Abstract
A human recombinant monoclonal Fab fragment that specifically recognizes all the influenza A virus strains tested was produced in transformed Escherichia coli using the phage display technique. No strain of influenza B virus reacted with it. It was purified after four cycles of panning and by a single passage through an immunoaffinity column. About 1 mg of pure monoclonal antibody was obtained from 1 liter of culture medium in 3 working days. The Fab fragment reacted with a viral 27-kDa protein, which could reasonably be a matrix protein. Indirect immunofluorescence tests performed on virus-infected MDCK cells showed that this Fab fragment was at least equally efficient as other commercial monoclonal antibody-based systems in detecting influenza A viral infections. The potential advantages of human recombinant Fabs on murine monoclonal antibodies are discussed.
Influenza is still one of the world's most common respiratory infections. Influenza viruses are classified into types A, B, and C on the basis of the nucleic acid structure and organization (33). In addition, influenza A (FLU-A) viruses are classified into subtypes by the analysis of the glycoproteins hemagglutinin (HA) and neuraminidase (NA). The HA and NA proteins are found in the viral surface, are highly variable, and are responsible for viral adsorption and penetration into the cell. Matrix proteins M1 and M2 form part of the FLU-A virus membrane. The M1 membrane protein is type specific, highly conserved within each species, and very abundant in some particular viruses, where it is found under the lipid layer (4, 33). Therefore, M1 protein seems to be an ideal target for the identification of FLU-A viral infections.
Influenza can cause quite relevant economic and social damage due to the high number of infections per year. Often the clinical symptoms are not so clear, and the disease can be confused with other respiratory microbial infections. Furthermore, new drugs are now available which allow a selective treatment of influenza viruses (3, 13, 18). Thus, for these reasons the use of a specific and rapid identification system for FLU-A virus in the diagnostic microbiology laboratory is considered important (22, 33). At present, some commercial systems for the identification of respiratory viruses and also the influenza virus exist, which use monoclonal antibodies (MAbs) with enzyme-linked immunosorbent assay (ELISA) and immunofluorescence assay (IFA) techniques (19, 29). About 20 years ago the discovery of MAbs opened a new era in human diagnostics and therapeutics. Their absolute specificity rendered them effective for the prophylaxis of infectious diseases and cancer therapy. But when mouse MAbs were used, several adverse effects were observed: they stimulated a human immune response which inactivated them, and furthermore, their biological half-life was shorter than that of human antibodies. On the other hand, human MAbs were difficult to obtain by the hybridoma technique (19). Genetically engineered antibodies appeared to overcome problems encountered with mouse antibodies and were found to be a useful tool for the diagnosis and therapy of many diseases (16, 19, 20, 23, 32). For many therapeutic uses antibody fragments have potential advantages over whole antibodies, because of their small size and potential better tissue penetration and clearance. They are rarely glycosylated, a fact that favors their expression in bacteria. Very high yields of antibody fragments can be obtained in Escherichia coli (1, 2, 10, 11, 12). Combinatorial immunoglobulin libraries representing the preimmune or immune antibody repertoires of a suitable animal can be created artificially (25, 26, 28, 30). However, the antigen screening method caused almost impracticable problems with combinatorial libraries. This difficulty was solved by using the phage display technique, through a procedure called panning (5, 6, 7, 9, 27, 30, 32). Phage and ribosomal display are fast and compatible with the possibility of high-throughput, automatic screening. Different antigens can be used to screen the same library several times (14, 15, 31).
In the present study the genes for human antibodies directed against FLU-A virus were cloned in E. coli. This was accomplished by identifying a subject with a high titer of anti-FLU antibodies and then cloning the subject's antibody repertoire in combinatorial vectors with phage exposure.
MATERIALS AND METHODS
mRNA preparation and construction of the library.
An asymptomatic subject was used as a serum donor in the search for specific antibodies against FLU-A virus. The anti-FLU-A virus antibodies were assayed by a commercial ELISA technique (Behring, Marburg, Germany). The library of antibody fragments (Fab) was prepared by the phage display technique (1), using the vector pComb3 TG (8), which contains the gene for ampicillin resistance. Ten milliliters of bone marrow was obtained from the same donor, according to the ethical and institutional national policies, and the lymphocytes were purified by centrifugation in a Ficoll gradient, for the elimination of the polynuclear cells (Ficoll-Paque; Pharmacia, Uppsala, Sweden). Total RNA was extracted with a commercial RNA isolation kit from Stratagene Inc. (La Jolla, Calif.); it was then used for the synthesis of cDNA by a retrotranscription system (Boehringer, Mannheim, Germany). The cDNA was employed as a template for the PCR amplification of the sequences which code for the regions Vh-Ch1 of the heavy chains (Hc) of immunoglobulin G1 (IgG1) isotype and the κ and λ light chains (Lc) by the use of a series of primers as described in Table 1. In addition, extension primers were used for the amplified chains in order to improve the efficiency of cutting the restriction enzymes during cloning (11). The PCR extension was performed in a Perkin-Elmer 9600 apparatus with a denaturation step at 94°C for 30 s, cooling to 60°C for 20 s, and extension at 72°C for 1 min. All the PCRs were done using the same temperature cycling profiles. The buffer was 10 mM MgCl2 (Promega) containing a 60 μM concentration of each of the four deoxynucleoside triphosphates, 5 U of DNA polymerase (Pfu; Stratagene), and 60 pmol of the 5′ and 3′ primers. Then, the amplified DNA was purified by gel electrophoresis, precipitated, and quantified before its insertion into the vector pComb3 (1), which was maintained in the host strain E. coli XL1-Blue. The light-chain library was calculated to be composed of 2 × 106 elements, while the heavy-chain library was estimated to contain 1 × 106 elements. This last number represents the total dimension of the library.
TABLE 1.
Human heavy and light chain primers
| Primer | Sequence |
|---|---|
| Heavy chain, variable, 5′ specific | |
| VH1a | 5′-CAG GTG CAG CTC GAG CAG TCT GGG-3 (24-mer) |
| VH1f | 5′-CAG GTG CAG CTG CTC GAG TCT GGG-3′ (24-mer) |
| VH2f | 5′-CAG GTG CAG CTA CTC GAG TCG GG-3′ (23-mer) |
| VH3a | 5′-GAG GTG CAG CTC GAG GAG TCT GGG-3′ (24-mer) |
| VH3f | 5′-GAG GTG CAG CTG CTC GAG TCT GGG-3′ (24-mer) |
| VH4f | 5′-CAG GTG CAG CTG CTC GAG TCG GG-3′ (23-mer) |
| VH6a | 5′-CAG GTA CAG CTC GAG CAG TCA GG-3′ (23-mer) |
| Light chain, variable, 5′ specific | |
| κ | |
| VK1a | 5′-GAC ATC GAG CTC ACC CAG TCT CCA-3′ (24-mer) |
| VK1s | 5′-GAC ATC GAG CTC ACC CAG TCT CC-3′ (23-mer) |
| VK2a | 5′-GAT ATT GAG CTC ACT CAG TCT CCA-3′ (24-mer) |
| VK3a | 5′-GAAATTGAGCTCACGCAGTCTCCA-3′ (24-mer) |
| VK3b* | 5′-GAAATTGAGCTCAC(G/A)CAG TCTCCA-3′ (24-mer) |
| λ | |
| VL1 | 5′-AAT TTT GAG CTC ACT CAG CCC CAC-3′(24-mer) |
| VL2 | 5′-TCT GCC GAG CTC CAG CCT GCC TCC GTG-3′ (27-mer) |
| VL3 | 5′-TCT GTG GAG CTC CAG CCG CCC TCA GTG-3′ (27-mer) |
| VL3′ | 5′-TCCTATGAGCTCACTCAGCCACCC-3′ (24-mer) |
| VL4 | 5′-TCTGAAGAGCTCCAGGACCCTGTTGTG TCTGTG-3′ |
| VL5 | 5′-CAG TCT GAG CTC ACG CAG CCG CCC-3′ (24-mer) |
| VL6 | 5′-CAG ACT GAG CTC ACT CAG GAG CCC-3′ (24-mer) |
| VL7 | 5′-CAG GTT GAG CTC ACT CAA CCG CCC-3′ (24-mer) |
| Heavy chain, constant, 3′ specific | |
| CG1z | 5′-GCA TGT ACT AGT TTT GTC ACA AGA TTT GGG-3′ (30-mer) |
| Light chain, constant, 3′ specific | |
| κ | |
| CK1d | 5′-GCG CCG TCT AGA ATT AAC ACT CTC CCC TGT TGA AGC TCT TTG TGACGG GCG AAC TCA G-3′ (57-mer) |
| λ | |
| CL2d | 5′-AGA GAG AGA GAG AGA GAG AGC GCC GTC TAG AAT TAT GAA CAT TCT GTA GG-3′ (50-mer) |
Selection of the antigen binding phage by repeated cycles of panning.
The panning of the combinatorial library was accomplished according to the technique described by Burton et al. (12). The human antibody Fab fragments were selected using commercial materials purchased from the Virion company; they were derived from a strain A2:England 23/78 virus (lot 225114.12 MDCK). Bovine serum albumin, 100 ng/well, was used as a negative control. The concentration of the antigen-specific phage in the repeated panning cycles was obtained by measuring the number of phages eluted per well using a selective plating assay as described by Barbas et al. (1). In total, four cycles of panning were performed. The positive clones were selected by an ELISA method as indicated by Williamson et al. (30).
Preparation of soluble Fab fragments.
After the last panning cycle, phage-bound Fab fragments were converted into soluble Fab by vector modification as described elsewhere (7). Strains of E. coli XL1-Blue (Stratagene) were transformed by the modified vector. After growing the strains at 37°C in a shaken bath to an optical density of 0.8 at a wavelength of 600 nm, they were induced with 1 mM isopropyl-β-d-thiogalactoside (IPTG) (Boehringer) and then grown overnight at 30°C. The cells were harvested and lysed. The Fab-rich supernatant was recovered after centrifuging the cell lysate at 14,000 rpm at 4°C for 25 min in an Eppendorf 5804 R centrifuge.
Viruses and cell lines.
Madin-Darby canine kidney cells (MDCK) were used for growing the influenza viruses. They were cultured in modified Eagle medium with 5% fetal calf serum supplemented with antibiotics and trypsin (2 μg/ml). The procedures of panning and screening were done with the viral strain A2:England 23/78. For the IFAs the following virus strains were employed: swine strain WSN (H1N1), A/Sydney (H3N2), and A/Mississippi (H3N2). These last strains were grown in MDCK cells in 24 multiwell plates (about 4 × 104 cells/well) with modified Eagle medium supplemented with 10% nonessential amino acids, trypsin, and 2% fetal serum; then, the cells were infected with influenza viruses at a high multiplicity of infection (high multiplicity of infection = 10). When the cytopathic effect was complete after 24 h of incubation at 37°C in 5% CO2, the cell monolayer was collected and used for slide preparation.
Influenza clinical strains were isolated from bronchial lavage specimens and pharyngeal swabs. Pathological specimens were assayed with an indirect immunofluorescence test for influenza viruses, before being processed for virus isolation on MDCK cells. FLU-A and -B virus strains were differentiated with the Imagen Influenza A-B kit (Dako Diagnostics, Carpinteria, Calif.). The FLU-A virus strains were subtyped using an agglutination inhibition test with MAbs obtained from Istituto Superiore di Sanita (Rome, Italy).
Immunofluorescence analysis of the soluble Fab fragments.
The recombinant Fab was assayed by the indirect immunofluorescence technique (IF), with a fluorescein isothiocyanate-conjugated anti-human IgG Fab-specific polyclonal antiserum (Sigma, Buchs, Switzerland), described by Cattani et al. (14). FLU-A virus-infected MDCK cells were fixed on glass slides; mock-infected and FLU-B virus-infected MDCK cells were also used as controls. All the assays were performed in comparison with an IF commercial kit (Dako, Carpinteria, Calif.).
Production and extraction of Fab.
The phagemid-containing bacterial colony was grown first in 10 ml of SB medium (Super broth: 5 g of NaCl, 20 g of Bacto Yeast extract, 35 g of tryptone, 0.5 ml of 10 M NaOH, 1,000 ml of distilled water) supplemented with ampicillin (final concentration, 100 μg/ml) and tetracycline (final concentration, 10 μg/ml). After overnight incubation in a shaken bath at 37°C, the bacterial culture was inoculated into 2 liters of the same medium. When the culture reached an optical density of about 0.8 at 600 nm, IPTG was added (final concentration, 250 μg/ml), and the culture was shaken overnight at 30°C. Then, it was centrifuged at 4,000 rpm for 25 min in an Eppendorf 5804 centrifuge, and the cells were suspended in 12 ml of phosphate-buffered saline, pH 7.2. The cells were lysed with three ultrasound cycles of 3 min each. Then the soluble Fab was harvested in a Beckman Ultracentrifuge at 18,000 rpm for 25 min at 4°C and sterilized by filtration.
Human Fab purification.
The purification of the monoclonal Fabs was obtained by an immunoaffinity chromatography. GammaBind G Sepharose (Pharmacia Biotech), bound to G protein and then conjugated with a polyclonal serum (anti-human IgG Fab-specific, Sigma), was used for the preparation of the chromatography column. After preparing the resin, the cleared sample was injected into the chromatographic column equilibrated with 20 to 30 ml of phosphate-buffered saline. The column-bound Fab was recovered with 10 ml of elution buffer (100 mM glycine, pH 2.5) and neutralized with Tris (1 M, pH 9). Total proteins were checked by the Bio-Rad protein microassay, and the antibody purity was assessed in a denaturing electrophoresis (sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis [SDS-8% PAGE]) assay as indicated by Laemmli (24). The Fab was also quantified by denaturating PAGE using a standard control of bovine serum albumin.
Western blot analysis.
The FLU-A virus A/Sydney (H3N2) was subjected to SDS-10% PAGE. The gel was blotted on nitrocellulose membrane (Bio-Rad) with a mini-protean apparatus (Bio-Rad) using a trans-buffer containing 25 mM Tris (pH 8.3), 192 mM glycine, 20% methanol, and 0.1% SDS and was incubated for 2 h at 37°C with the purified and concentrated (100 μg/ml) Fab fragment clone 9. The Fab was detected by a peroxidase-labeled anti-human antibody (Sigma) for 1 h at 37°C.
Neutralization and hemagglutination inhibition tests.
The purified Fab was used at twofold dilutions on a fixed number of infectious FLU-A virions (100 50% tissue culture infective doses in 0.5 ml of medium). Then, the virus suspension was titrated on MDCK monolayers according to the procedure described by Hierholzer and Killington (21). Chicken red blood cells (about 7 × 105/well) were used for the hemagglutination inhibition test. Seven twofold dilutions of the Fab fragment from 250 to 3.6 μg/ml were prepared in 96-well plates; in each well four hemagglutinating FLU-A viral units were added, and the plates (in triplicate) were incubated for 1 h at room temperature. Then, the red blood cells were injected into the wells and left for 1 h at 4°C. Hemagglutination was read macroscopically.
DNA sequence.
The DNA sequence was identified by the method described by Williamson et al., using their primers (30).
RESULTS
Selection and characterization of specific MAb-producing strains of E. coli.
After the preparation of the library, four panning cycles were performed, which permitted the selection of a positive clone (clone Fab 9) for FLU-A virus. This was selected by an ELISA technique and was identified using as antigen a commercial viral lysate of the strain A2 England 23/78. All further assays were made by an indirect immunofluorescence method. Microscope slides were prepared with cells infected with the two FLU-A virus serotypes H1N1 (WSN) and H3N2 (A/Sydney and A/Mississippi) and with a clinical FLU-B virus strain. The clone Fab 9 was specific for all of those FLU-A virus strains tested, while it did not react with the FLU-B virus or with other respiratory viruses. The assays were done either using the raw bacterial extract or the purified extract: no significant differences in the specificity and sensitivity of the assay were observed.
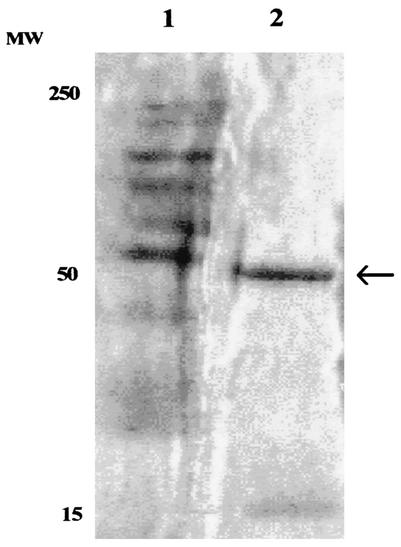
The quantitative analysis of the Fab present in the raw extract was performed using serial dilutions of a purified Fab, which had been previously quantified by SDS-PAGE. The raw extract was serially diluted, and the fluorescence was still present with a concentration of Fab of about 50 μg/ml. By this method it was possible to determine that from 1 liter of broth culture about 5 mg of raw extract was obtained, with approximately 1 mg of pure Fab (Fig. 1).
FIG. 1.
PAGE of purified Fab obtained from transformed E. coli clone 9. The clone Fab 9 appears as a clear band with a molecular weight (MW) of about 50,000 (lane 2); lane 1 contains size marker.
In order to define the target of the MAb, tests were performed on the inhibition of the viral hemagglutination and on the neutralization of infectivity of the influenza virus. Neither inhibition of the hemagglutination nor neutralization of the infectivity tests was observed (data not shown).
Western blot analysis of the recombinant MAb target viral protein.
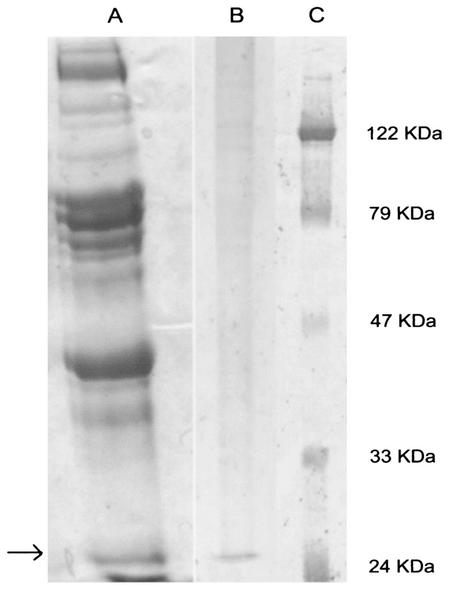
The purified virus, which still reacted in immunofluorescence tests with the recombinant Fab, was used for performing a Western blot assay to define the final target of the recombinant antibody. It was found that the Fab fragment interacted with a 27-kDa protein, which could actually correspond to the viral M1 protein (Fig. 2).
FIG. 2.
Western blot analysis of FLU-A virus PAGE protein profile using the Fab fragment from clone 9. Lane A, protein profile of FLU-A virus (strain WSN); lane B, Western blot using Fab clone 9; lane C, molecular marker. The arrow indicates a 27-kDa protein with affinity for the Fab fragment produced.
Determination of the amino acid sequence of the Fab fragment.
The amino acid sequences of both heavy and light chains of the Fab fragment were inferred from the DNA sequence (Fig. 3). The heavy chain was formed by seven domains with 119 amino acids, and the light chain was formed by seven domains containing 102 amino acids.
FIG. 3.
Amino acid sequence of both heavy and light chains of the anti-FLU-A virus Fab fragment produced in the recombinant E. coli clone 9.
Validation of specificity of Fab fragment.
The Fab fragment was validated on a series of clinical FLU-A virus strains. Twenty-three H3N2 A/Sydney clinical strains and one H3N2 A/Mississippi subtype were analyzed. The viruses were used for infecting MDCK cells. All the FLU-A viruses tested gave a positive result in the IFA with the recombinant Fab fragment. FLU-B virus always gave a negative result (Table 2 and Fig. 4). The antibody concentrate was able to give clear positive results in the immunofluorescence test up to a dilution of 50 μg/ml. All the tests were performed in parallel with the Imagen test for the identification of FLU-A and -B viruses (Dako).
TABLE 2.
Specific activity of recombinant MAb Fab clone 9 on clinical influenza virus strains
| Virus type | Subtype | No. of samples | No. of positive samplesa |
|---|---|---|---|
| Influenza A | H3N2 Sidney | 23b | 23 |
| Influenza A | H3N2 Mississippi | 1 | 1 |
| Influenza A | H1N1 WSN | 1 | 1 |
| Influenza B | 9b | 0 | |
| Total | 34 | 25 |
By indirect immunofluorescence test.
Clinical strains.
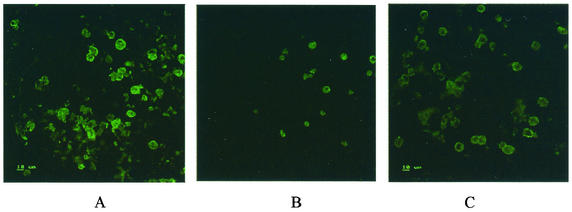
FIG. 4.
Indirect immunofluorescence of FLU-A virus-infected MDCK cells using recombinant human Fab produced in E. coli clone 9 (A). (B and C) The same antibody was tested with FLU-B virus-infected cells (B) and in mock-infected cells (C).
Conclusions.
In this work a human recombinant MAb has been described, which demonstrated a significant specificity only to FLU-A viral strains. It was obtained using combinatorial phage display technology, which can be considered a very useful tool for the production of both diagnostic and therapeutic MAbs, compared to traditional murine hybridoma MAbs. This technique appeared to be simple to perform, and a considerable amount of antibody could be obtained from laboratory-scale cultures; furthermore, in this work, only the Fab fragment of the IgG antibody was produced. It was claimed that antibody fragments have potential advantages over whole antibodies for some therapeutic uses, since they are smaller and can thus better penetrate into organs and tissues (17, 19). All the FLU-A viruses tested with this antibody by a fluorescence technique were rapidly recognized. No strain of FLU-B virus reacted with it. We believe that this product could actually be suitable for the preparation of a diagnostic kit for the rapid detection of FLU-A virus-infected patients.
MAbs are shown to be far more efficient in the recognition of many infectious agents than polyclonal antibodies. They are more selective and specific than polyclonal antibodies, show a high affinity for the antigens, and give rise to few false positive results (19, 22, 33). Murine MAbs have been produced for several years by the hybridoma technique. They are quite expensive, require animal handling, and can induce the production of inactivating antibodies when injected into heterologous subjects. In addition, cell hybridomas generally require delicate handling and are expensive to prepare. Human hybridomas cannot be easily obtained, and murine-human chimeric MAbs also induce the production of inactivating antibodies.
Our experience has upheld the claim of several authors that human recombinant MAbs are devoid of many of the problems that affect murine MAbs and are also easier to produce: in the present study at least 1 mg of specific antibody per liter of culture medium could be obtained and purified in about 3 working days. Fab fragments were concentrated in the periplasmic space of E. coli cells. They appeared to be very easy to extract by simple mild ultrasound disruption: they are obtained in a high concentration and can be purified by a single column passage. In fact their preparation showed no difficulties for concentration and purification, and they could also be manipulated for obtaining immunoconjugates suitable for direct immunofluorescence tests. In about 3 working days, a single technician could prepare at least 100 mg of Fab from clone 9, which allows the performance of about 2,000 tests for influenza virus. The MAbs obtained from clone 9 appeared to react with a viral protein of about 27 kDa, which showed the electrophoretic properties of the M1 matrix protein. This viral protein is type specific and well-conserved in all the FLU-A viruses. Since it is part of the viral membrane but is not exposed on the virion surface, the Fab type 9 did not show strong neutralizing properties on virus infectivity as anti-HA and anti-NA antibodies usually do. Thus, the clone Fab 9 described in this study could be mainly used for the preparation of a rapid diagnostic kit for FLU-A virus infections.
With the indirect immunofluorescence assay the clone Fab 9 was shown to be very sensitive and, in our hands, was found to have at least the same specificity and sensitivity as murine MAbs. A potential advantage of the diagnostic system based on the recombinant Fabs described above could be that the crude preparations suitable for indirect IF assays are easily and inexpensively prepared from E. coli cultures in a clinical microbiology laboratory, with virtually no need for any additional equipment or competence, other than those institutionally present. In this case, the cost of IF testing for FLU-A virus can be substantially reduced compared to that of commercial systems based on fluorescein-labeled murine MAbs. In addition, several authors (7, 14) have claimed that the introduction of a selectable marker between the Hc and Lc expression cassettes in the vector for soluble Fab expression increased the stability of the expression system, resulting in a more reproducible Fab yield in crude preparations. Another potential advantage of similar Fabs produced in bacteria is that they can easily be engineered by recombinant DNA technology to obtain immunoconjugates which, carrying suitable reporter moieties, could be useful for diagnostic purposes in direct immunoassays (5).
Acknowledgments
This work was supported by a grant from Biotecne, Cagliari, Italy, within the program of R&D for Biotechnologies sponsored by the Centro Regionale di Programmazione della Sardegna.
REFERENCES
- 1.Barbas III, C. F., A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas III, C. F., J. E. Crowe, D. Cabala, T. M. Jones, S. L. Zbedee, B. R. Murphy, R. Chanock, and D. R. Burton. 1992. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus f glycoprotein and neutralize infectivity. Proc. Natl. Acad. Sci. USA 89:10164-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethell, R., and P. Smith. 1998. Recent developments in sialidase inhibitors for the treatment of influenza. Drugs Future 23:1099-1109. [Google Scholar]
- 4.Bucher, D. J., A. Mikhail, S. Popple, P. Graves, G. Meiklojohn, D. S. Hodes, K. Johansson, and P. E. Halonen. 1991. Rapid detection of type A influenza viruses with monoclonal antibodies to the M protein (M1) by enzyme-linked immunosorbent assay and time-resolved fluoroimmunoassay. J. Clin. Microbiol. 29:2484-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugli, F., R. Bastidas, D. R. Burton, R. A. Williamson, M. Clementi, and R. Burioni. 2001. Molecular profile of a human monoclonal antibody Fab fragment specific for Epstein-Barr virus gp350/220 antigen. Hum. Immunol. 62:362-367. [DOI] [PubMed] [Google Scholar]
- 6.Burioni, R., P. Plaisant, F. Bugli, V. D. Carri, M. Candela, A. Gabrielli, and G. Fadda. 1998. Probing the natural antibody repertoire by combinatorial cloning of IgM and IgD isotypes in phage display vectors. Res. Virol. 149:321-325. [DOI] [PubMed] [Google Scholar]
- 7.Burioni, R., P. Plaisant, F. Bugli, V. Delli Carri, M. Clementi, and G. Fadda. 1998. A vector for the expression of recombinant monoclonal Fab fragments in bacteria. J. Immunol. Methods 217:195-199. [DOI] [PubMed] [Google Scholar]
- 8.Burioni, R., P. Plaisant, V. Delli Carri, A. Vannini, T. Spanu, M. Clementi, G. Fadda, and P. E. Varaldo. 1997. An improved phage display vector for antibody repertoire cloning by construction of combinatorial libraries. Res. Virol. 148:161-164. [DOI] [PubMed] [Google Scholar]
- 9.Burioni, R., P. Plaisant, A. Manzin, D. Rosa, V. Delli Carri, F. Bugli, L. Solforosi, S. Abrignani, P. E. Varaldo, G. Fadda, and M. Clementi. 1998. Dissection of human humoral immune response against hepatitis C virus E2 glycoprotein by repertoire cloning and generation of recombinant Fab fragments. Hepatology 28:810-814. [DOI] [PubMed] [Google Scholar]
- 10.Burioni, R., P. Plaisant, M. L. Riccio, G. M. Rossolini, R. Santangelo, A. Vannini, and G. Satta. 1995. Engineering human monoclonal antibody fragments: a recombinant enzyme-linked Fab. New Microbiol. 18:127-133. [PubMed] [Google Scholar]
- 11.Burioni, R., R. A. Williamson, P. P. Sanna, F. E. Bloom, and D. R. Burton. 1994. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc. Natl. acad. Sci. USA 91:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton, D. R., C. F. Barbas III, M. A. A. Persson, S. Koenig, R. M. Chanock, and R. A. Lerner. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 88:10134-10137. [DOI] [PMC free article] [PubMed]
- 13.Calfee, D. P., and F. G. Hayden. 1998. New approaches to influenza chemotherapy: neuraminidase inhibitors. Drugs 4:537-555. [DOI] [PubMed] [Google Scholar]
- 14.Cattani, P., G. M. Rossolini, S. Cresti, R. Santangelo, D. R. Burton, A. R. Williamson, P. P. Sanna, and G. Fadda. 1997. Detection and Typing of Herpes Simplex Viruses by using recombinant Immunoglobulin fragments produced in bacteria. J. Clin. Microbiol. 35:1504-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattani, P., P. Plaisant, S. Manzara, N. Capodicasa, R. Burioni, G. M. Rossolini, and G. Satta. 1995. Cloning and characterization of human recombinant antibody Fab fragments specific for types 1 and 2 herpes simplex virus. Microbiologica 18:135-142. [PubMed] [Google Scholar]
- 16.Chanock, R. M., J. E. Crowe, Jr., B. R. Murphy, and D. R. Burton. 1993. Human monoclonal antibody fragments cloned from combinatorial libraries: potential usefulness in prevention and/or treatment of major human viral diseases. Infect. Agents Dis. 2:118-131. [PubMed] [Google Scholar]
- 17.Colcher, D., G. Pvilinkova, G. Beresford, B. J. M. Booth, A. Choudhury, and S. K. Batra. 1998. Pharmacokinetics and distribution of genetically-engineered antibodies. Q. J. Nuclear Med. 42:225-241. [PubMed] [Google Scholar]
- 18.Colman, P. M. 1994. Influenza virus neuraminidase: structure, antibodies and inhibitors. Protein Sci. 3:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavilondo, J. V., and J. W. Larrick. 2000. Antibody engineering at the millennium. BioTechniques 29:128-145. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, A. D., S. C. Williams, O. Hartley, I. M. Tomlinson, P. Waterhouse, W. L. Crosby, R. E. Kontermann, P. T. Jones, N. M. Low, T. J. Allison, T. D. Prospero, H. R. Hoogenboom, A. Nissim, J. P. L. Cox, J. L. Harrison, M. Zaccolo, E. Gherardi, and G. Winter. 1994. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 13:3245-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hierholzer J. C., and R. A. Killington. 1996. Virus isolation and quantitation. In B. W. J. Mahy and H. O. Kangro (ed.), Virology methods manual, p. 35-36. Academic Press, London, United Kingdom.
- 22.Irmen, K. E., and J. J Kelleher. 2000. Use of monoclonal antibodies for rapid diagnosis of respiratory viruses in a community hospital. Clin. Diagn. Lab. Immunol. 7:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James, D. M., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins durino the assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.McCafferty, J., A. D. Griffiths, G. Winter, and D. J. Chiswell. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552-554. [DOI] [PubMed] [Google Scholar]
- 26.Persson, M. A. A., R. H. Caothien, and D. R. Burton. 1991. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc. Natl. Acad. Sci. USA 88:2432-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaisant, P., R. Burioni, A. Manzin, L. Solforosi, M. Candela, A. Gabrielli, G. Fadda, and M. Clementi. 1997. Human monoclonal recombinant Fabs specific for HCV antigens obtained by repertoire cloning in phage display combinatorial vectors. Res. Virol. 148:165-169. [DOI] [PubMed] [Google Scholar]
- 28.Sanna, P. P., R. A Williamson, A. De Logu, F. E. Bloom, and D. R. Burton. 1995. Direct selection of recombinant human monoclonal antibodies to herpes simplex virus glycoproteins from phage display libraries. Proc. Natl. Acad. Sci. USA 92:6439-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd, S. J., L. Minnich, and J. L. Warner. 1995. Comparison of rapid immunofluorescence procedure with test pack RSV and Directigen Flu-A for diagnosis of respiratory syncytial virus and influenza a virus. J. Clin. Microbiol. 33:1650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson, R. A., R. Burioni, P. P. Sanna, L. J. Partridge, C. F. Barbas III, and D. R. Burton. 1993. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc. Natl. Acad. Sci. USA 90:4141-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson, R. A., T. Lazzarotto, P. P. Sanna, R. B. Bastidas, B. Dalla Casa, G. Campisi, R. Burioni, M. P. Landini, and D. R. Burton. 1997. Use of recombinant human antibody fragments for detection of cytomegalovirus antigenemia. J. Clin. Microbiol. 35:2047-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter, G., A. D. Griffiths, R. E. Hawkins, and H. R. Hoogenboom. 1994. Making antibodies by phage display technology. Annu. Rev. Immunol. 17:433-455. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler, T., and N. J. Cox. 1999. Influenza viruses, p. 928-935. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.