Abstract
Neuropeptide Y (NPY) is one the most potent orexigenic peptides found in the brain. It stimulates food intake with a preferential effect on carbohydrate intake. It decreases latency to eat, increases motivation to eat and delays satiety by augmenting meal size. The effects on feeding are mediated through at least two receptors, the Y1 and Y5 receptors. The NPY system for feeding regulation is mostly located in the hypothalamus. It is formed of the arcuate nucleus (ARC), where the peptide is synthesized, and the paraventricular (PVN), dorsomedial (DMN) and ventromedial (VMN) nuclei and perifornical area where it is active. This activity is modulated by the hindbrain and limbic structures. It is dependent on energy availability, e.g. upregulation with food deprivation or restriction, and return to baseline with refeeding. It is also sensitive to diet composition with variable effects of carbohydrates and fats. Leptin signalling and glucose sensing which are directly linked to diet type are the most important factors involved in its regulation. Absence of leptin signalling in obesity models due to gene mutation either at the receptor level, as in the Zucker rat, the Koletsky rat or the db/db mouse, or at the peptide level, as in ob/ob mouse, is associated with increased mRNA abundance, peptide content and/or release in the ARC or PVN. Other genetic obesity models, such as the Otsuka–Long–Evans–Tokushima Fatty rat, the agouti mouse or the tubby mouse, are characterized by a diminution in NPY expression in the ARC nucleus and by a significant increase in the DMN. Further studies are necessary to determine the exact role of NPY in these latter models. Long-term exposure to high-fat or high-energy palatable diets leads to the development of adiposity and is associated with a decrease in hypothalamic NPY content or expression, consistent with the existence of a counter-regulatory mechanism to diminish energy intake and limit obesity development. On the other hand, an overactive NPY system (increased mRNA expression in the ARC associated with an upregulation of the receptors) is characteristic of rats or rodent strains sensitive to dietary-induced obesity. Finally, NPY appears to play an important role in body weight and feeding regulation, and while it does not constitute the only target for drug treatment of obesity, it may nevertheless provide a useful target in conjunction with others.
Keywords: feeding behaviour, dietary composition and preference, leptin resistance, insulin, hypothalamus, Zucker rat and ob/ob mouse
1. Introduction
Neuropeptide Y (NPY) was discovered over 20 years ago by Tatemoto & Mutt (Tatemoto et al. 1982). It is a 36 amino acid peptide that belongs to the pancreatic polypeptide family. Its name derives from the single-letter code (Y) for the amino acid tyrosine since it contains several tyrosine residues including an amidated C-terminal tyrosine residue. It is one of the most abundant peptides found in the brain, although it is also present in the peripheral nervous system (Everitt et al. 1984; Allen et al. 1987; Zukowska et al. 2003). It is one of the most conserved peptides during evolution (Larhammar 1996). These traits suggest that it is particularly important for the regulation of essential physiological functions.
In the brain, it is present in the cortex, hippocampus, hindbrain and hypothalamus (Chronwall et al. 1985). In the latter area, it is synthesized in abundance by neurons of the arcuate (ARC) nucleus (Chronwall 1985; Beck 2005). These neurons co-synthesize another orexigenic peptide, agouti-related peptide (AgRP; Hahn et al. 1998). They send their axons towards the paraventricular (PVN) nucleus as well as the dorsomedial (DMN) nucleus and the median preoptic area (Bai et al. 1985; Kerkerian & Pelletier 1986). The PVN also receives projections from NPY-synthesizing neurons present in the brainstem (Sahu et al. 1988b), particularly in the A1 and C1 areas (Sawchenko et al. 1985).
The widespread distribution of NPY is associated with diverse biological actions, including cardiovascular regulation, seizure and cognition, stress, modulation of neuroendocrine systems and appetite regulation (Walker et al. 1991; Cox 1993; Aubert et al. 1994; Redrobe et al. 1999; Thorsell & Heilig 2002; Zukowska et al. 2003). Biological actions of NPY are mediated by six receptors named Y1–Y6. The Y6 receptor is not present in all mammalian species (Widdowson et al. 1997b; Burkhoff et al. 1998). These receptors probably derive from three Y receptor genes leading to the Y1, Y2 and Y5 subfamilies (Larhammar & Salaneck 2004). All three subfamilies appear to play a role in the regulation of feeding behaviour (Henry et al. 2005).
This review will focus on NPY role in normal eating and in animal models with genetic and diet-induced obesity. It will only address the genetic models with a spontaneous gene mutation and not those resulting from human manipulations. Information on knockout and transgenic models can be found in recent reviews (Beck 2001; Herzog 2004).
2. NPY and normal feeding behaviour
(a) NPY is a potent orexigenic peptide
Soon after its discovery, several groups demonstrated that NPY injected into the brain either in the ventricles or in different hypothalamic nuclei induced a robust feeding response (Clark et al. 1984; Levine & Morley 1984; Stanley & Leibowitz 1984). It is effective throughout the day with strong effects observed at the beginning of the dark period (Tempel & Leibowitz 1990; Stanley & Thomas 1993). Food intake is increased several fold and the effect lasts 6–8 hours. The orexigenic effect of NPY has been noted in numerous species—both mammalian or non-mammalian (Parrott et al. 1986; Kuenzel et al. 1987; Morley et al. 1987b; Pau et al. 1988; Miner et al. 1989; Morris & Crews 1990). No effects have been described after peripheral infusion.
Feeding behaviour can be separated into two phases: an appetitive one consisting of searching and acquiring food and a consummatory one consisting of ingestion itself (Craig 1918). Consummatory behaviour may be tested in animals receiving a sucrose solution through an implanted intraoral cannula. There is general agreement that NPY injected in the lateral or third ventricle stimulates appetitive behaviour, e.g. eating pellets from a feeder or drinking sucrose from a bottle (Seeley et al. 1995; Ammar et al. 2000; Benoit et al. 2005). But NPY either stimulates (Benoit et al. 2005), fails to change (Seeley et al. 1995) or inhibits (Ammar et al. 2000) intraoral administration of sucrose. These discrepant observations on consummatory behaviour might be related to the training of the rats required to habituate them to the experimental procedure, as naive untrained rats ingest significantly more intraoral sucrose solution after NPY injection (Benoit et al. 2005). In hamsters, both appetitive and consummatory ingestive behaviours are stimulated (Day et al. 2005).
The stimulatory effect of NPY on food ingestion is confirmed by observations of the effect of blockade of the NPY system by various means. Continuous central infusion of NPY antibodies suppresses intake during the dark period in a dose-dependent fashion (Dube et al. 1994). Local injection of NPY antibodies into the PVN and ventromedial (VMN) nuclei leads to similar inhibitory effects on food ingestion (Shibasaki et al. 1993; Walter et al. 1994). Injection of NPY antisense oligodeoxynucleotides may also diminish food intake, meal size and duration (Hulsey et al. 1995), although in some studies no effects are reported (Dryden et al. 1998); in any case, the results obtained with this technique must be carefully checked because of the difficulties in using it (toxic effects, penetration, exact targeting). However, a recent study where NPY expression in the ARC was inhibited by a gene therapy approach showed that the treated animals released 50% less NPY, gained less weight and ate less than the controls up to 50 days after treatment (Gardiner et al. 2005).
A map of the hypothalamic areas responding to NPY has been established by injecting nanolitre volumes into well-determined nuclei. The perifornical area, the PVN and VMN are the areas most sensitive to administration of NPY injection (Stanley et al. 1985a,b, 1993; Morley et al. 1987a; Jolicoeur et al. 1995). There are, however, differences in the actions of NPY in different regions. In the PVN, NPY increases the respiratory quotient, in addition to its stimulatory effects on food intake (Menendez et al. 1990; Currie & Coscina 1995), consistent with an increased utilization of carbohydrates as energy source. On the other hand, in the perifornical area, NPY strongly stimulates intake without any modification of the respiratory quotient (Currie & Coscina 1995). Opposite effects have also been observed on body temperature following NPY injection in these two areas (Jolicoeur et al. 1995).
In the case of intraventricular administration, the precise site of administration of NPY does seem to be important. Thus, NPY stimulates food intake when it is injected into the third or lateral ventricles that are near the hypothalamic region, as well as when it is injected into the fourth ventricle (Corp et al. 1990; Steinman et al. 1994). This suggests that connections between hindbrain and hypothalamus are functionally important for the orexigenic properties of NPY. The importance of these links is indicated by observations of the induction of c-fos expression in the forebrain and in the PVN after NPY injection in the fourth ventricle (Xu et al. 1995), and by observations following brain section. Total transection leads to a decrease in NPY content in the PVN and an increased sensitivity of the operated rats to exogenous NPY injection (Sahu et al. 1988a).
Lesions of the ARC nucleus, either immunologically or chemically (Burlet et al. 1995; Stricker-Krongrad et al. 1996; Bugarith et al. 2005), modify feeding behaviour. A small but durable decrease in food intake is observed in lesioned rats following specific targeting of NPY neurons by neurotoxins, or by less selective destruction of NPY neurons by perinatal injection of toxic doses of monosodium glutamate (MSG; Burlet et al. 1995; Stricker-Krongrad et al. 1996). The decrease in food intake is not directly related to the size of the lesion with, for example, a 10% diminution of intake associated with a 40% or more diminution in NPY content in MSG rats (Stricker-Krongrad et al. 1996). Rats with a toxin-induced lesion in the basomedial hypothalamus remain responsive to NPY but not to leptin (Bugarith et al. 2005).
(b) Mode of action
Careful analyses of the patterns of feeding behaviour reveal that NPY reduces the latency to eat (Clark et al. 1984; Stanley & Leibowitz 1985; Morley et al. 1987b; Corp et al. 1990). Conversely, the absence of NPY in NPY knockout mice is associated with a pronounced delay in the initiation of feeding (Sindelar et al. 2005). NPY also delays satiety and therefore augments meal size, time spent eating and meal duration (Leibowitz & Alexander 1991; Lynch et al. 1994; Stricker-Krongrad et al. 1994). These effects are independent of the type of food, as they are observed with either solid dry food or sweetened condensed milk solution.
NPY also modifies the motivation to eat (Flood & Morley 1991). Its effect is comparable to that of a 36–48 hour food deprivation (Jewett et al. 1995). This has been shown in three paradigms: lever pressing, appetitive passive avoidance and quinine-adulterated milk. NPY injected into the third ventricle induces a higher consumption of milk when the animals have to work in a lever press apparatus to obtain it. The potency of NPY in inducing intake is evident even when substantial effort (number of lever-pressing events) is required to obtain small amounts of food (Jewett et al. 1992, 1995). NPY-injected mice also tolerate more electric shocks to the tongue while drinking milk and they overcome the unpleasant bitter taste of quinine added to the milk (Flood & Morley 1991). In addition, NPY presumably plays a role in mediating food anticipatory responses, as rats conditioned with daily NPY injections and a restricted feeding schedule eat more after saline injection into the third ventricle than rats conditioned with saline (Drazen et al. 2005).
(c) NPY modulates dietary preferences
Several studies have shown that centrally administered NPY modifies qualitative components of the ingested food in addition to increasing the ingested quantity. When the rats have the choice between three sources of pure macronutrient (casein, lard, mixture of sucrose–cornstarch–dextrose) correctly supplemented with vitamins and minerals, they preferentially increase their intake of carbohydrates after acute NPY injection (Stanley et al. 1985a,b; Stanley et al. 1989a). Similar results are obtained with a choice between two macronutrients, but only if carbohydrates constitute one of the two choices (Stanley et al. 1985a,b). These effects are partly dependent on the previous dietary preference of the rats (Welch et al. 1994). A preferential intake of carbohydrates is also observed when the rats have to choose between two nutritionally complete diets: a high-carbohydrate (HC) diet and a high-fat (HF) diet (Welch et al. 1994). When the rats have the same choice (HF diet versus HC diet), and when NPY is continuously injected into a brain ventricle through osmotic minipumps, both HF intake and HC intake are stimulated (Beck et al. 1992b), but the effect on HF intake is short (2 days), whereas that on HC intake is still observed after 9 days of injection (Beck et al. 1992b).
The nature of the carbohydrate plays a role in the orexigenic effects of NPY. When rats can choose between a HF diet and a HC diet, the animals eat more of the HC diet if the source of carbohydrate is sucrose or polycose compared with cornstarch (Glass et al. 1997). The change in palatability due to increased sweetness of the sucrose or polycose diets versus cornstarch might be an element of this preference. This is supported by an experiment where different solutions either sweetened with sucrose or saccharin were tested in fully sated rats. NPY increased the intake of the different sucrose solutions (2–10%) as well as the preferred saccharin solution (Lynch et al. 1993). Orosensory mechanisms might therefore play a role on the stimulatory effects of NPY on carbohydrate intake.
(d) Orexigenic effects are mediated through Y1 and Y5 receptors
At the time of early studies on the NPY system, only two receptor subtypes were known: Y1 and Y2. These were differentiated on the basis of their affinity for NPY fragments (Leibowitz & Alexander 1991; Widdowson 1993). The maximal stimulation is obtained with the complete amino acid sequence (McLaughlin et al. 1991). The loss of several amino acids at either end of the peptide is associated with a diminution of the orexigenic effect. NPY1–27 is, for example, less potent than the entire NPY (Paez et al. 1991). There is, however, an exception since NPY2–36 induces the same or an even greater feeding response than intact NPY (Jolicoeur et al. 1991; Kalra et al. 1991a; Stanley et al. 1992). Y1 agonists like [D-Arg25]-NPY stimulate food ingestion (Mullins et al. 2001). Potent Y1 antagonists such as 1229U91 and BIBO 3304 are able to inhibit fasting- and NPY-stimulated food intake (Kanatani et al. 1996, 2001; Wieland et al. 1998; Daniels et al. 2001). Comparable effects are observed after injection of Y1 antisense oligodeoxynucleotides (Lopez-Valpuesta et al. 1996; Schaffhauser et al. 1998).
NPY13–36 does not bind to Y1 receptors, but it is a full agonist at the Y2 receptor. It does not stimulate food intake but inhibits NPY release from hypothalamic slices in vitro (King et al. 1999, 2000). This might contribute to an anorexigenic response. In this context, it is worth noting the inhibitory role of the Y2 ligands, such as PYY3–36 on food intake in humans and rodents that has been recently emphasized (see Tschop et al. 2004; leRoux & Bloom 2005 for more details). Chronic activation of Y2 receptors leads to hypophagia and transient weight loss (Henry et al. 2005) and a normal feeding behaviour requires the presence of the Y2 receptor (Naveilhan et al. 1999).
The variable efficiency of NPY fragments in stimulating food intake, and more particularly that of NPY2–36, suggested that feeding may be elicited through a variant of the Y1 receptor. Subsequently, a further receptor, Y5, was cloned (Gerald et al. 1996; Hu et al. 1996) that is present in areas involved in feeding regulation (PVN, lateral hypothalamus (LH)), sometimes in association with the Y1 receptor (Parker & Herzog 1999; Durkin et al. 2000). It is now well established that the Y5 subtype is implicated in feeding behaviour (for review see Balasubramaniam 1997; Blomqvist & Herzog 1997; Inui 1999). Like the Y1 receptor, it is present in the parvocellular part of the PVN (Wolak et al. 2003). The effects of selective receptor agonists suggest that the Y5 receptor might mediate the consummatory behaviour, whereas the appetitive behaviour might be more related to the Y1 receptor (Day et al. 2005). The Y5 receptor could also play a role in the NPY-induced reduction of energy expenditure (Hwa et al. 1999) and it is downregulated by NPY release induced by diet restriction (Widdowson et al. 1997a).
The importance of the Y5 receptor is indicated by several different lines of evidence, including the injection of Y5 antisense oligonucleotides (Schaffhauser et al. 1997; Tang-Christensen et al. 1998; Flynn et al. 1999; Campbell et al. 2001), the injection of specific Y5 agonists (Haynes et al. 1998; Wyss et al. 1998a,b; Cabrele et al. 2000; Parker et al. 2000; Lecklin et al. 2003) and antagonists (Polidori et al. 2000; Kask et al. 2001; Yokosuka et al. 2001; Daniels et al. 2002). In contrast, several studies have suggested a minor role of the Y5 receptor in feeding behaviour. First, the Y5 subtype is expressed at low levels in the hypothalamus (Widdowson et al. 1997b; Dumont et al. 1998; Naveilhan et al. 1998). Second, injection of a highly selective Y5 antagonist fails to inhibit NPY-induced feeding and has no effect on food intake when it is chronically administered (Kanatani et al. 2000b; Turnbull et al. 2002)—this contrasts with the effects of other Y5 antagonists that have affinity for receptors that do not belong to the Y family (DellaZuana et al. 2001). Moreover, NPY-induced feeding is markedly reduced in Y1-knockout mice, whereas it is not altered in Y5-knockout mice (Kanatani et al. 2000a), although others have noted that these differences are limited to low doses of NPY (Marsh et al. 1998). Since administration of Y5 agonists induces food intake in Y5-knockout mice, it would seem that either these compounds exhibit some agonist activity at Y1 receptor, or there exists yet another (novel) receptor subtype (Kanatani et al. 2000a). Studies using different NPY analogues support the latter hypothesis (O'Shea et al. 1997; Dumont et al. 2005). Overlapping of the gene structure of the Y1 and Y5 receptors suggests an exclusion of the co-expression of the two genes in certain cell types; coordinate expression might be important for NPY activity (Herzog et al. 1997). This might explain some differences between studies using animals with different genetic backgrounds.
(e) Feeding factors influencing endogenous NPY
(i) Fasting and associated changes in blood parameters
The most important factor that influences the hypothalamic content of NPY is food deprivation. When rats are deprived of food for 24–96 hours, NPY content and expression are markedly increased in the ARC nucleus as shown by numerous studies (Sahu et al. 1988c; Beck et al. 1990e; Brady et al. 1990; Sanacora et al. 1990; White & Kershaw 1990; Chua et al. 1991a; O'Shea & Gundlach 1991; Marks et al. 1992; Lewis et al. 1993; Schwartz et al. 1993; Davies & Marks 1994). Chronic food restriction induces similar changes (McShane et al. 1993; White et al. 1994b; Kim et al. 1998; McShane et al. 1999), but in addition increases NPY expression in the hindbrain which is not observed after fasting (Chua et al. 1991a; Ishizaki et al. 2003). Parallel increases in NPY content are observed in the PVN after fasting (Sahu et al. 1988c; Calza et al. 1989; Beck et al. 1990e). Refeeding rapidly returns the abundance of NPY in the ARC to initial values (Sahu et al. 1988c; Beck et al. 1990e; Davies & Marks 1994). In the PVN, NPY remains elevated after 6 hour refeeding, which may be related to the fact that body weight of the rats has not returned to normal in this period (Beck et al. 1990e); normalization is, however, achieved after 24 hours.
The changes in NPY content are also reflected in measurements of NPY release both in vitro and in vivo using the push-pull (PP) cannula technique. In vitro, NPY release from microdissected PVN of fasted rats is higher than that measured from ad libitum fed rats (Dube et al. 1992). In vivo, the PP technique allows measurements of peptide in the extracellular space in specific brain regions. Fasting is associated with increased NPY release in the PVN and food ingestion induces a diminution in NPY release (Kalra et al. 1991b; Stricker-Krongrad et al. 1993; Lambert et al. 1994). A similar situation exists in rats used to a daily scheduled feeding for a few hours. Thus, NPY release and hypothalamic expression are enhanced in advance of scheduled feeding time and food ingestion rapidly decreases NPY release in the PVN (Sahu et al. 1992; Yoshihara et al. 1996). The NPY release in response to hyper-osmotic KCl is also associated with feeding episodes (Stricker-Krongrad et al. 1993). All these experiments provide support for the idea of a primary role of the ARC–PVN axis in the regulation of food intake by NPY (cf. figure 1) and a modulatory role of the brainstem in this regulation.
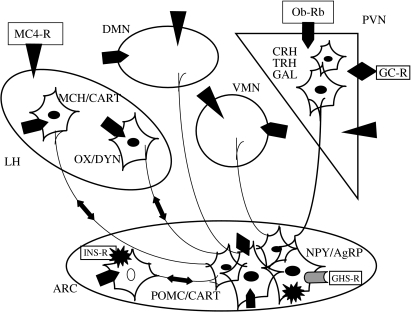
Figure 1.
The hypothalamic neuropeptide Y (NPY) system. Names of the areas involved in the system are indicated in italics: ARC, arcuate nucleus; PVN, paraventricular nucleus; LH, lateral hypothalamus; VMN, ventromedial nucleus; DMN, dorsomedial nucleus. The location of specific receptors is shown. The different receptors are identified once in an area by a sign and the receptor name tied to this sign (e.g. black triangle for MC4-R, black explosion for insulin, diamond for glucocorticoids, etc.). In the other nuclei, their presence is indicated by signs only. Ob-Rb, long form of the leptin receptor; MC4-R, type 4 melanocortin receptor; GHS-R, growth hormone secretagogue (ghrelin) receptor; INS-R, insulin receptor; GC-R, glucocorticoid receptor. The names of the neuronal populations connected to NPY neurons in the ARC are given in small capitals: POMC, proopiomelanocortin; CART, cocaine and amphetamine-related peptide; DYN, dynorphin; GAL, galanin; CRH, corticotropin-releasing hormone; MCH, melanin concentrating hormone; OX, orexins; TRH, thyrotropin-releasing hormone. Agouti-related peptide (AgRP) is co-localized with NPY (for more details, see Beck 2005).
Diurnal variations in hypothalamic NPY in the rat are consistent with this view, as NPY content peaks one hour before dark onset (Jhanwar-Uniyal et al. 1990) in the PVN and decreases one hour after lights off. There is also a pre-dark period rise in NPY mRNA in the mediobasal hypothalamus, including the ARC nucleus (Akabayashi et al. 1994a). Light transition is a triggering signal for food intake and the beginning of the dark period is characterized by a greater intake of carbohydrates when the rats have to choose between the three macronutrients (Tempel et al. 1989). Food ingested during the first few hours of darkness is associated with a peak in NPY release in the PVN (Stricker-Krongrad et al. 1997) and a sharp decrease in NPY mRNA (Akabayashi et al. 1994a). When NPY is absent, as in the NPY knockout mice, intake during the first four hours of the dark period is reduced by one-third (Sindelar et al. 2005).
Fasting leads to changes in numerous plasma parameters. A decrease in blood glucose concentrations is one of these, and diminished glucose availability to the brain could be a factor in NPY regulation. Glucose-sensitive neurons that contain NPY are present in the ARC nucleus (Muroya et al. 1999), and may have an integrative role in the regulation of energy balance (Wang et al. 2004). After induction of a moderate hypoglycaemia by insulin, the feeding response is much larger in normal mice than in mice lacking NPY (Sindelar et al. 2004). Glucoprivation induced by injection of the metabolic blocker, 2-deoxyglucose (2DG) also induces feeding (Smith & Epstein 1969). It is associated with the activation of hypothalamic NPY neurons (Minami et al. 1995) and increased NPY expression and content in the ARC (Akabayashi et al. 1993; Giraudo et al. 1998; Sergeyev et al. 2000) and in the hindbrain (Li & Ritter 2004). Moreover, 2DG-induced feeding is attenuated by injection of NPY antibodies in the PVN (He et al. 1998), and by deletion of the NPY gene (Sindelar et al. 2004).
Further evidence for the importance of glucose availability comes from experiments in which blood glucose concentrations are increased. After an intravenous (IV) injection of glucose, the stimulation of food intake by NPY is less that after an IV injection of saline (Rowland 1988). This is not observed after fructose injection probably because fructose is not metabolized by the brain. Intraperitoneal injection of 10% glucose solution 3 hours before onset of the dark period induces a diminution in NPY expression during the first hour after dark (Chang et al. 2005). A similar result is obtained after injection of microlitre volumes of a 5% glucose solution in the third ventricle.
Changes in food intake also influence plasma insulin concentrations. The latter are diminished by fasting, and can also be suppressed by administration of streptozotocin (STZ) to destroy insulin-secreting pancreatic beta-cells. Streptozotocin-induced diabetic rats are hyperphagic and less sensitive to NPY (Morgan et al. 1996), while the abundance of NPY, and/or NPY expression and release are markedly increased in the hypothalamus and particularly in the ARC–PVN (Williams et al. 1988, 1989; Sahu et al. 1990; White et al. 1990; Abe et al. 1991; McKibbin et al. 1992; Marks et al. 1993; Lambert et al. 1994; Frankish et al. 1995; Morris & Pavia 2004). These increases occur before the onset of hyperphagia (Sahu et al. 1997). Moreover, NPY receptor number is diminished in STZ rats (Frankish et al. 1993). NPY expression is normalized after insulin treatment. In addition, insulin administered peripherally or centrally attenuates the fasting-induced NPY increase in the ARC (Malabu et al. 1992; Schwartz et al. 1992). Furthermore, hyperphagia is not observed in STZ-treated NPY-deficient mice (Sindelar et al. 2002).
Other relevant factors modified by fasting include glucocorticoids and leptin. Leptin receptors are present in NPY neurons in the ARC nucleus (Mercer et al. 1996a). Expression in adipose tissue and plasma concentrations of leptin are decreased by fasting (Saladin et al. 1995; Hardie et al. 1996), which leads to upregulation of the NPY system with an increased mRNA expression and peptide production in the ARC and an increased release of NPY in the PVN. On the other hand, chronic intracerebroventricular (ICV) administration of NPY augments leptin mRNA in the adipose tissue (Sainsbury et al. 1996). The loop existing between leptin and NPY has been discussed in detail in several recent reviews (Ahima & Flier 2000; Woods & Seeley 2000; Porte et al. 2002; Kalra & Kalra 2003; Sahu 2003; Woods et al. 2006).
Glucocorticoids are involved in body weight regulation (Dallman et al. 2004). There are discrepancies in the published literature on the relation between NPY and glucocorticoids. Glucocorticoid receptors are found on almost all NPY neurons in the ARC (Ceccatelli et al. 1989), and there is evidence of a positive association between NPY and glucocorticoids (Akabayashi et al. 1994a; Tempel & Leibowitz 1994; Wang et al. 1998a, 1999). Thus, adrenalectomy (ADX) has been reported to be associated with a decrease (Pralong et al. 1993; Akabayashi et al. 1994b; Watanabe et al. 1995; Savontaus et al. 2002) or no change (Ponsalle et al. 1992; Larsen et al. 1994; Baker & Herkenham 1995) in NPY mRNA abundance and/or peptide content. Dexamethasone treatment or corticosterone replacement after ADX upregulates, or restores to normal, NPY expression (Corder et al. 1988; Larsen et al. 1994; White et al. 1994a). NPY-induced food intake is diminished in ADX rats and restored by corticosterone treatment or co-injection with dexamethasone (Stanley et al. 1989b; Zakrzewska et al. 1999). Glucocorticoid receptor inactivation produces lean animals. It is associated with decreased food intake and, perhaps as a compensatory mechanism, with increased NPY levels (Kellendonk et al. 2002). During fasting, corticosterone levels increase before the upregulation of NPY mRNA (Dallman et al. 1999). Some authors claim that the presence of glucocorticoids is necessary for the upregulation of NPY (Ponsalle et al. 1992), whereas others find that elevated corticosterone is not required for the fasting-induced increase of NPY (Hanson et al. 1997; Savontaus et al. 2002).
(ii) Influence of diet composition
In addition to energy intake per se, macronutrient composition of the diet also influences NPY abundance in the hypothalamus. Concentration in the parvocellular part of the PVN is lower after two weeks of ingestion of a HC diet than after the ingestion of a HF diet (Beck et al. 1990d). A significant negative correlation exists between NPY concentrations and the carbohydrate-to-fat ratio in the diet (Beck et al. 1992c). When rats have a choice between HC and HF diets, their choice is reflected in PVN NPY concentrations (Beck et al. 1992c). Over longer periods (two months), when the rats are fed from weaning with an obesogenic diet, e.g. a high-starch diet associated with a 25% sucrose solution to drink, the hyperphagia present at the end of the experiment is associated with a significant decrease of NPY content in the ARC nucleus. A further decrease is noted in the ARC as well as in the PVN in normophagic, but obese rats fed a HF diet (Beck et al. 1994a). These changes can be considered as part of a counter-regulatory mechanism necessary for limiting over-consumption of food as well as fat accretion.
These findings are consistent with data obtained in rats exhibiting marked dietary preferences (Beck et al. 2001a). When Long–Evans rats can choose between a HC and a HF diet, food choice among individuals follows a Gaussian distribution with the majority of the animals having no or minor dietary preferences. At each end of the distribution, there are rats showing a clear preference for either carbohydrates or fats. The former are characterized by lower NPY concentrations in the parvocellular part of the PVN (Beck et al. 2001a) in the basal state. In feeding periods, such as the beginning of the dark period, carbohydrate-preferring rats have higher hypothalamic NPY concentrations (Jhanwar-Uniyal et al. 1993). During this period, there is also a spontaneous preferential consumption of carbohydrates (Tempel et al. 1989). In relation to these data, it seems likely that the amplitude of NPY variations rather than the absolute content better characterizes preference for macronutrients. Each dietary preference presumably reflects the result of a balance (Beck et al. 2001a) between the actions of NPY and those of other hormones and neuropeptides.
Macronutrient composition has also an impact on the NPY system during development. In the hypothalamus, NPY neurons in the ARC are already present during the last week of gestation and the maturation of the system goes on postnatally during the suckling period (Kagotani et al. 1989; Bouret et al. 2004). The offspring of dams fed on unbalanced diets (HF or HC) during these periods have persistent alterations in the functioning of the NPY system in adulthood (Kozak et al. 1998, 2000, 2005). The changes in sensitivity to NPY injection and an increased peptide release after a glucoprivic challenge are associated with delays in the establishment of the dietary preferences (Kozak et al. 2000, 2005). Under- and over-nutrition during early life or gestational diabetes also strongly influence the NPY system by boosting it whatever the type of the adverse condition (Plagemann et al. 1999a,b, 2000; Lopez et al. 2005). All these data support an important effect of nutritional factors on NPY.
(iii) Interaction with other modulators of feeding
Macronutrient composition not only influences the energy content of diets, but is also an essential element in determining palatability. By manipulating it, it is possible to study the hedonic/reward aspects of food. Two systems, the opioid system and the cannabinoid system have been linked to these aspects of feeding behaviour (Glass et al. 1999; Harrold & Williams 2003; Di Marzo & Matias 2005). Both systems operate in areas such as nucleus accumbens and hypothalamus (Harrold et al. 2002; Bodnar 2004) and in the hippocampus, which is also linked to food reward (Tracy et al. 2001). Owing to the widespread distribution of NPY in the brain (Everitt et al. 1984; Chronwall et al. 1985) and particularly in these areas, and as NPY is sensitive to diet composition and modulates motivation for food ingestion, relationships among the three systems appear plausible. In support of this, it has recently been demonstrated that NPY release from rat hypothalamic explants in vitro is stimulated by the cannabinoid agonist, anandamide, and inhibited by the antagonist AM 251 (Gamber et al. 2005). Furthermore, NPY-induced feeding can be strongly decreased with pre-treatment of rats with different (mu, kappa and delta) opioid receptor antagonists (Israel et al. 2005).
Finally, the NPY system interacts with a number of other orexigenic systems (reviewed in Beck 2005). These include the melanocortin system which is also represented in the ARC nucleus. Both systems interact through axons and axonal terminals that form synaptic contacts on each type of neurons (Csiffary et al. 1990). Y1 receptors have also been detected on neurons positive for melanocortin type 4 receptor (MC4-R) in the PVN (Kishi et al. 2005). The precise relations between the two systems are detailed in recent interesting reviews (Seeley et al. 2004; Cone 2005). NPY neurons in the ARC are connected with another orexigenic system: the orexin neurons in the lateral hypothalamus (Elias et al. 1998; Horvath et al. 1999). Orexins are sensitive to fat ingestion (Park et al. 2004) and there are orexin receptors on NPY neurons (Funahashi et al. 2003). These connections may link feeding with the wake/sleep cycle (Willie et al. 2001). A recently discovered peptide, ghrelin, also interacts with the NPY system. Ghrelin is the only peripheral peptide that stimulates food intake (Nakazato et al. 2001). It is also linked to diet composition, as it preferentially stimulates fat intake and conversely fat ingestion decreases both expression and content in the stomach as well as circulating levels (Beck et al. 2002; Moesgaard et al. 2004; Shimbara et al. 2004). Anatomical and functional relationships have been established with the NPY system. Ghrelin is produced in abundance in the stomach, but also at lower levels in the hypothalamus (Horvath et al. 2001; Beck et al. 2003). The growth hormone secretagogue receptors that bind ghrelin are expressed by ARC NPY-expressing neurons (Willesen et al. 1999). Hypothalamic ghrelin neurons send efferent projections on ARC NPY neurons (Cowley et al. 2003). Peripheral as well as central injections of ghrelin activate NPY neurons (Hewson & Dickson 2000; Kamegai et al. 2001; Cowley et al. 2003). In addition, the orexigenic effects of ghrelin are markedly reduced by pre-treatment with a Y1 receptor antagonist (Shintani et al. 2001). The ghrelin–NPY pathway is one of the links between the gastrointestinal tract and the brain (Kojima & Kangawa 2005).
In summary, in normal conditions, NPY strongly stimulates food intake, even in satiated rats, when it is administered intraventricularly, or directly to the PVN, VMN or perifornical area. It acts on multiple components of the microstructure of feeding (decreased latency to eat, increased meal size and duration). It not only augments the quantity of ingested food, but also influences food choice by stimulating a preferential intake of carbohydrates. Its orexigenic effects are mediated by at least two receptors, the Y1 and Y5 receptors. A negative energy balance with its associated hormonal and metabolic changes is the main factor activating the NPY system. On the other hand, diet composition can influence the hypothalamic abundance of NPY mainly in the PVN. Carbohydrate-rich diets influence NPY in the PVN over both short and long terms, whereas fat-rich diets have predominantly long-term influences. Dietary preference for either carbohydrate or fat is associated with NPY status in the PVN. The NPY system is in close interaction with other brain modulators (including orexins, ghrelin, glucocorticoids) to adapt feeding behaviour to the individual needs and situations.
3. NPY in genetic models of obesity
The identification of leptin and its receptor in the mid-1990s is a pivotal point in studies of genetic models of obesity (Zhang et al. 1994; Tartaglia et al. 1995). It is now clear that leptin is a primary factor regulating body weight (Ahima & Flier 2000) and that genetic models of obesity may include faults in leptin signalling as well as other mediators.
(a) Models with a leptin-signalling failure
The Zucker fa/fa rat, the db/db mouse and the ob/ob mouse are among the most widely used rodent models of obesity (Bray 1977; Argiles 1989; Robinson et al. 2000). Zucker (fa/fa) rats become obese due to a missense mutation in the leptin receptor gene, which diminishes but does not completely eliminate responsiveness to leptin (Chua et al. 1996a,b; Iida et al. 1996; Phillips et al. 1996). The obese Zucker does not respond to ICV injections of leptin (Seeley et al. 1996), except at very high doses (Dryden et al. 1999), and leptin inhibits a much lower proportion of the ARC neurons (Nagamori et al. 2003).
The mouse homologue of the Zucker rat is the db/db mouse (Truett et al. 1991; Chua et al. 1996a). The obese cp/cp JCR : LA corpulent rat and the Koletsky rat are other models with mutations in the leptin receptor genes (Takaya et al. 1996; Kahle et al. 1997). However, the nonsense mutation in the Koletsky rat results in a premature stop codon in the extracellular domain of the leptin receptor gene. All isoforms of the receptor are affected (Takaya et al. 1996; Wu-Peng et al. 1997), leading to a total insensitivity of the Koletsky rat to exogenous leptin (Wildman et al. 2000).
In the obese ob/ob mouse, a nonsense mutation in the coding region of the leptin gene prevents the production of a functional peptide (Zhang et al. 1994). However, when leptin is injected ICV in ob/ob mice, it regulates feeding behaviour (Schwartz et al. 1996; Smith et al. 1998), proving that receptor mechanisms are functional. It is worth noting that all these models share with human obesity the characteristics of hyperphagia, hypertriglyceridemia, hyperleptinemia and hyperinsulinemia (Bray 1977; Argiles 1989; Williams et al. 1992; Hiraoka et al. 1997; Robinson et al. 2000; Keen-Rhinehart et al. 2004a).
(i) The obese Zucker rat
In the obese Zucker rat, the central NPY system is profoundly altered. This dysregulation was described several years before the discovery of leptin, and this was thought at the time to be the primary cause for the development of obesity. The NPY changes in the brain are site-specific. Thus, while studies of blocks of hypothalamic tissue failed to detect differences in NPY content between lean and obese phenotypes (Williams et al. 1991a), microdissection of individual hypothalamic nuclei revealed significantly higher NPY concentrations in the PVN and ARC nuclei of obese rats (Beck et al. 1990b,c, 1993; McKibbin et al. 1991). Parallel studies have demonstrated that the mRNA encoding NPY is also increased in abundance in the ARC nucleus of the fa/fa rat (Sanacora et al. 1990; Mercer et al. 1996b), suggesting that with respect to NPY expression obese rats appear to be constantly in a deprivation state. Leptin-receptor gene transfer into the ARC nucleus of female fatty Zucker rats induced a diminution of NPY mRNA abundance in this nucleus (Keen-Rhinehart et al. 2004b), supporting the site specificity of changes in the NPY system. The hyperphagia of Zucker rats might also relate to the GABAergic system since NPY inhibits the firing of GABA (gamma amino butyric acid) neurons in the ARC and PVN (Pronchuk & Colmers 2004; Acuna-Goycolea et al. 2005). Moreover, muscimol, a GABA agonist stimulates food intake when it is injected into hypothalamic nuclei (Kelly et al. 1979). Interestingly, the obese rat has a similar feeding response to lean rats after injection of muscimol in the PVN (Tsujii & Bray 1991), but NPY is less effective at PVN GABA synapses in obese compared with lean Zucker rats (Pronchuk & Colmers 2004). NPY could therefore modulate feeding through this pathway.
The main metabolic changes are exhibited very early (three to five weeks of age) in the life of these animals (Krief & Bazin 1991). The NPY increases are also apparent early in life. In terms of content and expression, the increase is significant 30 days after birth when weaned rats begin to overeat (Bchini-Hooft van Huijsduijnen et al. 1993; Beck et al. 1993). These increases are still evident later in life up to 40 weeks of age (Sanacora et al. 1992; Bchini-Hooft van Huijsduijnen et al. 1993; Jhanwar-Uniyal & Chua 1993). New molecular genetic methods (Smoller et al. 1993; Chua et al. 1996b) have allowed studies of the fa genotype at earlier stages than more classic approaches, such as oxygen consumption or inguinal fat pad weights (Kaplan 1979; Lavau & Bazin 1982). They reveal that NPY mRNA abundance in the ARC nucleus of fa/fa pups is increased as early as post-natal day 9 (Kowalski et al. 1999), whereas hypothalamic concentration is decreased (Ster et al. 2003), consistent with an augmented NPY release. However, it is difficult to link food intake and NPY changes before weaning, as discordant results have been reported concerning the onset of hyperphagia during this period (Buchberger & Schmidt 1996; Kowalski et al. 1998). This discrepancy may simply be related to the method of determination of food intake in the pups: one study in which suckling and pup weight were measured found no increase, whereas another examining ingestion independent of the dam found that hyperphagia emerges between post-natal days 9 and 12. Nonetheless, during this early period, NPY could contribute to fat deposition by its metabolic and antithermogenic effects (Godbole et al. 1978).
Early increases in NPY content and expression in the Zucker fatty rat have physiological consequences for NPY receptor function. First, receptor-binding studies have shown that the hypothalamic receptor density is reduced in obese rats (McCarthy et al. 1991). Studies employing antagonists and masking techniques were able to show that binding to Y5 receptors was likely to be reduced in obese rats (Widdowson 1997), and this was subsequently confirmed by measurement of NPY receptor expression by RT-PCR (Beck et al. 2001b). It appears that both Y1 and Y5 receptors are downregulated. The downregulation of NPY receptors leads to a decreased sensitivity of obese rats to exogenous NPY, so that higher NPY doses are required to induce the same food intake as in lean rats (McCarthy et al. 1991; Stricker-Krongrad et al. 1994), and in some cases, NPY is even unable to stimulate feeding (Brief et al. 1992). The decrease in NPY binding and receptors is, however, not sufficient to normalize food intake.
The diminution of the receptor-binding capacity in obese rats also accounts for the fact that weak antagonists or agonists are unable to modify the feeding behaviour in obese rats (Beck et al. 1994b), even if they are Y5-specific (Wyss et al. 1998a; B. Beck, unpublished observations). However, in circumstances where there is extreme overeating, a potent NPY antagonist was able to markedly diminish food intake up to 24 hours after central injection (Ishihara et al. 1998). Intracerebroventricular NPY antiserum also curtailed daily food intake and promoted weight loss in female obese Zucker rats (MarinBivens et al. 1998).
The functionality of the NPY system is also dependent on nutritional and hormonal state. Thus, in lean rats, food deprivation induces an increase in hypothalamic NPY mRNA abundance (Sanacora et al. 1990; Berelowitz et al. 1992). Peptide content is not changed even after restriction, indicating clear differences with the Sprague–Dawley rat (McKibbin et al. 1991; Beck et al. 1992a). The same situation occurs in the obese Zucker rat, although at higher levels for the peptide content (McKibbin et al. 1991; Beck et al. 1992a). The high NPY mRNA abundance in obese rats is either further increased by deprivation (Korner et al. 2001) or shows a tendency to increase (Sanacora et al. 1990; Berelowitz et al. 1992), after long (two weeks) and severe (50%) food restriction (Pesonen et al. 1992). These data suggest increased NPY synthesis, transport and release in the Zucker rat. Measurements of the in vivo NPY release in the PVN by the PP cannula technique support this hypothesis. Whereas the basal NPY efflux measured in vitro is not different between lean and obese rats (Kalra et al. 1994), the in vivo release at different times in the light/dark cycle is significantly enhanced in obese rats (Dryden et al. 1995; Stricker-Krongrad et al. 1997). Moreover, at the light-to-dark transition, which is characterized by peak concentrations in the PVN and ARC nucleus (Jhanwar-Uniyal et al. 1990) and by feeding episodes (Tempel et al. 1989), the obese rat does not show the typical peak of NPY release observed in its lean counterpart (Stricker-Krongrad et al. 1997). It seems then that NPY is released in an unsynchronized manner in the obese rat which may contribute to the disappearance of the dark/light rhythm of feeding behaviour in Zucker fa/fa rats (Becker & Grinker 1977; Alingh Prins et al. 1986).
A similar NPY profile is found in the other animal models with a leptin receptor mutation. NPY mRNA abundance is increased in db/db mice (Chua et al. 1991b; Schwartz et al. 1996; Yamamoto et al. 1999; Bates et al. 2003), and this is further augmented by food deprivation or restriction (Chua et al. 1991a; Yamamoto et al. 2000). In cp/cp rats, NPY concentrations are elevated in the ARC nucleus as in Zucker rats, although food restriction does not further enhance NPY in the ARC (Williams et al. 1992). In the Koletsky rat, there is also a large augmentation of both NPY mRNA and peptide content (Keen-Rhinehart et al. 2004a). Gene therapy which restores leptin receptor expression in this rat leads to a significant reduction in NPY mRNA expression (Morton et al. 2003), adding weight to view that the leptin receptor is important in NPY regulation. Moreover, in keeping with this idea, deletion of the leptin receptor in mice induces an increase in NPY mRNA expression and obesity (Cohen et al. 2001).
Chronic NPY infusion in brain ventricles of normal rats through osmotic minipumps mimics the constant elevated central NPY levels measured in the obese Zucker rat and Koletsky rat, and induces hyperphagia, disrupts nycthemeral feeding rhythms and leads to increased body weight and adiposity (Beck et al. 1990a, 1992b) as well as numerous other hormonal and metabolic changes, including elevated plasma insulin, leptin and corticosterone concentrations (McMinn et al. 1998; Raposinho et al. 2001; Baran et al. 2002). Adrenalectomy prevents the deleterious effects induced by chronic NPY administration (Sainsbury et al. 1997). In contrast to Zucker rats, however, NPY mRNA abundance is reduced by overfeeding in NPY-injected Long–Evans rats, which might be related to the high concentrations of insulin and active leptin in these rats (McMinn et al. 1998; Raposinho et al. 2001) unlike obese fa/fa rats (Schwartz et al. 1991). It should also be noted that NPY effects diminish after 9–10 days of infusion, reflecting a possible downregulation of receptors. This extinction of the effects is not observed in overweight rats after repeated (three times per day) injection of NPY (Stanley et al. 1989a) that allows a return to basal state between injections. Similar effects on body weight, adiposity and respiratory quotient are observed after chronic infusion for 6 days of Y1 and Y5 agonists (Mashiko et al. 2003; Henry et al. 2005), thereby confirming the role of both receptors in this system.
(ii) The obese ob/ob mouse
The hypothalamic NPY status of obese mice is somewhat comparable to that of the Zucker fa/fa rat. Hypothalamic NPY gene expression is upregulated (Wilding et al. 1993; Schwartz et al. 1996; Mercer et al. 1997; Stricker-Krongrad et al. 2002) and peptide content in the ARC nucleus is significantly increased (Jang & Romsos 1998), although no change in the peptide concentrations are noted in either the whole hypothalamus or the PVN in the fed state (Williams et al. 1991b; Jang & Romsos 1998; Stricker-Krongrad et al. 2002). Peptide content is neither modified by food deprivation nor by refeeding in obese mice unlike their lean counterparts (Jang & Romsos 1998). There is a downregulation of the Y5 receptor level (Xin & Huang 1998), but no change in the feeding response to ICV NPY (Morley et al. 1987b; Walker & Romsos 1993). These data are consistent with an increased transport and release of NPY. The importance of NPY in ob/ob mice is furthermore demonstrated by the attenuation of the obese phenotype in the ob/ob mouse crossed with NPY null mice (Erickson et al. 1996; Marsh et al. 1998). However, the ob/ob phenotype is unlikely to be mediated by NPY signalling through Y5 receptors because Y5 receptor-deficient ob/ob mice are equally obese (Marsh et al. 1998). Delivery of biologically active leptin through gene therapy in ob/ob mice induces a decrease of NPY expression in the ARC associated with body weight loss and diminished food intake (Dhillon et al. 2000).
(b) Other genetic models
(i) The Otsuka–Long–Evans–Tokushima Fatty rat
The Otsuka–Long–Evans–Tokushima Fatty (OLETF) rat is characterized by a lack of functional cholecystokinin (CCK-A) receptors (Miyasaka et al. 1994; Takiguchi et al. 1998; Moran & Bi 2006). The OLETF rats have therefore a peripheral satiety deficit that results in increased meal size and overeating (Moran et al. 1998), together with decreased responsiveness to ingested fat (Schwartz et al. 1999) and increased preference for sucrose (DeJonghe et al. 2005). It is moderately obese and diabetic and at least in part decreased physical activity favours weight gain (Sei et al. 1999). Defects in CCK-1 receptor signalling appear to be the major cause of hyperphagia in this rat. But it should be noted that a similar phenotype is not found in CCK-1 receptor null mice which are normoglycaemic and have a normal body weight (Kopin et al. 1999). This suggests the existence of either species differences or additional mutations independent of CCK in the OLETF rat. A very recent paper has described a mutated GPCR10 receptor in OLETF rats as responsible for its hyperphagia (Watanabe et al. 2005) even if the ligand of this receptor, prolactin-releasing peptide, is a poor mediator of food intake (Beck et al. 2004).
The NPY status in the OLETF rat is interesting. NPY mRNA expression is decreased in the ARC nucleus, whereas it is increased in the dorsomedial hypothalamus (DMH). The latter over-expression is already evident at five-week-old preobese rats (Bi et al. 2001). Food deprivation does not modify NPY mRNA in either area (Bi & Moran 2003), but exercise induces an increase in the ARC nucleus only (Bi et al. 2005). The OLETF rat is more sensitive to NPY injection than its lean control (Moran et al. 2002). This is in accordance with the observations made in rats with a NPY decrease in the ARC–PVN axis (Sahu et al. 1988a; Stricker-Krongrad et al. 1996). The role of NPY in the DMH remains to be clarified.
(ii) The yellow agouti mouse
The yellow agouti Ay mouse is another well-studied model of obesity. The product of the agouti gene interacts with α-melanocyte stimulating hormone (α-MSH) at the level of melanocortin type 1 receptors to determine the coat colour of the animals. The agouti gene is expressed throughout the body of the animals. In the hypothalamus, its product, AgRP, interacts with α-MSH at MC4-R. This leads to the development of obesity associated with moderate hyperphagia and decreased thermogenesis (Moustaïd Moussa & Claycombe 1999). Feeding behaviour of the obese agouti mouse is characterized by a durable preference for fat (Koegler et al. 1999). Over-expression of AgRP in transgenic mice results in the same syndrome (Graham et al. 1997) as well as the genetic deletion of the MC4-R (Huszar et al. 1997) and the long-term blockage of the MC4-R through HS014, a specific antagonist (Kask et al. 1999). The abundance of NPY mRNA in the ARC nucleus is similar to that in the lean mice, but NPY is expressed at high levels in the DMN nucleus (Kesterson et al. 1997). The role of NPY in this model remains to be fully explored since AY-NPY knockout mice exhibit greater food intake and body weight then Ay mice (Hollopeter et al. 1998).
(iii) The fat and tubby mice
In contrast to the yellow agouti mouse, the other obesity mutations in mouse models are recessive. The tubby mouse is characterized by an autosomal recessive mutation, which results in maturity-onset obesity (Coleman & Eicher 1990). The tub gene is well expressed in the brain (Kleyn et al. 1996; Nobentrauth et al. 1996). Its deletion is sufficient to give rise to the full spectrum of the tubby phenotype (Stubdal et al. 2000). In old mice, when obesity is present, NPY mRNA abundance is increased in the DMN and VMN nuclei and diminished in the ARC nucleus (Guan et al. 1998). The number of NPY-immunoreactive fibres is also decreased in the ARC (Backberg et al. 2004). It is unlikely that the decreased abundance of NPY mRNA in the ARC has an impact on the development of the obese phenotype because NPY mRNA is also decreased in the ARC in young non-obese animals. Thus the downregulation of the proopiomelanocortin (POMC) mRNA in the ARC (Guan et al. 1998) is thought to be more important in the development of the obese tub phenotype.
The fat mutation maps to the gene encoding carboxypeptidase E (CPE). CPE is widely distributed in the brain and is involved in the processing of peptides by selective cleavage at sites containing basic amino acids (Fricker & Leiter 1999). The CPE (fat) mouse has extremely low levels of CPE which together with other carboxypeptidases apparently are evidently sufficient for viability. The fat mouse only develops late-onset overeating and its obesity might therefore be related to defects in nutrient partitioning (Leiter et al. 1999). To our knowledge, the NPY status of this mouse is not known even though changes in some other regulatory peptides, such as thyrotropin-releasing hormone, neurotensin, CCK8, POMC, opioids or melanin-concentrating hormone, have been described (Rovere et al. 1996; Wang et al. 1998b; Shen et al. 1999; Boudarine et al. 2002; Nillni et al. 2002). AgRP which is co-localized with NPY in the ARC is not modified (Wilson et al. 1999).
In summary (cf. table 1), the animal models of obesity with a deficiency in leptin signalling are characterized by an upregulation of the NPY system which contributes to their hyperphagia and excessive weight gain. The other genetic models of obesity present different and distinctive pictures with respect to NPY status. They are indeed characterized by a diminution in NPY expression in the ARC nucleus and by a significant increase in the DMN. A similar profile is also described in the obese hyperphagic UCP-DTA mice (Tritos et al. 1998). ARC, PVN and DMN belong to complex pathways involved in the control of food intake. NPY neurons in the ARC nucleus project their axons towards the DMH and PVN (Bai et al. 1985) and DMN neurons directly innervate the PVN (Thompson et al. 1996). NPY neurons in the DMH are also differentially regulated as they do not co-express leptin receptors (Bi et al. 2003) unlike NPY neurons in the ARC (Mercer et al. 1996a). In the other genetic models, an NPY increase in the DMH might therefore be a compensatory mechanism for the ARC deficiency in order to maintain adequate NPY levels in the PVN for the feeding regulation. Measurement of NPY release in the PVN could indicate if the peptide has a real importance in the hyperphagia of OLETF rats, agouti and tubby mice.
Table 1.
Characteristics of the hypothalamic NPY system in some genetic animal models. (ARC, arcuate nucleus; PVN, paraventricular rat; OLETF rat, Otsuka–Long–Evans–Tokushima Fatty rat; CPE, carboxypeptidase E; n.d., not described to our knowledge.)
| model | food intake | ARC content | ARC mRNA expression | Y1/Y5 receptors | PVN release |
|---|---|---|---|---|---|
| mutation at: | |||||
| leptin receptor gene | |||||
| Zucker fa/fa rat | ↑++ | ↑+++ | ↑+++ | Y1:↓/Y5:↓+ | ↑++ |
| db/db mouse | ↑++ | ↑++ | ↑+++ | n.d. | n.d. |
| cp/cp JCR : LA rat | ↑++ | ↑++ | ↑++ | n.d. | n.d. |
| Koletsky rat | ↑++ | ↑++ | ↑++ | n.d. | n.d. |
| leptin gene | |||||
| ob/ob mouse | ↑++ | ↑ or → | ↑+++ | Y5:↓+ | n.d. |
| CCK-A receptor | |||||
| OLETF rat | ↑++ | n.d. | ↓ | n.d. | n.d. |
| agouti gene | |||||
| yellow Ay mouse | ↑ | n.d. | → | n.d. | n.d. |
| tub gene | |||||
| tubby mouse | ↓ | n.d. | ↓ | n.d. | n.d. |
| CPE gene | |||||
| fat mouse | ↑late | n.d | n.d | n.d | n.d |
4. NPY in diet-induced obesity (DIO)
Single gene mutations at the level of either the leptin gene or the NPY gene are very rare in human obesity (Hung et al. 2004; Farooqi & O'Rahilly 2005). Obesity is for the most part a polygenic disease (Perusse et al. 2005) and the environment, especially the nutritional conditions, in which genes function is particularly important. A plethora of studies have shown that obesity can be induced by ingestion of unbalanced diets either HF, HC or high-energy (HE) palatable (‘cafeteria’) diets (Rothwell & Stock 1979; Louis-Sylvestre et al. 1984; Sclafani 1987; Warwick & Schiffman 1992). The fat content is a primary factor for the development of metabolic syndrome (Ghibaudi et al. 2002), and a 40–45% fat diet is already obesogenic as shown in multiple studies described below. The nature of the fat source is important and lower effects on weight gain are measured after a diet containing 32% fish oil than after the same diet containing either maize oil or beef tallow (Jang et al. 2003).
Several approaches have been used to study the relation between NPY and dietary-induced obesity. One approach consists in feeding animals with unbalanced diets (mostly HF diets) and then following peptide variations in parallel with body weight, adiposity and metabolic factors. A second depends on the selection of individuals with a susceptibility to obesity and their comparison with individuals resistant to obesity. A third is similar but uses rodent strains either resistant or sensitive to HF diets instead of individuals of the same strain.
As already indicated, hypothalamic NPY is sensitive to diet composition. In rats fed either HC or HF diets, it varies differentially according to the duration of the dietary exposure. There is a decline in NPY levels after short (two weeks) and long (two months) periods of ingestion of carbohydrate-rich diets by Long–Evans rats (Beck et al. 1990d, 1992c, 1994a). A transient decrease in NPY mRNA is noted after 2 days of ingestion of a HF diet (Ziotopoulou et al. 2000). During these 2 days, the rats are hyperphagic. After one or two weeks, NPY expression or content are unchanged or return to baseline in HF rats (Beck et al. 1992c; Lin et al. 2000b; Ziotopoulou et al. 2000; Hansen et al. 2004; Tabb et al. 2004), except when the HF diet consists of highly saturated fat (Wang et al. 2002) or when the fat content is increased to 77% (Giraudo et al. 1994). An absence of change in NPY mRNA expression is also noted after one week of a high-palatable (fat and sweet) diet (Kim et al. 1998). A significant decrease is once again observed after 8–10 weeks in HF diet-fed rats when the fat content is 45% or greater than 60% (Beck et al. 1994a; Lin et al. 2000b). The reduction in mRNA abundance is probably related to the substantial (more than 200%) increase in circulating leptin concentrations at eight weeks and the progressive development of leptin insensitivity with age (Qian et al. 1998). After 12 weeks, a decrease in NPY overflow from hypothalamic slices is observed (Hansen et al. 2004). An upregulation of Y5 receptors in adult rats fed for six weeks on HE diet is consistent with a diminished NPY release (Widdowson et al. 1997a). At 16–17 weeks, NPY concentration is diminished in HF fed rats in the PVN (Hansen et al. 2004; Velkoska et al. 2005) and in the ARC with a 30% fat diet (Hansen et al. 2004). At 19 weeks, however, when central leptin resistance is well established, NPY mRNA expression is not further decreased (Stricker-Krongrad et al. 1998; Lin et al. 2000a). Leptin resistance is specifically located in the ARC (Munzberg et al. 2004) and the sensitivity of the leptin system appears to be an essential element in the development of obesity (Ren et al. 2005). This link with leptin resistance is further supported by the absence of effect of HF diets in the obese Zucker rat (Mercer et al. 1996b).
A comparable diminution is present in rats fed a HE palatable diet (Widdowson et al. 1999; Archer et al. 2004) but in this case somewhat earlier, e.g. after five weeks. This is probably related to the greater sensitivity of the NPY system to the sucrose content of the diet. The interaction of NPY with palatable diets is supported by the decreased intake of such a diet in NPY knockout mice (Sindelar et al. 2005).
All these dietary treatments induce increased adiposity although they do not systematically lead to increased body weight or overeating, when given early in life e.g. from weaning (Oscai et al. 1984; Beck et al. 1994a; Archer et al. 2004). All these data are consistent with the existence of a counter-regulatory mechanism mediated at least partly by depressed NPY diminishing energy intake and limiting the development of obesity in rats consuming HF or HE diets. This is not, however, sufficient to totally counteract the obesogenic drive. Reducing energy intake from HF diets can attenuate but not suppress obesity (Petro et al. 2004). Data obtained in STZ diabetic rats support the downregulation of the NPY system by HF diets. When the STZ rats are fed a HF diet, they reduce their caloric intake in association with a decrease in NPY mRNA expression in the hypothalamus (Chavez et al. 1998). An intact hepatic vagus nerve is necessary for these changes (laFleur et al. 2003).
Further support to this hypothesis is provided by the study of individuals or strains either susceptible (DIO-S) or resistant (DIO-R) to dietary obesity. The Sprague–Dawley rat strain has been widely used for obesity studies. It has been characterized by the existence of obesity-prone and obesity-resistant rats (Levin et al. 1983, 1985; Levin 2006). These rats were detected after several months of exposure to HF diets. This bimodal distribution has, however, not always been confirmed (Archer et al. 2003) perhaps because of the different origin of the rats. Therefore, in some studies (Chang et al. 1990; Gao et al. 2002; Farley et al. 2003), rats characterized as DIO-S or DIO-R are taken at each end of the body weight distribution of a large population.
The DIO-S rats are hyperphagic and their hyperphagia is due to an increased meal size (Farley et al. 2003). They have also a different energy metabolism with a lower energy expenditure and an increased respiratory quotient indicating a lower use of fat as fuel substrate favouring therefore fat storage (Chang et al. 1990; Gao et al. 2002).
Cross-breeding of high weight gaining rats over several generations reinforces their characteristics and leads to a strain of DIO-S rats that are heavier than DIO-R rats even on a low-fat (LF) diet (Levin et al. 1997, 2003; Levin & Dunn-Meynell 2002c). Obesity induced during gestation in DIO-S dams fed a HE diet further enhances obesity in offspring (Levin & Govek 1998). The DIO-S rats are characterized by higher (greater than 40%) NPY mRNA expression in the ARC in the preobese state (Levin & Dunn-Meynell 1997; Levin 1999; Gao et al. 2002) and by the absence of regulation of this expression by 48 hour fast or 50% food restriction (Levin & Dunn-Meynell 2002b; Levin et al. 2004), or insulin-induced hypoglycaemia (Tkacs & Levin 2004). These changes might be due to the early loss of sensitivity to leptin (Levin & Dunn-Meynell 2002b; Levin et al. 2004) and to a decreased responsiveness of hypothalamic neurons to glucose (Levin et al. 1998). However, when obesity is well established in 22-week-old rats, NPY mRNA expression is about 20% lower than in DIO-R rats (Levin 1999), although the NPY system becomes responsive to diet restriction and palatability (Levin 1999; Levin & Dunn-Meynell 2002a) in order to defend body weight. Leptin delivery directly in the PVN through gene therapy reverses dietary obesity and normalizes the metabolic parameters (Bagnasco et al. 2003). Exercise, which induces a lower weight gain, is not associated with any NPY change in the ARC and DMN in DIO-S rats (Levin & Dunn-Meynell 2004). However, in the case of the selected DIO-S mice, there is leptin-resistance after 22 weeks on a 40% fat diet, NPY mRNA expression is increased and the whole NPY system is disturbed as Y5 mRNA expression is 36% higher (Huang et al. 2003).
Increased activity of the NPY system has been described in other rodent strains. It is well known that some rat (Schemmel et al. 1970; Bray et al. 1987) and mouse (West et al. 1992; Kobayashi et al. 2004) strains are more susceptible to develop obesity on a HF diet. This susceptibility is often associated with an increased consumption of fat in a food choice paradigm (Smith et al. 2000). The Osborne–Mendel rat is one of these DIO-S rats. Its NPY mRNA expression is about 50% higher than in S5B/P1 rats (Schaffhauser et al. 2002). Despite this increase, Y1 and Y5 mRNA expression is raised by 16–71% with the highest augmentation measured on a LF diet (Schaffhauser et al. 2002). This is the opposite to the receptor downregulation existing in the Zucker rat (Beck et al. 2001b). This rat remains, however, responsive to central leptin injection even after five weeks on HF diet (Lin et al. 1998).
The C57BL/6J and DBA mouse strains belong to the group of DIO-S strains whereas the A/J and SWR/J mice are DIO-resistant (West et al. 1992; Surwit et al. 1995; Tortoriello et al. 2004). On a HF diet for 14 weeks, NPY mRNA expression decreases in A/J mice but not in C57BL/6J mice (Bergen et al. 1999). This is not observed after a shorter exposure to HF diet (Bullen et al. 2004). The NPY system is also upregulated in DBA/2J mice (Tortoriello et al. 2004). These central NPY changes might be related to the high circulating concentrations of leptin (Surwit et al. 1997) and the maintenance of leptin responsiveness in A/J mice (Watson et al. 2000) while C57BL/6J mice are less responsive to leptin in the fed state (Takahashi et al. 2002). Leptin responsiveness is also normal in the DIO-R SWR/J mice (Takahashi et al. 2002), leading to a suppression of NPY mRNA expression when leptin levels are elevated.
In summary, it is evident that there is an adaptation of the NPY system when normal rats are fed an energy-dense food. The diminution in hypothalamic NPY is, however, not sufficient to totally stop excessive weight gain in most cases. In DIO-S strains or individuals however, there is a clear link between the obesity of these animals and increased activity of the NPY system and disturbed receptor regulation. Decreased sensitivity to leptin appears to be a major factor explaining these changes. Other factors are, however, probably involved in the short term resistance to DIO in some strains (Bullen et al. 2004). Some of these factors can be linked to energy expenditure through the uncoupling proteins whereas some others remain unknown (Surwit et al. 1998; Bullen et al. 2004).
5. Conclusion
This review is focused on the role of NPY in feeding regulation in normal and obese animals (figure 2). Notwithstanding evidence for the importance of NPY, it is obvious that feeding results from interaction between numerous neuropeptides and neurotransmitters in the central nervous system as well as peripheral hormones (Kalra et al. 1999; Schwartz et al. 2000). NPY is implicated in the changes that occur in many obesity models (Beck 2000). The melanocortins have a particular and direct interaction with NPY (Seeley et al. 2004; Cone 2005) and there are also strong interactions with the stress hormone corticotropin-releasing hormone (Heilig 2004). All these systems exhibit plasticity in the face of changes in nutritional and environmental factors, the specific response depending on the situation and on individual sensitivity. Given the complexity of this system it is not particularly surprising to find discrepancies between studies as described in this review. Or, for that matter, between different models—for example, animals with hypothalamic lesions induced by gold thioglucose (GTG) or MSG develop obesity in the presence of lower hypothalamic NPY content and/or mRNA expression (Kerkerian & Pelletier 1986; Marks et al. 1996; Stricker-Krongrad et al. 1996; Bergen et al. 1998), and are associated with different feeding behaviours (hyperphagia in the GTG model and hypophagia in MSG-treated animals). The compensatory mechanisms that are likely to be involved in these cases are also well exemplified in knockout models (Beck 2001; Herzog 2004) and double knockout mice (Qian et al. 2002). For this reason, efficacious treatment of obesity through a specific agonist or antagonist may be difficult to achieve; instead, it may be more profitable to consider simultaneously targeting several systems—of which the NPY system is likely to be a prime component.
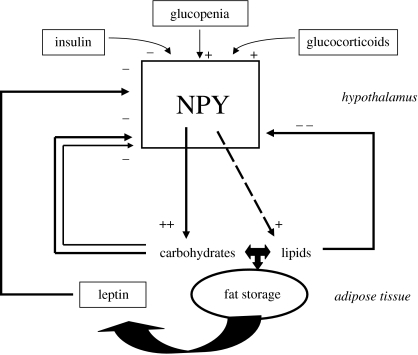
Figure 2.
Schematic of neuropeptide Y (NPY) regulation of food intake and body weight in relation to main hormones and macronutrients. Glucopenia includes hypoglycemia and cellular glucoprivation. Long-term and short-term regulation by macronutrients are indicated by thick and thin arrows, respectively.
Acknowledgments
The author wishes to thank Ms Lydie Poirson for her help during the preparation of the present manuscript. Research of the author is supported by the European Commission, Quality of Life and Management of Living Resources, Key action 1 ‘Food, nutrition and health’ programme (QLK1-2000-00515), INSERM and Institut Benjamin Delessert (Paris).
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Abe M, Saito M, Ikeda H, Shimazu T. Increased neuropeptide-Y content in the arcuato-paraventricular hypothalamic neuronal system in both insulin-dependent and non-insulin-dependent diabetic rats. Brain Res. 1991;539:223–227. doi: 10.1016/0006-8993(91)91624-a. doi:10.1016/0006-8993(91)91624-A [DOI] [PubMed] [Google Scholar]
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol A. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. doi:10.1523/JNEUROSCI.1008-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima R.S, Flier J.S. Leptin. Annu. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. doi:10.1146/annurev.physiol.62.1.413 [DOI] [PubMed] [Google Scholar]
- Akabayashi A, Zaia C.T.B.V, Silva I, Chae H.J, Leibowitz S.F. Neuropeptide-Y in the arcuate nucleus is modulated by alterations in glucose utilization. Brain Res. 1993;621:343–348. doi: 10.1016/0006-8993(93)90125-7. doi:10.1016/0006-8993(93)90125-7 [DOI] [PubMed] [Google Scholar]
- Akabayashi A, Levin N, Paez X, Alexander J.T, Leibowitz S.F. Hypothalamic neuropeptide Y and its gene expression: relation to light/dark cycle and circulating corticosterone. Mol. Cell. Neurosci. 1994a;5:210–218. doi: 10.1006/mcne.1994.1025. doi:10.1006/mcne.1994.1025 [DOI] [PubMed] [Google Scholar]
- Akabayashi A, Watanabe Y, Wahlestedt C, Mcewen B.S, Paez X, Leibowitz S.F. Hypothalamic neuropeptide Y, its gene expression and receptor activity: relation to circulating corticosterone in adrenalectomized rats. Brain Res. 1994b;665:201–212. doi: 10.1016/0006-8993(94)91339-0. doi:10.1016/0006-8993(94)91339-0 [DOI] [PubMed] [Google Scholar]
- Alingh Prins A.B, De Jong-Nagelsmit A.M, Keijser I, Strubbe J.H. Daily rhythms of feeding in the genetically obese and lean Zucker rats. Physiol. Behav. 1986;38:423–426. doi: 10.1016/0031-9384(86)90115-0. doi:10.1016/0031-9384(86)90115-0 [DOI] [PubMed] [Google Scholar]
- Allen J.M, Hughes J, Bloom S.R. Presence, distribution, and pharmacological effects of neuropeptide Y in mammalian gastrointestinal tract. Dig. Dis. Sci. 1987;32:506–512. doi: 10.1007/BF01296034. doi:10.1007/BF01296034 [DOI] [PubMed] [Google Scholar]
- Ammar A.A, Sederholm F, Saito T.R, Scheurink A.J.W, Johnson A.E, Sodersten P. NPY–leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am. J. Physiol. 2000;278:R1627–R1633. doi: 10.1152/ajpregu.2000.278.6.R1627. [DOI] [PubMed] [Google Scholar]
- Archer Z.A, Rayner D.V, Rozman J, Klingenspor M, Mercer J.G. Normal distribution of body weight gain in male Sprague–Dawley rats fed a high-energy diet. Obes. Res. 2003;11:1376–1383. doi: 10.1038/oby.2003.186. [DOI] [PubMed] [Google Scholar]
- Archer Z.A, Rayner D.V, Mercer J.G. Hypothalamic gene expression is altered in underweight but obese juvenile male Sprague–Dawley rats fed a high-energy diet. J. Nutr. 2004;134:1369–1374. doi: 10.1093/jn/134.6.1369. [DOI] [PubMed] [Google Scholar]
- Argiles J.M. The obese Zucker rat: a choice for fat metabolism. Prog. Lipid Res. 1989;28:53–66. doi: 10.1016/0163-7827(89)90007-6. doi:10.1016/0163-7827(89)90007-6 [DOI] [PubMed] [Google Scholar]
- Aubert M.L, Gruaz N.M, Pierroz D.D, Dalleves V, Sizonenko P.C. Developmental aspects of metabolic control of sexual function: role of neuropeptide Y. In: Sizonenko P.C, Aubert M.L, Vassalli J.D, editors. Developmental endocrinology. Ares-Serono Symposia Publications; Rome, Italy: 1994. pp. 147–159. [Google Scholar]
- Backberg M, Madjid N, Ogren S.O, Meister B. Down-regulated expression of agouti-related protein (AGRP) mRNA in the hypothalamic arcuate nucleus of hyperphagic and obese tub/tub mice. Mol. Brain Res. 2004;125:129–139. doi: 10.1016/j.molbrainres.2004.03.012. doi:10.1016/j.molbrainres.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Bagnasco M, Dube M.G, Katz A, Kalra P.S, Kalra S.P. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obes. Res. 2003;11:1463–1470. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- Bai F.L, Yamano M, Shiotani Y, Emson P.C, Smith A.D, Powell J.F, Toyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. doi:10.1016/0006-8993(85)90730-9 [DOI] [PubMed] [Google Scholar]
- Baker R.A, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J. Comp. Neurol. 1995;358:518–530. doi: 10.1002/cne.903580405. doi:10.1002/cne.903580405 [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445–457. doi: 10.1016/s0196-9781(96)00347-6. doi:10.1016/S0196-9781(96)00347-6 [DOI] [PubMed] [Google Scholar]
- Baran K, Preston E, Wilks D, Cooney G.J, Kraegen E.W, Sainsbury A. Chronic central melanocortin-4 receptor antagonism and central neuropeptide-Y infusion in rats produce increased adiposity by divergent pathways. Diabetes. 2002;51:152–158. doi: 10.2337/diabetes.51.1.152. [DOI] [PubMed] [Google Scholar]
- Bates S.H, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. doi:10.1038/nature01388 [DOI] [PubMed] [Google Scholar]
- Bchini-Hooft van Huijsduijnen O, Rohner-Jeanrenaud F, Jeanrenaud B. Hypothalamic neuropeptide-Y messenger ribonucleic acid levels in pre-obese and genetically obese (fa/fa) rats—potential regulation thereof by corticotropin-releasing factor. J. Neuroendocrinol. 1993;5:381–386. doi: 10.1111/j.1365-2826.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptides and obesity. Nutrition. 2000;16:916–923. doi: 10.1016/s0899-9007(00)00410-x. doi:10.1016/S0899-9007(00)00410-X [DOI] [PubMed] [Google Scholar]
- Beck B. KO's and organisation of peptidergic feeding behavior mechanisms. Neurosci. Biobehav. Rev. 2001;25:143–158. doi: 10.1016/s0149-7634(01)00003-3. doi:10.1016/S0149-7634(01)00003-3 [DOI] [PubMed] [Google Scholar]
- Beck B. The arcuate nucleus: its special place in the central networks that regulate feeding behavior. In: Zempleni J, Dakshinamurti K, editors. Nutrient and cell signalling. Marcel Dekker; New York, NY: 2005. pp. 665–699. [Google Scholar]
- Beck B, Stricker-Krongrad A, Nicolas J.P, Burlet C. Chronic and continuous ICV infusion of neuropeptide Y disrupts the nycthemeral feeding patterns in rats. Ann. NY Acad. Sci. 1990a;611:491–494. [Google Scholar]
- Beck B, Burlet A, Nicolas J.P, Burlet C. Hyperphagia in obesity is associated with a central peptidergic dysregulation in rats. J. Nutr. 1990b;120:806–811. doi: 10.1093/jn/120.7.806. [DOI] [PubMed] [Google Scholar]
- Beck B, Burlet A, Nicolas J.P, Burlet C. Hypothalamic neuropeptide Y (NPY) in obese Zucker rats: implications in feeding and sexual behaviors. Physiol. Behav. 1990c;47:449–453. doi: 10.1016/0031-9384(90)90107-f. doi:10.1016/0031-9384(90)90107-F [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Burlet A, Nicolas J.P, Burlet C. Influence of diet composition on food intake and hypothalamic neuropeptide Y (NPY) in the rat. Neuropeptides. 1990d;17:197–203. doi: 10.1016/0143-4179(90)90036-x. doi:10.1016/0143-4179(90)90036-X [DOI] [PubMed] [Google Scholar]
- Beck B, Jhanwar-Uniyal M, Burlet A, Chapleur-Chateau M, Leibowitz S.F, Burlet C. Rapid and localized alterations of neuropeptide Y in discrete hypothalamic nuclei with feeding status. Brain Res. 1990e;528:245–249. doi: 10.1016/0006-8993(90)91664-3. doi:10.1016/0006-8993(90)91664-3 [DOI] [PubMed] [Google Scholar]
- Beck B, Burlet A, Nicolas J.P, Burlet C. Unexpected regulation of hypothalamic neuropeptide-Y by food deprivation and refeeding in the Zucker rat. Life Sci. 1992a;50:923–930. doi: 10.1016/0024-3205(92)90169-p. doi:10.1016/0024-3205(92)90169-P [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Nicolas J.P, Burlet C. Chronic and continuous intracerebroventricular infusion of neuropeptide Y in Long–Evans rats mimics the feeding behaviour of obese Zucker rats. Int. J. Obes. 1992b;16:295–302. [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Burlet A, Nicolas J.P, Burlet C. Specific hypothalamic neuropeptide-Y variation with diet parameters in rats with food choice. Neuroreport. 1992c;3:571–574. doi: 10.1097/00001756-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Beck B, Burlet A, Bazin R, Nicolas J.P, Burlet C. Elevated neuropeptide-Y in the arcuate nucleus of young obese Zucker rats may contribute to the development of their overeating. J. Nutr. 1993;123:1168–1172. doi: 10.1093/jn/123.6.1168. [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Burlet A, Max J.P, Musse N, Nicolas J.P, Burlet C. Macronutrient type independently of energy intake modulates hypothalamic neuropeptide Y in Long–Evans rats. Brain Res. Bull. 1994;34:85–91. doi: 10.1016/0361-9230(94)90002-7. doi:10.1016/0361-9230(94)90002-7 [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Musse N, Nicolas J.P, Burlet C. Putative neuropeptide Y antagonist failed to decrease overeating in obese Zucker rats. Neurosci. Lett. 1994b;181:126–128. doi: 10.1016/0304-3940(94)90575-4. doi:10.1016/0304-3940(94)90575-4 [DOI] [PubMed] [Google Scholar]
- Beck B, Stricker-Krongrad A, Burlet A, Cumin F, Burlet C. Plasma leptin and hypothalamic neuropeptide Y and galanin levels in Long–Evans rats with marked dietary preferences. Nutr. Neurosci. 2001a;4:39–50. doi: 10.1080/1028415x.2001.11747349. [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Dimitrov T, Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem. Biophys. Res. Commun. 2001b;286:518–523. doi: 10.1006/bbrc.2001.5420. doi:10.1006/bbrc.2001.5420 [DOI] [PubMed] [Google Scholar]
- Beck B, Musse N, Stricker-Krongrad A. Ghrelin, macronutrient intake and dietary preferences in Long–Evans rats. Biochem. Biophys. Res. Commun. 2002;292:1031–1035. doi: 10.1006/bbrc.2002.6737. doi:10.1006/bbrc.2002.6737 [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Stricker-Krongrad A. Ghrelin and body weight regulation in the obese Zucker rat in relation to feeding state and dark/light cycle. Exp. Biol. Med. 2003;228:1124–1131. doi: 10.1177/153537020322801005. [DOI] [PubMed] [Google Scholar]
- Beck B, Max J.P, Richy S, Stricker-Krongrad A. Feeding response to a potent prolactin-releasing peptide agonist in lean and obese Zucker rats. Brain Res. 2004;1016:135–138. doi: 10.1016/j.brainres.2004.05.002. doi:10.1016/j.brainres.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Becker E.E, Grinker J.A. Meal patterns in the genetically obese Zucker rat. Physiol. Behav. 1977;18:685–692. doi: 10.1016/0031-9384(77)90067-1. doi:10.1016/0031-9384(77)90067-1 [DOI] [PubMed] [Google Scholar]
- Benoit S.C, Clegg D.J, Woods S.C, Seeley R.J. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides. 2005;26:751–757. doi: 10.1016/j.peptides.2004.12.012. doi:10.1016/j.peptides.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Berelowitz M, Bruno J.F, White J.D. Regulation of hypothalamic neuropeptide expression by peripheral metabolism. Trends Endocrinol. Metab. 1992;3:127–133. doi: 10.1016/1043-2760(92)90101-6. doi:10.1016/1043-2760(92)90101-6 [DOI] [PubMed] [Google Scholar]
- Bergen H.T, Mizuno T.M, Taylor J, Mobbs C.V. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology. 1998;139:4483–4488. doi: 10.1210/endo.139.11.6324. doi:10.1210/en.139.11.4483 [DOI] [PubMed] [Google Scholar]
- Bergen H.T, Mizuno T, Taylor J, Mobbs C.V. Resistance to diet-induced obesity is associated with increased proopiomelanocortin mRNA and decreased neuropeptide Y mRNA in the hypothalamus. Brain Res. 1999;851:198–203. doi: 10.1016/s0006-8993(99)02186-1. doi:10.1016/S0006-8993(99)02186-1 [DOI] [PubMed] [Google Scholar]
- Bi S, Moran T.H. Response to acute food deprivation in OLETF rats lacking CCK-A receptors. Physiol. Behav. 2003;79:655–661. doi: 10.1016/s0031-9384(03)00173-2. doi:10.1016/S0031-9384(03)00173-2 [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim E.E, Schwartz G.J, Moran T.H. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am. J. Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson B.M, Moran T.H. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am. J. Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott K.A, Hyun J, Ladenheim E.E, Moran T.H. Running wheel activity prevents hyperphagia and obesity in Otsuka Long–Evans Tokushima fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. doi:10.1210/en.2004-1441 [DOI] [PubMed] [Google Scholar]
- Blomqvist A.G, Herzog H. Y-receptor subtypes—how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. doi:10.1016/S0166-2236(96)01057-0 [DOI] [PubMed] [Google Scholar]
- Bodnar R.J. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. doi:10.1016/j.peptides.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Boudarine M, Yegorov O, Sterling-Dubrovsky A, Devi L, Berman Y. Developmental changes in opioid peptides and their receptors in Cpefat/Cpefat mice lacking peptide processing enzyme carboxypeptidase E. J. Pharmacol. Exp. Ther. 2002;303:1317–1324. doi: 10.1124/jpet.102.037663. doi:10.1124/jpet.102.037663 [DOI] [PubMed] [Google Scholar]
- Bouret S.G, Draper S.J, Simerly R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. doi:10.1523/JNEUROSCI.5369-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L.S, Smith M.A, Gold P.W, Herkenham M. Altered expression of hypothalamic neuropeptide messenger RNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Bray G.A. The Zucker-fatty rat: a review. Fed. Proc. 1977;36:146–153. [PubMed] [Google Scholar]
- Bray G.A, Teague R.J, Lee C.K. Brain uptake of ketones in rats with differing susceptibility to dietary obesity. Metabolism. 1987;36:27–30. doi: 10.1016/0026-0495(87)90058-8. doi:10.1016/0026-0495(87)90058-8 [DOI] [PubMed] [Google Scholar]
- Brief D.J, Sipols A.J, Woods S.C. Intraventricular neuropeptide Y injections stimulate food intake in lean, but not obese Zucker rats. Physiol. Behav. 1992;51:1105–1110. doi: 10.1016/0031-9384(92)90294-c. doi:10.1016/0031-9384(92)90294-C [DOI] [PubMed] [Google Scholar]
- Buchberger P, Schmidt I. Is the onset of obesity in suckling fa/fa rats linked to a potentially larger milk intake? Am. J. Physiol. 1996;40:R472–R476. doi: 10.1152/ajpregu.1996.271.2.R472. [DOI] [PubMed] [Google Scholar]
- Bugarith K, Dinh T.T, Li A.J, Speth R.C, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–1191. doi: 10.1210/en.2004-1166. doi:10.1210/en.2004-1166 [DOI] [PubMed] [Google Scholar]
- Bullen J.W, Ziotopoulou M, Ungsunan L, Misra J, Alevizos I, Kokkotou E, MaratosFlier E, Stephanopoulos G, Mantzoros C.S. Short-term resistance to diet-induced obesity in A/J mice is not associated with regulation of hypothalamic neuropeptides. Am. J. Physiol. 2004;287:E662–E670. doi: 10.1152/ajpendo.00114.2004. [DOI] [PubMed] [Google Scholar]
- Burkhoff A.M, Linemeyer D.L, Salon J.A. Distribution of a novel hypothalamic neuropeptide Y receptor gene and its absence in rat. Mol. Brain Res. 1998;53:311–316. doi: 10.1016/s0169-328x(97)00302-1. doi:10.1016/S0169-328X(97)00302-1 [DOI] [PubMed] [Google Scholar]
- Burlet A, Grouzmann E, Musse N, Fernette B, Nicolas J.P, Burlet C. The immunological impairment of arcuate neuropeptide Y neurons by ricin a chain produces persistent decrease of food intake and body weight. Neuroscience. 1995;66:151–159. doi: 10.1016/0306-4522(94)00573-n. doi:10.1016/0306-4522(94)00573-N [DOI] [PubMed] [Google Scholar]
- Cabrele C, Langer M, Bader R, Wieland H.A, Doods H.N, Zerbe O, BeckSickinger A.G. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J. Biol. Chem. 2000;275:36 043–36 048. doi: 10.1074/jbc.M000626200. doi:10.1074/jbc.M000626200 [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Battistini N, Zanni M, Galetti S, Protopapa F, Velardo A. Increase of neuropeptide Y-like immunoreactivity in the paraventricular nucleus of fasting rats. Neurosci. Lett. 1989;104:99–104. doi: 10.1016/0304-3940(89)90336-4. doi:10.1016/0304-3940(89)90336-4 [DOI] [PubMed] [Google Scholar]
- Campbell R.E, FfrenchMullen J.M.H, Cowley M.A, Smith M.S, Grove K.L. Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology. 2001;74:106–119. doi: 10.1159/000054676. doi:10.1159/000054676 [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Cintra A, Hokfelt T, Fuxe K, Wikstrom A, Gustafsson J. Coexistence of glucocorticoid receptor-like immunoreactivity with neuropeptides in the hypothalamic paraventricular nucleus. Exp. Brain Res. 1989;78:33–42. doi: 10.1007/BF00230684. doi:10.1007/BF00230684 [DOI] [PubMed] [Google Scholar]
- Chang S, Graham B, Yakubu F, Lin D, Peters J.C, Hill J.O. Metabolic differences between obesity-prone and obesity-resistant rats. Am. J. Physiol. 1990;259:R1103–R1110. doi: 10.1152/ajpregu.1990.259.6.R1103. [DOI] [PubMed] [Google Scholar]
- Chang G.Q, Karatayev O, Davydova Z, Wortley K, Leibowitz S.F. Glucose injection reduces neuropeptide Y and agouti-related protein expression in the arcuate nucleus: a possible physiological role in eating behavior. Mol. Brain Res. 2005;135:69–80. doi: 10.1016/j.molbrainres.2004.12.017. doi:10.1016/j.molbrainres.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Chavez M, Seeley R.J, Havel P.J, Friedman M.I, Matson C.A, Woods S.C, Schwartz M.W. Effect of a high-fat diet on food intake and hypothalamic neuropeptide gene expression in streptozotocin diabetes. J. Clin. Invest. 1998;102:340–346. doi: 10.1172/JCI603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall B.M. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl. 2):1–11. doi: 10.1016/0196-9781(85)90128-7. doi:10.1016/0196-9781(85)90128-7 [DOI] [PubMed] [Google Scholar]
- Chronwall B.M, Di Maggio D.A, Massari V.J, Pickel V.M, Ruggiero D.A, O'Donohue T.L. The anatomy of neuropeptide Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. doi:10.1016/0306-4522(85)90260-X [DOI] [PubMed] [Google Scholar]
- Chua S.C, Leibel R.L, Hirsch J. Food deprivation and age modulate neuropeptide gene expression in the murine hypothalamus and adrenal gland. Mol. Brain Res. 1991a;9:95–101. doi: 10.1016/0169-328x(91)90134-j. doi:10.1016/0169-328X(91)90134-J [DOI] [PubMed] [Google Scholar]
- Chua S.C, Brown A.W, Kim J.H, Hennessey K.L, Leibel R.L, Hirsch J. Food deprivation and hypothalamic neuropeptide gene expression—effects of strain background and the diabetes mutation. Mol. Brain Res. 1991b;11:291–299. doi: 10.1016/0169-328x(91)90038-y. doi:10.1016/0169-328X(91)90038-Y [DOI] [PubMed] [Google Scholar]
- Chua S.C, Chung W.K, Wupeng X.S, Zhang Y.Y, Liu S.M, Tartaglia L, Leibel R.L. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996a;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Chua S.C, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (lepr) Diabetes. 1996b;45:1141–1143. doi: 10.2337/diab.45.8.1141. [DOI] [PubMed] [Google Scholar]
- Clark J.T, Kalra P.S, Crowley W.R, Kalra S.P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X.L, Montez J.M, Rohani S.C, Feinstein P, Mombaerts P, Friedman J.M. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. doi:10.1172/JCI200113914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D.L, Eicher E.M. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J. Hered. 1990;81:424–427. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. doi:10.1038/nn1455 [DOI] [PubMed] [Google Scholar]
- Corder R, Pralong F, Turnill D, Saudan P, Muller A, Gaillard R. Dexamethasone treatment increases neuropeptide Y levels in rat hypothalamic neurones. Life Sci. 1988;43:1879–1886. doi: 10.1016/s0024-3205(88)80005-5. doi:10.1016/S0024-3205(88)80005-5 [DOI] [PubMed] [Google Scholar]
- Corp E.S, Melville L.D, Greenberg D, Gibbs J, Smith G.P. Effect of 4th ventricular neuropeptide-Y and peptide-YY on ingestive and other behaviors. Am. J. Physiol. 1990;259:R317–R323. doi: 10.1152/ajpregu.1990.259.2.R317. [DOI] [PubMed] [Google Scholar]
- Cowley M.A, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. doi:10.1016/S0896-6273(03)00063-1 [DOI] [PubMed] [Google Scholar]
- Cox H.M. The role of NPY and related peptides in the control of gastrointestinal function. In: Colmers W.F, Wahlestedt C, editors. Biology of neuropeptide Y and related peptides. Humana Press; Totowa, NJ: 1993. pp. 273–313. [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biol. Bull. 1918;34:91–107. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiffary A, Gorcs T, Palkovits M. Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res. 1990;506:215–222. doi: 10.1016/0006-8993(90)91253-d. doi:10.1016/0006-8993(90)91253-D [DOI] [PubMed] [Google Scholar]
- Currie P.J, Coscina D.V. Dissociated feeding and hypothermic effects of neuropeptide Y in the paraventricular and perifornical hypothalamus. Peptides. 1995;16:599–604. doi: 10.1016/0196-9781(95)00020-k. doi:10.1016/0196-9781(95)00020-K [DOI] [PubMed] [Google Scholar]
- Dallman M.F, et al. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. doi:10.1210/en.140.9.4015 [DOI] [PubMed] [Google Scholar]
- Dallman M.F, LaFleur S.E, Pecoraro N.C, Gomez F, Houshyar H, Akana S.F. Minireview: glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. doi:10.1210/en.2004-0037 [DOI] [PubMed] [Google Scholar]
- Daniels A.J, Chance W.T, Grizzle M.K, Heyer D, Matthews J.E. Food intake inhibition and reduction in body weight gain in rats treated with GI264879A, a non-selective NPY–Y1 receptor antagonist. Peptides. 2001;22:483–491. doi: 10.1016/s0196-9781(01)00358-8. doi:10.1016/S0196-9781(01)00358-8 [DOI] [PubMed] [Google Scholar]
- Daniels A.J, Grizzle M.K, Wiard R.P, Matthews J.E, Heyer D. Food intake inhibition and reduction in body weight gain in lean and obese rodents treated with GW438014A, a potent and selective NPY–Y5 receptor antagonist. Regul. Pept. 2002;106:47–54. doi: 10.1016/s0167-0115(02)00034-4. doi:10.1016/S0167-0115(02)00034-4 [DOI] [PubMed] [Google Scholar]
- Davies L, Marks J.L. Role of hypothalamic neuropeptide Y gene expression in body weight regulation. Am. J. Physiol. 1994;266:R1687–R1691. doi: 10.1152/ajpregu.1994.266.5.R1687. [DOI] [PubMed] [Google Scholar]
- Day D.E, Keen-Rhinehart E, Bartness T.J. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am. J. Physiol. 2005;289:R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- DeJonghe B.C, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am. J. Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- DellaZuana O, et al. Reduced food intake in response to CCP 71683A may be due to mechanisms other than NPYY5 receptor blockade. Int. J. Obes. 2001;25:84–94. doi: 10.1038/sj.ijo.0801472. doi:10.1038/sj.ijo.0801472 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Ge Y.L, Minter R.M, Prima V, Moldawer L.L, Muzyczka N, Zolotukhin S, Kalra P.S, Kalra S.P. Long-term differential modulation of genes encoding orexigenic and anorexigenic peptides by leptin delivered by rAAV vector in ob/ob mice—relationship with body weight change. Regul. Pept. 2000;92:97–105. doi: 10.1016/s0167-0115(00)00155-5. doi:10.1016/S0167-0115(00)00155-5 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. doi:10.1038/nn1457 [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Wortman M.D, Seeley R.J, Woods S.C. Neuropeptide Y prepares rats for scheduled feeding. Am. J. Physiol. 2005;288:R1606–R1611. doi: 10.1152/ajpregu.00817.2004. [DOI] [PubMed] [Google Scholar]
- Dryden S, Pickavance L, Frankish H.M, Williams G. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995;690:185–188. doi: 10.1016/0006-8993(95)00628-4. doi:10.1016/0006-8993(95)00628-4 [DOI] [PubMed] [Google Scholar]
- Dryden S, Pickavance L, Tidd D, Williams G. The lack of specificity of neuropeptide Y (NPY) antisense oligodeoxynucleotides administered intracerebroventricularly in inhibiting food intake and NPY gene expression in the rat hypothalamus. J. Endocrinol. 1998;157:169–175. doi: 10.1677/joe.0.1570169. doi:10.1677/joe.0.1570169 [DOI] [PubMed] [Google Scholar]
- Dryden S, King P, Pickavance L, Doyle P, Williams G. Divergent effects of intracerebroventricular and peripheral leptin administration on feeding and hypothalamic neuropeptide Y in lean and obese (fa/fa) Zucker rats. Clin. Sci. 1999;96:307–312. doi: 10.1042/cs0960307. doi:10.1042/CS19980226 [DOI] [PubMed] [Google Scholar]
- Dube M.G, Sahu A, Kalra P.S, Kalra S.P. Neuropeptide-Y release is elevated from the microdissected paraventricular nucleus of food-deprived rats—an in vitro study. Endocrinology. 1992;131:684–688. doi: 10.1210/endo.131.2.1639015. doi:10.1210/en.131.2.684 [DOI] [PubMed] [Google Scholar]
- Dube M.G, Xu B, Crowley W.R, Kalra P.S, Kalra S.P. Evidence that neuropeptide Y is a physiological signal for normal food intake. Brain Res. 1994;646:341–344. doi: 10.1016/0006-8993(94)90103-1. doi:10.1016/0006-8993(94)90103-1 [DOI] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, Quirion R. Expression and characterization of the neuropeptide Y Y-5 receptor subtype in the rat brain. J. Neurosci. 1998;18:5565–5574. doi: 10.1523/JNEUROSCI.18-15-05565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Moyse E, Fournier A, Quirion R. Evidence for the existence of an additional class of neuropeptide Y receptor sites in the rat brain. J. Pharmacol. Exp. Ther. 2005;315:99–108. doi: 10.1124/jpet.105.089300. doi:10.1124/jpet.105.089300 [DOI] [PubMed] [Google Scholar]
- Durkin M.M, Walker M.W, Smith K.E, Gustafson E.L, Gerald C, Branchek T.A. Expression of a novel neuropeptide Y receptor subtype involved in food intake: an in situ hybridization study of Y5 mRNA distribution in rat brain. Exp. Neurol. 2000;165:90–100. doi: 10.1006/exnr.2000.7446. doi:10.1006/exnr.2000.7446 [DOI] [PubMed] [Google Scholar]
- Elias C.F, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998;402:442–459. doi:10.1002/(SICI)1096-9861(19981228)402:4<442::AID-CNE2>3.0.CO;2-R [PubMed] [Google Scholar]
- Erickson J.C, Hollopeter G, Palmiter R.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. doi:10.1126/science.274.5293.1704 [DOI] [PubMed] [Google Scholar]
- Everitt B.J, Hökfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential coexistence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11:443–462. doi: 10.1016/0306-4522(84)90036-8. doi:10.1016/0306-4522(84)90036-8 [DOI] [PubMed] [Google Scholar]
- Farley C, Cook J.A, Spar B.D, Austin T.M, Kowalski T.J. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes. Res. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- Farooqi I.S, O'Rahilly S. Monogenic obesity in humans. Annu. Rev. Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. doi:10.1146/annurev.med.56.062904.144924 [DOI] [PubMed] [Google Scholar]
- Flood J.F, Morley J.E. Increased food intake by neuropeptide Y is due to an increased motivation to eat. Peptides. 1991;12:1329–1332. doi: 10.1016/0196-9781(91)90215-b. doi:10.1016/0196-9781(91)90215-B [DOI] [PubMed] [Google Scholar]
- Flynn M.C, Turrin N.P, PlataSalaman C.R, FfrenchMullen J.M.H. Feeding response to neuropeptide Y-related compounds in rats treated with Y5 receptor antisense or sense phosphothio-oligodeoxynucleotide. Physiol. Behav. 1999;66:881–884. doi: 10.1016/s0031-9384(99)00031-1. doi:10.1016/S0031-9384(99)00031-1 [DOI] [PubMed] [Google Scholar]
- Frankish H.M, Mc Carthy H.D, Dryden S, Kilpatrick A, Williams G. Neuropeptide-Y receptor numbers are reduced in the hypothalamus of streptozotocin-diabetic and food-deprived rats—further evidence of increased activity of hypothalamic NPY-containing pathways. Peptides. 1993;14:941–948. doi: 10.1016/0196-9781(93)90070-w. doi:10.1016/0196-9781(93)90070-W [DOI] [PubMed] [Google Scholar]
- Frankish H.M, Dryden S, Hopkins D, Wang Q, Williams G. Neuropeptide Y, the hypothalamus, and diabetes: insights into the central control of metabolism. Peptides. 1995;16:757–771. doi: 10.1016/0196-9781(94)00200-p. doi:10.1016/0196-9781(94)00200-P [DOI] [PubMed] [Google Scholar]
- Fricker L.D, Leiter E.H. Peptides, enzymes and obesity: new insights from a ‘dead’ enzyme. Trends Biochem. Sci. 1999;24:390–393. doi: 10.1016/s0968-0004(99)01448-6. doi:10.1016/S0968-0004(99)01448-6 [DOI] [PubMed] [Google Scholar]
- Funahashi H, Yamada S, Kageyama H, Takenoya F, Guan J.L, Shioda S. Co-existence of leptin- and orexin-receptors in feeding-regulating neurons in the hypothalamic arcuate nucleus-a triple labeling study. Peptides. 2003;24:687–694. doi: 10.1016/s0196-9781(03)00130-x. doi:10.1016/S0196-9781(03)00130-X [DOI] [PubMed] [Google Scholar]
- Gamber K.M, MacArthur H, Westfall T.C. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gao J, Ghibaudi L, vanHeek M, Hwa J.J. Characterization of diet-induced obese rats that develop persistent obesity after 6 months of high-fat followed by 1 month of low-fat diet. Brain Res. 2002;936:87–90. doi: 10.1016/s0006-8993(02)02493-9. doi:10.1016/S0006-8993(02)02493-9 [DOI] [PubMed] [Google Scholar]
- Gardiner J.V, Kong W.M, Ward H, Murphy K.G, Dhillo W.S, Bloom S.R. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem. Biophys. Res. Commun. 2005;327:1088–1093. doi: 10.1016/j.bbrc.2004.12.113. doi:10.1016/j.bbrc.2004.12.113 [DOI] [PubMed] [Google Scholar]
- Gerald C, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. doi:10.1038/382168a0 [DOI] [PubMed] [Google Scholar]
- Ghibaudi L, Cook J, Farley C, vanHeek M, Hwa J.J. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague–Dawley rats. Obes. Res. 2002;10:956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- Giraudo S.Q, Kotz C.M, Grace M.K, Levine A.S, Billington C.J. Rat hypothalamic NPY mRNA and brown fat uncoupling protein mRNA after high-carbohydrate or high-fat diets. Am. J. Physiol. 1994;266:R1578–R1583. doi: 10.1152/ajpregu.1994.266.5.R1578. [DOI] [PubMed] [Google Scholar]
- Giraudo S.Q, Kim E.M, Grace M.K, Billington C.J, Levine A.S. Effect of peripheral 2-DG on opioid and neuropeptide Y gene expression. Brain Res. 1998;792:136–140. doi: 10.1016/s0006-8993(98)00197-8. doi:10.1016/S0006-8993(98)00197-8 [DOI] [PubMed] [Google Scholar]
- Glass M.J, Cleary J.P, Billington C.J, Levine A.S. Role of carbohydrate type on diet selection in neuropeptide Y-stimulated rats. Am. J. Physiol. 1997;42:R2040–R2045. doi: 10.1152/ajpregu.1997.273.6.R2040. [DOI] [PubMed] [Google Scholar]
- Glass M.J, Billington C.J, Levine A.S. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. doi:10.1054/npep.1999.0050 [DOI] [PubMed] [Google Scholar]
- Godbole V, York D.A, Bloxham D.P. Developmental changes in the fatty (fa/fa) rat: evidence for defective thermogenesis preceding the hyperlipogenesis and hyperinsulinemia. Diabetologia. 1978;15:41–44. doi: 10.1007/BF01219327. doi:10.1007/BF01219327 [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter J.R, Sarmiento U, Sarosi I, Stark K.L. Overexpression of Agrt leads to obesity in transgenic mice. Nat. Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Guan X.M, Yu H, VanderPloeg L.H.T. Evidence of altered hypothalamic pro-piomelanocortin/neuropeptide Y mRNA expression in tubby mice. Mol. Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. doi:10.1016/S0169-328X(98)00150-8 [DOI] [PubMed] [Google Scholar]
- Hahn T.M, Breininger J.F, Baskin D.G, Schwartz M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998;1:271–272. doi: 10.1038/1082. doi:10.1038/1082 [DOI] [PubMed] [Google Scholar]
- Hansen M.J, Jovanovska V, Morris M.J. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J. Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- Hanson E.S, Levin N, Dallman M.F. Elevated corticosterone is not required for the rapid induction of neuropeptide Y gene expression by an overnight fast. Endocrinology. 1997;138:1041–1047. doi: 10.1210/endo.138.3.4995. doi:10.1210/en.138.3.1041 [DOI] [PubMed] [Google Scholar]
- Hardie L.J, Rayner D.V, Holmes S, Trayhurn P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem. Biophys. Res. Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. doi:10.1006/bbrc.1996.0951 [DOI] [PubMed] [Google Scholar]
- Harrold J.A, Williams G. The cannabinoid system: a role in both the homeostatic and hedonic control of eating? Br. J. Nutr. 2003;90:729–734. doi: 10.1079/bjn2003942. doi:10.1079/BJN2003942 [DOI] [PubMed] [Google Scholar]
- Harrold J.A, Elliott J.C, King P.J, Widdowson P.S, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. doi:10.1016/S0006-8993(02)03245-6 [DOI] [PubMed] [Google Scholar]
- Haynes A.C, Arch J.R.S, Wilson S, McClue S, Buckingham R.E. Characterisation of the neuropeptide Y receptor that mediates feeding in the rat: a role for the Y5 receptor? Regul. Pept. 1998;75–76:355–361. doi: 10.1016/s0167-0115(98)00088-3. doi:10.1016/S0167-0115(98)00088-3 [DOI] [PubMed] [Google Scholar]
- He B, White B.D, Edwards G.L, Martin R.J. Neuropeptide Y antibody attenuates 2-deoxy-d-glucose induced feeding in rats. Brain Res. 1998;781:348–350. doi: 10.1016/s0006-8993(97)01310-3. doi:10.1016/S0006-8993(97)01310-3 [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. doi:10.1016/j.npep.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Henry M, Ghibaudi L, Gao J, Hwa J.J. Energy metabolic profile of mice after chronic activation of central NPYY1, Y2, or Y5 receptors. Obes. Res. 2005;13:36–47. doi: 10.1038/oby.2005.6. [DOI] [PubMed] [Google Scholar]
- Herzog H. Transgenic and knockout models in NPY research. In: Michel M.C, editor. Neuropeptide Y and related peptides. Springer; Berlin, Germany: 2004. pp. 447–478. [Google Scholar]
- Herzog H, Darby K, Ball H, Hort Y, BeckSickinger A, Shine J. Overlapping gene structure of the human neuropeptide Y receptor subtypes Y1 and Y5 suggests coordinate transcriptional regulation. Genomics. 1997;41:315–319. doi: 10.1006/geno.1997.4684. doi:10.1006/geno.1997.4684 [DOI] [PubMed] [Google Scholar]
- Hewson A.K, Dickson S.L. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J. Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. doi:10.1046/j.1365-2826.2000.00584.x [DOI] [PubMed] [Google Scholar]
- Hiraoka J, et al. Augmentation of obese (ob) gene expression and leptin secretion in obese spontaneously hypertensive rats (obese SHR or Koletsky rats) Biochem. Biophys. Res. Commun. 1997;231:582–585. doi: 10.1006/bbrc.1997.6145. doi:10.1006/bbrc.1997.6145 [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Erickson J.C, Palmiter R.D. Role of neuropeptide Y in diet-, chemical- and genetic-induced obesity of mice. Int. J. Obes. 1998;22:506–512. doi: 10.1038/sj.ijo.0800615. doi:10.1038/sj.ijo.0800615 [DOI] [PubMed] [Google Scholar]
- Horvath T.L, Diano S, vandenPol A.N. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T.L, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance—a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. doi:10.1210/en.142.10.4163 [DOI] [PubMed] [Google Scholar]
- Hu Y.H, et al. Identification of a novel hypothalamic neuropeptide Y receptor associated with feeding behavior. J. Biol. Chem. 1996;271:26 315–26 319. doi:10.1074/jbc.271.42.26315 [PubMed] [Google Scholar]
- Huang X.F, Han M, Storlien L.H. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Mol. Brain Res. 2003;115:21–28. doi: 10.1016/s0169-328x(03)00174-8. doi:10.1016/S0169-328X(03)00174-8 [DOI] [PubMed] [Google Scholar]
- Hulsey M.G, Pless C.M, White B.D, Martin R.J. ICV administration of anti-NPY antisense oligonucleotide: effects on feeding behavior, body weight, peptide content and peptide release. Regul. Pept. 1995;59:207–214. doi: 10.1016/0167-0115(95)00110-w. doi:10.1016/0167-0115(95)00110-W [DOI] [PubMed] [Google Scholar]
- Hung C.C.C, et al. Studies of the peptide YY and neuropeptide Y2 receptor genes in relation to human obesity and obesity-related traits. Diabetes. 2004;53:2461–2466. doi: 10.2337/diabetes.53.9.2461. [DOI] [PubMed] [Google Scholar]
- Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. doi:10.1016/S0092-8674(00)81865-6 [DOI] [PubMed] [Google Scholar]
- Hwa J.J, et al. Activation of the NPYY5 receptor regulates both feeding and energy expenditure. Am. J. Physiol. 1999;277:R1428–R1434. doi: 10.1152/ajpregu.1999.277.5.R1428. [DOI] [PubMed] [Google Scholar]
- Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine→proline) of the leptin receptor (OB-r) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem. Biophys. Res. Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. doi:10.1006/bbrc.1996.1070 [DOI] [PubMed] [Google Scholar]
- Inui A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharmacol. Sci. 1999;20:43–46. doi: 10.1016/s0165-6147(99)01303-6. doi:10.1016/S0165-6147(99)01303-6 [DOI] [PubMed] [Google Scholar]
- Ishihara A, Tanaka T, Kanatani A, Fukami T, Ihara M, Fukuroda T. A potent neuropeptide Y antagonist, 1229U91, suppressed spontaneous food intake in Zucker fatty rats. Am. J. Physiol. 1998;43:R1500–R1504. doi: 10.1152/ajpregu.1998.274.5.R1500. [DOI] [PubMed] [Google Scholar]
- Ishizaki K, Honma S, Katsuno Y, Abe H, Masubuchi S, Namihira M, Honma K. Gene expression of neuropeptide Y in the nucleus of the solitary tract is activated in rats under restricted daily feeding but not under 48-h food deprivation. Eur. J. Neurosci. 2003;17:2097–2105. doi: 10.1046/j.1460-9568.2003.02672.x. doi:10.1046/j.1460-9568.2003.02672.x [DOI] [PubMed] [Google Scholar]
- Israel Y, Kandov Y, Khaimova E, Kest A, Lewis S.R, Pasternak G.W, Pan Y.X, Rossi G.C, Bodnar R.J. NPY-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. Peptides. 2005;26:1167–1175. doi: 10.1016/j.peptides.2005.01.017. doi:10.1016/j.peptides.2005.01.017 [DOI] [PubMed] [Google Scholar]
- Jang M.Y, Romsos D.R. Neuropeptide Y and corticotropin-releasing hormone concentrations within specific hypothalamic regions of lean but not ob/ob mice respond to food-deprivation and refeeding. J. Nutr. 1998;128:2520–2525. doi: 10.1093/jn/128.12.2520. [DOI] [PubMed] [Google Scholar]
- Jang I.S, et al. Role of dietary fat type in the development of adiposity from dietary obesity-susceptible Sprague–Dawley rats. Br. J. Nutr. 2003;89:429–437. doi: 10.1079/BJN2002801. doi:10.1079/BJN2002801 [DOI] [PubMed] [Google Scholar]
- Jewett D.C, Cleary J, Levine A.S, Schaal D.W, Thompson T. Effects of neuropeptide-Y on food-reinforced behavior in satiated rats. Pharmacol. Biochem. Behav. 1992;42:207–212. doi: 10.1016/0091-3057(92)90517-j. doi:10.1016/0091-3057(92)90517-J [DOI] [PubMed] [Google Scholar]
- Jewett D.C, Cleary J, Levine A.S, Schaal D.W, Thompson T. Effects of neuropeptide Y, insulin, a 2-deoxyglucose, and food deprivation on food motivated behavior. Psychopharmacology. 1995;120:267–271. doi: 10.1007/BF02311173. doi:10.1007/BF02311173 [DOI] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Chua S.C. Critical effects of aging and nutritional state on hypothalamic neuropeptide-Y and galanin gene expression in lean and genetically obese Zucker rats. Mol. Brain Res. 1993;19:195–202. doi: 10.1016/0169-328x(93)90026-l. doi:10.1016/0169-328X(93)90026-L [DOI] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Beck B, Burlet C, Leibowitz S.F. Diurnal rhythm of neuropeptide Y-like immunoreactivity in the suprachiasmatic, arcuate and paraventricular nuclei and other hypothalamic sites. Brain Res. 1990;536:331–334. doi: 10.1016/0006-8993(90)90045-d. doi:10.1016/0006-8993(90)90045-D [DOI] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Beck B, Jhanwar Y.S, Burlet C, Leibowitz S.F. Neuropeptide Y projection from arcuate nucleus to parvocellular division of paraventricular nucleus—specific relation to the ingestion of carbohydrate. Brain Res. 1993;631:97–106. doi: 10.1016/0006-8993(93)91192-u. doi:10.1016/0006-8993(93)91192-U [DOI] [PubMed] [Google Scholar]
- Jolicoeur F.B, Michaud J.N, Menard D, Fournier A. In vivo structure activity study supports the existence of heterogeneous neuropeptide-Y receptors. Brain Res. Bull. 1991;26:309–311. doi: 10.1016/0361-9230(91)90243-d. doi:10.1016/0361-9230(91)90243-D [DOI] [PubMed] [Google Scholar]
- Jolicoeur F.B, Bouali S.M, Fournier A, St Pierre S. Mapping of hypothalamic sites involved in the effects of NPY on body temperature and food intake. Brain Res. Bull. 1995;36:125–129. doi: 10.1016/0361-9230(94)00176-2. doi:10.1016/0361-9230(94)00176-2 [DOI] [PubMed] [Google Scholar]
- Kagotani Y, Hashimoto T, Tsuruo Y, Kawano H, Daikoku S, Chihara K. Development of the neuronal system containing neuropeptide Y in the rat hypothalamus. Int. J. Dev. Neurosci. 1989;7:359–374. doi: 10.1016/0736-5748(89)90057-9. doi:10.1016/0736-5748(89)90057-9 [DOI] [PubMed] [Google Scholar]
- Kahle E.B, Butz K.G, Chua S.C, Kershaw E.E, Leibel R.L, Fenger T.W, Hansen C.T, Michaelis O.E. The rat corpulent (cp) mutation maps to the same interval on (Pgm1–Glut1) rat chromosome 5 as the fatty (fa) mutation. Obes. Res. 1997;5:142–145. doi: 10.1002/j.1550-8528.1997.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Kalra S.P, Kalra P.S. Neuropeptide Y—a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49–55. doi: 10.1385/ENDO:22:1:49. doi:10.1385/ENDO:22:1:49 [DOI] [PubMed] [Google Scholar]
- Kalra S.P, Dube M.G, Fournier A, Kalra P.S. Structure–function analysis of stimulation of food intake by neuropeptide-Y—effects of receptor agonists. Physiol. Behav. 1991a;50:5–9. doi: 10.1016/0031-9384(91)90490-f. doi:10.1016/0031-9384(91)90490-F [DOI] [PubMed] [Google Scholar]
- Kalra S.P, Dube M.G, Sahu A, Phelps C.P, Kalra P.S. Neuropeptide-Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc. Natl Acad. Sci. USA. 1991b;88:10 931–10 935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, S. P., Dube, M. G., Finkelstein, J. & Sahu, A. 1994 Neuropeptide Y (NPY) release from the paraventricular nucleus (PVN) of genetically obese and lean Zucker rats. A-18 Second Independent SSIB Meeting, McMaster University, Hamilton, Canada.
- Kalra S.P, Dube M.G, Pu S.Y, Xu B, Horvath T.L, Kalra P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. doi:10.1210/er.20.1.68 [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide YY1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. doi:10.1210/en.137.8.3177 [DOI] [PubMed] [Google Scholar]
- Kanatani A, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000a;141:1011–1016. doi: 10.1210/endo.141.3.7387. doi:10.1210/en.141.3.1011 [DOI] [PubMed] [Google Scholar]
- Kanatani A, et al. L-152,804: orally active and selective neuropeptide YY5 receptor antagonist. Biochem. Biophys. Res. Commun. 2000b;272:169–173. doi: 10.1006/bbrc.2000.2696. doi:10.1006/bbrc.2000.2696 [DOI] [PubMed] [Google Scholar]
- Kanatani A, et al. A typical Y1 receptor regulates feeding behaviors: effects of a potent and selective Y1 antagonist, J-115814. Mol. Pharmacol. 2001;59:501–505. doi: 10.1124/mol.59.3.501. [DOI] [PubMed] [Google Scholar]
- Kaplan M.L. Consumption of O2 and early detection of fa/fa genotype in rats. Metabolism. 1979;28:1147–1151. doi: 10.1016/0026-0495(79)90154-9. doi:10.1016/0026-0495(79)90154-9 [DOI] [PubMed] [Google Scholar]
- Kask A, Pahkla R, Irs A, Rago L, Wikberg J.E.S, Schioth H.B. Long-term administration of MC4 receptor antagonist HS014 causes hyperphagia and obesity in rats. Neuroreport. 1999;10:707–711. doi: 10.1097/00001756-199903170-00009. [DOI] [PubMed] [Google Scholar]
- Kask A, Vasar E, Heidmets L.T, Allikmets L, Wikberg J.E.S. Neuropeptide YY5 receptor antagonist CGP71683A: the effects on food intake and anxiety-related behavior in the rat. Eur. J. Pharmacol. 2001;414:215–224. doi: 10.1016/s0014-2999(01)00768-3. doi:10.1016/S0014-2999(01)00768-3 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Kalra S.P, Kalra P.S. Neuropeptidergic characterization of the leptin receptor mutated obese Koletsky rat. Regul. Pept. 2004a;119:3–10. doi: 10.1016/j.regpep.2003.12.016. doi:10.1016/j.regpep.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Kalra S.P, Kalra P.S. Leptin-receptor gene transfer into the arcuate nucleus of female fatty Zucker rats using recombinant adeno-associated viral vectors stimulates the hypothalamo–pituitary–gonadal axis. Biol. Reprod. 2004b;71:266–272. doi: 10.1095/biolreprod.103.025858. doi:10.1095/biolreprod.103.025858 [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Eiden S, Kretz O, Schutz G, Schmidt I, Tronche F, Simon E. Inactivation of the GR in the nervous system affects energy accumulation. Endocrinology. 2002;143:2333–2340. doi: 10.1210/endo.143.6.8853. doi:10.1210/en.143.6.2333 [DOI] [PubMed] [Google Scholar]
- Kelly J, Rothstein J, Grossman S. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol. Behav. 1979;23:1123–1134. doi: 10.1016/0031-9384(79)90306-8. doi:10.1016/0031-9384(79)90306-8 [DOI] [PubMed] [Google Scholar]
- Kerkerian L, Pelletier G. Effects of monosodium l-glutamate administration on neuropeptide Y-containing neurons in the rat hypothalamus. Brain Res. 1986;369:388–390. doi: 10.1016/0006-8993(86)90557-3. doi:10.1016/0006-8993(86)90557-3 [DOI] [PubMed] [Google Scholar]
- Kesterson R.A, Huszar D, Lynch C.A, Simerly R.B, Cone R.D. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the Agouti obesity syndrome. Mol. Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. doi:10.1210/me.11.5.630 [DOI] [PubMed] [Google Scholar]
- Kim E.M, Welch C.C, Grace M.K, Billington C.J, Levine A.S. Effects of palatability-induced hyperphagia and food restriction on mRNA levels of neuropeptide-Y in the arcuate nucleus. Brain Res. 1998;806:117–121. doi: 10.1016/s0006-8993(98)00755-0. doi:10.1016/S0006-8993(98)00755-0 [DOI] [PubMed] [Google Scholar]
- King P.J, Widdowson P.S, Doods H.N, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J. Neurochem. 1999;73:641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. doi:10.1046/j.1471-4159.1999.0730641.x [DOI] [PubMed] [Google Scholar]
- King P.J, Williams G, Doods H, Widdowson P.S. Effect of a selective neuropeptide YY2 receptor antagonist, BIIE0246 on neuropeptide Y release. Eur. J. Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. doi:10.1016/S0014-2999(00)00230-2 [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi C.J, Choi B.J, Lopez M.E, Lee C.E, Liu H.Y, Hollenberg A.N, Friedman J.M, Elmquist J.K. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J. Comp. Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. doi:10.1002/cne.20432 [DOI] [PubMed] [Google Scholar]
- Kleyn P.W, et al. Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell. 1996;85:281–290. doi: 10.1016/s0092-8674(00)81104-6. doi:10.1016/S0092-8674(00)81104-6 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohno T, Tsuchiya T, Horio F. Characterization of diabetes-related traits in MSM and JF1 mice on high-fat diet. J. Nutr. Biochem. 2004;15:614–621. doi: 10.1016/j.jnutbio.2004.05.001. doi:10.1016/j.jnutbio.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Koegler F.H, Schaffhauser A.O, Mynatt R.L, York D.A, Bray G.A. Macronutrient diet intake of the lethal yellow agouti (A(Y)/a) mouse. Physiol. Behav. 1999;67:809–812. doi: 10.1016/s0031-9384(99)00104-3. doi:10.1016/S0031-9384(99)00104-3 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. doi:10.1152/physrev.00012.2004 [DOI] [PubMed] [Google Scholar]
- Kopin A.S, Mathes W.F, McBride E.W, Nguyen M, AlHaider W, Schmitz F, BonnerWeir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua S.C, Leibel R.L, Wardlaw S.L. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J. Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. doi:10.1046/j.1365-2826.2001.00716.x [DOI] [PubMed] [Google Scholar]
- Kowalski T.J, Ster A.M, Smith G.P. Ontogeny of hyperphagia in the Zucker (fa/fa) rat. Am. J. Physiol. 1998;44:R1106–R1109. doi: 10.1152/ajpregu.1998.275.4.R1106. [DOI] [PubMed] [Google Scholar]
- Kowalski T.J, Houpt T.A, Jahng J, Okada N, Liu S.M, Chua S.C, Smith G.P. Neuropeptide Y overexpression in the preweanling Zucker (fa/fa) rat. Physiol. Behav. 1999;67:521–525. doi: 10.1016/s0031-9384(99)00095-5. doi:10.1016/S0031-9384(99)00095-5 [DOI] [PubMed] [Google Scholar]
- Kozak R, Mercer J.G, Burlet A, Moar K.M, Burlet C, Beck B. Hypothalamic neuropeptide Y content and mRNA expression in weanling rats subjected to dietary manipulations during fetal and neonatal life. Regul. Pept. 1998;75–76:397–402. doi: 10.1016/s0167-0115(98)00094-9. doi:10.1016/S0167-0115(98)00094-9 [DOI] [PubMed] [Google Scholar]
- Kozak R, Burlet A, Burlet C, Beck B. Dietary composition during fetal and neonatal life affects neuropeptide Y functioning in adult offspring. Dev. Brain Res. 2000;125:75–82. doi: 10.1016/s0165-3806(00)00120-6. doi:10.1016/S0165-3806(00)00120-6 [DOI] [PubMed] [Google Scholar]
- Kozak R, Richy S, Beck B. Persistent alterations in neuropeptide Y release in the paraventricular nucleus of rats subjected to dietary manipulation during early life. Eur. J. Neurosci. 2005;21:2887–2892. doi: 10.1111/j.1460-9568.2005.04101.x. doi:10.1111/j.1460-9568.2005.04101.x [DOI] [PubMed] [Google Scholar]
- Krief S, Bazin R. Genetic obesity—is the defect in the sympathetic nervous system?—a review through developmental studies in the preobese Zucker rat. Proc. Soc. Exp. Biol. Med. 1991;198:528–538. doi: 10.3181/00379727-198-43286c. [DOI] [PubMed] [Google Scholar]
- Kuenzel W.J, Douglass L.W, Davison B.A. Robust feeding following central administration of neuropeptide Y or peptide YY in chicks, Gallus domesticus. Peptides. 1987;8:823–828. doi: 10.1016/0196-9781(87)90066-0. doi:10.1016/0196-9781(87)90066-0 [DOI] [PubMed] [Google Scholar]
- laFleur S.E, Ji H, Manalo S.L, Friedman M.I, Dallman M.F. The hepatic vagus mediates fat-induced inhibition of diabetic hyperphagia. Diabetes. 2003;52:2321–2330. doi: 10.2337/diabetes.52.9.2321. [DOI] [PubMed] [Google Scholar]
- Lambert P.D, Wilding J.P.H, Turton M.D, Ghatei M.A, Bloom S.R. Effect of food deprivation and streptozotocin-induced diabetes on hypothalamic neuropeptide Y release as measured by a radioimmunoassay-linked microdialysis procedure. Brain Res. 1994;656:135–140. doi: 10.1016/0006-8993(94)91374-9. doi:10.1016/0006-8993(94)91374-9 [DOI] [PubMed] [Google Scholar]
- Larhammar D. Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996;62:1–11. doi: 10.1016/0167-0115(95)00169-7. doi:10.1016/0167-0115(95)00169-7 [DOI] [PubMed] [Google Scholar]
- Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38:141–151. doi: 10.1016/j.npep.2004.06.002. doi:10.1016/j.npep.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Larsen P.J, Jessop D.S, Chowdrey H.S, Lightman S.L, Mikkelsen J.D. Chronic administration of glucocorticoids directly upregulates prepro-neuropeptide Y and Y-1-receptor mRNA levels in the arcuate nucleus of the rat. J. Neuroendocrinol. 1994;6:153–159. doi: 10.1111/j.1365-2826.1994.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Lavau M, Bazin R. Inguinal fat pad weight plotted versus body weight as a method of genotype identification in 16-day-old Zucker rats. J. Lipid Res. 1982;23:941–943. [PubMed] [Google Scholar]
- Lecklin A, Lundell I, Salmela S, Mannisto P.T, BeckSickinger A.G, Larhammar D. Agonists for neuropeptide Y receptors Y-1 and Y-5 stimulate different phases of feeding in guinea pigs. Br. J. Pharmacol. 2003;139:1433–1440. doi: 10.1038/sj.bjp.0705389. doi:10.1038/sj.bjp.0705389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz S.F, Alexander J.T. Analysis of neuropeptide Y-induced feeding—dissociation of Y1-receptor and Y2-receptor effects on natural meal patterns. Peptides. 1991;12:1251–1260. doi: 10.1016/0196-9781(91)90203-2. doi:10.1016/0196-9781(91)90203-2 [DOI] [PubMed] [Google Scholar]
- Leiter E.H, Kintner J, Flurkey K, Beamer W.G, Naggert J.K. Physiologic and endocrinologic characterization of male sex-biased diabetes in C57BLKS/J mice congenic for the fat mutation at the carboxypeptidease E locus. Endocrine. 1999;10:57–66. doi: 10.1385/endo:10:1:57. doi:10.1385/ENDO:10:1:57 [DOI] [PubMed] [Google Scholar]
- leRoux C.W, Bloom S.R. Peptide YY, appetite and food intake. Proc. Nutr. Soc. 2005;64:213–216. doi: 10.1079/pns2005427. doi:10.1079/PNS2005427 [DOI] [PubMed] [Google Scholar]
- Levin B.E. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am. J. Physiol. 1999;45:R382–R387. doi: 10.1152/ajpregu.1999.276.2.R382. [DOI] [PubMed] [Google Scholar]
- Levin B.E. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Phil. Trans. R. Soc. B. 2006;361 doi: 10.1098/rstb.2006.1851. doi:10.1098/rstb.2006.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. Am. J. Physiol. 1997;41:R1365–R1370. doi: 10.1152/ajpregu.1997.272.5.R1365. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. 2002a;282:R46–R54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A. Reduced central leptin sensitivity in rats with diet-induced obesity. Am. J. Physiol. 2002b;283:R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am. J. Physiol. 2002c;283:R1087–R1093. doi: 10.1152/ajpregu.00402.2002. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am. J. Physiol. 2004;286:R771–R778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am. J. Physiol. 1998;44:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- Levine A.S, Morley J.E. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. doi:10.1016/0196-9781(84)90165-7 [DOI] [PubMed] [Google Scholar]
- Levin B.E, Triscari J, Sullivan A.C. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am. J. Physiol. 1983;245:R364–R371. doi: 10.1152/ajpregu.1983.245.3.R364. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Finnegan M, Triscari J, Sullivan A.C. Brown adipose and metabolic features of chronic diet-induced obesity. Am. J. Physiol. 1985;248:R717–R723. doi: 10.1152/ajpregu.1985.248.6.R717. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A, Balkan B, Keesey R.E. Selective breeding for diet-induced obesity and resistance in Sprague–Dawley rats. Am. J. Physiol. 1997;42:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Govek E.K, Dunn-Meynell A.A. Reduced glucose-induced neuronal activation in the hypothalamus of diet-induced obese rats. Brain Res. 1998;808:317–319. doi: 10.1016/s0006-8993(98)00839-7. doi:10.1016/S0006-8993(98)00839-7 [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A, McMinn J.E, Alperovich M, CunninghamBussel A, Chua S.C. A new obesity-prone, glucose-intolerant rat strain (F.DIO) Am. J. Physiol. 2003;285:R1184–R1191. doi: 10.1152/ajpregu.00267.2003. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A, Banks W.A. Obesity-prone rats have normal blood–brain barrier transport but defective central leptin signaling before obesity onset. Am. J. Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- Lewis D.E, Shellard L, Koeslag D.G, Boer D.E, McCarthy H.D, McKibbin P.E, Russell J.C, Williams G. Intense exercise and food restriction cause similar hypothalamic neuropeptide-Y increases in rats. Am. J. Physiol. 1993;264:E279–E284. doi: 10.1152/ajpendo.1993.264.2.E279. [DOI] [PubMed] [Google Scholar]
- Li A.J, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur. J. Neurosci. 2004;19:2147–2154. doi: 10.1111/j.1460-9568.2004.03287.x. doi:10.1111/j.1460-9568.2004.03287.x [DOI] [PubMed] [Google Scholar]
- Lin X, Chavez M.R, Bruch R.C, Kilroy G.E, Simmons L.A, Lin L, Braymer H.D, Bray G.A, York D.A. The effects of a high fat diet on leptin mRNA, serum leptin and the response to leptin are not altered in a rat strain susceptible to high fat diet-induced obesity. J. Nutr. 1998;128:1606–1613. doi: 10.1093/jn/128.10.1606. [DOI] [PubMed] [Google Scholar]
- Lin S, Thomas T.C, Storlien L.H, Huang X.F. Development of high fat diet-induced obesity and leptin resistance in C57BI/6J mice. Int. J. Obes. 2000a;24:639–646. doi: 10.1038/sj.ijo.0801209. doi:10.1038/sj.ijo.0801209 [DOI] [PubMed] [Google Scholar]
- Lin S, Storlien L.H, Huang X.F. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000b;875:89–95. doi: 10.1016/s0006-8993(00)02580-4. doi:10.1016/S0006-8993(00)02580-4 [DOI] [PubMed] [Google Scholar]
- Lopez M, Seoane L.M, Tovar S, Garcia M, Nogueiras R, Dieguez C, Senaris R.M. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–148. doi: 10.1007/s00125-004-1596-z. doi:10.1007/s00125-004-1596-z [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta F.J, Nyce J.W, Griffinbiggs T.A, Ice J.C, Myers R.D. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypothalamus underlie NPY hypothermia and feeding in rats. Proc. R. Soc. B. 1996;263:881–886. doi: 10.1098/rspb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Louis-Sylvestre J, Giachetti I, Le Magnen J. Sensory versus dietary factors in cafeteria-induced overweight. Physiol. Behav. 1984;32:901–905. doi: 10.1016/0031-9384(84)90275-0. doi:10.1016/0031-9384(84)90275-0 [DOI] [PubMed] [Google Scholar]
- Lynch W.C, Grace M, Billington C.J, Levine A.S. Effects of Neuropeptide-Y on ingestion of flavored solutions in nondeprived rats. Physiol. Behav. 1993;54:877–880. doi: 10.1016/0031-9384(93)90295-q. doi:10.1016/0031-9384(93)90295-Q [DOI] [PubMed] [Google Scholar]
- Lynch W.C, Hart P, Babcock A.M. Neuropeptide Y attenuates satiety—evidence from a detailed analysis of patterns ingestion. Brain Res. 1994;636:28–34. doi: 10.1016/0006-8993(94)90171-6. doi:10.1016/0006-8993(94)90171-6 [DOI] [PubMed] [Google Scholar]
- Malabu U.H, Mc Carthy H.D, Mc Kibbin P.E, Williams G. Peripheral insulin administration attenuates the increase in neuropeptide-Y concentrations in the hypothalamic arcuate nucleus of fasted rats. Peptides. 1992;13:1097–1102. doi: 10.1016/0196-9781(92)90013-s. doi:10.1016/0196-9781(92)90013-S [DOI] [PubMed] [Google Scholar]
- MarinBivens C.L, Kalra S.P, Olster D.H. Intraventricular injection of neuropeptide Y antisera curbs weight gain and feeding, and increases the display of sexual behaviors in obese Zucker female rats. Regul. Pept. 1998;75–76:327–334. doi: 10.1016/s0167-0115(98)00085-8. doi:10.1016/S0167-0115(98)00085-8 [DOI] [PubMed] [Google Scholar]
- Marks J.L, Li M, Schwartz M, Porte D, Baskin D.G. Effect of fasting on regional levels of neuropeptide-Y messenger RNA and insulin receptors in the rat hypothalamus—an autoradiographic study. Mol. Cell. Neurosci. 1992;3:199–205. doi: 10.1016/1044-7431(92)90039-5. doi:10.1016/1044-7431(92)90039-5 [DOI] [PubMed] [Google Scholar]
- Marks J.L, Waite K, Li M. Effects of streptozotocin-induced diabetes-mellitus and insulin treatment on neuropeptide-Y messenger RNA in the rat hypothalamus. Diabetologia. 1993;36:497–502. doi: 10.1007/BF02743264. [DOI] [PubMed] [Google Scholar]
- Marks J.L, Waite K, CameronSmith D, Blair S.C, Cooney G.J. Effects of gold thioglucose on neuropeptide Y messenger RNA levels in the mouse hypothalamus. Am. J. Physiol. 1996;39:R1208–R1214. doi: 10.1152/ajpregu.1996.270.6.R1208. [DOI] [PubMed] [Google Scholar]
- Marsh D.J, Hollopeter G, Kafer K.E, Palmiter R.D. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat. Med. 1998;4:718–721. doi: 10.1038/nm0698-718. doi:10.1038/nm0698-718 [DOI] [PubMed] [Google Scholar]
- Mashiko S, et al. Characterization of neuropeptide Y (NPY) Y5 receptor-mediated obesity in mice: chronic intracerebroventricular infusion of D-Trp(34)NPY. Endocrinology. 2003;144:1793–1801. doi: 10.1210/en.2002-0119. doi:10.1210/en.2002-0119 [DOI] [PubMed] [Google Scholar]
- McCarthy H.D, McKibbin P.E, Holloway B, Mayers R, Williams G. Hypothalamic neuropeptide-Y receptor characteristics and NPY-induced feeding responses in lean and obese Zucker rats. Life Sci. 1991;49:1491–1497. doi: 10.1016/0024-3205(91)90049-h. doi:10.1016/0024-3205(91)90049-H [DOI] [PubMed] [Google Scholar]
- McKibbin P.E, Cotton S.J, McMillan S, Holloway B, Mayers R, McCarthy H.D, Williams G. Altered neuropeptide-Y concentrations in specific hypothalamic regions of obese (fa/fa) Zucker rats—possible relationship to obesity and neuroendocrine disturbances. Diabetes. 1991;40:1423–1429. doi: 10.2337/diab.40.11.1423. [DOI] [PubMed] [Google Scholar]
- McKibbin P.E, McCarthy H.D, Shaw P, Williams G. Insulin deficiency is a specific stimulus to hypothalamic Neuropeptide-Y—a comparison of the effects of insulin replacement and food restriction in streptozocin-diabetic rats. Peptides. 1992;13:721–727. doi: 10.1016/0196-9781(92)90178-6. doi:10.1016/0196-9781(92)90178-6 [DOI] [PubMed] [Google Scholar]
- McLaughlin C.L, Tou J.S, Rogan G.J, Baile C.A. Full amino acid sequence of centrally administered NPY required for maximal food intake response. Physiol. Behav. 1991;49:521–526. doi: 10.1016/0031-9384(91)90274-r. doi:10.1016/0031-9384(91)90274-R [DOI] [PubMed] [Google Scholar]
- McMinn J.E, Seeley R.J, Wilkinson C.W, Havel P.J, Woods S.C, Schwartz M.W. NPY-induced overfeeding suppresses hypothalamic NPY mRNA expression: potential roles of plasma insulin and leptin. Regul. Pept. 1998;75–76:425–431. doi: 10.1016/s0167-0115(98)00098-6. doi:10.1016/S0167-0115(98)00098-6 [DOI] [PubMed] [Google Scholar]
- McShane T.M, Petersen S.L, McCrone S, Keisler D.H. Influence of food restriction on neuropeptide-Y, proopiomelanocortin, and luteinizing hormone-releasing hormone gene expression in sheep hypothalami. Biol. Reprod. 1993;49:831–839. doi: 10.1095/biolreprod49.4.831. doi:10.1095/biolreprod49.4.831 [DOI] [PubMed] [Google Scholar]
- McShane T.M, Wilson M.E, Wise P.M. Effects of lifelong moderate caloric restriction on levels of neuropeptide Y, proopiomelanocortin, and galanin mRNA. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B14–B21. doi: 10.1093/gerona/54.1.b14. [DOI] [PubMed] [Google Scholar]
- Menendez J.A, Mc Gregor I.A, Healey P.A, Atrens D.M, Leibowitz S.F. Metabolic effects of neuropeptide Y injections into the paraventricular nucleus of the hypothalamus. Brain Res. 1990;516:8–14. doi: 10.1016/0006-8993(90)90890-n. doi:10.1016/0006-8993(90)90890-N [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Hoggard N, Williams L.M, Lawrence C.B, Hannah L.T, Morgan P.J, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J. Neuroendocrinol. 1996a;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. doi:10.1046/j.1365-2826.1996.05161.x [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Lawrence C.B, Atkinson T. Regulation of galanin gene expression in the hypothalamic paraventricular nucleus of the obese Zucker rat by manipulation of dietary macronutrients. Mol. Brain Res. 1996b;43:202–208. doi: 10.1016/s0169-328x(96)00174-x. doi:10.1016/S0169-328X(96)00174-X [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Moar K.M, Rayner D.V, Trayhurn P, Hoggard N. Regulation of leptin receptor and NPY gene expression in hypothalamus of leptin-treated obese (ob/ob) and cold-exposed lean mice. FEBS Lett. 1997;402:185–188. doi: 10.1016/s0014-5793(96)01525-6. doi:10.1016/S0014-5793(96)01525-6 [DOI] [PubMed] [Google Scholar]
- Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by administration of 2-deoxy-d-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Mol. Brain Res. 1995;33:305–310. doi: 10.1016/0169-328x(95)00151-h. doi:10.1016/0169-328X(95)00151-H [DOI] [PubMed] [Google Scholar]
- Miner J.L, Della-Fera M.A, Paterson J.A, Baile C.A. Lateral cerebroventricular injection of neuropeptide Y stimulates feeding in the sheep. Am. J. Physiol. 1989;257:R383–R387. doi: 10.1152/ajpregu.1989.257.2.R383. [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Kanai S, Ohta M, Kawanami T, Kono A, Funakoshi A. Lack of satiety effect of cholecystokinin (CCK) in a new rat model not expressing the CCK-A receptor gene. Neurosci. Lett. 1994;180:143–146. doi: 10.1016/0304-3940(94)90507-x. doi:10.1016/0304-3940(94)90507-X [DOI] [PubMed] [Google Scholar]
- Moesgaard S.G, Ahren B, Carr R.D, Gram D.X, Brand C.L, Sundler F. Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regul. Pept. 2004;120:261–267. doi: 10.1016/j.regpep.2004.03.018. doi:10.1016/j.regpep.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK1 receptors. Phil. Trans. R. Soc. B. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1857. doi:10.1098/rstb.2006.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T.H, Katz L.F, PlataSalaman C.R, Schwartz G.J. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am. J. Physiol. 1998;43:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- Moran T.H, Lee P, Ladenheim E.E, Schwartz G.J. Responsivity to NPY and melanocortins in obese OLETF rats lacking CCK-A receptors. Physiol. Behav. 2002;75:397–402. doi: 10.1016/s0031-9384(01)00667-9. doi:10.1016/S0031-9384(01)00667-9 [DOI] [PubMed] [Google Scholar]
- Morgan D.G.A, Lambert P.D, Smith D.M, Wilding J.P.H, Bloom S.R. Reduced NPY induced feeding in diabetic but not steroid-treated rats: lack of evidence for changes in receptor number or affinity. J. Neuroendocrinol. 1996;8:283–290. doi: 10.1046/j.1365-2826.1996.04565.x. doi:10.1046/j.1365-2826.1996.04565.x [DOI] [PubMed] [Google Scholar]
- Morley J.E, Levine A.S, Gosnell B.A, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am. J. Physiol. 1987a;252:R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- Morley J.E, Hernandez E.N, Flood J.F. Neuropeptide Y increases food intake in mice. Am. J. Physiol. 1987b;253:R516–R522. doi: 10.1152/ajpregu.1987.253.3.R516. [DOI] [PubMed] [Google Scholar]
- Morris Y.A, Crews D. The effects of exogenous neuropeptide-Y on feeding and sexual behavior in the red-sided garter snake (Thamnophis sirtalis parietalis) Brain Res. 1990;530:339–341. doi: 10.1016/0006-8993(90)91307-3. doi:10.1016/0006-8993(90)91307-3 [DOI] [PubMed] [Google Scholar]
- Morris M.J, Pavia M.M. Increased endogenous noradrenaline and neuropeptide Y release from the hypothalamus of streptozotocin diabetic rats. Brain Res. 2004;1006:100–106. doi: 10.1016/j.brainres.2004.02.002. doi:10.1016/j.brainres.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Morton G.J, Niswender K.D, Rhodes C.J, Myers M.G, Blevins J.E, Baskin D.G, Schwartz M.W. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. doi:10.1210/en.2002-0115 [DOI] [PubMed] [Google Scholar]
- Moustaïd Moussa N, Claycombe K.J. The yellow mouse obesity syndrome and mechanisms of Agouti-induced obesity. Obes. Res. 1999;7:506–514. doi: 10.1002/j.1550-8528.1999.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Mullins D, Kirby D, Hwa J, Guzzi M, Rivier J, Parker E. Identification of potent and selective neuropeptide YY1 receptor agonists with orexigenic activity in vivo. Mol. Pharmacol. 2001;60:534–540. [PubMed] [Google Scholar]
- Munzberg H, Flier J.S, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. doi:10.1210/en.2004-0726 [DOI] [PubMed] [Google Scholar]
- Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci. Lett. 1999;264:113–116. doi: 10.1016/s0304-3940(99)00185-8. doi:10.1016/S0304-3940(99)00185-8 [DOI] [PubMed] [Google Scholar]
- Nagamori K, Ishibashi M, Shiraishi T, Oomura Y, Sasaki K. Effects of leptin on hypothalamic arcuate neurons in Wistar and Zucker rats: an in vitro study. Exp. Biol. Med. 2003;228:1162–1167. doi: 10.1177/153537020322801010. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. doi:10.1038/35051587 [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. doi:10.1016/S0306-4522(98)00141-9 [DOI] [PubMed] [Google Scholar]
- Naveilhan P, et al. Normal feeding behavior, body weight and leptin response require the neuropeptide YY2 receptor. Nat. Med. 1999;5:1188–1193. doi: 10.1038/13514. doi:10.1038/13514 [DOI] [PubMed] [Google Scholar]
- Nillni E.A, Xie W.H, Mulcahy L, Sanchez V.C, Wetsel W.C. Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpe(fat/fat) mice. J. Biol. Chem. 2002;277:48 587–48 595. doi: 10.1074/jbc.M206702200. doi:10.1074/jbc.M206702200 [DOI] [PubMed] [Google Scholar]
- Nobentrauth K, Naggert J.K, North M.A, Nishina P.M. A candidate gene for the mouse mutation tubby. Nature. 1996;380:534–538. doi: 10.1038/380534a0. doi:10.1038/380534a0 [DOI] [PubMed] [Google Scholar]
- Oscai L.B, Brown M.M, Miller W.C. Effect of dietary fat on food intake, growth and body composition in rats. Growth. 1984;48:415–424. [PubMed] [Google Scholar]
- O'Shea R.D, Gundlach A.L. Preproneuropeptide-Y messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat is increased by food deprivation or dehydration. J. Neuroendocrinol. 1991;3:11–14. doi: 10.1111/j.1365-2826.1991.tb00232.x. [DOI] [PubMed] [Google Scholar]
- O'Shea D, et al. Neuropeptide Y induced feeding in the rat is mediated by a novel receptor. Endocrinology. 1997;138:196–202. doi: 10.1210/endo.138.1.4899. doi:10.1210/en.138.1.196 [DOI] [PubMed] [Google Scholar]
- Paez X.M, Nyce J.W, Myers R.D. Differential feeding responses evoked in the rat by NPY and NPY1-27 injected intracerebroventricularly. Pharmacol. Biochem. Behav. 1991;38:379–384. doi: 10.1016/0091-3057(91)90295-d. doi:10.1016/0091-3057(91)90295-D [DOI] [PubMed] [Google Scholar]
- Park E.S, Yi S.J, Kim J.S, Lee H.S, Lee I.S, Seong J.K, Jin H.K, Yoon Y.S. Changes in orexin-A and neuropeptide Y expression in the hypothalamus of the fasted and high-fat diet fed rats. J. Vet. Sci. 2004;5:295–302. [PubMed] [Google Scholar]
- Parker R.M.C, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. doi:10.1046/j.1460-9568.1999.00553.x [DOI] [PubMed] [Google Scholar]
- Parker E.M, Balasubramaniam A, Guzzi M, Mullins D.E, Salisbury B.G, Sheriff S, Witten M.B, Hwa J.J. [D-Trp(34)] neuropeptide Y is a potent and selective neuropeptide YY5 receptor agonist with dramatic effects on food intake. Peptides. 2000;21:393–399. doi: 10.1016/s0196-9781(00)00156-x. doi:10.1016/S0196-9781(00)00156-X [DOI] [PubMed] [Google Scholar]
- Parrott R.F, Heavens R.P, Baldwin B.A. Stimulation of feeding in the satiated pig by intracerebroventricular injection of neuropeptide Y. Physiol. Behav. 1986;36:523–525. doi: 10.1016/0031-9384(86)90325-2. doi:10.1016/0031-9384(86)90325-2 [DOI] [PubMed] [Google Scholar]
- Pau M.Y.C, Pau K.Y.F, Spies H.G. Characterization of central actions of neuropeptide Y on food and water intake in rabbits. Physiol. Behav. 1988;44:797–802. doi: 10.1016/0031-9384(88)90065-0. doi:10.1016/0031-9384(88)90065-0 [DOI] [PubMed] [Google Scholar]
- Perusse L, Rankinen T, Zuberi A, Chagnon Y.C, Weisnagel S.J, Argyropoulos G, Walts B, Snyder E.E, Bouchard C. The human obesity gene map: the 2004 update. Obes. Res. 2005;13:381–490. doi: 10.1038/oby.2005.50. [DOI] [PubMed] [Google Scholar]
- Pesonen U, Huupponen R, Rouru J, Koulu M. Hypothalamic neuropeptide expression after food restriction in Zucker rats—evidence of persistent neuropeptide-Y gene activation. Mol. Brain Res. 1992;16:255–260. doi: 10.1016/0169-328x(92)90233-2. doi:10.1016/0169-328X(92)90233-2 [DOI] [PubMed] [Google Scholar]
- Petro A.E, Cotter J, Cooper D.A, Peters J.C, Surwit S.J, Surwit R.S. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. doi:10.1016/j.metabol.2003.11.018 [DOI] [PubMed] [Google Scholar]
- Phillips M.S, Liu Q.Y, Hammond H.A, Dugan V, Hey P.J, Caskey C.T, Hess J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. doi:10.1038/ng0596-18 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J. Neuroendocrinol. 1999a;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. doi:10.1046/j.1365-2826.1999.00357.x [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Melchior K, Rake A, Rohde W, Dorner G. Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport. 1999b;10:3211–3216. doi: 10.1097/00001756-199910190-00016. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides. 2000;34:1–6. doi: 10.1054/npep.1999.0778. doi:10.1054/npep.1999.0778 [DOI] [PubMed] [Google Scholar]
- Polidori C, Ciccocioppo R, Regoli D, Massi M. Neuropeptide Y receptor(s) mediating feeding in the rat: characterization with antagonists. Peptides. 2000;21:29–35. doi: 10.1016/s0196-9781(99)00170-9. doi:10.1016/S0196-9781(99)00170-9 [DOI] [PubMed] [Google Scholar]
- Ponsalle P, Srivastava L.S, Uht R.M, White J.D. Glucocorticoids are required for food deprivation-induced increases in hypothalamic neuropeptide-Y expression. J. Neuroendocrinol. 1992;4:585–591. doi: 10.1111/j.1365-2826.1992.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Porte D, Baskin D.G, Schwartz M.W. Leptin and insulin action in the central nervous system. Nutr. Rev. 2002;60:S20–S29. doi: 10.1301/002966402320634797. doi:10.1301/002966402320634797 [DOI] [PubMed] [Google Scholar]
- Pralong F.P, Corder R, Gaillard R.C. The effects of chronic glucocorticoid excess, adrenalectomy and stress on neuropeptide-Y in individual rat hypothalamic nuclei. Neuropeptides. 1993;25:223–231. doi: 10.1016/0143-4179(93)90107-l. doi:10.1016/0143-4179(93)90107-L [DOI] [PubMed] [Google Scholar]
- Pronchuk N, Colmers W.F. NPY presynaptic actions are reduced in the hypothalamic mpPVN of obese (fa/fa), but not lean, Zucker rats in vitro. Br. J. Pharmacol. 2004;141:1032–1036. doi: 10.1038/sj.bjp.0705699. doi:10.1038/sj.bjp.0705699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Azain M.J, Hartzell D.L, Baile C.A. Increased leptin resistance as rats grow to maturity. Proc. Soc. Exp. Biol. Med. 1998;219:160–165. doi: 10.3181/00379727-219-44330. [DOI] [PubMed] [Google Scholar]
- Qian S, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. doi:10.1128/MCB.22.14.5027-5035.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposinho P.D, Pierroz D.D, Broqua P, White R.B, Pedrazzini T, Aubert M.L. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol. Cell. Endocrinol. 2001;185:195–204. doi: 10.1016/s0303-7207(01)00620-7. doi:10.1016/S0303-7207(01)00620-7 [DOI] [PubMed] [Google Scholar]
- Redrobe J.P, Dumont Y, StPierre J.A, Quirion R. Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res. 1999;848:153–166. doi: 10.1016/s0006-8993(99)02119-8. doi:10.1016/S0006-8993(99)02119-8 [DOI] [PubMed] [Google Scholar]
- Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. doi:10.1016/j.cmet.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Robinson S.W, Dinulescu D.M, Cone R.D. Genetic models of obesity and energy balance in the mouse. Annu. Rev. Genet. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. doi:10.1146/annurev.genet.34.1.687 [DOI] [PubMed] [Google Scholar]
- Rothwell N, Stock M. Regulation of energy balance in two models of reversible obesity in the rat. J. Comp. Physiol. Psychol. 1979;93:1024–1034. doi: 10.1037/h0077631. [DOI] [PubMed] [Google Scholar]
- Rovere C, Viale A, Nahon J.L, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology. 1996;137:2954–2958. doi: 10.1210/endo.137.7.8770919. doi:10.1210/en.137.7.2954 [DOI] [PubMed] [Google Scholar]
- Rowland N.E. Peripheral and central satiety factors in neuropeptide Y-induced feeding in rats. Peptides. 1988;9:989–992. doi: 10.1016/0196-9781(88)90078-2. doi:10.1016/0196-9781(88)90078-2 [DOI] [PubMed] [Google Scholar]
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. doi:10.1016/j.yfrne.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Sahu A, Dube M.G, Kalra S.P, Kalra P.S. Bilateral neural transections at the level of mesencephalon increase food intake and reduce latency to onset of feeding in response to neuropeptide Y. Peptides. 1988a;9:1269–1273. doi: 10.1016/0196-9781(88)90191-x. doi:10.1016/0196-9781(88)90191-X [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra S.P, Crowley W.R, Kalra P.S. Evidence that NPY-containing neurons in the brainstem project into selected hypothalamic nuclei: implication in feeding behavior. Brain Res. 1988b;457:376–378. doi: 10.1016/0006-8993(88)90710-x. doi:10.1016/0006-8993(88)90710-X [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra P.S, Kalra S.P. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988c;9:83–86. doi: 10.1016/0196-9781(88)90013-7. doi:10.1016/0196-9781(88)90013-7 [DOI] [PubMed] [Google Scholar]
- Sahu A, Sninsky C.A, Kalra P.S, Kalra S.P. Neuropeptide-Y concentration in microdissected hypothalamic regions and in vitro release from the medial basal hypothalamus-preoptic area of streptozotocin-diabetic rats with and without insulin substitution therapy. Endocrinology. 1990;126:192–198. doi: 10.1210/endo-126-1-192. [DOI] [PubMed] [Google Scholar]
- Sahu A, White J.D, Kalra P.S, Kalra S.P. Hypothalamic neuropeptide-Y gene expression in rats on scheduled feeding regimen. Mol. Brain Res. 1992;15:15–18. doi: 10.1016/0169-328x(92)90145-2. doi:10.1016/0169-328X(92)90145-2 [DOI] [PubMed] [Google Scholar]
- Sahu A, Sninsky C.A, Kalra S.P. Evidence that hypothalamic neuropeptide Y gene expression and NPY levels in the paraventricular nucleus increase before the onset of hyperphagia in experimental diabetes. Brain Res. 1997;755:339–342. doi: 10.1016/s0006-8993(97)00192-3. doi:10.1016/S0006-8993(97)00192-3 [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Cusin I, Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B. Intracerebroventricular administration of neuropeptide Y to normal rats increases obese gene expression in white adipose tissue. Diabetologia. 1996;39:353–356. doi: 10.1007/BF00418353. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Cusin I, RohnerJeanrenaud F, Jeanrenaud B. Adrenalectomy prevents the obesity syndrome produced by chronic central neuropeptide Y infusion in normal rats. Diabetes. 1997;46:209–214. doi: 10.2337/diab.46.2.209. [DOI] [PubMed] [Google Scholar]
- Saladin R, Devos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. doi:10.1038/377527a0 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kershaw M, Finkelstein J.A, White J.D. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in genetically obese Zucker rats and its regulation by food deprivation. Endocrinology. 1990;127:730–737. doi: 10.1210/endo-127-2-730. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Finkelstein J.A, White J.D. Developmental aspects of differences in hypothalamic preproneuropeptide Y messenger ribonucleic acid content in lean and genetically obese Zucker rats. J. Neuroendocrinol. 1992;4:353–357. doi: 10.1111/j.1365-2826.1992.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Savontaus E, Conwell I.M, Wardlaw S.L. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. doi: 10.1016/s0006-8993(02)03674-0. doi:10.1016/S0006-8993(02)03674-0 [DOI] [PubMed] [Google Scholar]
- Sawchenko P.E, Swanson L.W, Grzanna R, Howe P.R.C, Bloom S.R, Polak J.M. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. doi:10.1002/cne.902410203 [DOI] [PubMed] [Google Scholar]
- Schaffhauser A.O, Stricker-Krongrad A, Brunner L, Cumin F, Gerald C, Whitebread S, Criscione L, Hofbauer K.G. Inhibition of food intake by neuropeptide Y Y5 receptor antisense oligodeoxynucleotides. Diabetes. 1997;46:1792–1798. doi: 10.2337/diab.46.11.1792. [DOI] [PubMed] [Google Scholar]
- Schaffhauser A.O, Whitebread S, Haener R, Hofbauer K.G, Stricker-Krongrad A. Neuropeptide Y YI receptor antisense oligodeoxynucleotides enhance food intake in energy-deprived rats. Regul. Pept. 1998;75–76:417–423. doi: 10.1016/s0167-0115(98)00097-4. doi:10.1016/S0167-0115(98)00097-4 [DOI] [PubMed] [Google Scholar]
- Schaffhauser A.O, Madiehe A.M, Braymer H.D, Bray G.A, York D.A. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes. Res. 2002;10:1188–1196. doi: 10.1038/oby.2002.161. [DOI] [PubMed] [Google Scholar]
- Schemmel R, Mickelsen O, Gill J.L. Dietary obesity in rats: body weight and body fat accretion in seven strains of rats. J. Nutr. 1970;100:1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Marks J.L, Sipols A.J, Baskin D.G, Woods S.C, Kahn S.E, Porte D. Central insulin administration reduces neuropeptide-Y messenger RNA expression in the arcuate nucleus of food-deprived lean (FA/FA) but not obese (fa/fa) Zucker rats. Endocrinology. 1991;128:2645–2647. doi: 10.1210/endo-128-5-2645. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, et al. Inhibition of hypothalamic neuropeptide-Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. doi:10.1210/en.130.6.3608 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Sipols A.J, Grubin C.E, Baskin D.G. Differential effect of fasting on hypothalamic expression of genes encoding neuropeptide-Y, galanin, and glutamic acid decarboxylase. Brain Res. Bull. 1993;31:361–367. doi: 10.1016/0361-9230(93)90228-4. doi:10.1016/0361-9230(93)90228-4 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Schwartz G.J, Whitney A, Skoglund C, Castonguay T.W, Moran T.H. Decreased responsiveness to dietary fat in Otsuka Long–Evans Tokushima fatty rats lacking CCK-A receptors. Am. J. Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Woods S.C, Porte D, Seeley R.J, Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci. Biobehav. Rev. 1987;11:155–162. doi: 10.1016/s0149-7634(87)80020-9. doi:10.1016/S0149-7634(87)80020-9 [DOI] [PubMed] [Google Scholar]
- Seeley R.J, Payne C.J, Woods S.C. Neuropeptide Y fails to increase intraoral intake in rats. Am. J. Physiol. 1995;37:R423–R427. doi: 10.1152/ajpregu.1995.268.2.R423. [DOI] [PubMed] [Google Scholar]
- Seeley R.J, et al. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm. Metab. Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- Seeley R.J, Drazen D.L, Clegg D.J. The critical role of the melanocortin system in the control of energy balance. Annu. Rev. Nutr. 2004;24:133–149. doi: 10.1146/annurev.nutr.24.012003.132428. doi:10.1146/annurev.nutr.24.012003.132428 [DOI] [PubMed] [Google Scholar]
- Sei M, Sei H, Shima K. Spontaneous activity, sleep, and body temperature in rats lacking the CCK-A receptor. Physiol. Behav. 1999;68:25–29. doi: 10.1016/s0031-9384(99)00146-8. doi:10.1016/S0031-9384(99)00146-8 [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-d-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11:117–121. doi: 10.1097/00001756-200001170-00023. [DOI] [PubMed] [Google Scholar]
- Shen F.S, Aguilera G, Loh Y.P. Altered biosynthesis and secretion of pro-opiomelanocortin in the intermediate and anterior pituitary of carboxpyeptidase E-deficient, Cpe(Fat)/Cpe(Fat) mice. Neuropeptides. 1999;33:276–280. doi: 10.1054/npep.1999.0045. doi:10.1054/npep.1999.0045 [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Oda T, Imaki T, Ling N, Demura H. Injection of antineuropeptide-Y gamma-globulin into the hypothalamic paraventricular nucleus decreases food intake in rats. Brain Res. 1993;601:313–316. doi: 10.1016/0006-8993(93)91727-a. doi:10.1016/0006-8993(93)91727-A [DOI] [PubMed] [Google Scholar]
- Shimbara T, Mondal M.S, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, Date Y, Nakazato M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci. Lett. 2004;369:75–79. doi: 10.1016/j.neulet.2004.07.060. doi:10.1016/j.neulet.2004.07.060 [DOI] [PubMed] [Google Scholar]
- Shintani M, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- Sindelar D.K, Mystkowski P, Marsh D.J, Palmiter R.D, Schwartz M.W. Attenuation of diabetic hyperphagia in neuropeptide Y-deficient mice. Diabetes. 2002;51:778–783. doi: 10.2337/diabetes.51.3.778. [DOI] [PubMed] [Google Scholar]
- Sindelar D.K, SteMarie L, Miura G.I, Palmiter R.D, McMinn J.E, Morton G.J, Schwartz M.W. Neuropeptide Y is required for hyperphagic feeding in response to neuroglucopenia. Endocrinology. 2004;145:3363–3368. doi: 10.1210/en.2003-1727. doi:10.1210/en.2003-1727 [DOI] [PubMed] [Google Scholar]
- Sindelar D.K, Palmiter R.D, Woods S.C, Schwartz M.W. Attenuated feeding responses to circadian and palatability cues in mice lacking neuropeptide Y. Peptides. 2005;26:2597–2602. doi: 10.1016/j.peptides.2005.04.018. doi:10.1016/j.peptides.2005.04.018 [DOI] [PubMed] [Google Scholar]
- Smith G.P, Epstein A.N. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am. J. Physiol. 1969;217:1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- Smith F.J, Campfield L.A, Moschera J.A, Bailon P.S, Burn P. Brain administration of OB protein (leptin) inhibits neuropeptide-Y-induced feeding in ob/ob mice. Regul. Pept. 1998;75–76:433–439. doi: 10.1016/s0167-0115(98)00099-8. doi:10.1016/S0167-0115(98)00099-8 [DOI] [PubMed] [Google Scholar]
- Smith B.K, Andrews P.K, West D.B. Macronutrient diet selection in thirteen mouse strains. Am. J. Physiol. 2000;278:R797–R805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- Smoller J.W, Truett G.E, Hirsch J, Leibel R.L. A molecular genetic method for genotyping fatty (fa/fa) rats. Am. J. Physiol. 1993;264:R8–R11. doi: 10.1152/ajpregu.1993.264.1.R8. [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Leibowitz S.F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. doi:10.1016/0024-3205(84)90032-8 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Leibowitz S.F. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc. Natl Acad. Sci. USA. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley B.G, Thomas W.J. Feeding responses to perifornical hypothalamic injection of neuropeptide-Y in relation to circadian rhythms of eating behavior. Peptides. 1993;14:475–481. doi: 10.1016/0196-9781(93)90135-4. doi:10.1016/0196-9781(93)90135-4 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Chin A.S, Leibowitz S.F. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res. Bull. 1985a;14:521–524. doi: 10.1016/0361-9230(85)90100-5. doi:10.1016/0361-9230(85)90100-5 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Daniel D.R, Chin A.S, Leibowitz S.F. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985b;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. doi:10.1016/0196-9781(85)90452-8 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Anderson K.C, Grayson M.H, Leibowitz S.F. Repeated hypothalamic stimulation with neuropeptide Y increases daily carbohydrate and fat intake and body weight gain in female rats. Physiol. Behav. 1989;46:173–177. doi: 10.1016/0031-9384(89)90251-5. doi:10.1016/0031-9384(89)90251-5 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Lanthier D, Chin A.S, Leibowitz S.F. Suppression of neuropeptide Y-elicited eating by adrenalectomy or hypophysectomy: reversal with corticosterone. Brain Res. 1989b;501:32–36. doi: 10.1016/0006-8993(89)91023-8. doi:10.1016/0006-8993(89)91023-8 [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Magdalin W, Seirafi A, Nguyen M.M, Leibowitz S.F. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1-receptor mediating this peptide's effect. Peptides. 1992;13:581–587. doi: 10.1016/0196-9781(92)90093-i. doi:10.1016/0196-9781(92)90093-I [DOI] [PubMed] [Google Scholar]
- Stanley B.G, Magdalin W, Seirafi A, Thomas W.J, Leibowitz S.F. The perifornical area—the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s) Brain Res. 1993;604:304–317. doi: 10.1016/0006-8993(93)90382-w. doi:10.1016/0006-8993(93)90382-W [DOI] [PubMed] [Google Scholar]
- Steinman J.L, Gunion M.W, Morley J.E. Forebrain and hindbrain involvement of neuropeptide Y in ingestive behaviors of rats. Pharmacol. Biochem. Behav. 1994;47:207–214. doi: 10.1016/0091-3057(94)90001-9. doi:10.1016/0091-3057(94)90001-9 [DOI] [PubMed] [Google Scholar]
- Ster A.M, Kowalski T.J, Dube M.G, Kalra S.P, Smith G.P. Decreased hypothalamic concentration of neuropeptide Y correlates with onset of hyperphagia fa/fa rats on postnatal day 12. Physiol. Behav. 2003;78:517–520. doi: 10.1016/s0031-9384(03)00079-9. doi:10.1016/S0031-9384(03)00079-9 [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Barbanel G, Beck B, Burlet A, Nicolas J.P, Burlet C. K+-stimulated neuropeptide-Y release into the paraventricular nucleus and relation to feeding behavior in free-moving rats. Neuropeptides. 1993;24:307–312. doi: 10.1016/0143-4179(93)90020-b. doi:10.1016/0143-4179(93)90020-B [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Max J.P, Musse N, Nicolas J.P, Burlet C, Beck B. Increased threshold concentrations of neuropeptide Y for a stimulatory effect on food intake in obese Zucker rats—changes in the microstructure of the feeding behavior. Brain Res. 1994;660:162–166. doi: 10.1016/0006-8993(94)90851-6. doi:10.1016/0006-8993(94)90851-6 [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Beck B, Burlet C. Enhanced feeding response to neuropeptide Y in hypothalamic neuropeptide Y-depleted rats. Eur. J. Pharmacol. 1996;295:27–34. doi: 10.1016/0014-2999(95)00647-8. doi:10.1016/0014-2999(95)00647-8 [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Kozak R, Burlet C, Nicolas J.P, Beck B. Physiological regulation of hypothalamic neuropeptide Y release in lean and obese rats. Am. J. Physiol. 1997;42:R2112–R2116. doi: 10.1152/ajpregu.1997.273.6.R2112. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Cumin F, Burlet C, Beck B. Hypothalamic neuropeptide Y and plasma leptin after long-term high-fat feeding in the rat. Neurosci. Lett. 1998;254:157–160. doi: 10.1016/s0304-3940(98)00678-8. doi:10.1016/S0304-3940(98)00678-8 [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul. Pept. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. doi:10.1016/S0167-0115(01)00344-5 [DOI] [PubMed] [Google Scholar]
- Stubdal H, et al. Targeted deletion of the tub mouse obesity gene reveals that tubby is a loss-of-function mutation. Mol. Cell. Biol. 2000;20:878–882. doi: 10.1128/mcb.20.3.878-882.2000. doi:10.1128/MCB.20.3.878-882.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit R.S, Feinglos M.N, Rodin J, Sutherland A, Petro A.E, Opara E.C, Kuhn C.M, Rebuffescrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. doi:10.1016/0026-0495(95)90123-X [DOI] [PubMed] [Google Scholar]
- Surwit R.S, Petro A.E, Parekh P, Collins S. Low plasma leptin in response to dietary fat in diabetes- and obesity-prone mice. Diabetes. 1997;46:1516–1520. doi: 10.2337/diab.46.9.1516. [DOI] [PubMed] [Google Scholar]
- Surwit R.S, Wang S.Y, Petro A.E, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc. Natl Acad. Sci. USA. 1998;95:4061–4065. doi: 10.1073/pnas.95.7.4061. doi:10.1073/pnas.95.7.4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb K, Szot P, White S.S, Liles L.C, Weinshenker D. The ketogenic diet does not alter brain expression of orexigenic neuropeptides. Epilepsy Res. 2004;62:35–39. doi: 10.1016/j.eplepsyres.2004.08.002. doi:10.1016/j.eplepsyres.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Patel H.R, Qi Y, Dushay J, Ahima R.S. Divergent effects of leptin in mice susceptible or resistant to obesity. Horm. Metab. Res. 2002;34:691–697. doi: 10.1055/s-2002-38251. doi:10.1055/s-2002-38251 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Hiraoka J, Hosoda K, Yamori Y, Nakao K, Koletsky R.J. Nonsense mutation of leptin receptor in the obese spontaneously hypertensive Koletsky rat. Nat. Genet. 1996;14:130–131. doi: 10.1038/ng1096-130. doi:10.1038/ng1096-130 [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Takahashi N, Kataoka K, Hirashima T, Kawano K, Miyasaka K, Funakoshi A, Kono A. A disrupted cholecystokinin A receptor gene induces diabetes in obese rats synergistically with ODB1 gene. Am. J. Physiol. 1998;37:E265–E270. doi: 10.1152/ajpendo.1998.274.2.E265. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Kristensen P, Stidsen C.E, Brand C.L, Larsen P.J. Central administration of Y5 receptor antisense decreases spontaneous food intake and attenuates feeding in response to exogenous neuropeptide Y. J. Endocrinol. 1998;159:307–312. doi: 10.1677/joe.0.1590307. doi:10.1677/joe.0.1590307 [DOI] [PubMed] [Google Scholar]
- Tartaglia L.A, et al. Identification and expression cloning of a leptin receptor, OB-r. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. doi:10.1016/0092-8674(95)90151-5 [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. doi:10.1038/296659a0 [DOI] [PubMed] [Google Scholar]
- Tempel D.L, Leibowitz S.F. Diurnal variations in the feeding responses to norepinephrine, neuropeptide-Y and galanin in the PVN. Brain Res. Bull. 1990;25:821–825. doi: 10.1016/0361-9230(90)90177-2. doi:10.1016/0361-9230(90)90177-2 [DOI] [PubMed] [Google Scholar]
- Tempel D.L, Leibowitz S.F. Adrenal steroid receptors: interactions with brain neuropeptide systems in relation to nutrient intake and metabolism. J. Neuroendocrinol. 1994;6:479–501. doi: 10.1111/j.1365-2826.1994.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Tempel D.L, Shor-Posner G, Dwyer D, Leibowitz S.F. Nocturnal patterns of macronutrient intake in freely feeding and food-deprived rats. Am. J. Physiol. 1989;256:R541–R548. doi: 10.1152/ajpregu.1989.256.2.R541. [DOI] [PubMed] [Google Scholar]
- Thompson R.H, Canteras N.S, Swanson L.W. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-l study in the rat. J. Comp. Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. doi:10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Thorsell A, Heilig M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides. 2002;36:182–193. doi: 10.1054/npep.2002.0897. doi:10.1054/npep.2002.0897 [DOI] [PubMed] [Google Scholar]
- Tkacs N.C, Levin B.E. Obesity-prone rats have preexisting defects in their counterregulatory response to insulin-induced hypoglycemia. Am. J. Physiol. 2004;287:R1110–R1115. doi: 10.1152/ajpregu.00312.2004. [DOI] [PubMed] [Google Scholar]
- Tortoriello D.V, McMinn J, Chua S.C. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–1247. doi: 10.1210/en.2003-1406. doi:10.1210/en.2003-1406 [DOI] [PubMed] [Google Scholar]
- Tracy A.L, Jarrard L.E, Davidson T.L. The hippocampus and motivation revisited: appetite and activity. Behav. Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. doi:10.1016/S0166-4328(01)00364-3 [DOI] [PubMed] [Google Scholar]
- Tritos N.A, Elmquist J.K, Mastaitis J.W, Flier J.S, MaratosFlier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein–promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. doi:10.1210/en.139.11.4634 [DOI] [PubMed] [Google Scholar]
- Truett G.E, Bahary N, Friedman J.M, Leibel R.L. Rat obesity gene fatty (fa) maps to chromosome-5—evidence for homology with the mouse gene diabetes (db) Proc. Natl Acad. Sci. USA. 1991;88:7806–7809. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, et al. Does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:U1–U3. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Bray G.A. GABA-related feeding control in genetically obese rats. Brain Res. 1991;540:48–54. doi: 10.1016/0006-8993(91)90491-d. doi:10.1016/0006-8993(91)90491-D [DOI] [PubMed] [Google Scholar]
- Turnbull A.V, et al. Selective antagonism of the NPYY5 receptor does not have a major effect on feeding in rats. Diabetes. 2002;51:2441–2449. doi: 10.2337/diabetes.51.8.2441. [DOI] [PubMed] [Google Scholar]
- Velkoska E, Cole T.J, Morris M.J. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am. J. Physiol. 2005;288:E1236–E1243. doi: 10.1152/ajpendo.00505.2004. [DOI] [PubMed] [Google Scholar]
- Walker H.C, Romsos D.R. Similar effects of NPY on energy metabolism and on plasma insulin in adrenalectomized ob/ob and lean mice. Am. J. Physiol. 1993;264:E226–E230. doi: 10.1152/ajpendo.1993.264.2.E226. [DOI] [PubMed] [Google Scholar]
- Walker P, Grouzmann E, Burnier M, Waeber B. The role of neuropeptide-Y in cardiovascular regulation. Trends Pharmacol. Sci. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. doi:10.1016/0165-6147(91)90518-W [DOI] [PubMed] [Google Scholar]
- Walter M.J, Scherrer J.F, Flood J.F, Morley J.E. Effects of localized injections of neuropeptide Y antibody on motor activity and other behaviors. Peptides. 1994;15:607–613. doi: 10.1016/0196-9781(94)90083-3. doi:10.1016/0196-9781(94)90083-3 [DOI] [PubMed] [Google Scholar]
- Wang J, Akabayashi A, Dourmashkin J, Yu H.J, Alexander J.T, Chae H.J, Leibowitz S.F. Neuropeptide Y in relation to carbohydrate intake, corticosterone and dietary obesity. Brain Res. 1998a;802:75–88. doi: 10.1016/s0006-8993(98)00551-4. doi:10.1016/S0006-8993(98)00551-4 [DOI] [PubMed] [Google Scholar]
- Wang W.G, Cain B.M, Beinfeld M.C. Adult carboxypeptidase E-deficient fat/fat mice have a near-total depletion of brain CCK 8 accompanied by a massive accumulation of glycine and arginine extended CCK—identification of CCK 8 Gly as the immediate precursor of CCK 8 in rodent brain. Endocrine. 1998b;9:329–332. doi: 10.1385/ENDO:9:3:329. doi:10.1385/ENDO:9:3:329 [DOI] [PubMed] [Google Scholar]
- Wang J, Dourmashkin J.T, Yun R, Leibowitz S.F. Rapid changes in hypothalamic neuropeptide Y produced by carbohydrate-rich meals that enhance corticosterone and glucose levels. Brain Res. 1999;848:124–136. doi: 10.1016/s0006-8993(99)02040-5. doi:10.1016/S0006-8993(99)02040-5 [DOI] [PubMed] [Google Scholar]
- Wang H.Q, Storlien L.H, Huang X.F. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am. J. Physiol. 2002;282:E1352–E1359. doi: 10.1152/ajpendo.00230.2001. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu X, Hentges S.T, Dunn-Meynell A.A, Levin B.E, Wang W, Routh V.H. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- Warwick Z.S, Schiffman S.S. Role of dietary fat in calorie intake and weight gain. Neurosci. Biobehav. Rev. 1992;16:585–596. doi: 10.1016/s0149-7634(05)80198-8. doi:10.1016/S0149-7634(05)80198-8 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Akabayashi A, McEwen B.S. Adrenal steroid regulation of neuropeptide Y(NPY) mRNA: differences between dentate hilus and locus coeruleus and arcuate nucleus. Mol. Brain Res. 1995;28:135–140. doi: 10.1016/0169-328x(94)00192-h. doi:10.1016/0169-328X(94)00192-H [DOI] [PubMed] [Google Scholar]
- Watanabe T.K, et al. Mutated G-protein-coupled receptor GPR10 is responsible for the hyperphagia/dyslipidaemia/obesity locus of Dmo1 in the OLETF rat. Clin. Exp. Pharmacol. Physiol. 2005;32:355–366. doi: 10.1111/j.1440-1681.2005.04196.x. doi:10.1111/j.1440-1681.2005.04196.x [DOI] [PubMed] [Google Scholar]
- Watson P.M, Commins S.P, Beiler R.J, Hatcher H.C, Gettys T.W. Differential regulation of leptin expression and function in A/J vs. C57BL/6J mice during diet-induced obesity. Am. J. Physiol. 2000;279:E356–E365. doi: 10.1152/ajpendo.2000.279.2.E356. [DOI] [PubMed] [Google Scholar]
- Welch C.C, Grace M.K, Billington C.J, Levine A.S. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am. J. Physiol. 1994;266:R426–R433. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- West D.B, Boozer C.N, Moody D.L, Atkinson R.L. Dietary obesity in 9 inbred mouse strains. Am. J. Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- White J.D, Kershaw M. Increased hypothalamic neuropeptide Y expression following food deprivation. Mol. Cell. Neurosci. 1990;1:41–48. doi: 10.1016/1044-7431(90)90040-b. doi:10.1016/1044-7431(90)90040-B [DOI] [PubMed] [Google Scholar]
- White J.D, Olchovsky D, Kershaw M, Berelowitz M. Increased hypothalamic content of preproneuropeptide-Y messenger ribonucleic acid in streptozotocin-diabetic rats. Endocrinology. 1990;126:765–772. doi: 10.1210/endo-126-2-765. [DOI] [PubMed] [Google Scholar]
- White B.D, Dean R.G, Edwards G.L, Martin R.J. Type II corticosteroid receptor stimulation increases NPY gene expression in basomedial hypothalamus of rats. Am. J. Physiol. 1994a;266:R1523–R1529. doi: 10.1152/ajpregu.1994.266.5.R1523. [DOI] [PubMed] [Google Scholar]
- White B.D, He B, Dean R.G, Martin R.J. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J. Nutr. 1994b;124:1152–1160. doi: 10.1093/jn/124.8.1152. [DOI] [PubMed] [Google Scholar]
- Widdowson P.S. Quantitative receptor autoradiography demonstrates a differential distribution of neuropeptide-Y Y-1 and Y-2 receptor subtypes in human and rat brain. Brain Res. 1993;631:27–38. doi: 10.1016/0006-8993(93)91182-r. doi:10.1016/0006-8993(93)91182-R [DOI] [PubMed] [Google Scholar]
- Widdowson P.S. Regionally-selective down-regulation of NPY receptor subtypes in the obese Zucker rat. Relationship to the Y5 ‘feeding’ receptor. Brain Res. 1997;758:17–25. doi: 10.1016/s0006-8993(97)00160-1. doi:10.1016/S0006-8993(97)00160-1 [DOI] [PubMed] [Google Scholar]
- Widdowson P.S, Upton R, Henderson L, Buckingham R, Wilson S, Williams G. Reciprocal regional changes in brain NPY receptor density during dietary restriction and dietary-induced obesity in the rat. Brain Res. 1997a;774:1–10. doi: 10.1016/s0006-8993(97)81680-0. doi:10.1016/S0006-8993(97)81680-0 [DOI] [PubMed] [Google Scholar]
- Widdowson P.S, Buckingham R, Williams G. Distribution of [Leu(31),Pro(34)]NPY-sensitive, BIBP3226-insensitive [I-125]PYY(3-36) binding sites in rat brain: possible relationship to Y-5 NPY receptors. Brain Res. 1997b;778:242–250. doi: 10.1016/s0006-8993(97)01102-5. doi:10.1016/S0006-8993(97)01102-5 [DOI] [PubMed] [Google Scholar]
- Widdowson P.S, Henderson L, Pickavance L, Buckingham R, Tadayyon M, Arch J.R.S, Williams G. Hypothalamic NPY status during positive energy balance and the effects of the NPY antagonist, BW1229U91, on the consumption of highly palatable energy-rich diet. Peptides. 1999;20:367–372. doi: 10.1016/s0196-9781(99)00044-3. doi:10.1016/S0196-9781(99)00044-3 [DOI] [PubMed] [Google Scholar]
- Wieland H.A, Engel W, Eberlein W, Rudolf K, Doods H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. doi:10.1038/sj.bjp.0702084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J.P.H, Gilbey S.G, Bailey C.J, Batt R.A, Williams G, Ghatei M.A, Bloom S.R. Increased neuropeptide-Y messenger ribonucleic acid (messenger RNA) and decreased neurotensin messenger RNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132:1939–1944. doi: 10.1210/endo.132.5.7682936. doi:10.1210/en.132.5.1939 [DOI] [PubMed] [Google Scholar]
- Wildman H.F, Chua S, Leibel R.L, Smith G.P. Effects of leptin and cholecystokinin in rats with a null mutation of the leptin receptor Lepr(Fak) Am. J. Physiol. 2000;278:R1518–R1523. doi: 10.1152/ajpregu.2000.278.6.R1518. [DOI] [PubMed] [Google Scholar]
- Willesen M.G, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. doi:10.1159/000054491 [DOI] [PubMed] [Google Scholar]
- Williams G, Steel J.H, Cardoso H, Ghatei M.A, Lee Y.C, Gill J.S, Burrin J.M, Polak J.M, Bloom S.R. Increased hypothalamic neuropeptide Y concentrations in diabetic rat. Diabetes. 1988;37:763–772. doi: 10.2337/diab.37.6.763. [DOI] [PubMed] [Google Scholar]
- Williams G, Gill J.S, Lee Y.C, Cardoso H.M, Okpere B.E, Bloom S.R. Increased neuropeptide Y concentrations in specific hypothalamic regions of streptozocin-induced diabetic rats. Diabetes. 1989;38:321–327. doi: 10.2337/diab.38.3.321. [DOI] [PubMed] [Google Scholar]
- Williams G, Cardoso H.M, Lee Y.C, Ball J.M, Ghatei M.A, Stock M.J, Bloom S.R. Hypothalamic regulatory peptides in obese and lean Zucker rats. Clin. Sci. 1991a;80:419–426. doi: 10.1042/cs0800419. [DOI] [PubMed] [Google Scholar]
- Williams G, Cardoso H, Lee Y.C, Ghatei M.A, Flatt P.R, Bailey C.J, Bloom S.R. Reduced hypothalamic neurotensin concentrations in the genetically obese diabetic (ob/ob) mouse—possible relationship to obesity. Metabolism. 1991b;40:1112–1116. doi: 10.1016/0026-0495(91)90139-n. doi:10.1016/0026-0495(91)90139-N [DOI] [PubMed] [Google Scholar]
- Williams G, Shellard L, Lewis D.E, McKibbin P.E, McCarthy H.D, Koeslag D.G, Russell J.C. Hypothalamic neuropeptide-Y disturbances in the obese (cp/cp) JCR–LA corpulent rat. Peptides. 1992;13:537–540. doi: 10.1016/0196-9781(92)90085-h. doi:10.1016/0196-9781(92)90085-H [DOI] [PubMed] [Google Scholar]
- Willie J.T, Chemelli R.M, Sinton C.M, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. doi:10.1146/annurev.neuro.24.1.429 [DOI] [PubMed] [Google Scholar]
- Wilson B.D, Bagnol D, Kaelin C.B, Ollmann M.M, Gantz I, Watson S.J, Barsh G.S. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology. 1999;140:2387–2397. doi: 10.1210/endo.140.5.6728. doi:10.1210/en.140.5.2387 [DOI] [PubMed] [Google Scholar]
- Wolak M.L, deJoseph M.R, Cator A.D, Mokashi A.S, Brownfield M.S, Urban J.H. Comparative distribution of neuropeptide YY1 and Y5 receptors in the rat brain by using immunohistochemistry. J. Comp. Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. doi:10.1002/cne.10823 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Seeley R.J. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16:894–902. doi: 10.1016/s0899-9007(00)00454-8. doi:10.1016/S0899-9007(00)00454-8 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Lutz T.A, Geary N, Langhans W. Pancreatic signals controlling food intake—insulin, glucagon and amylin. Phil. Trans. R. Soc. B. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. doi:10.1098/rspb.2006.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Peng X.S, Chua S.C, Okada N, Liu S.M, Nicolson M, Leibel R.L. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (Lepr): evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46:513–518. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- Wyss P, Levens N, Stricker-Krongrad A. Stimulation of feeding in lean but not in obese Zucker rats by a selective neuropeptide Y Y5 receptor agonist. Neuroreport. 1998a;9:2675–2677. doi: 10.1097/00001756-199808030-00046. [DOI] [PubMed] [Google Scholar]
- Wyss P, Stricker-Krongrad A, Brunner L, Miller J, Crossthwaite A, Whitebread S, Criscione L. The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not Y2 or Y4 subtypes. Regul. Pept. 1998b;75–76:363–371. doi: 10.1016/s0167-0115(98)00089-5. doi:10.1016/S0167-0115(98)00089-5 [DOI] [PubMed] [Google Scholar]
- Xin X.G, Huang X.F. Down-regulated NPY receptor subtype-5 mRNA expression in genetically obese mouse brain. Neuroreport. 1998;9:737–741. doi: 10.1097/00001756-199803090-00032. [DOI] [PubMed] [Google Scholar]
- Xu B, Li B.H, Rowland N.E, Kalra S.P. Neuropeptide Y injection into the fourth cerebroventricle stimulates c-fos expression in the paraventricular nucleus and other nuclei in the forebrain: effect of food consumption. Brain Res. 1995;698:227–231. doi: 10.1016/0006-8993(95)00905-6. doi:10.1016/0006-8993(95)00905-6 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, et al. Down regulation of the prepro-orexin gene expression in genetically obese mice. Mol. Brain Res. 1999;65:14–22. doi: 10.1016/s0169-328x(98)00320-9. doi:10.1016/S0169-328X(98)00320-9 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ueta Y, Serino R, Nomura M, Shibuya I, Yamashita H. Effects of food restriction on the hypothalamic prepro-orexin gene expression in genetically obese mice. Brain Res. Bull. 2000;51:515–521. doi: 10.1016/s0361-9230(99)00271-3. doi:10.1016/S0361-9230(99)00271-3 [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Dube M.G, Kalra P.S, Kalra S.P. The mPVN mediates blockade of NPY-induced feeding by a Y5 receptor antagonist: a c-FOS analysis. Peptides. 2001;22:507–514. doi: 10.1016/s0196-9781(01)00360-6. doi:10.1016/S0196-9781(01)00360-6 [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Honma S, Honma K. Effects of restricted daily feeding on neuropeptide Y release in the rat paraventricular nucleus. Am. J. Physiol. 1996;33:E589–E595. doi: 10.1152/ajpendo.1996.270.4.E589. [DOI] [PubMed] [Google Scholar]
- Zakrzewska K.E, Sainsbury A, Cusin I, Rouru J, Jeanrenaud B, RohnerJeanrenaud F. Selective dependence of intracerebroventricular neuropeptide Y-elicited effects on central glucocorticoids. Endocrinology. 1999;140:3183–3187. doi: 10.1210/endo.140.7.6874. doi:10.1210/en.140.7.3183 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. doi:10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- Ziotopoulou M, Mantzoros C.S, Hileman S.M, Flier J.S. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am. J. Physiol. 2000;279:E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
- Zukowska Z, Pons J, Lee E, Li L.J. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can. J. Physiol. Pharmacol. 2003;81:89–94. doi: 10.1139/y03-006. doi:10.1139/y03-006 [DOI] [PubMed] [Google Scholar]