Abstract
Forty patients with severe sepsis or septic shock recently received C1 inhibitor. In the present study we studied the effect of C1 inhibitor therapy on circulating elastase-α1-antitrypsin complex (EA) and lactoferrin (LF) levels in these patients to gain further insight about agonists involved in the activation of neutrophils in human sepsis. Elevated levels of EA and LF were found in 65 and 85% of the septic patients, respectively. Patients with elevated EA levels had higher organ dysfunction scores, higher levels of cytokines, and higher levels of complement activation products than patients with normal EA levels. C1 inhibitor therapy reduced EA as well as complement activation and IL-8 release in the patients with elevated EA on admission. We conclude that neutrophil activation in human sepsis correlates with the severity of organ dysfunction and involves complement and interleukin-8 as agonists. The effect of C1 inhibitor therapy on neutrophils may provide an explanation for the beneficial, although mild, effects of this treatment on organ dysfunction in sepsis.
Sepsis is still a leading cause of mortality in noncardiologic intensive care units (37). Sepsis is suggested to result from an extensive triggering of body defense mechanisms. This includes the release of cytokines, activation of polymorphonuclear neutrophils (PMN), and activation of plasma protein cascade systems such as the complement and contact phase systems (4, 8, 21).
The development of multiple organ dysfunction syndrome is a frequent complication of sepsis. There is evidence suggesting that PMN are involved in the pathogenesis of sepsis and multiple organ dysfunction syndrome (12, 25, 30, 35). Release of interleukin-8 (IL-8), which is strongly chemotactic for PMN and induces the expression of adhesion molecules on endothelial cells, may encourage activated PMN to adhere to endothelial cells, thereby inducing endothelial damage (1, 10, 21, 33, 51). However, other agonists, such as C5a, activated factor XII (FXIIa), or kallikrein, may be involved as well (15, 18, 38, 41, 48, 49).
Elastase released from azurophilic granules of activated PMN is a serine protease, which in plasma is rapidly inactivated by its main inhibitor, α1-antitrypsin, to form elastase-α1-antitrypsin complex (EA). However, due to inactivation of α1-antitrypsin by toxic oxygen species, some of the elastase may escape inhibition and promote tissue injury (7, 11, 50). Lactoferrin (LF) is an iron-binding glycoprotein that is released from PMN-specific granules upon stimulation. LF can modulate the inflammatory process and exerts antimicrobial activity (3).
Circulating levels of EA and LF are often measured to assess activation of PMN in vivo (6, 12, 30). Elevated EA levels have been demonstrated in human volunteers upon endotoxin administration and in patients with sepsis (13, 14, 42, 45). In the latter, EA levels correlate well with organ dysfunction scores, disease severity, and outcome, supporting a role for PMN in the pathogenesis of severe sepsis (18, 26, 30, 35). It is unknown, however, which agonists mediate the activation of PMN in sepsis.
C1 inhibitor inhibits the classical pathway of the complement system by neutralization of C1r and C1s activities and is the main inhibitor of the contact phase system by inhibition of factor FXIIa, kallikrein, and FXIa (8). Because of these anti-inflammatory activities, administration of C1 inhibitor constitutes a potential therapy to treat inflammatory diseases such as sepsis. Indeed, in septic baboons, C1 inhibitor substitution had a beneficial effect on the clinical course, presumably by inhibiting the activation of the complement and the contact phase systems and by attenuating cytokine release (27).
Recently we performed a randomized, double-blinded, placebo-controlled trial on the effect of C1 inhibitor in a limited number of sepsis patients. We found C1 inhibitor to significantly improve renal function. However, the precise mechanisms by which C1 inhibitor improves renal function remain unclear (9). As C1 inhibitor can inhibit the formation and activation of various agonists for PMN, this trial provided a unique opportunity to study the activation of PMN in human sepsis. Hence, we studied the effect of C1 inhibitor administration on the levels of EA and LF in septic patients. The observed effects were related to the effects of C1 inhibitor on agonists of PMN.
(Part of the data were presented during the XVIIIth Congress of the International Society of Thrombosis and Haemostasis, Paris, France, July 2001.)
MATERIALS AND METHODS
Patients.
The protocol was approved by the local ethics committee. Informed consent was obtained from all patients. In the case of impaired consciousness, informed consent was obtained from a family member or the closest relative or partner of the patient, according to national legal guidelines.
Patients in the medical and surgical intensive care units were eligible if they met the inclusion criteria for severe sepsis and septic shock according to the definitions of the American College of Chest Physicians consensus conference (5). The patient evaluation is described in detail elsewhere (9).
Treatment.
The patients reported in this study participated in a randomized, double-blinded, placebo-controlled trial to study the efficacy and safety of C1 inhibitor in severe sepsis and septic shock. Twenty patients each received C1 inhibitor or placebo. The study medication consisted of a purified and sterilized lyophilisate of human C1 inhibitor (Berinert HS; Aventis Behring AG, Zürich, Switzerland) dissolved in 0.9% (wt/vol) sodium chloride. The patients were given 6,000 IU, followed by, in order, 3,000, 2,000, and 1,000 IU, every 12 h. The 28- and 90-day mortalities were 25 and 32%, respectively, with no difference between the treatment and placebo groups. Except for one patient who died because of an intraoperative aortic rupture, all patients died from the septic process.
Patient evaluation.
Patients were monitored for 90 days or until death. The logistic organ dysfunction (LOD) (29) and sepsis-related organ failure (SOFA) (47) scores were calculated at entry. Diagnostic investigations of suspected foci of infection (e.g., cultures of blood and tissue) were done according to the decisions of the attending physician.
Blood collection.
Arterial blood was obtained at study inclusion and on days 1, 2, 3, and 4 and was collected for cytokine and complement analysis in vials containing EDTA (4 mM K2EDTA; Sarsted Monovettes, Nümbrecht, Germany) and for determination of the clotting factors and neutrophil activation products in citrate-containing vials (0.106 M Na3citrate; Sarsted Monovettes). Plasma was prepared by centrifuging blood vials twice for 10 min at 1,500 × g at room temperature. Small aliquots of plasma were stored in polypropylene tubes at −70°C until analysis.
Routine parameters.
Routine hematology, chemistry, and coagulation parameters were obtained on admission and daily thereafter until day 4.
Assays.
(i) Neutrophil activation products.
EA and LF levels were measured with an enzyme-linked immunosorbent assay (ELISA) as previously described (39). Based on levels in healthy controls, EA levels of >100 ng/ml and LF levels of >200 ng/ml were considered to be elevated.
(ii) Cytokines.
Concentrations of tumor necrosis factor alpha, IL-6, IL-8, and IL-10 in plasma were measured with ELISAs as previously described (24, 28, 44).
(iii) Complement assays.
Levels of C3a were determined by a radioimmunoassay (22), and levels of C4 activation products (C4b/c) were determined by an ELISA (52). C1 inhibitor antigen was determined with a nephelometric assay, and C1 inhibitor activity was determined with a commercial chromogenic assay (Immuno AG, Vienna, Austria).
(iv) Coagulation and (anti)fibrinolytic parameters.
Thrombin-antithrombin (TAT) complexes were determined by using a commercial assay (Dade Behring, Liederbach, Germany). Plasminogen activator inhibitor type 1 (PAI-1) was measured as previously described (36).
Statistical analysis.
Results are expressed as means ± standard errors of the means or as median and range when appropriate. Statistical analysis was performed with a commercial statistical package (SigmaStat; SAS Institute Inc.). Differences between groups at a given time point were analyzed by using the Mann-Whitney rank sum test. Comparison of differences within a group was performed with the Wilcoxon signed rank test. Statistical significance was designated as a P value of <0.05 (two-sided P value). Relative risk analysis was performed by calculating crude odds ratios.
RESULTS
Forty patients were enrolled in the study; 32 had severe sepsis and 8 had septic shock. Among the 32 patients with severe sepsis, 7 were female (22%; median age [range], 69.4 [50.3 to 74.0] years) and 25 were males (78%; median age [range], 63.6 [28.7 to 73.9] years). All eight patients with septic shock were males (median age [range], 51.9 [29.1 to 73.7] years). Clinical data for these patients are described elsewhere (9).
PMN activation in the patients.
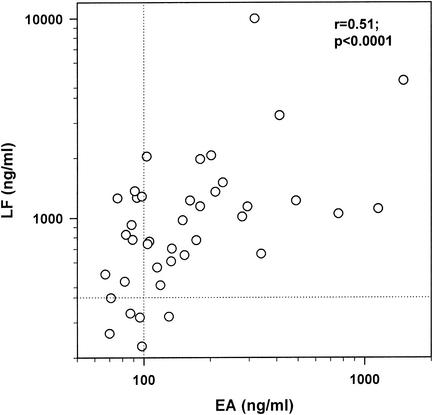
The median EA and LF levels (range) in all patients were 131.5 (67 to 1,501) ng/ml and 949 (228 to 10,000) ng/ml, respectively. Twenty-six patients (65%) had elevated EA levels (median [range], 180 [103 to 1,501] ng/ml), whereas 34 patients (85%) had increased LF levels (median [range], 1,080 [461 to 10,000] ng/ml), at study entry (Fig. 1). The EA levels correlated well with the LF levels in all patients (r = 0.51; P < 0.0001). The EA and LF levels in patients suffering from severe sepsis and septic shock are shown in Fig. 2 and 3, respectively. Relative risk analysis indicated that higher EA levels constituted an increased risk for septic shock; i.e., there was a 6-fold (95% confidence interval, 1.1- to 31.3-fold) increased relative risk for septic shock at EA levels of above 200 ng/ml.
FIG. 1.
EA and LF levels in patients with severe sepsis and septic shock. Dotted lines indicate normal values (EA, 100 ng/ml; LF, 400 ng/ml). r, Spearman rank correlation coefficient.
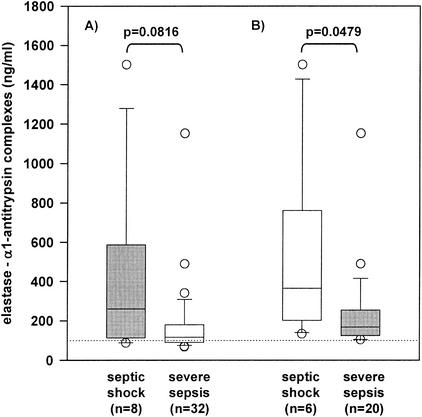
FIG. 2.
EA levels in patients with severe sepsis and septic shock. No statistical significant difference in EA levels between patients with severe sepsis (n = 32) and with septic shock (n = 8) was found (P = 0.0816) (A). In patients with elevated EA levels on inclusion (day 0), a significant difference between patients with severe sepsis (n = 20) and with septic shock (n = 6) was observed (P = 0.0479) (B).
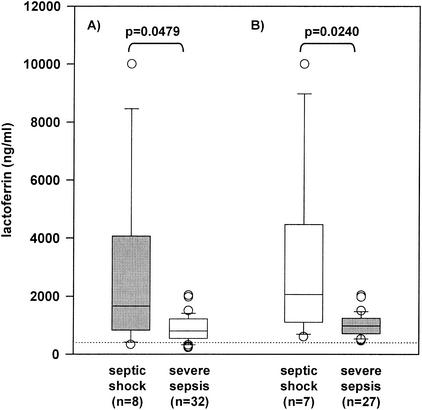
FIG. 3.
LF levels in patients with severe sepsis and septic shock. No statistical significant difference in LF levels between patients with severe sepsis (n = 32) and with septic shock (n = 8) was found (P = 0.0479) (A). In patients with elevated EA levels on inclusion (day 0), a significant difference between patients with severe sepsis (n = 27) and with septic shock (n = 7) was observed (P = 0.0240) (B).
Nonsurvivors (n = 13) had higher EA (median [range], 202 [87 to 1,501] ng/ml) and LF (median [range], 1,049 [33 to 4,869] ng/ml) levels than survivors (n = 27; median [range], 115 [67 to 1,152 ng/ml] and 774 [228 to 10,000] ng/ml, respectively) (not significant). Relative risk analysis revealed a 5.1-fold (95% confidence interval, 1.2- to 22.1-fold) increased mortality risk in patients with EA levels of above 200 ng/ml. Mortality in patients with EA levels of >200 ng/ml was 58%, compared to 21% in patients with levels below 200 ng/ml. A similar relationship was observed for LF: patients with LF levels of ≤1,000 ng/ml (n = 24) had a mortality of 21%, whereas patients with levels of >1,000 ng/ml had a mortality of 42%.
PMN activation and organ dysfunction at inclusion.
LOD and SOFA scores in patients with elevated EA levels on day 0 were significantly higher (median [range], 5 [2 to 14] and 8.5 [6 to 18], respectively) than those in patients with normal EA levels (median [range], 3.5 [1 to 6] and 7.0 [4 to 12], respectively; P < 0.05). Patients with elevated EA levels on day 0 had significant higher creatinine levels (median [range], 180.5 [73 to 717] μmol/liter) and lower pH levels (median [range], 7.33 [7.09 to 7.44]) than patients with normal EA levels (median [range], 91.5 [67 to 413] μmol/liter and 7.39 [7.27 to 7.43], respectively; P < 0.05), whereas no differences in leukocyte or platelet numbers, aspartate aminotransferase levels, or alanine aminotransferase levels between the two groups were observed. A significant correlation was found between elevated EA levels and organ dysfunction scores, such as LOD and SOFA scores (r, >0.57; P < 0.05), and with clinical parameters, such as creatinine, aspartate aminotransferase, and alanine aminotransferase levels (r, >0.42; P < 0.05).
Although no significant difference in organ dysfunction scores or clinical parameters was found between patients with elevated and normal LF levels on admission, elevated LF levels significantly correlated with LOD and SOFA scores (r, 0.365 and 0.481, respectively; P < 0.05).
PMN activation and inflammatory and coagulation parameters at inclusion.
Comparisons of cytokine and complement levels in patients with elevated and normal EA levels are given in Tables 1 and 2, respectively. Levels of cytokines, such as IL-8 and IL-10, correlated well (r, >0.5; P < 0.05) with elevated EA levels. Elevated EA levels significantly correlated with parameters predictive for a poor outcome, such as C3a, PAI-1, and TAT (r, >0.58; P < 0.05). Moreover, patients with elevated EA levels showed significantly (P = 0.01) higher PAI-1 levels (median [range], 433 [55 to 5,788] ng/ml) than patients with normal EA levels (median [range], 216.5 [53 to 616] ng/ml) on admission.
TABLE 1.
Cytokine levels in patients with severe sepsis and septic shock with normal or elevated EA levels on inclusion (day 0)
| Group (n) | Median pg/ml (range)
|
||
|---|---|---|---|
| IL-6 | IL-8 | IL-10 | |
| Elevated EA (26) | 257 (<7-151050) | 197.5 (<20-66,910) | 46 (<20-6,356) |
| Normal EA (14) | 52.5 (<7-378) | 83.5 (<20-202) | <20 (<20-215) |
For all three cytokines the difference between the two groups was statistically significant (P < 0.05).
TABLE 2.
Complement parameters in patients with normal and elevated EA levels on inclusion (day 0)a
| Group (n) | C1 inhibitor antigen (mg/ml) | C1 inhibitor activity (%)b | C3a (nM)b | C4b/c (nM) |
|---|---|---|---|---|
| Elevated EA (26) | 0.3 (0.16-0.6) | 81.5 (40-168) | 18 (5-54) | 40 (9-165) |
| Normal EA (14) | 0.32 (0.2-0.53) | 93.5 (41-209) | 10.5 (<5-17) | 47 (21-138) |
All values are given as medians and ranges.
The difference between the two groups was statistically significant (P < 0.05).
Except for TAT levels, which were significantly (P < 0.05) higher in patients with elevated LF levels (median [range], 9.3 [0.7 to 117] ng/ml) than in those with normal LF levels (4.75 [3.2 to 8.6] ng/ml), cytokine levels and other inflammatory or coagulation parameters were equal in patients with elevated and normal LF levels on admission. Correlation analysis revealed significant correlations between elevated LF and IL-6, IL-8, and IL-10 (r, >0.40; P < 0.02) as well as TAT complexes (r, 0.391; P < 0.05).
Effect of C1 inhibitor on PMN activation.
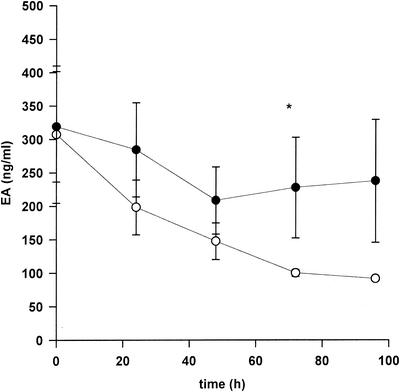
C1 inhibitor treatment in patients with elevated EA levels led to a decrease of EA levels compared to those of patients with elevated EA in the placebo group, with the difference being statistically significant on day 3 (Fig. 4). Furthermore, EA levels in the treatment group were significantly decreased at days 1 to 4 compared to baseline levels at day 0 (P < 0.05), whereas such a significant decrease was not observed in the placebo group. In contrast, no effect of C1 inhibitor administration on LF release was observed.
FIG. 4.
Influence of C1 inhibitor treatment on EA in patients with elevated EA levels. Mean EA levels (± standard errors of the means) are shown. In the placebo group, EA levels significantly decreased from day 1 to 4 compared to day 0 (Wilcoxon signed rank test, P < 0.05). *, statistically significant difference between the C1 inhibitor group (open circles) and the placebo group (closed circles) (Mann-Whitney rank sum test, P < 0.05).
Effect of C1 inhibitor on complement and cytokines in patients with elevated EA.
As described in the previous paragraph, C1 inhibitor therapy significantly reduced PMN degranulation in the patients with elevated EA levels. It is likely that this effect occurred via reduction of the formation or release of PMN agonists. To investigate the nature of these agonists, the effects of C1 inhibitor on IL-8 release and on complement activation in the patients with elevated EA levels were investigated. In these patients, IL-8 levels were significantly higher at days 1 (median [range], 262 [36 to 1,435] pg/ml) and 3 (146.5 [22 to 1,199] pg/ml) in those receiving the placebo than in those receiving C1 inhibitor (76 [36 to 44,344] and 52.5 [30 to 152] pg/ml, respectively), although levels at inclusion were lower in the treatment group on day 0 (85 [36 to 66,910] versus 228.5 [<20 to 28,214] pg/ml). No difference was found between the treatment group (median [range], 100 [30 to 36,342] and 64.5 [25 to 120] pg/ml, respectively) and the placebo group (202.5 [31 to 1,066] and 121.5 [<20 to 1,442] pg/ml, respectively) on days 2 and 4. In addition, the decrease of IL-8 in the treatment group was significant (P < 0.05) on days 1, 3, and 4 compared to day 0. This decrease apparently was not due to the natural course of IL-8 in sepsis, since in the placebo group, levels did not significantly decrease until day 4. Analysis of relative rather then absolute levels yielded the same result: a significant decrease in IL-8 levels was seen on days 1, 2, and 4 compared to day 0 in the C1 inhibitor group, whereas no such effect was observed in the placebo group. In contrast, no such differences were found for IL-6 or IL-10 levels. When a similar analysis comparing patients with elevated LF levels was done, no effect of C1 inhibitor therapy on cytokine levels was observed (data not shown).
As we recently showed, C1 inhibitor substitution significantly increased C1 inhibitor activity and antigen levels and significantly attenuated the activation of the classical pathway of the complement system (9). Similarly, in patients with elevated EA or LF levels, C1 inhibitor substitution significantly (P < 0.05) increased C1 inhibitor activity and antigen levels on days 1 to 4. C4b/c levels, which were equal on day 0 in the treatment group (median [range], 30 [9 to 165] nM) and the placebo group (46.5 [15 to 164] nM), had been significantly (P < 0.05) reduced by C1 inhibitor on day 1 (18 [7 to 35] nM) and on day 2 (20 [7 to 37] nM) compared to the placebo group (37.5 [13 to 113] and 34.5 [14 to 97] nM, respectively). No difference between the treatment group (median [range], 30 [4 to 56] and 45.5 [18 to 100] nM, respectively) and the placebo group (38 [13 to 99] and 40.5 [14 to 84] nM, respectively) was found on days 3 and 4. C1 inhibitor also affected C4b/c levels in patients with elevated LF levels. Whereas no difference between the treatment group (median [range], 30 [9 to 165] nM) and the placebo group (56 [15 to 164] nM) was found on day 0, C1 inhibitor significantly decreased (P < 0.01) C4b/c levels on day 1 (21 [7 to 37] nM), on day 2 (18.5 [4 to 51] nM), and on day 3 (27 [4 to 55] nM) compared to the placebo group (46.5 [13 to 165], 40 [14 to 97], and 45 [13 to 142] nM, respectively). No effect of treatment was found on day 4 (38.5 [6 to 100] versus 44 [14 to 94] nM). However, C1 inhibitor did not show any effect on C3a levels in patients with elevated EA or LF. No effect of C1 inhibitor was found on contact phase parameters in patients with elevated EA or LF (data not shown).
DISCUSSION
There is evidence that neutrophils play an important role in the pathogenesis of sepsis and multiple organ failure. Patients suffering from sepsis showed elevated EA levels (13, 14, 30), which predict clinical outcome (6, 30) and correlate with organ dysfunction scores (18, 26, 35). In this study we confirmed that neutrophils are activated in sepsis; this activation is associated with advanced organ dysfunction. Moreover, we showed that C1 inhibitor reduces EA release, in particular in patients with activated neutrophils. Finally, this decrease in EA levels was accompanied by reduced IL-8 release and attenuated complement activation.
In the patients with severe sepsis or septic shock, 65 and 85% had elevated EA and LF levels, respectively. Elastase and LF are both released by neutrophils upon activation. Detection of elevated EA and LF levels in septic patients was reported to reflect neutrophil activation (30). Low-dose infusion of endotoxin to healthy volunteers led to an increase in EA levels (42, 45). For patients suffering from sepsis, elevated EA levels have been described by various authors (13, 14, 30, 40).
We found EA and LF levels to increase with disease severity and to correlate with outcome; however, such a relationship was much weaker for LF, as was reported in other studies as well (30). On admission, patients with elevated EA levels showed significantly higher LOD and SOFA scores and had significantly worse renal function than those with normal EA levels, in accordance to published observations reporting EA levels to increase with disease severity and to predict mortality (6, 18, 30). In contrast, patients with normal EA levels had only mild organ dysfunction. Furthermore, we found EA levels of above 200 ng/ml to be associated with a fivefold-increased mortality risk or with a sixfold-increased risk for development of septic shock. Moreover, patients with elevated EA levels had significantly higher levels of parameters associated with poor outcome, such as C3a, PAI-1, and IL-6 (8, 21, 46), than those with normal EA levels. In contrast, except for significant correlations with organ dysfunction scores, no associations between organ dysfunction and circulating LF were found. In vitro studies indicate that stronger neutrophil stimulation is needed for EA release than for LF release (2, 16, 19). EA levels in septic patients were shown to increase with disease severity and to correlate with clinical parameters, whereas no such relationship was found for LF (6, 30). We now found that the correlation of EA levels with disease severity and clinical parameters was stronger than that of LF levels. Thus, increased EA levels in sepsis likely reflect potentially toxic activation of PMN.
Patients with elevated EA levels on admission showed significantly higher levels of cytokines (IL-6, IL-8, and IL-10) and C3a than patients with normal EA levels. In contrast, no difference in levels of contact phase proteins was found between patients with elevated and normal EA levels (data not shown). During sepsis, the complement and contact phase systems are activated and proinflammatory cytokines, such as tumor necrosis factor alpha, IL-6, and IL-8, are released (8, 21). Cytokines (such as IL-8), complement activation products (such as C3a), and contact phase factors (such as FXIIa and kallikrein), may be involved in the activation of neutrophils (1, 15, 38, 48, 49). IL-8 and C3a levels were significantly higher in patients with elevated EA levels than in those with normal EA levels. In addition, elevated EA levels correlated well with IL-8 and C3a levels. In contrast, no difference in contact phase parameters between the two groups was noted. This suggests that neutrophil activation in septic patients occurs mainly via the complement system and/or via IL-8. Furthermore, the significantly higher levels of cytokines (not only IL-8 but also IL-6 and IL-10), C3a, and PAI-1 in the patients with elevated EA levels compared to those with normal EA levels suggest that these patients have a stronger inflammatory response pattern than patients with normal EA levels. This hypothesis is supported by the in vitro observation that compared to LF release, a much stronger neutrophil stimulation is needed to release elastase (2, 16, 19). Therefore, neutrophil activation as measured by assessing EA levels may be a good indicator for the severity of the inflammatory response during sepsis.
Neutrophils may contribute to the development of organ dysfunction during sepsis (18, 26, 35) and play a crucial role in the pathogenesis of acute respiratory distress syndrome and acute renal failure (17, 20, 25). However, the molecular mechanisms leading to organ damage by PMN are not precisely known, although a number of mechanisms has been proposed based on in vitro studies. For example, vascular leakage due to endothelial damage induced by toxic mediators of neutrophils may explain the link between organ dysfunction and PMN (23). Vascular leakage syndrome induced by IL-2 or after bone marrow transplantation has been suggested to result from an activation of inflammatory mediator systems, such as cytokines, neutrophils, and the complement and contact phase systems (8). Administration of C1 inhibitor inhibited the classical complement pathway and improved the clinical picture of vascular leakage syndrome (31, 32, 34). Hence, the beneficial, although mild, effect of C1 inhibitor on organ dysfunction in the patients included in this study (9) may be mediated via its action on PMN. Support for this notion is that only patients with elevated EA levels on admission showed a benefit from C1 inhibitor therapy, whereas patients with normal EA levels showed no difference in organ dysfunction scores and creatinine levels upon treatment (data not shown).
There are several molecular explanations for the effect of C1 inhibitor on PMN activation. Administration of this inhibitor to septic baboons resulted in decreased IL-8 levels and inhibition of activation of the classical pathway of complement and of the contact phase system (27). Thus, reduction of complement or contact system-derived PMN agonists, or reduced IL-8 responses, might explain reduced PMN activation in patients receiving C1 inhibitor. As previously reported, C1 inhibitor substitution significantly inhibited the classical pathway of the complement system, whereas no effect on C3a or contact phase parameters was found (9). In accordance, we found C1 inhibitor to attenuate the activation of the classical pathway of the complement system in patients with elevated EA on admission, whereas no such effect on C3a was found. Moreover, no effect of C1 inhibitor administration on the contact system in patients with elevated EA was observed (data not shown). These results exclude the possibility that activated contact factors such as factor XIIa or kallikrein are among the major agonists for PMN in our sepsis patients. However, the involvement of activated complement products in the activation of PMN in our patients cannot be ruled out, because C3a is rapidly cleared from circulation and the level of C3a in plasma may represent only 10% of the generated C3a (43). Therefore, an effect of C1 inhibitor on C3a release may have escaped detection. C1 inhibitor significantly decreased IL-8 levels in patients with elevated EA on admission. In septic baboons such an attenuation of IL-8 release was also observed (27). The effect of C1 inhibitor on IL-8 may explain its effects on PMN. Nevertheless, the precise mechanism by which C1 inhibitor attenuates IL-8 release remains to be elucidated. As IL-8 as well as complement can act as an agonist for neutrophils, one may speculate that both of these mediators (IL-8 and complement) can act as agonists contributing to neutrophil activation in sepsis. However, our data do not exclude involvement of other agonists not affected by C1 inhibitor as well.
In spite of its effect on several PMN agonists, administration of C1 inhibitor did not significantly affect PMN activation in the baboons lethally challenged with Escherichia coli (27), which is in contrast to the changes of PMN observed in this study. In our patients C1 inhibitor was given at a time when PMN already were activated, whereas the baboons received the first dose of C1 inhibitor before the lethal E. coli challenge. Hence, stimulation by different agonists may well explain the differences between the effects of C1 inhibitor on neutrophil activation in the patients and those in the baboons. For example, in the initial phase of baboon sepsis, extremely high numbers of E. coli and very high levels of endotoxin circulate, which may activate PMN but are not affected by C1 inhibitor. Thus, these bacterial agonists may have blurred an effect of C1 inhibitor therapy on PMN function.
In this study we show that activation of PMN in patients with sepsis is reduced upon administration of C1 inhibitor. We suggest that this effect of C1 inhibitor on PMN provides a rationale for its favorable effect on organ function in sepsis. Moreover, our results point to a role for IL-8 and/or complement in the activation of PMN in patients with sepsis.
Acknowledgments
This study was supported by a grant (32-55312.98) from the Swiss National Foundation for Scientific Research and by a unrestricted grant from Aventis Behring Switzerland.
REFERENCES
- 1.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainton, D. F. 1973. Sequential degranulation of two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J. Cell Biol. 58:249-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baveye, S., E. Elass, J. Mazurier, G. Spik, and D. Legrand. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37:281-286. [DOI] [PubMed] [Google Scholar]
- 4.Bone, R. C. 1996. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit. Care Med. 24:163-172. [DOI] [PubMed] [Google Scholar]
- 5.Bone, R. C., R. A. Balk, F. B. Cerra, R. P. Dellinger, A. M. Fein, W. A. Knaus, R. M. Schein, W. J. Sibbald, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101:1644-1655. [DOI] [PubMed] [Google Scholar]
- 6.Bossink, A. W. J., A. B. J. Groeneveld, and L. G. Thijs. 1999. Prediction of microbial infection and mortality in medical patients with fever: plasma procalcitonin, neotrophilic elastase-alpha1-antitrypsin, and lactoferrin compared with clinical variables. Clin. Infect. Dis. 29:398-407. [DOI] [PubMed] [Google Scholar]
- 7.Brower, M. S., and P. C. Harpel. 1982. Proteolytic cleavage and inactivation of alpha-2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J. Biol. Chem. 257:9849-9854. [PubMed] [Google Scholar]
- 8.Caliezi, C., W. A. Wuillemin, S. Zeerleder, S. Redondo, B. Eisele, and C. E. Hack. 2000. C1-esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol. Rev. 52:91-112. [PubMed] [Google Scholar]
- 9.Caliezi, C., S. Zeerleder, M. Redondo, B. Regli, H. U. Rothen, R. Zürcher-Zenklusen, R. Rieben, J. Devay, C. E. Hack, B. Lämmle, and W. A. Wuillemin. 2002. C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit. Care Med. 30:1722-1728. [DOI] [PubMed] [Google Scholar]
- 10.Carden, D., F. Xiao, C. Moak, B. H. Willis, S. Robinson-Jackson, and S. Alexander. 1998. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 275:H385-H392. [DOI] [PubMed] [Google Scholar]
- 11.Carrell, R. W., and M. C. Owen. 1985. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature 317:730-732. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly, S. C., I. MacGregor, A. Zamani, M. W. G. Gordon, C. E. Robertson, D. J. Steedman, K. Little, and C. Haslett. 1995. Plasma elastase levels and the development of adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 151:1428-1433. [DOI] [PubMed] [Google Scholar]
- 13.Duswald, K. H., M. Jochum, W. Schramm, and H. Fritz. 1985. Released granulocytic elastase: an indicator of pathobiochemical alterations in septicaemia after abdominal surgery. Surgery 98:892-899. [PubMed] [Google Scholar]
- 14.Egbring, R., W. Schmidt, G. Fuchs, and K. Havemann. 1977. Demonstration of granulocytic proteases in plasma of patients with acute leukemia and septicaemia with coagulation defects. Blood 49:219-231. [PubMed] [Google Scholar]
- 15.Ehrengruber, M. U., T. Geiser, and D. A. Deranleau. 1994. Activation of human neutrophils by C3a and C5a. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 346:181-184. [DOI] [PubMed] [Google Scholar]
- 16.Estensen, R. D., J. G. White, and B. Holmes. 1974. Specific degranulation of human polymorphonuclear leukocytes. Nature 248:347-348. [DOI] [PubMed] [Google Scholar]
- 17.Gando, S., T. Kameue, S. Nanzaki, T. Hayakawa, and Y. Nakanishi. 1997. Increased neutrophil elastase, persistent intravascular coagulation, and decreased fibrinolytic activity in patients with posttraumatic acute respiratory distress syndrome. J. Trauma 42:1068-1072. [DOI] [PubMed] [Google Scholar]
- 18.Gardinali, M., P. Padalino, S. Vesconi, A. Calcagno, S. Ciappellano, L. Conciato, O. Chiara, A. Agostini, and A. Nespoli. 1992. Complement activation and polymorphonuclear neutrophil leukocyte elastase in sepsis. Arch. Surg. 127:1219-1224. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, I. M., S. T. Hoffstein, and G. Weissmann. 1975. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J. Cell Biol. 66:647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groeneveld, A. B., P. G. Raijmakers, C. E. Hack, and L. G. Thijs. 1995. Interleukin 8-related neutrophil elastase and the severity of adult respiratory distress syndrome. Cytokine 7:746-752. [DOI] [PubMed] [Google Scholar]
- 21.Hack, C. E., L. A. Aarden, and L. G. Thijs. 1997. Role of cytokines in sepsis. Adv. Immunol. 66:101-195. [DOI] [PubMed] [Google Scholar]
- 22.Hack, C. E., J. Paardekooper, A. J. Eerenberg, G. O. Navis, M. W. Nijsten, L. G. Thijs, and J. H. Nuijens. 1988. A modified competitive radioimmunoassay for the detection of C3a. Use of 125I-C3 instead of 125I-C3a. J. Immunol. Methods 108:77-84. [DOI] [PubMed] [Google Scholar]
- 23.Hazelzet, J. A., R. De Groot, G. Van Mierlo, K. F. M. Joosten, E. Van der Voort, A. Eerenberg, M. H. Suur, W. C. Hop, and C. E. Hack. 1998. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 66:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helle, M., L. Boeije, E. de Groot, A. de Vos, and L. Aarden. 1991. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J. Immunol. Methods 138:47-56. [DOI] [PubMed] [Google Scholar]
- 25.Hörl, W. H., R. M. Schäfer, M. Hörl, and A. Heidland. 1990. Neutrophil activation in acute renal failure and sepsis. Arch. Surg. 125:651-654. [DOI] [PubMed] [Google Scholar]
- 26.Ikei, S., M. Ogawa, and Y. Yamaguchi. 1998. Blood concentrations of polymorphonuclear leucocyte elastase and interleukin-6 are indicators for the occurrence of multiple organ failures at the early stage of acute pancreatitis. J. Gastroenterol. Hepatol. 13:1274-1283. [PubMed] [Google Scholar]
- 27.Jansen, P. M., B. Eisele, I. W. de Jong, A. Chang, U. Delvos, F. B. J. Taylor, and C. E. Hack. 1998. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J. Immunol. 160:475-484. [PubMed] [Google Scholar]
- 28.Jansen, P. M., T. C. T. M. van der Pouw Kraan, I. W. de Jong, G. van Mierlo, J. Wijdenes, A. A. Chang, L. A. Aarden, F. B. J. Taylor, and C. E. Hack. 1996. Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon-gamma. Blood 87:5144-5151. [PubMed] [Google Scholar]
- 29.LeGall, J. R., J. Klar, S. Lemeshow, F. Saulnier, C. Alberti, A. Artigas, and D. Teres. 1996. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. JAMA 276:802-810. [DOI] [PubMed] [Google Scholar]
- 30.Nuijens, J. H., J. J. Abbink, Y. T. Wachtfogel, R. W. Colman, A. J. Eerenberg, D. Dors, A. J. Kamp, R. J. Strack van Schijndel, L. G. Thijs, and C. E. Hack. 1992. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J. Lab. Clin. Med. 119:159-168. [PubMed] [Google Scholar]
- 31.Nürnberger, W., U. G”bel, H. Stannigel, B. Eisele, A. Janssen, and U. Delvos. 1992. C1-inhibitor concentrate for sepsis-related capillary leak syndrome. Lancet 339:990. [DOI] [PubMed] [Google Scholar]
- 32.Nürnberger, W., R. Heying, S. Burdach, and U. G”bel. 1997. C1 esterase inhibitor concentrate for capillary leakage syndrome following bone marrow transplantation. Ann. Hematol. 75:95-101. [DOI] [PubMed] [Google Scholar]
- 33.Ofosu-Appiah, W., G. Sfeir, D. Smith, and T. Richard. 1997. Neutrophil-mediated damage to vascular endothelium in the spontaneously hypertensive rat. Clin. Immunol. Immunopathol. 83:293-301. [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie, A. C., J. W. Baars, A. J. Eerenberg, C. E. Hack, H. M. Pinedo, L. G. Thijs, and J. Wagstaff. 1994. A pilot study to evaluate the effects of C1 esterase inhibitor on the toxicity of high-dose interleukin-2. Br. J. Cancer 69:596-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacher, R., H. Redl, M. Frass, D. H. Petzl, E. Schuster, and W. Woloszczuk. 1989. Relationship between neopterin and granulocyte elastase plasma levels and severity of multiple organ failure. Crit. Care Med. 17:221-226. [DOI] [PubMed] [Google Scholar]
- 36.Portielje, J. E. A., W. H. J. Kruit, A. J. M. Eerenberg, M. Schuler, A. Sparreboom, C. H. J. Lamers, R. L. H. Bolhuis, G. Stoter, C. Huber, and C. E. Hack. 2001. Interleukin 12 induces activation of fibrinolysis and coagulation in humans. Br. J. Haematol. 112:499-505. [DOI] [PubMed] [Google Scholar]
- 37.Rangel-Frausto, M. S., D. Pittet, M. Costigan, T. Hwang, C. S. Davis, and R. P. Wenzel. 1995. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273:117-123. [PubMed] [Google Scholar]
- 38.Schapira, M., E. Despland, C. F. Scott, L. A. Boxer, and R. W. Colman. 1982. Purified human plasma kallikrein aggregates human blood neutrophils. J. Clin. Investig. 69:1199-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz, M. J., P. Speelman, C. E. Hack, W. A. Buurman, S.J.H. van Deventer, and T. van der Poll. 2000. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:235-240. [DOI] [PubMed] [Google Scholar]
- 40.Seitz, R., M. Wolf, R. Egbring, K. P. Radtke, A. Liesenfeld, P. Pittner, and K. Havemann. 1987. Participation and interactions of neutrophil elastase in haemostatic disorders of patients with severe infections. Eur. J. Haematol. 38:231-240. [DOI] [PubMed] [Google Scholar]
- 41.Smedly, L. A., M. G. Tonnesen, R. A. Sandhaus, C. Haslett, L. A. Guthrie, R. B. Johnston, P. M. Henson, and G. S. Worthen. 1986. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J. Clin. Investig. 77:1233-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suffredini, A. F., P. C. Harpel, and J. E. Parillo. 1989. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N. Engl. J. Med. 320:1165-1172. [DOI] [PubMed] [Google Scholar]
- 43.Te Velthuis, H., P. G. Jansen, C. E. Hack, L. Eijsman, and C. R. Wildevuur. 1996. Specific complement inhibition with heparin-coated extracorporeal circuits. Ann. Thorac. Surg. 61:1153-1157. [DOI] [PubMed] [Google Scholar]
- 44.Van der Pouw Kraan, T. C., L. C. Boeije, R. J. Smeek, J. Wijdenes, and L. A. Aarden. 1995. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 181:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Deventer, S. J., H. R. Buller, J.W. ten Cate, L. A. Aarden, C. E. Hack, and A. Sturk. 1990. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76:2520-2526. [PubMed] [Google Scholar]
- 46.Vervloet, M. G., L. G. Thijs, and C. E. Hack. 1998. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin. Thromb. Hemost. 24:33-44. [DOI] [PubMed] [Google Scholar]
- 47.Vincent, J. L., R. Moreno, J. Takala, S. Willatts, A. De Mendonca, H. Bruining, C. K. Reinhart, P. M. Suter, and L. G. Thijs. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 22:707-710. [DOI] [PubMed] [Google Scholar]
- 48.Wachtfogel, Y. T., U. Kucich, H. L. James, C. F. Scott, M. Schapira, M. Zimmerman, A. B. Cohen, and R. W. Colman. 1983. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J. Clin. Investig. 72:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wachtfogel, Y. T., R. A. Pixley, U. Kucich, W. Abrams, G. Weinbaum, M. Schapira, and R. W. Colman. 1986. Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood 67:1731-1737. [PubMed] [Google Scholar]
- 50.Weiss, S. J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365-376. [DOI] [PubMed] [Google Scholar]
- 51.Westlin, W. F., and M. A. Gimbrone. 1993. Neutrophil-mediated damage to human vascular endothelium. Role of cytokine activation. Am. J. Pathol. 142:117-128. [PMC free article] [PubMed] [Google Scholar]
- 52.Wolbink, G. J., J. Bollen, J. W. Baars, R.J. ten Berge, A. J. Swaak, J. Paardekooper, and C. E. Hack. 1993. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for quantification of complement activation via the classical pathway. J. Immunol. Methods 163:67-76. [DOI] [PubMed] [Google Scholar]