% cystein hydrochloride and 0.5% glucose. Anaerobic conditions were obtained in hermetic jars with Anaerogen (Oxoid, Basingstoke, United Kingdom). Selective enumeration of L. plantarum NCIMB8826 or Lactococcus lactis MG1363 was performed by plating the organ suspensions on MRS-chloramphenicol or GM17-erythromycin agar, respectively. Whenever the situation warranted, the mice were weighed daily and their appearance was evaluated according to an activity score (51) ranging from 1 to 3 (1, lazy, slow movement; 2, intermediate level of activity; 3, active movement or searching). The colon and the small intestine were macroscopically and histologically scored as previously described (9), the histological scoring being performed in a blind manner. We used both the scoring described by Wallace et al. (49), which reflects the level of inflammation and the extent of ulceration (score range, 0 to 10), and the criteria described by Ameho et al. (2), which take into account the degree of the inflammatory infiltrate and cell alterations (score range, 0 to 6). Quantification of myeloperoxidase (MPO) in ileal and colonic samples was performed by immunoblotting as described previously (9).
Statistics.
The comparisons were analyzed by the nonparametric Kruskal-Wallis one-way analysis of variance test or by the Mann-Whitney U test. Differences were judged to be statistically significant when the P value was <0.05.
RESULTS
Persistence of LAB in the mouse intestine.
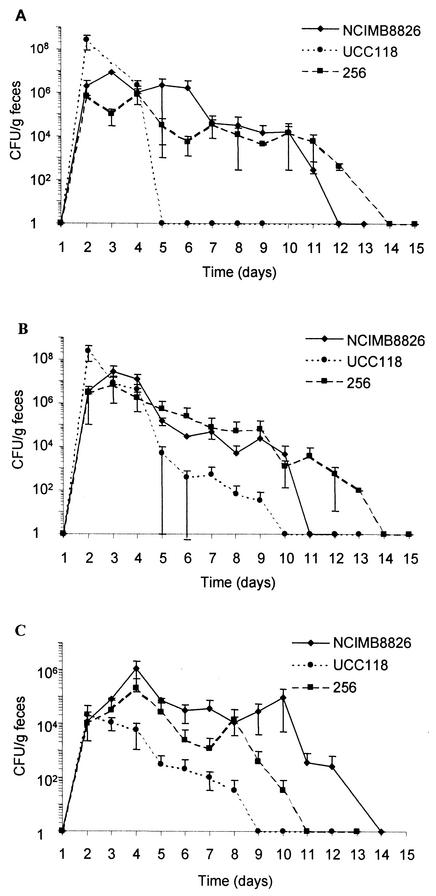
Four strains of LAB were evaluated for their ability to persist in the GITs of mice after 3 days of oral, intragastric, or rectal administration. After the oral and intragastric administrations (Fig. 1A and B, respectively), the two L. plantarum strains were able to persist for 7 to 10 days after the last dose (day 3), with bacterial levels ranging from 104 to 106 CFU/g of feces during 1 week. After intragastric administration, L. salivarius UCC118 was detected in feces at lower counts and for fewer days than L. plantarum. This difference was even more pronounced after oral administration, as L. salivarius was recovered from the feces only 1 day after the last administration. The three Lactobacillus strains given by the intrarectal route were able to persist for several days (Fig. 1C). It is noteworthy that L. plantarum, especially strain NCIMB8826, was still found in the feces 10 days after the last administration and maintained itself at levels of approximately 105 CFU/g of feces from days 4 to 10.
FIG. 1.
Fecal counts of L. plantarum NCIMB8826, L. plantarum 256, and L. salivarius UCC118 given orally (A), intragastrically (B), or intrarectally (C) for three consecutive days (days 1 to 3). Data represented (numbers of CFU/gram of feces) are the arithmetic means ± standard errors of the means of results from three separate experiments.
Growth of the mouse endogenous intestinal flora (enterobacteria and streptococci) on GM17-erythromycin as well as on other selective lactococcal media prevented us from obtaining precise persistence data for Lactococcus lactis. However, colonization studies from Gruzza et al. indicate that Lactococcus lactis only passively transits through the digestive tracts of mice colonized with a human microflora (19). In view of the duration and high level of persistence of L. plantarum NCIMB8826 in mice, this strain was selected for the next experiments. Oral administration was retained, as it is less stress-inducing for the mice than the intragastric route and as it is similar to natural food intake. Moreover, this mode of administration corresponds to the one used in human clinical trials. Lactococcus lactis MG1363 was also chosen as a prototype of a noncolonizing strain (19, 47) that exhibits in vitro immunomodulation properties that are quite different from those of L. plantarum (32).
Cytokine expression in the intestinal mucosa.
We quantified TNF-α, IFN-γ, IL-4, and IL-10 mRNA in the ilea and colons of mice receiving a single daily dose of L. plantarum NCIMB8826 or Lactococcus lactis for four consecutive days (Fig. 2). Among the four cytokines, TNF-α (levels 10- to 100-fold higher than those of the other cytokines) was dominant in healthy mice. IFN-γ was found in larger amounts in the colon than in the ileum, while IL-4 and IL-10 were more predominant in the ileum than in the colon. However, only IL-4 levels appeared significantly higher in the ileum than in the colon (P = 0.009). This result indicates that the Th1/Th2 balance may differ between these two subcompartments. As shown in Fig. 2, no significant variation in the cDNA levels of TNF-α, IL-4, or IL-10 was observed after oral administration of LAB. Only the level of IFN-γ was significantly increased in the ilea of mice receiving Lactococcus lactis. However, the cDNA amounts were detected in only four of the eight samples and were so close to the detection limit of the method (0.2 pg/PCR) that the physiological significance of this increase in the level of IFN-γ can be questioned. When heat-killed L. plantarum NCIMB8826 or Lactococcus lactis MG1363 was given to the mice, the levels of the four cytokines remained identical to those observed with a buffer or live bacteria (data not shown).
FIG. 2.
Cytokine mRNA levels in the ilea (A) and the colons (B) of mice after oral administration for 4 days of carbonate buffer, L. plantarum NCIMB8826, or Lactococcus lactis MG1363. Data correspond to the means of results from eight samples and are representative of two separate experiments.
Effects of repeated oral administration of LAB in healthy and TNBS-treated mice.
Oral administrations of 109 CFU of L. plantarum NCIMB8826 or Lactococcus lactis MG1363 were performed daily for 4 days, and different parameters were monitored to evaluate the potential adverse effect of repeated bacterial ingestion (Table 1). No variation in mouse activity and weight was observed in comparison to the activity and weight of the buffer control group. No sign of macroscopic or histological inflammation was detected (Wallace and Ameho scores of 0) either in the ileum or in the colon. MPO amounts, which reflect the levels of neutrophil recruitment in tissues, remained undetectable by Western blot analysis in all mice. The MLN and spleen cultures were negative for both L. plantarum NCIMB8826 and Lactococcus lactis MG1363, indicating that repeated ingestion of high doses of either strain did not induce abnormal BT or dissemination in healthy mice.
TABLE 1.
Effect on mice of oral administration of NaHCO3 buffer, L. plantarum NCIMB8826, or Lactococcus lactis MG1363a
| Buffer or strain | No. of mice | Activity score | % Variation in wt (day 5 − day 1) | Macroscopic score | Histological score | NCIMB8826 or MG1363 translocation or disseminationb | OD with MPO |
|---|---|---|---|---|---|---|---|
| NaHCO3 | 8 | 3 ± 0 | +1.7 ± 1.9 | 0 | 0 | ND | 0 |
| NC1MB8826 | 9 | 3 ± 0 | +1.1 ± 2.2 | 0 | 0 | No | 0 |
| MG1363 | 8 | 3 ± 0 | +6.4 ± 7.9 | 0 | 0 | No | 0 |
Values are means ± standard errors of the means. OD, optical density; ND, not determined.
Detection level, ≥100 CFU/organ.
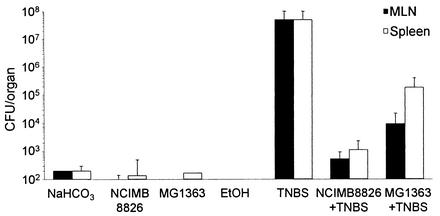
The BT of the global endogenous flora was also determined for healthy mice and for mice with TNBS-induced colitis. Rectal administration of TNBS resulted in increased BT in the MLN and spleen, as only 20% of the organs remained free of bacteria in these mice, whereas 50 to 75% of the organs remained sterile in healthy animals (Table 2). Bacteria were then enumerated in each positive organ (Fig. 3). In healthy mice receiving buffer, ethanol, L. plantarum NCIMB8826, or Lactococcus lactis MG1363, low numbers of bacteria were found, reflecting the normal basal translocation level. In TNBS-treated mice the level of translocation was much higher (107-fold increase). Interestingly, the total number of intestinal bacteria that translocated was greatly reduced in the TNBS-treated mice that preventively received L. plantarum NCIMB8826 (105-fold decrease) or Lactococcus lactis MG1363 (103-fold decrease) compared to numbers in the TNBS-treated mice that received no preventive dose of bacteria. As observed with healthy mice, the orally given Lactobacillus or Lactococcus strains were not found in the MLN or spleens of the TNBS-treated mice in spite of the dramatic increase in the level of translocation of the endogenous microflora.
TABLE 2.
BT of the intestinal microbiota in healthy mice and in mice with TNBS-induced colitis
| Pretreatment (oral) | Treatment (i.r.a) | nb | Colitis | MLNc | Spleenc |
|---|---|---|---|---|---|
| NaHCO3 | None | 5 | No | 25 | 50 |
| NCIMB8826 (in NaHCO3) | None | 9 | No | 20 | 33 |
| MG1363 (in NaHCO3) | None | 8 | No | 0 | 37 |
| None | TNBS-ethanol | 5 | Yes | 80 | 80 |
| None | Ethanol | 5 | No | 0 | 25 |
| NCIMB8826 (in NaHCO3) | TNBS-ethanol | 8 | Yes | 75 | 62 |
| MG1363 (in NaHCO3) | TNBS-ethanol | 8 | Yes | 86 | 43 |
i.r., intrarectal administration.
n, number of animals per group.
Data are the percentages of organs positive for bacterial cultures (detection limit, ≥100 CFU/organ).
FIG. 3.
BT of the intestinal microflora in MLN and spleen. Mice received carbonate, L. plantarum NCIMB8826, or Lactococcus lactis MG1363 orally and were treated or not with TNBS-ethanol or ethanol (EtOH) by intrarectal administration. Data correspond to mean numbers of CFU per positive organ ± standard errors of the means.
DISCUSSION
According to the current definition (40), a probiotic strain is expected to transiently persist in the GIT of the host and to reach high numbers of viable cells in the targeted part of the gut. Persistence is thought to be dependent on the mode of administration and on the intrinsic properties of the bacterial strain, including resistance to gastric acid and bile and adhesion to the intestinal epithelium or mucus (44). The pharmacokinetic properties of potential probiotic strains should determine at which dose and for how long the strains have to be administered to the host. This determination, in turn, will allow correlation of the observed biological effect with the presence of the probiotic strain under study.
In this work we first compared the levels of persistence of four LAB strains administered to mice by different routes (oral, intragastric, and rectal). In addition to L. plantarum NCIMB8826 and Lactococcus lactis MG1363, we chose to include the well-described probiotic strain L. salivarius UCC118 as well as another L. plantarum strain of nonhuman origin (L. plantarum 256). Irrespective of the route of administration, the L. plantarum strains were detected for longer periods than was L. salivarius UCC118 in the feces of mice. Interestingly, the best-surviving strain in our mouse experiments also performed very well in human feeding trials. Vesa et al. have shown that L. plantarum NCIMB8826 reached the human ileum with a survival rate of 7%, which was far superior to the survival rate of the other tested LAB (47). We also evaluated the intrarectal route as it might allow an increase in the concentrations of probiotics in the colon, which may be advantageous for specific applications, such as the treatment of pouchitis. Indeed, Madsen et al. reported that rectal administration of Lactobacillus reuteri in IL-10 gene-deficient mice prevents colitis and normalizes the colonic Lactobacillus levels (28). To the best of our knowledge, our study was the first to analyze the persistence rate of LAB delivered by the rectal route. The three Lactobacillus strains were found to establish themselves, though at variable levels. Again, L. plantarum NCIMB8826 was found in feces for especially long periods.
Previous work in the laboratory demonstrated that heat-inactivated L. plantarum NCIMB8826 and Lactococcus lactis MG1363 differ substantially in their capacities to modulate cytokine production by human peripheral blood mononuclear cells. While the former leads to strong IL-10 production, the latter is characterized by the stimulation of TNF-α, IFN-γ, and IL-12 (32). In the present study, we verified whether substantial in vivo cytokine modulation could also be measured after mice were fed with L. plantarum NCIMB8826 or Lactococcus lactis MG1363. For this purpose, we used quantitative reverse transcriptase PCR rather than other in vitro or ex vivo techniques (for example, enzyme-linked immunosorbent assay), as this method was successfully employed in previous studies to measure mRNA expression directly in the intestinal mucosa (8-11). No major immunomodulatory effects were induced in the ileal and colonic mucosae of mice after the administration of L. plantarum or Lactococcus lactis. As an additional control, we administered heat-killed L. plantarum or Lactococcus lactis to mice to verify whether the cytokine stimulation profile observed in vitro could be reproduced in vivo. This was not found to be the case. Nevertheless, these results were coherent with the data reported by Maassen et al. which show that L. plantarum NCIMB8826 is a weaker cytokine inducer than other Lactobacillus species (27). In the human feeding trial described by Dunne et al., the systemic cytokine levels remained unchanged also after the consumption of L. salivarius UCC118 (12). It might be argued that in healthy individuals, probiotic strains should not definitely or significantly affect the natural cytokine balance as this might, for example, increase the risk of uncontrolled inflammation. All together, these data indicate that immunomodulatory effects observed in vitro need to be corroborated by animal studies.
Finally, we examined the safety of the two LAB strains since a probiotic should, above all else, be innocuous even when the integrity of the host intestinal barrier is partly impaired. Even though dietary LAB benefit from the GRAS (generally recognized as safe) status, some reports have identified Lactobacillus species associated with bacteremia or endocarditis (1, 3, 16, 24, 30, 48). We therefore chose to examine the rates of BT in a TNBS-induced acute colitis model that leads to important lesions and necrosis in the mouse GIT. This model displays immunological features common to the chronic stage of Crohn's disease. It is characterized by an inflammation of the colon marked by a transmural cellular infiltration with T cells and macrophages in a pattern similar to that described for the human situation (33, 42). It is noteworthy that we purposely used high doses of TNBS (150 mg/kg) in order to exacerbate the risk of BT, yet these experimental conditions are less appropriate for evaluating the capacity of probiotic LAB strains to prevent the onset of colitis. Significantly, in healthy as well as in TNBS-treated mice, feeding live L. plantarum or Lactococcus lactis induced no detrimental effects and no abnormal translocation of the administered bacteria. On the contrary, prefeeding TNBS-treated mice with L. plantarum NCIMB8826 and, to a lesser extent (2-log difference), with Lactococcus lactis MG1363 significantly reduced the BT of the global intestinal microflora. Other studies have reported the preventive effect of Lactobacillus rhamnosus GG (26), Lactobacillus reuteri R2LC and L. plantarum DSM9843 (29), and Bifidobacterium longum (43) on BT, but the studies were performed mainly with healthy mice. In the present study, we showed that even when the intestinal mucosa was severely injured, L. plantarum and Lactococcus lactis exerted a beneficial effect which might be critical for the restoration of the intestinal homeostasis. Under our experimental conditions, this effect did not seem to be mediated by strong in vivo cytokine modifications. At present, it is difficult to assess whether the greater efficacy of L. plantarum than that of Lactococcus lactis is linked to its superior persistence capacity rather than to its intrinsic immunomodulatory properties.
Acknowledgments
This work was supported by Rhodia Food S.A.S., France, FEDER funds, and Institut Pasteur de Lille funding. This work was also supported by a grant from the EUQLK1-2000-00146 research program.
We acknowledge P. Pouwels and J. K. Collins for providing L. plantarum 256 and L. salivarius UCC118 strains, respectively. We warmly thank B. Pot for critical reading of the manuscript.
REFERENCES
- 1.Aguirre, M., and M. D. Collins. 1993. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 75:95-107. [DOI] [PubMed] [Google Scholar]
- 2.Ameho, C. K., A. A. Adjei, E. K. Harrison, K. Takeshita, T. Morioka, Y. Arakaki, E. Ito, I. Suzuki, A. D. Kulkarni, A. Kawajiri, and S. Yamamoto. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 41:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, A. S., A. W. Chow, D. Betts, and L. B. Guze. 1978. Lactobacillemia—report of nine cases. Important clinical and therapeutic considerations. Am. J. Med. 64:808-813. [DOI] [PubMed] [Google Scholar]
- 4.Berg, R. D. 1999. Bacterial translocation from the gastrointestinal tract. Adv. Exp. Med. Biol. 473:11-30. [DOI] [PubMed] [Google Scholar]
- 5.Berg, R. D., and A. W. Garlington. 1979. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 23:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campieri, M., and P. Gionchetti. 1999. Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative? Gastroenterology 116:1246-1249. [DOI] [PubMed] [Google Scholar]
- 7.de Ruyter, P. G. G. A., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desreumaux, P., E. Brandt, L. Gambiez, D. Emilie, K. Geboes, O. Klein, N. Ectors, A. Cortot, M. Capron, and J. F. Colombel. 1997. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 113:118-126. [DOI] [PubMed] [Google Scholar]
- 9.Desreumaux, P., L. Dubuquoy, S. Nutten, M. Peuchmaur, W. Englaro, K. Schoonjans, B. Derijard, B. Desvergne, W. Wahli, P. Chambon, M. D. Leibowitz, J. F. Colombel, and J. Auwerx. 2001. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 193:827-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desreumaux, P., O. Ernst, K. Geboes, L. Gambiez, D. Berrebi, H. Muller-Alouf, S. Hafraoui, D. Emilie, N. Ectors, M. Peuchmaur, A. Cortot, M. Capron, J. Auwerx, and J. F. Colombel. 1999. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology 117:73-81. [DOI] [PubMed] [Google Scholar]
- 11.Dombrowicz, D., S. Nutten, P. Desreumaux, C. Neut, G. Torpier, M. Peeters, J. F. Colombel, and M. Capron. 2001. Role of the high affinity immunoglobulin E receptor in bacterial translocation and intestinal inflammation. J. Exp. Med. 193:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne, C., L. Murphy, S. Flynn, L. O'Mahony, S. O'Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly, B. Kiely, E. M. Quigley, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 1999. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Leeuwenhoek 76:279-292. [PubMed] [Google Scholar]
- 13.Dunne, C., L. O'Mahony, L. Murphy, G. Thornton, D. Morrissey, S. O'Halloran, M. Feeney, S. Flynn, G. Fitzgerald, C. Daly, B. Kiely, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S-392S. [DOI] [PubMed]
- 14.Erickson, K. L., and N. E. Hubbard. 2000. Probiotic immunomodulation in health and disease. J. Nutr. 130:403S-409S. [DOI] [PubMed]
- 15.Garcia-Lafuente, A., M. Antolin, F. Guarner, E. Crespo, A. Salas, P. Forcada, M. Laguarda, J. Gavalda, J. A. Baena, J. Vilaseca, and J. R. Malagelada. 1997. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am. J. Physiol. 272:G10-G15. [DOI] [PubMed] [Google Scholar]
- 16.Gasser, F. 1994. Safety of lactic acid bacteria and their occurrence in human clinical infections. Bull. Inst. Pasteur 92:45-67. [Google Scholar]
- 17.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gionchetti, P., F. Rizzello, A. Venturi, P. Brigidi, D. Matteuzzi, G. Bazzocchi, G. Poggioli, M. Miglioli, and M. Campieri. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305-309. [DOI] [PubMed] [Google Scholar]
- 19.Gruzza, M., M. Fons, M. F. Ouriet, Y. Duval-Iflah, and R. Ducluzeau. 1994. Study of gene transfer in vitro and in the digestive tract of gnotobiotic mice from Lactococcus lactis strains to various strains belonging to human intestinal flora. Microb. Releases 2:183-189. [PubMed] [Google Scholar]
- 20.Guslandi, M., G. Mezzi, M. Sorghi, and P. A. Testoni. 2000. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig. Dis. Sci. 45:1462-1464. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson, B. A., R. Gokhale, and J. H. Cho. 2002. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 15:79-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann, P., J. M. Zeeh, J. Lakshmanan, V. S. Wu, F. Procaccino, M. Reinshagen, J. A. McRoberts, and V. E. Eysselein. 1997. Increased expression of transforming growth factor alpha precursors in acute experimental colitis in rats. Gut 41:195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hols, P., P. Slos, P. Dutot, J. Reymund, P. Chabot, B. Delplace, J. Delcour, and A. Mercenier. 1997. Efficient secretion of the model antigen M6-gp41E in Lactobacillus plantarum NCIMB 8826. Microbiology 143:2733-2741. [DOI] [PubMed] [Google Scholar]
- 24.Husni, R. N., S. M. Gordon, J. A. Washington, and D. L. Longworth. 1997. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin. Infect. Dis. 25:1048-1055. [DOI] [PubMed] [Google Scholar]
- 25.Kruis, W., E. Schutz, P. Fric, B. Fixa, G. Judmaier, and M. Stolte. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 11:853-858. [DOI] [PubMed] [Google Scholar]
- 26.Lee, D. J., R. A. Drongowski, A. G. Coran, and C. M. Harmon. 2000. Evaluation of probiotic treatment in a neonatal animal model. Pediatr. Surg. Int. 16:237-242. [DOI] [PubMed] [Google Scholar]
- 27.Maassen, C. B., C. van Holten-Neelen, F. Balk, M. J. den Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 28.Madsen, K. L., J. S. Doyle, L. D. Jewell, M. M. Tavernini, and R. N. Fedorak. 1999. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116:1107-1114. [DOI] [PubMed] [Google Scholar]
- 29.Mao, Y., S. Nobaek, B. Kasravi, D. Adawi, U. Stenram, G. Molin, and B. Jeppsson. 1996. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology 111:334-344. [DOI] [PubMed] [Google Scholar]
- 30.Maskell, R., and L. Pead. 1992. 4-Fluoroquinolones and Lactobacillus spp as emerging pathogens. Lancet 339:929. [DOI] [PubMed] [Google Scholar]
- 31.Morris, G. P., P. L. Beck, M. S. Herridge, W. T. Depew, M. R. Szewczuk, and J. L. Wallace. 1989. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96:795-803. [PubMed] [Google Scholar]
- 32.Müller-Alouf, H., C. Grangette, D. Goudercourt, N. Reveneau, and A. Mercenier. 1999. Comparative cytokine inducing pattern of lactic acid bacteria used for mucosal vaccine development. Immunol. Lett. 69:33. [Google Scholar]
- 33.Neurath, M. F., I. Fuss, B. L. Kelsall, E. Stuber, and W. Strober. 1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Mahony, L., M. Feeney, S. O'Halloran, L. Murphy, B. Kiely, J. Fitzgibbon, G. Lee, G. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment. Pharmacol. Ther. 15:1219-1225. [DOI] [PubMed] [Google Scholar]
- 35.Rath, H. C., H. H. Herfarth, J. S. Ikeda, W. B. Grenther, T. E. Hamm, Jr., E. Balish, J. D. Taurog, R. E. Hammer, K. H. Wilson, and R. B. Sartor. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human β2 microglobulin transgenic rats. J. Clin. Investig. 98:945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rath, H. C., J. S. Ikeda, H. J. Linde, J. Scholmerich, K. H. Wilson, and R. B. Sartor. 1999. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology 116:310-319. [DOI] [PubMed] [Google Scholar]
- 37.Rath, H. C., M. Schultz, R. Freitag, L. A. Dieleman, F. Li, H.-J. Linde, J. Scholmerich, and R. B. Sartor. 2001. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect. Immun. 69:2277-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rath, H. C., K. H. Wilson, and R. B. Sartor. 1999. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect. Immun. 67:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rembacken, B. J., A. M. Snelling, P. M. Hawkey, D. M. Chalmers, and A. T. Axon. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635-639. [DOI] [PubMed] [Google Scholar]
- 40.Schrezenmeir, J., and M. de Vrese. 2001. Probiotics, prebiotics, and synbiotics—approaching a definition. Am. J. Clin. Nutr. 73:361S-364S. [DOI] [PubMed]
- 41.Shanahan, F. 2002. Crohn's disease. Lancet 359:62-69. [DOI] [PubMed] [Google Scholar]
- 42.Strober, W., B. R. Ludviksson, and I. J. Fuss. 1998. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann. Intern. Med. 128:848-856. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, T., K. Itoh, T. Kaneko, and H. Suzuki. 1997. Inhibition of bacterial translocation from the gastrointestinal tract of mice by oral administration of a culture condensate of Bifidobacterium longum. J. Vet. Med. Sci. 59:665-669. [DOI] [PubMed] [Google Scholar]
- 44.Tuomola, E., R. Crittenden, M. Playne, E. Isolauri, and S. Salminen. 2001. Quality assurance criteria for probiotic bacteria. Am. J. Clin. Nutr. 73:393S-398S. [DOI] [PubMed]
- 45.Venturi, A., P. Gionchetti, F. Rizzello, R. Johansson, E. Zucconi, P. Brigidi, D. Matteuzzi, and M. Campieri. 1999. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment. Pharmacol. Ther. 13:1103-1108. [DOI] [PubMed] [Google Scholar]
- 46.Verdu, E. F., P. Bercik, B. Cukrowska, M. A. Farre-Castany, H. Bouzourene, E. Saraga, A. L. Blum, I. Corthesy-Theulaz, H. Tlaskalova-Hogenova, and P. Michetti. 2000. Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin. Exp. Immunol. 120:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]
- 48.Wagner, R., and E. Balish. 1998. Potential hazards of probiotic bacteria for immunodeficient patients. Bull. Inst. Pasteur 96:165-170. [Google Scholar]
- 49.Wallace, J. L., W. K. MacNaughton, G. P. Morris, and P. L. Beck. 1989. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology 96:29-36. [DOI] [PubMed] [Google Scholar]
- 50.Wells, J., and K. Schofield. 1996. Cloning and expression vectors for lactococci, p. 37-62. In T. F. Bozoglu and R. Bibek (ed.), Lactic acid bacteria: current advances in metabolism, genetics, and applications. NATO ASI Series H, vol. H 98. Springer-Verlag, Heidelberg, Germany.
- 51.Zhou, J. S., Q. Shu, K. J. Rutherfurd, J. Prasad, P. K. Gopal, and H. S. Gill. 2000. Acute oral toxicity and bacterial translocation studies on potentially probiotic strains of lactic acid bacteria. Food Chem. Toxicol. 38:153-161. [DOI] [PubMed] [Google Scholar]