Abstract
This pilot study was designed to determine the serum cytokine profile of acute otitis media (AOM) due to Streptococcus pneumoniae and the impact of clarithromycin (Abbott Laboratories, Inc). Serum levels of interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 were measured at diagnosis and 3 to 5 days after start of antibiotic treatment in 10 patients (mean age, 18.3 ± 13.9 months) who had middle ear fluid culture positive for S. pneumoniae. The mean concentrations of all cytokines were elevated at diagnosis of AOM compared to levels in healthy controls, yet only IL-6 reached statistical significance (P = 0.05). IL-6 showed a statistically significant decrease in mean serum concentration at visit 2 (P = 0.03). IL-8 displayed a similar pattern to IL-6, but the difference between samples from day 1 and day 2 did not reach statistical significance. The cytokines IL-1β and TNF-α appear to be elevated in the serum of patients with S. pneumoniae AOM, but there was no significant change between mean serum levels obtained pre- and postinitiation of antibiotic treatment in the time frame studied. The results suggest a systemic inflammatory response as evidenced by increased IL-6. A significant decrease of IL-6 and improvement of clinical symptoms were observed. Determining cytokine levels, especially IL-6, in AOM could offer a powerful tool for objective assessment of response to treatment, minimizing unnecessary treatment of asymptomatic children who may still have some otoscopic findings suggestive of AOM at follow-up visits.
The impact of acute otitis media (AOM) on the health care system in the United States is significant. AOM was responsible for approximately 24.5 million office visits in 1990 (12) and constituted the most common diagnosis during pediatric office visits between 1993 and 1995 among 1 to 4 year olds (6). AOM is estimated to cost the public health care system more than $5 billion dollar annually (7) and accounts for more than 25% of all prescriptions for oral antibiotics (3). Although there is an up to 60 to 80% spontaneous cure rate for AOM at late follow-up (11), AOM due to Streptococcus pneumoniae is less likely to resolve without antibiotics (4, 13). S. pneumoniae is also the most common pathogen isolated in middle ear fluid from patients who have failed antibiotic therapy (10). These findings underscore the need for a stepwise approach to treatment of AOM aimed at documenting inflammation as it relates to acute infection, and they attempt to identify specific pathogens. An understanding of the interactions between pathogen and host defense, as well as individual differences in immune modulation, should help us to better explain the correlation between clinical and microbiological success or failure.
While extensive research has been done investigating local inflammatory processes in the middle ear, little is known about the systemic immune response during AOM.
Cytokines are glycoproteins synthesized by a variety of cells and are known to modulate cellular functions in inflammatory and immune reactions. Interleukin-1β (IL-1β) is known to induce synthesis of tumor necrosis factor alpha (TNF-α) (14) and the growth of osteoclasts and fibroblasts and to cause hemodynamic changes and fever. IL-6 is a potent inducer of C-reactive protein and has been shown in vitro and in animal models to inhibit TNF-α (1). IL-8, a potent chemoattractant, is produced predominantly by monocytes, macrophages, and endothelial cells in response to stimuli such as lipopolysaccharide and TNF-α (2). Numerous cytokines and growth factors have been detected in middle ear fluid of children with otitis media (9, 14). In a previous study (8), serum IL-6 levels were positively correlated with bacterial AOM, especially when caused by S. pneumoniae. Our pilot study was designed to investigate the systemic inflammatory response, and the impact of macrolide antibiotic therapy on this response, in children with AOM due to S. pneumoniae.
From September 1999 to January 2000, patients seen at our ambulatory care center with symptoms suggestive of AOM were screened for eligibility by the investigators. Patients were included if they had at least one clinical sign of inflammation (ear pain, fever >100.1°F or 37.8°C, redness of the tympanic membrane [TM], or bulging of the TM) and at least two signs of middle ear effusion (decreased TM mobility, visible air-fluid level, or yellow discoloration or opacification of the TM). Children with a history of chronic or recurrent AOM were excluded. Patients (n = 12) who met inclusion criteria and whose parents gave consent received a comprehensive clinical evaluation at enrollment, which included an AOM severity score previously described by Dagan et al. (5). Subsequently, middle ear fluid was collected by tympanocentesis and immediately inoculated into brain heart infusion broth and sent for culture. Prior to the first dose of antibiotic, a venous blood sample was obtained.
To investigate the systemic inflammatory response in children with AOM, we measured serum levels of IL-1β, TNF-α, IL-6, and IL-8 at baseline. Study subjects were then started on clarithromycin, 15 mg/kg/day (first dose was given in clinic) in two divided doses, to complete 10 days of treatment. A follow-up visit was done 3 to 5 days after treatment was started, consisting of a limited clinical evaluation. The AOM severity score was again established. Patients (n = 10; mean age, 18.3 ± 13.9 months) who had middle ear fluid culture positive for S. pneumoniae underwent a second blood sampling for determination of serum cytokine levels. If the middle ear fluid isolate was resistant to macrolides, the antibiotic regimen was modified accordingly and the patient (n = 1) was excluded from further analysis. Patients (n = 2) whose cultures grew a pathogen different from S. pneumoniae (Haemophilus influenzae, Moraxella catarrhalis) from middle ear fluid were also excluded from further analysis. Subjects remaining in study (n = 9) had a late follow-up visit 13 to 21 days after enrollment to evaluate for late treatment failure. In addition, five healthy age-matched controls without significant past medical history (identified during well-child visits in our ambulatory care center) were included for baseline cytokine studies (one blood sample). Blood specimens from study patients, collected on visits 1 and 2 and specimens from controls were centrifuged at 2,000 rpm (Beckman model TJ-6) at 6°C for 10 min. Serum was separated and immediately stored as aliquots at −70°C until assayed. Serum IL-1β, TNF-α, IL-6, and IL-8 concentrations were quantified by enzyme-linked immunoassays (BioSource International Inc., Camarillo, Calif.). Standard curves were based on dilutions of the human recombinant cytokines. The minimum detectable concentration of cytokines was between 2 and 5 pg/ml, with an intraassay variance between 4 and 7%. Power analysis was done using paired and unpaired t test calculations. The statistical analysis included both paired analysis for each patient (sick versus convalescent) and comparison of the magnitude of change of mean values. Comparisons between patients and healthy controls included means and standard deviations.
After obtaining informed consent, a total of 12 children were enrolled in this pilot study. One study patient with a culture positive for S. pneumoniae resistant to clarithromycin (Abbott Laboratories, Inc.) (MIC for erythromycin, >1 μg/ml) showed no improvement in clinical symptoms at visit 2 (primary failure).
The child was started on alternative treatment and excluded from the study. One study patient with AOM due to another pathogen was followed for clinical response but also excluded from the study. One patient was noncompliant with the treatment plan and did not provide a second blood sample. Nine children, with a mean age of 19.3 months, completed the study protocol. At visit 2, patients had demonstrated significant clinical improvement as indicated by the change in the mean AOM severity scores (Table 1).
TABLE 1.
Clinical severity scores and mean cytokine levels
| Patient group | Result (P value)a
|
||||
|---|---|---|---|---|---|
| Severity scoreb | IL-1β (pg/ml) | TNF-α (pg/ml) | IL-6 (pg/ml) | IL-8 (pg/ml) | |
| Visit 1 | 8.4 ± 1.8 (<0.001) | 1,201 ± 985 (0.32) | 13.5 ± 17.8 (0.15) | 54.5 ± 38.1 (0.05) | 45.5 ± 39.4 (0.47) |
| Visit 2 | 2.2 ± 2.2 (0.001) | 1,530 ± 871 (0.51) | 15.2 ± 17.5 (0.76) | 15.9 ± 24.0 (0.03) | 28.8 ± 52.0 (0.69) |
| Visit 3 | 1.6 ± 2.1 | N/Ac | N/A | N/A | N/A |
| Controls | 0 | 737 ± 477 | 1.5 ± 2.22 | 9.7 ± 8.2 | 7.1 ± 8.6 |
Results are means ± standard deviations (n = 9). P values for data from visit 1 are based on comparisons with controls; P values for data from visit 2 are based on comparisons with visit 1 data.
Severity scores were determined according to the method of Dagan et al. (5). The clinical score by Dagan et al. was based on the temperature measured at the clinic, report of irritability and ear tugging by the parents, in response to specific questions addressed during visits 1 and 2, and the appearance (bulging) and redness of the eardrum observed by the examiner. The categories of irritability, tugging, redness, and bulging were classified as absent (0), mild (1), moderate (2), or severe (3). If the eardrum was perforated at the time of the second visit and pus was draining, this was scored by definition as “severe bulging.” We did not provide a definition for severity but let the parents and the examiner freely decide which level of severity to choose. Temperatures were scored as mildly (38.0-38.5°C = 1), moderately (38.6-39°C = 2), and severely (>39°C = 3) elevated. The maximum score was 15 (when the temperature was >39.0°C and all other categories were judged “severe”), and the minimum score was 0 (when the temperature was <38.0°C and all other categories were judged “absent”).
N/A, not applicable.
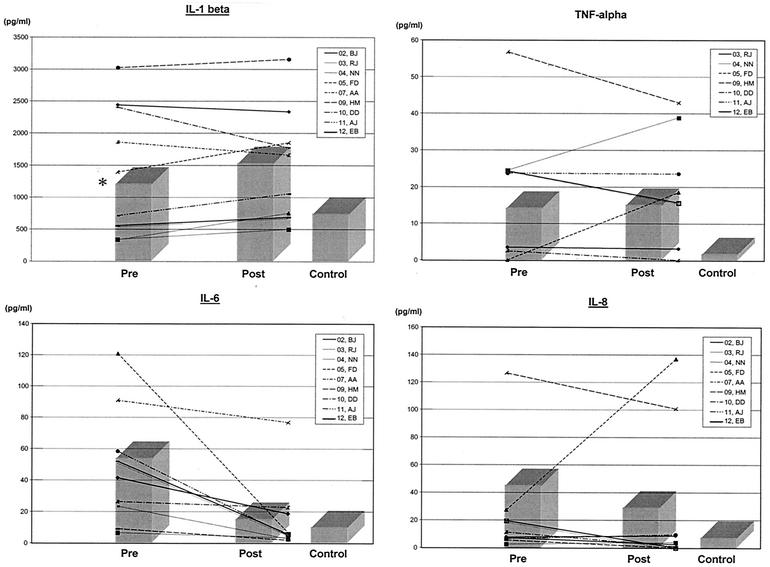
The mean concentration of all cytokines was elevated at diagnosis of AOM compared to levels in healthy controls, yet only IL-6 reached statistical significance (P = 0.05) (Table 1). IL-6 showed a statistically significant decrease in mean serum concentration at visit 2 (P = 0.03) (Table 1). IL-8 displayed a similar pattern to IL-6, but the difference between samples from day 1 and day 2 did not reach statistical significance. The cytokines IL-1β and TNF-α appear to be elevated in the serum of patients with S. pneumoniae AOM, but there was no significant change between mean serum levels obtained pre- and postinitiation of antibiotic treatment in the time frame studied. Of note, the pattern of change between the two time frames studied did not show a consistent decline for IL-1β, IL-8, or TNF-α (Fig. 1).
FIG. 1.
Serum cytokine levels at diagnosis and early follow-up visit.
Appropriate choice of antibiotics for treatment of AOM in children has become more complex. It is imperative that we develop strategies to minimize antibiotic overuse that has led to emergence of resistance among bacterial pathogens responsible for respiratory tract infections. Cytokines are inflammatory mediators produced in response to infection in a predictable fashion. IL-1β and TNF are produced early and sequentially after infection as local or systemic responses. IL-6 peaks shortly thereafter and rapidly returns to normal levels with resolution of infection. Our data show these mediators to be elevated at the time of diagnosis.
In our study, only IL-6 declined consistently with successful treatment. It is possible that peak IL-1 and TNF levels occurred after initiation of antibiotics, some time between our two sampling times, which could explain the highly variable levels noted at visit 2. Another possibility is that IL-6 might decrease in some patients on its own, or with spontaneous resolution of AOM. This is difficult to verify, as there was no untreated control group in this study. Although the sample size in this pilot study was small, our results highlight the importance of establishing a profile of inflammatory markers that could aid in the appropriate diagnosis and management of AOM in children. This pilot study allows us to specify the objectives of a larger study with this goal. Our study suggests a significant systemic inflammatory response in children with AOM due to S. pneumoniae. Measurement of IL-6 in serum of patients with presumed AOM may add information regarding the microbiological etiology of the infection, allowing for more objective criteria when choosing antibiotics. The results support the findings previously noted by Heikkinen et al. (8). While that study had a larger study population, it focused on the role of IL-6 in distinguishing certain bacterial and viral etiologies for AOM. By including more cytokine markers and by establishing profiles, we might be able to make more specific predictions and eventually identify a more specific marker or markers for AOM due to S. pneumoniae. Therefore, determining cytokine levels in AOM could offer a powerful tool for objective assessment of response to treatment, minimizing unnecessary treatment of asymptomatic children who may still have some otoscopic findings suggestive of AOM at follow-up visits. The in vivo test of cure has been advocated as the most reliable way to evaluate antibiotics for AOM by documenting microbiological success. Although scientifically sound, this approach involves a minimum of two tympanocentesis procedures to establish sterilization of the middle ear fluid, which is unacceptable in terms of time, cost, and discomfort, considering the large number of affected children. Serial measurements of IL-6 could potentially be used as a marker of acute infection in the middle ear, minimizing the discomfort to patients in double tympanocentesis studies. Our preliminary data do suggest that two measurements would be required to confirm effective treatment, because of the considerable overlap between acute and convalescent levels in different patients. Macrolide antibiotics are known to have an immune-modulating antiinflammatory effect. The changes noted in this study may reflect this to a certain degree. Further studies are necessary to evaluate the impact of other antibiotic classes on systemic inflammation in AOM due to S. pneumoniae and other organisms and the potential beneficial effect of macrolide-mediated attenuation of inflammation in AOM.
Acknowledgments
This study was funded by a grant from Abbott Laboratories, Inc.
REFERENCES
- 1.Aderka, D., J. M. Le, and J. Vilcek. 1989. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J. Immunol. 143:3517-3523. [PubMed] [Google Scholar]
- 2.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone, C. D. 1989. Modern management of otitis media. Pediatr. Clin. North Am. 36:1371-1387. [DOI] [PubMed] [Google Scholar]
- 4.Chonmaitree, T., M. J. Owen, J. A. Patel, D. Hedgpeth, D. Horlick, and V. M. Howie. 1992. Effect of viral respiratory tract infection on outcome of acute otitis media. J. Pediatr. 120:856-862. [DOI] [PubMed] [Google Scholar]
- 5.Dagan, R., E. Leibovitz, D. Greenberg, P. Yagupsky, D. M. Fliss, and A. Leiberman. 1998. Early eradication of pathogens from middle ear fluid during antibiotic treatment of acute otitis media is associated with improved clinical outcome. Pediatr. Infect. Dis. J. 17:776-782. [DOI] [PubMed] [Google Scholar]
- 6.Freid, V. M., D. M. Mukuc, and R. N. Rooks. 1998. Ambulatory health care visits by children: principal diagnosis and place of visit. Vital Health Stat. 13:1-23. [PubMed] [Google Scholar]
- 7.Gates, G. A. 1996. Cost-effectiveness considerations in otitis media treatment. Otolaryngol. Head Neck Surg. 114:525-530. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen, T., F. Ghaffar, A. O. Okorodudu, and T. Chonmaitree. 1998. Serum interleukin-6 in bacterial and nonbacterial acute otitis media. Pediatrics 102:296-299. [DOI] [PubMed] [Google Scholar]
- 9.Nassif, P. S., S. Q. Simpson, A. A. Izzo, and P. J. Nicklaus. 1998. Epidermal growth factor and transforming growth factor-alpha in middle ear effusion. Otolaryngol. Head Neck Surg. 119:564-568. [DOI] [PubMed] [Google Scholar]
- 10.Pichichero, M. E., and C. L. Pichichero. 1995. Persistent acute otitis media. I. Causative pathogens. Pediatr. Infect. Dis. J. 14:178-183. [DOI] [PubMed] [Google Scholar]
- 11.Roark, R., and S. Berman. 1997. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr. Infect. Dis. J. 16:376-381. [DOI] [PubMed] [Google Scholar]
- 12.Schappert, S. M. 1992. Office visits for otitis media: United States, 1975-1990. Adv. Data Vital Health Stat. 214:1-20. [PubMed] [Google Scholar]
- 13.Wald, E. R., E. O. Mason, Jr., J. S. Bradley, W. J. Barson, and S. L. Kaplan. 2001. Acute otitis media caused by Streptococcus pneumoniae in children's hospitals between 1994 and 1997. Pediatr. Infect. Dis. J. 20:34-39. [DOI] [PubMed] [Google Scholar]
- 14.Yellon, R. F., W. J. Doyle, T. L. Whiteside, W. F. Diven, A. R. March, and P. Fireman. 1995. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Arch. Otolaryngol. Head Neck Surg. 121:865-869. [DOI] [PubMed] [Google Scholar]