Abstract
Enzyme-linked immunosorbent assays using antigens extracted from Brucella abortus with n-lauroylsarcosine differentiated natural Brucella-infected animals from Brucella-vaccinated or Yersinia enterocolitica O9-infected animals. A field trial in Mongolia showed cattle, sheep, goat, reindeer, camel, and human sera without infection could be distinguished from Brucella-infected animals by conventional serological tests.
Brucellosis is a worldwide infectious disease of domestic animals, and the causative agent, Brucella spp., is transmitted to humans by contact with infected animals or by contaminated dairy products (4). Serodiagnosis of acute and recent infections with Brucella and Yersinia enterocolitica O9 by using the commonly used microagglutination assay is seriously impaired by the well-documented and strong serological cross-reactivity between these bacteria (2, 6-11, 13, 14). The Rose Bengal test and complement fixation test are the most accepted tests worldwide (5). These tests are based on a reaction between a Brucella whole-cell antigen and antibodies produced in response to the infection. Differentiating between animals infected with Brucella and animals vaccinated against Brucella is too difficult by conventional serological tests, such as the Rose Bengal test, tube agglutination test, and complement fixation test (13), because vaccinated animals have a high titer against Brucella antigens. Therefore, we tried to find an easy serological method to differentiate Brucella-infected animals from vaccinated or Y. enterocolitica O9-infected animals.
To differentiate natural Brucella-infected animals from Y. enterocolitica O9-infected animals, antigens extracted from the virulent Brucella abortus strain 544 (15) with n-lauroylsarcosine were used for an enzyme-linked immunosorbent assay (ELISA), and the specificity of the ELISA was tested. The antigens were extracted as follows. B. abortus strain 544 cells were grown to A600 = 3.0 in brucella broth (Becton Dickinson, Sparks, Md.), and bacterial cells were harvested by centrifugation and washed once with distilled water (DW). For whole bacterial cell antigens, bacteria were inactivated by formalin (0.5% final concentration) and were concentrated to 1.5 (the optical density at 600 nm [OD600]) in DW at this step. For n-lauroylsarcosine-extracted antigens, n-lauroylsarcosine (0.5% final concentration) was added to the bacterial suspension and the cells were incubated at room temperature for 30 min with shaking. The bacterial suspension was centrifuged and filtrated, and then the supernatant was transferred to a new centrifuge tube for use with the antigens. The protein concentration of antigen was checked by Bio-Rad protein assay, and the antigen was also checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Western blotting was done with anti-B. abortus or anti-Y. enterocolitica O9 rabbit serum for each preparation. To coat the antigens on Immuno plates for the ELISA, 50 μl of the antigens (sarcosine extracts; 4 μg/ml in DW) was added onto a 96-well Immuno plate (Nunc, Rochester, N.Y.) and left overnight. Then, the wells were blocked by 0.5% bovine serum albumin (BSA) for 30 min. Sera diluted 1/50 to 1/3,200 were applied to the wells. The wells were incubated at 37°C for 1 h and washed, and then horseradish peroxidase-labeled protein G was added. The wells were incubated at 37°C for 1 h and washed, and the substrate o-phenylenediamine was added. The absorbance was measured at 492 nm by using an ELISA reader (model 450; Bio-Rad, Hercules, Calif.).
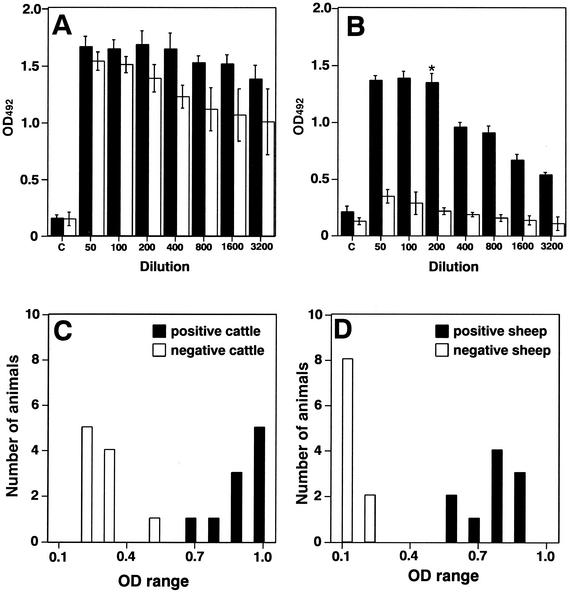
Rabbit serum immunized with B. abortus 544 or Y. enterocolitica O9 had a strong positive reaction with whole bacterial cell antigens inactivated with formalin, as shown by ELISA. The OD492 values were 1.676 ± 0.12 or 1.38 ± 0.14 at 200-fold dilution, respectively (Fig. 1A). Serum immunized with B. abortus was also positive with sarcosine extracts, as shown by ELISA (OD492 = 1.344 ± 0.12 at 200-fold dilution) (Fig. 1B). In contrast, serum immunized with Y. enterocolitica O9 was negative with sarcosine extracts as shown by ELISA (OD492 = 0.210 ± 0.04 at 200-fold dilution) (Fig. 1B). The competitive indices of 1/200 and 1/400 dilutions of anti-B. abortus sera were significantly different (Fig. 1B). Therefore, the 1/200 dilution was used as a single dilution.
FIG. 1.
ELISA absorbance values of sera by using whole bacterial cell antigens or n-lauroylsarcosine-extracted antigens. Whole bacterial cell antigens (A) or n-lauroylsarcosine-extracted antigens (B) were reacted with rabbit serum immunized with B. abortus (black columns) or Y. enterocolitica O9 (white columns) at the indicated dilutions. The BSA-coated control well results are in the columns marked “c.” Values are averages and standard deviations of triplicate wells from three identical experiments. Three rabbits were immunized with B. abortus or Y. enterocolitica O9, and their sera showed similar results. Typical data of one rabbit serum are shown. Significant differences between competitive indices of 1/200 and 1/400 dilutions were compared by using the Student t test. *, P < 0.001. n-Lauroylsarcosine-extracted antigens were reacted with sera from 10 Brucella-positive (black columns) cattle (C) or sheep (D) and 10 negative (white columns) cattle (C) or sheep (D) at a 1/200 dilution.
To further investigate the serological reactivity of the sarcosine extracts, sera from unvaccinated Brucella-infected or noninfected animals, which were judged by conventional serological tests, were tested by using an ELISA. The sarcosine extracts strongly reacted with sera from positive cattle and sheep, but not with sera from negative cattle, except for one serum, or sheep at a single serum dilution (1/200) (Fig. 1C and D). Anti-B. abortus or anti-Y. enterocolitica O9 rabbit serum at a 1/200 dilution was used as a standard for each assay. These results suggested that the ELISA with sarcosine extracts would be useful for specific detection of Brucella-infected animals.
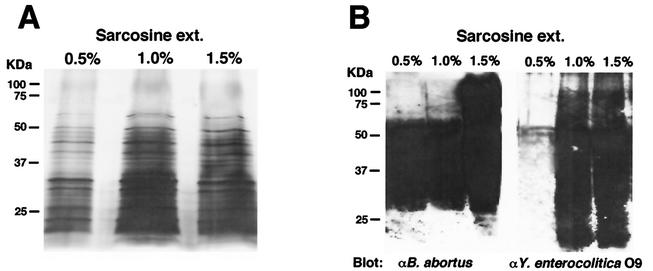
To confirm the serological reactivity of the sarcosine extracts, antigens were tested by silver staining and Western blotting with anti-B. abortus or anti-Y. enterocolitica O9 rabbit serum at a 1/200 dilution. The 0.5, 1.0, and 1.5% sarcosine extracts reacted strongly with anti-B. abortus rabbit serum. The sarcosine extracts also reacted with anti-Y. enterocolitica O9 serum, but 0.5% sarcosine extracts showed much less reactivity (Fig. 2B). As the 0.5, 1.0, and 1.5% sarcosine extracts showed similar protein band patterns by silver staining and smear band patterns by Western blotting, the polysaccharide would have reacted with the antiserum (Fig. 2). Presumably, the differences in reactivity against anti-B. abortus or anti-Y. enterocolitica O9 serum depend on the concentration of polysaccharide in the sarcosine extracts.
FIG. 2.
Analysis of sarcosine extracts of B. abortus. Antigens were extracted by 0.5, 1.0, and 1.5% sarcosine and were analyzed by 12% SDS- PAGE and silver staining (A) or by Western blotting with anti-B. abortus or anti-Y. enterocolitica O9 serum (B).
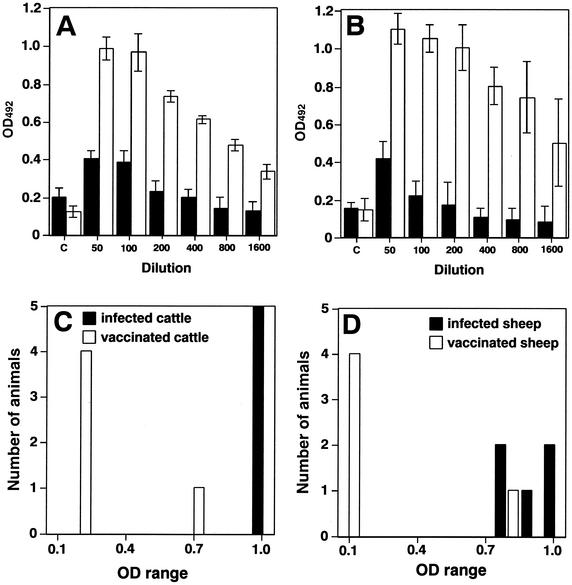
To investigate if an ELISA with sarcosine extracts can differentiate vaccinated animals from naturally infected animals, sera from vaccinated and infected animals in Mongolia were tested by using an ELISA. Vaccinated animals were usually positive by the Rose Bengal test with 1/40 diluted serum. Forty-fold-diluted sera from vaccinated animals did not exceed an OD492 of 0.5, and 1/50- to 1/800-diluted sera from infected animals had OD492 values higher than 0.5 (Fig. 3A and B). Therefore, when the absorbance exceeded an OD492 of 0.5, the serum sample was judged as positive. Sera from cattle vaccinated with strain S-19 (1), which were positive by conventional serological tests, were negative with sarcosine extracts as shown by ELISA (Fig. 3C). Similar results were obtained with sheep sera vaccinated with strain Rev-1 (1) (Fig. 3D). For both strains, one serum from a vaccinated animal was judged as positive because the absorbance exceeded an OD492 of 0.5. But both animals were suspected of having brucellosis, because these animals were kept in Brucella-contaminated farms. These results showed that an ELISA with sarcosine extracts is useful for differentiating vaccinated animals from naturally infected animals.
FIG. 3.
ELISA absorbance values of sera from vaccinated or infected animals in Mongolia by using n-lauroylsarcosine-extracted antigens. Sera from vaccinated (black columns) cattle (A) or sheep (B) and infected (white columns) cattle (A) or sheep (B) were tested at the indicated dilution. The BSA-coated control well results are in the columns marked “c.” Sera from three vaccinated or infected animals showed similar results. Typical data of one animal serum are shown. Values are averages and standard deviations of triplicate wells from three identical experiments. Sera from five infected (black columns) cattle (C) or sheep (D) and vaccinated (white columns) cattle (C) or sheep (D) were tested at a 1/200 dilution.
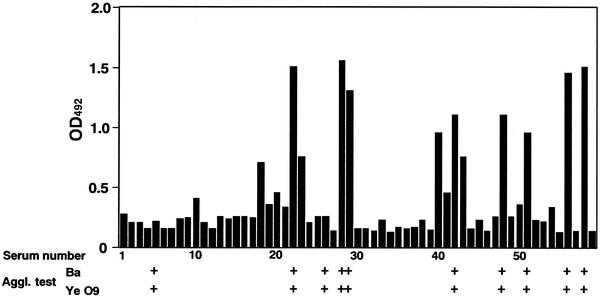
To investigate if the ELISA with sarcosine extracts can be used to diagnose brucellosis, a field trial of the assay was done in Mongolia. Fifty-nine sera from unvaccinated cattle were collected from various places in Mongolia and were tested by using ELISA. Ten of the 59 sera were positive by conventional serological test, and 2 of 59 sera (sample numbers 4 and 26) were negative with sarcosine extracts as shown by ELISA (20% discrimination), suggesting that both cattle might be infected with Y. enterocolitica O9 (Fig. 4). Although four sera (serum numbers 18, 23, 40, and 43) were negative by conventional serological tests but were positive using sarcosine extracts by ELISA, they were not infected with Brucella and would have an unknown antibody that cross-reacted with sarcosine extracts. Other sera were negative by both ELISA and conventional serological tests (Fig. 4). Serological cross-reactions between Brucella species and organisms of other genera have been reported (3), including cross-reactions with Pasteurella species, Salmonella serotypes including Salmonella enterica serotype Urbana and S. enterica serotype Pullorum, Francisella tularensis, and EsAcherichia coli O:157. In Mongolia, Y. enterocolitica O9, F. tularensis, and Salmonella contaminations are a problem. The strongest cross-reaction is with Y. enterocolitica O9, which is the most important problem. As F. tularensis and Salmonella are partially cross-reactive with Brucella, sera of agglutination tests that are negative and of ELISA that are positive may react with these pathogens. In this study, by using an ELISA with sarcosine extracts, the cutoff absorbance value of 0.5 (OD492) for brucellosis produced a sensitivity and specificity (serodiagnostic indices) of 66.7 and 92.2%, respectively. However, when sera were checked by the Rose Bengal test and then positive sera were assayed by ELISA, the sensitivity and specificity increased to 80 and 100%, respectively. Thus, ELISA with sarcosine extracts will be better when used together with the conventional serological tests.
FIG. 4.
ELISA absorbance values of sera from nonvaccinated cattle in Mongolia by using n-lauroylsarcosine-extracted antigens. Fifty-nine sera from nonvaccinated cattle were tested at a 1/200 dilution. Positive reactions by the tube agglutination test with B. abortus antigen (Ba) or Y. enterocolitica O9 antigen (Ye O9) are shown in columns marked with a “+.”
We also investigated 162 sheep, 95 goat, 20 reindeer, 17 camel, and 29 human sera in Mongolia (Table 1). To eliminate Y. enterocolitica O9-infected animals from those with suspected brucellosis, the Rose Bengal test-positive sera were tested by ELISA with sarcosine extracts. The results showed that 17.5% of sheep, 27.3% of goat, 0% of reindeer, 0% of camel, and 46.1% of human sera were differentiated from suspected Brucella-infected animals by conventional serological tests (Table 1).
TABLE 1.
Serological tests of domestic animals and humans in Mongolia
| Animal | No. of animals tested | % of RBT-positive animalsa | % ELISA positive in RBT-positive animalsb | % Eliminationc |
|---|---|---|---|---|
| Sheep | 162 | 35.2 | 82.5 | 17.5 |
| Goat | 95 | 34.7 | 72.7 | 27.3 |
| Reindeer | 20 | 15.0 | 100 | 0 |
| Camel | 17 | 23.5 | 100 | 0 |
| Human | 29 | 44.8 | 53.9 | 46.1 |
RBT, Rose Bengal test.
RBT-positive sera were tested by ELISA with sarcosine extracts.
Percent elimination represents percentage of animals that were negative for Brucella infection in RBT-positive animals by using ELISA. The percentage of elimination was determined as the percentage of ELISA positives in RBT-positive animals subtracted from 100% (RBT-positive animals).
The agar gel immunodiffusion test that uses polysaccharide antigen differentiates infected and vaccinated cattle (12), but it does not differentiate Brucella-infected animals from Y. enterocolitica O9-infected animals. We believe our study is the first that uses an ELISA with sarcosine extracts to differentiate Brucella-infected animals from Y. enterocolitica O9-infected animals. The ELISA with sarcosine extracts in this study is an easier method than other conventional serological tests, and the ELISA can be done within 2 h after coating the antigen. Therefore, the ELISA with sarcosine extracts can be used to diagnose brucellosis and to identify Brucella-infected animals by using it together with other conventional serological tests.
Acknowledgments
This work was part of the project “Improvement of the Technology on Diagnosis of Animal Infectious Diseases in Mongolia” sponsored by the Japan International Cooperation Agency (JICA) and also was partly supported by a Grant-in Aid for Scientific Research from the Japanese Society for the Promotion of Science (12470062) and by a grant from the Ministry of Health, Labour and Welfare (Research on Emerging and Re-emerging Infectious Diseases). J. Erdenebaatar was a JICA scholarship researcher.
REFERENCES
- 1.Bosseray, N., and M. Plommet. 1990. Brucella suis S2, Brucella melitensis Rev. 1 and Brucella abortus S19 living vaccines: residual virulence and immunity induced against three Brucella species challenge strains in mice. Vaccine 8:462-468. [DOI] [PubMed] [Google Scholar]
- 2.Bundle, D. R., M. A. J. Gidney, M. B. Perry, J. R. Duncan, and J. W. Cherwonogrodzky. 1984. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect. Immun. 46:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbel, J. M. 1985. Recent advances in the study of Brucella antigens and their serological cross-reactions. Vet. Bull. 55:927-942. [Google Scholar]
- 4.Corbel, J. M. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 2:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, G. 1971. The Rose Bengal test. Vet. Rec. 88:447-449. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Aparicio, E., V. Aragon, C. Marin, B. Alonso, M. Font, E. Moreno, S. Perez-Ortiz, J. M. Blasco, R. Diaz, and I. Moriyon. 1993. Comparative analysis of Brucella serotype A and M and Yersinia enterocolitica O:9 polysaccharides for serological diagnosis of brucellosis in cattle, sheep, and goats. J. Clin. Microbiol. 31:3136-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurvell, B. 1972. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Acta Vet. Scand. 13:472-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kittelberger, R., F. Hilbink, M. F. Hansen, M. Penrose, G. W. de Lisle, J. J. Letesson, B. Garin-Bastuji, J. Searson, C. A. Fossati, A. Cloeckaert, et al. 1995. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. I. Immunoblot analysis of the antibody response to Brucella protein antigens in bovine brucellosis. Vet. Microbiol. 47:257-270. [DOI] [PubMed] [Google Scholar]
- 9.Kittelberger, R., F. Hilbink, M. F. Hansen, G. P. Ross, M. A. Joyce, S. Fenwick, J. Heesemann, H. Wolf-Watz, and K. Nielsen. 1995. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. II. The use of Yersinia outer proteins for the specific detection of Yersinia enterocolitica infections in ruminants. Vet. Microbiol. 47:271-280. [DOI] [PubMed] [Google Scholar]
- 10.Kittelberger, R., M. P. Reichel, M. A. Joyce, and C. Staak. 1997. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. III. Specificity of the in vitro antigen-specific gamma interferon test for bovine brucellosis diagnosis in experimentally Yersinia enterocolitica O:9-infected cattle. Vet. Microbiol. 57:361-371. [DOI] [PubMed] [Google Scholar]
- 11.Kittelberger, R., P. G. Bundesen, A. Cloeckaert, I. Greiser-Wilke, and J. J. Letesson. 1998. Serological cross-reactivity between Brucella abortus and Yersinia enterocolitica O:9. IV. Evaluation of the M- and C-epitope antibody response for the specific detection of B. abortus infections. Vet. Microbiol. 60:45-57. [DOI] [PubMed] [Google Scholar]
- 12.Lord, V. R., M. R. Rolo, and J. W. Cherwonogrodzky. 1989. Evaluation of humoral immunity to Brucella sp. in cattle by use of an agar-gel immunodiffusion test containing a polysaccharide antigen. Am. J. Vet. Res. 50:1813-1816. [PubMed] [Google Scholar]
- 13.Samartino, L., D. Gall, R. Gregoret, and K. Nielsen. 1999. Validation of enzyme-linked immunosorbent assays for the diagnosis of bovine brucellosis. Vet. Microbiol. 70:193-200. [DOI] [PubMed] [Google Scholar]
- 14.Schoerner, C., K. Wartenberg, and M. Rollinghoff. 1990. Differentiation of serological responses to Yersinia enterocolitica serotype O9 and Brucella species by immunoblot or enzyme-linked immunosorbent assay using whole bacteria and Yersinia outer membrane proteins. J. Clin. Microbiol. 28:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watarai, M., S.-I. Makino, and T. Shirahata. 2002. An essential virulence protein of Brucella abortus, VirB4, requires an intact nucleoside triphosphate-binding domain. Microbiology 148:1439-1446. [DOI] [PubMed] [Google Scholar]