Abstract
Complementary neurophysiological recordings in macaques and functional neuroimaging in humans show that the primary taste cortex in the rostral insula and adjoining frontal operculum provides separate and combined representations of the taste, temperature and texture (including viscosity and fat texture) of food in the mouth independently of hunger and thus of reward value and pleasantness. One synapse on, in the orbitofrontal cortex, these sensory inputs are for some neurons combined by learning with olfactory and visual inputs. Different neurons respond to different combinations, providing a rich representation of the sensory properties of food. In the orbitofrontal cortex, feeding to satiety with one food decreases the responses of these neurons to that food, but not to other foods, showing that sensory-specific satiety is computed in the primate (including human) orbitofrontal cortex. Consistently, activation of parts of the human orbitofrontal cortex correlates with subjective ratings of the pleasantness of the taste and smell of food. Cognitive factors, such as a word label presented with an odour, influence the pleasantness of the odour and the activation produced by the odour in the orbitofrontal cortex. These findings provide a basis for understanding how what is in the mouth is represented by independent information channels in the brain; how the information from these channels is combined; and how and where the reward and subjective affective value of food is represented and is influenced by satiety signals. Activation of these representations in the orbitofrontal cortex may provide the goal for eating, and understanding them helps to provide a basis for understanding appetite and its disorders.
Keywords: sensory-specific satiety, fat, food texture, taste, olfaction, viscosity
1. Introduction
The aims of this paper are to describe the rules of the cortical processing of taste and smell, how the pleasantness or affective value of taste and smell are represented in the brain and to relate this to the brain mechanisms underlying the control of appetite and food intake. To make the results relevant to understanding the control of human food intake, complementary evidence is provided by neurophysiological studies in non-human primates, and by functional neuroimaging studies in humans. A broad perspective on brain processing involved in emotion and in hedonic aspects of the control of food intake is provided by Rolls (2005).
2. Taste processing in the primate brain
(a) Pathways
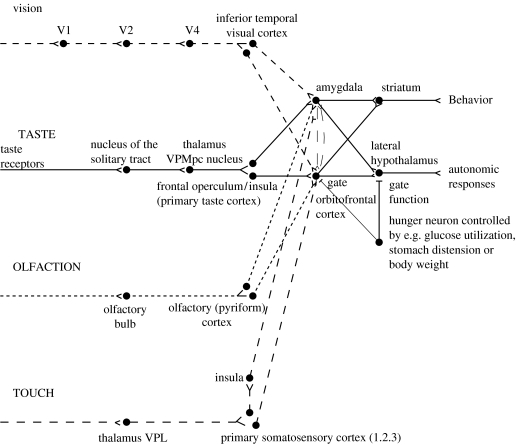
A diagram of the taste and related olfactory, somatosensory and visual pathways in primates is shown in figure 1. Of particular interest is that in primates there is a direct projection from the rostral part of the nucleus of the solitary tract (NTS) to the taste thalamus and thus to the primary taste cortex in the frontal operculum and adjoining insula, with no pontine taste area and associated subcortical projections as in rodents (Norgren 1984; Pritchard et al. 1986). This emphasis on cortical processing of taste in primates may be related to the great development of the cerebral cortex in primates. There is an advantage of using extensive and similar cortical analysis of inputs from every sensory modality before the analysed representations from each modality are brought together in multimodal regions, as is described below. The multimodal convergence that enables single neurons to respond to different combinations of taste, olfactory, texture, temperature and visual inputs to represent different flavours produced often by new combinations of sensory input is a theme of recent research that will be described.
Figure 1.
Schematic diagram of the taste and olfactory pathways in primates showing how they converge with each other and with visual pathways. The gate functions shown refer to the finding that the responses of taste neurons in the orbitofrontal cortex and the lateral hypothalamus are modulated by hunger. VPMpc, ventralposteromedial thalamic nucleus; V1, V2, V4, visual cortical areas.
(b) The primary taste cortex
The primary taste cortex in the primate anterior insula and adjoining frontal operculum contains not only taste neurons tuned to sweet, salt, bitter, sour (Scott et al. 1986; Yaxley et al. 1990; Rolls & Scott 2003) and umami as exemplified by monosodium glutamate (MSG; Baylis & Rolls 1991; Rolls et al. 1996b), but also other neurons that encode oral somatosensory stimuli including viscosity, fat texture, temperature and capsaicin (Verhagen et al. 2004). Some neurons in the primary taste cortex respond to particular combinations of taste and oral texture stimuli, but do not respond to olfactory stimuli or visual stimuli such as the sight of food (Verhagen et al. 2004). Neurons in the primary taste cortex do not represent the reward value of taste, i.e. the appetite for a food, in that their firing is not decreased to zero by feeding the taste to satiety (Rolls et al. 1988; Yaxley et al. 1988).
(c) The secondary taste cortex
A secondary cortical taste area in primates was discovered by Rolls et al. (1990) in the caudolateral orbitofrontal cortex, extending several millimetres in front of the primary taste cortex. One principle of taste processing is that by the secondary taste cortex, the tuning of neurons can become quite specific, with some neurons responding for example only to sweet taste. This specific tuning (especially when combined with olfactory inputs) helps to provide a basis for changes in appetite for some but not other foods eaten during a meal.
(d) Five prototypical tastes, including umami
In the primary and secondary taste cortex, there are many neurons that respond best to each of the four classical prototypical tastes sweet, salt, bitter and sour (Rolls 1997; Rolls & Scott 2003), but also there are many neurons that respond best to umami tastants such as glutamate (which is present in many natural foods such as tomatoes, mushrooms and milk; Baylis & Rolls 1991) and inosine monophosphate (which is present in meat and some fish such as tuna; Rolls et al. 1996b). This evidence, taken together with the identification of a glutamate taste receptor (Chaudhari et al. 2000), leads to the view that there are five prototypical types of taste information channels, with umami contributing, often in combination with corresponding olfactory inputs (Rolls et al. 1998), to the flavour of protein. In addition, other neurons respond to water, and others to the somatosensory stimuli astringency as exemplified by tannic acid (Critchley & Rolls 1996a), and to capsaicin (Rolls et al. 2003b; Kadohisa et al. 2004).
(e) The pleasantness of the taste of food
The modulation of the reward value of a sensory stimulus such as the taste of food by motivational state, e.g. hunger, is one important way in which motivational behaviour is controlled (Rolls 1999, 2005). The subjective correlate of this modulation is that food tastes pleasant when hungry, and tastes hedonically neutral when it has been eaten to satiety. We have found that the modulation of taste-evoked signals by motivation is not a property found in early stages of the primate gustatory system. The responsiveness of taste neurons in the NTS (Yaxley et al. 1985) and in the primary taste cortex (frontal opercular, Rolls et al. 1988; insular, Yaxley et al. 1988) is not attenuated by feeding to satiety. In contrast, in the secondary taste cortex, in the caudolateral part of the orbitofrontal cortex, it has been shown that the responses of the neurons to the taste of glucose decreased to zero while the monkey ate it to satiety, during the course of which the behaviour turned from avid acceptance to active rejection (Rolls et al. 1989). This modulation of responsiveness of the gustatory responses of the orbitofrontal cortex neurons by satiety could not have been due to peripheral adaptation in the gustatory system or to altered efficacy of gustatory stimulation after satiety was reached, because modulation of neuronal responsiveness by satiety was not seen at the earlier stages of the gustatory system, including the NTS, the frontal opercular taste cortex and the insular taste cortex.
(f) Sensory-specific satiety
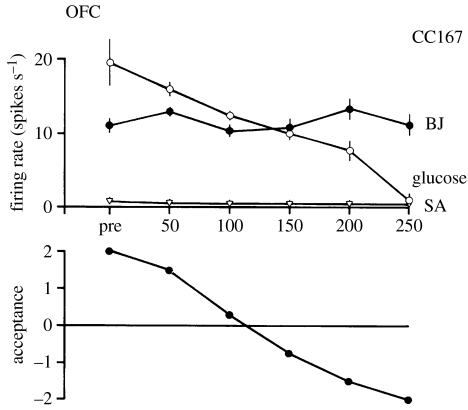
In the secondary taste cortex, it was also found that the decreases in the responsiveness of the neurons were relatively specific to the food with which the monkey had been fed to satiety. For example, in seven experiments in which the monkey was fed glucose solution, neuronal responsiveness decreased to the taste of the glucose but not to the taste of blackcurrant juice (e.g. figure 2). Conversely, in two experiments in which the monkey was fed to satiety with fruit juice, the responses of the neurons decreased to fruit juice but not to glucose (Rolls et al. 1989).
Figure 2.
The effect of feeding to satiety with glucose solution on the responses of two neurons in the secondary taste cortex to the taste of glucose and of blackcurrant juice (BJ). The spontaneous firing rate is also indicated (SA). Below the neuronal response data for each experiment, the behavioural measure of the acceptance or rejection of the solution on a scale from +2 to −2 (see text) is shown. The solution used to feed to satiety was 20% glucose. The monkey was fed 50 ml of the solution at each stage of the experiment as indicated along the abscissa, until he was satiated as shown by whether he accepted or rejected the solution. Pre—the firing rate of the neuron before the satiety experiment started. The values shown are the mean firing rate and its s.e. (After Rolls et al. 1989).
This evidence shows that the reduced acceptance of food which occurs when food is eaten to satiety, and the reduction in the pleasantness of its taste (Cabanac 1971; Rolls & Rolls 1977, 1982; Rolls et al. 1981a,b, 1982, 1983a) are not produced by a reduction in the responses of neurons in the NTS or frontal opercular or insular gustatory cortices to gustatory stimuli. Indeed, after feeding to satiety, humans reported that the taste of the food on which they had been satiated tasted almost as intense as when they were hungry, though much less pleasant (Rolls et al. 1983c). This comparison is consistent with the possibility that activity in the frontal opercular and insular taste cortices as well as the NTS does not reflect the pleasantness of the taste of a food, but rather its sensory qualities independently of motivational state. On the other hand, the responses of the neurons in the caudolateral orbitofrontal cortex taste area and in the lateral hypothalamus (Rolls et al. 1986) are modulated by satiety, and it is presumably in areas such as these that neuronal activity may be related to whether a food tastes pleasant, and to whether the food should be eaten (see further Scott et al. 1995; Critchley & Rolls 1996c; Rolls 1996, 1999, 2000a,b; Rolls & Scott 2003). In addition to providing an implementation of sensory-specific satiety (probably by habituation of the synaptic afferents to orbitofrontal neurons with a time course of the order of the length of a course of a meal), it is likely that visceral and other satiety-related signals reach the orbitofrontal cortex (as indicated in figure 1; from the NTS, via thalamic areas) and there modulate the representation of food, resulting in an output that reflects the reward (or appetitive) value of each food (Rolls 2005).
It is an important principle that the identity of a taste, and its intensity, are represented separately from its pleasantness. Thus it is possible to represent what a taste is, and to learn about it, even when we are not hungry.
3. The representation of flavour: convergence of olfactory and taste inputs
At some stage in taste processing, it is likely that taste representations are brought together with inputs from different modalities, e.g. with olfactory inputs to form a representation of flavour (see figure 1). We found (Rolls & Baylis 1994) that in the orbitofrontal cortex taste areas, of 112 single neurons which responded to any of these modalities, many were unimodal (taste 34%, olfactory 13%, visual 21%), but were found in close proximity to each other. Some single neurons showed convergence, responding for example to taste and visual inputs (13%), taste and olfactory inputs (13%) and olfactory and visual inputs (5%). Some of these multimodal single neurons had corresponding sensitivities in the two modalities, in that they responded best to sweet tastes (e.g. 1 M glucose), and responded more in a visual discrimination task to the visual stimulus which signified sweet fruit juice than to that which signified saline; or responded to sweet taste, and in an olfactory discrimination task to fruit odour. The different types of neurons (unimodal in different modalities, and multimodal) were frequently found close to one another in tracks made into this region, consistent with the hypothesis that the multimodal representations are actually being formed from unimodal inputs to this region.
It thus appears to be in these orbitofrontal cortex areas that flavour representations are built, where flavour is taken to mean a representation which is evoked best by a combination of gustatory and olfactory input. This orbitofrontal region does appear to be an important region for convergence, for there is only a low proportion of bimodal taste and olfactory neurons in the primary taste cortex (Rolls & Baylis 1994).
4. The rules underlying the formation of olfactory representations in the primate cortex
Critchley & Rolls (1996c) showed that 35% of orbitofrontal cortex olfactory neurons categorized odours based on their taste association in an olfactory-to-taste discrimination task. Rolls et al. (1996b) found that 68% of orbitofrontal cortex odour-responsive neurons modified their responses in some way following changes in the taste reward associations of the odourants during olfactory–taste discrimination learning and its reversal. (In an olfactory discrimination experiment, if a lick response to one odour, the S+, is made a drop of glucose taste reward is obtained; if incorrectly a lick response is made to another odour, the S−, a drop of aversive saline is obtained. At some time in the experiment, the contingency between the odour and the taste is reversed, and when the ‘meaning’ of the two odours alters, so does the behaviour. It is of interest to investigate in which parts of the olfactory system the neurons show reversal, for where they do, it can be concluded that the neuronal response to the odour depends on the taste with which it is associated, and does not depend primarily on the physico-chemical structure of the odour). These findings demonstrate directly a coding principle in primate olfaction whereby the responses of some orbitofrontal cortex olfactory neurons are modified by, and depend upon, the taste with which the odour is associated (Rolls 2001, 2002a,b).
It was of interest, however, that this modification was less complete, and much slower, than the modifications found for orbitofrontal visual neurons during visual–taste reversal (Rolls et al. 1996a). This relative inflexibility of olfactory responses is consistent with the need for some stability in odour–taste associations to facilitate the formation and perception of flavours. In addition, some orbitofrontal cortex olfactory neurons did not encode information in relation to the taste with which the odour was associated (Critchley & Rolls 1996c), showing that there is also a taste-independent representation of odour in this region.
5. The representation of the pleasantness of odour in the brain: olfactory and visual sensory-specific satiety, their representation in the primate orbitofrontal cortex and the role of sensory-specific satiety in appetite
It has also been possible to investigate whether the olfactory representation in the orbitofrontal cortex is affected by hunger, and thus whether the pleasantness of odour is represented in the orbitofrontal cortex. In satiety experiments, Critchley & Rolls (1996b) showed that the responses of some olfactory neurons to a food odour are decreased during feeding to satiety with a food (e.g. fruit juice) containing that odour. In particular, seven of nine olfactory neurons that were responsive to the odours of foods, such as blackcurrant juice, were found to decrease their responses to the odour of the satiating food. The decrease was typically at least partly specific to the odour of the food that had been eaten to satiety, potentially providing part of the basis for sensory-specific satiety. It was also found for eight of nine neurons that had selective responses to the sight of food that they demonstrated a sensory-specific reduction in their visual responses to foods following satiation. These findings show that the olfactory and visual representations of food, as well as the taste representation of food, in the primate orbitofrontal cortex are modulated by hunger. Usually a component related to sensory-specific satiety can be demonstrated.
These findings link at least part of the processing of olfactory and visual information in this brain region to the control of feeding-related behaviour. This is further evidence that part of the olfactory representation in this region is related to the hedonic value of the olfactory stimulus, and in particular that at this level of the olfactory system in primates, the pleasure elicited by the food odour is at least part of what is represented.
As a result of the neurophysiological and behavioural observations showing the specificity of satiety in the monkey (originally made by E. T. Rolls in 1974 and illustrated e.g. in Rolls 1981), experiments were performed to determine whether satiety was specific to foods eaten in humans. It was found that the pleasantness of the taste of food eaten to satiety decreased more than for foods that had not been eaten (Rolls et al. 1981a). One consequence of this is that if one food is eaten to satiety, appetite reduction for other foods is often incomplete, and this will lead to enhanced eating when a variety of foods is offered (Rolls et al. 1981a,b, 1984). Since sensory factors such as similarity of colour, shape, flavour and texture are usually more important than metabolic equivalence in terms of protein, carbohydrate and fat content in influencing how foods interact in this type of satiety, it has been termed ‘sensory-specific satiety’ (Rolls & Rolls 1977, 1982; Rolls et al. 1981a,b; Rolls 1990). It should be noted that this effect is distinct from alliesthesia, in that alliesthesia is a change in the pleasantness of sensory inputs produced by internal signals (such as glucose in the gut; see Cabanac & Duclaux 1970; Cabanac 1971; Cabanac & Fantino 1977), whereas sensory-specific satiety is a change in the pleasantness of sensory inputs which is accounted for at least partly by the external sensory stimulation received (such as the taste of a particular food), in that as shown above it is at least partly specific to the external sensory stimulation received.
To investigate whether the sensory-specific reduction in the responsiveness of the orbitofrontal olfactory neurons might be related to a sensory-specific reduction in the pleasure produced by the odour of a food when it is eaten to satiety, Rolls & Rolls (1997) measured humans' responses to the smell of a food which was eaten to satiety. It was found that the pleasantness of the odour of a food, but much less significantly its intensity, was decreased when the subjects ate it to satiety. It was also found that the pleasantness of the smell of other foods (i.e. foods not eaten in the meal) showed much less decrease. This finding has clear implications for the control of food intake; for ways to keep foods presented in a meal appetitive; and for effects on odour pleasantness ratings that could occur following meals. In an investigation of the mechanisms of this odour-specific sensory-specific satiety, Rolls & Rolls (1997) allowed humans to chew a food without swallowing, for approximately as long as the food is normally in the mouth during eating. They demonstrated sensory-specific satiety with this procedure, showing that the sensory-specific satiety does not depend on food reaching the stomach. Thus at least part of the mechanism is likely to be produced by a change in processing in the olfactory pathways. It is not yet known which is the earliest stage of olfactory processing at which this modulation occurs. It is unlikely to be in the receptors, because the change in pleasantness found was much more significant than the change in the intensity (Rolls & Rolls 1997).
The increase of food intake that can occur when a variety of foods is available, as a result of the operation of sensory-specific satiety, may have been advantageous in evolution in ensuring that different foods with important different nutrients were consumed. However, today in humans, when a wide variety of foods are readily available, this may be a factor that can lead to overeating and obesity. In a test of this in the rat, it has been found that variety itself can lead to obesity (Rolls et al. 1983b; Rolls & Hetherington 1989).
6. The responses of orbitofrontal cortex taste and olfactory neurons to the sight, texture and temperature of food
Many of the neurons with visual responses in this region also show olfactory or taste responses (Rolls & Baylis 1994), reverse rapidly in visual discrimination reversal, see above and Rolls et al. (1996c), and only respond to the sight of food if hunger is present (Critchley & Rolls 1996b). This part of the orbitofrontal cortex thus seems to implement a mechanism which can flexibly alter the responses to visual stimuli depending on the reinforcement (e.g. the taste) associated with the visual stimulus (see Thorpe et al. 1983; Rolls 1996). This enables prediction of the taste associated with ingestion of what is seen, and thus in the visual selection of foods (see Rolls 1993, 1994, 1999, 2000b). It also provides a mechanism for the sight of a food to influence its flavour.
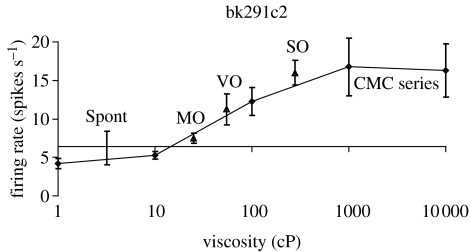
The orbitofrontal cortex of primates is also important as an area of convergence for somatosensory inputs, related for example to the texture of food including fat in the mouth. We have shown for example in recent recordings that single neurons influenced by taste in this region can in some cases have their responses modulated by the texture of the food. This was shown in experiments in which the texture of food was manipulated by the addition of methyl cellulose or gelatine, or by puréeing a semi-solid food (Rolls 1998, 1999). It has been shown that some of these neurons with texture-related responses encode parametrically the viscosity of food in the mouth (using a methyl cellulose series in the range 1–10 000 cP; see figure 3), and that others independently encode the particulate quality of food in the mouth, produced quantitatively e.g. by adding 20–100 μm microspheres to methyl cellulose (Rolls et al. 2003b).
Figure 3.
A neuron in the primate orbitofrontal cortex responding to texture and in particular viscosity in the mouth. The cell (bk291c2) had a firing rate response that increased parametrically with the viscosity of methyl cellulose (CMC) in the mouth. The neuron encoded viscosity, in that its response to fats and oils (shown by triangles) depended on their viscosity. CMC series, carboxymethylcellulose of different viscosities; MO, mineral oil; SO, silicone oil; VO, vegetable oil. Some of these neurons have taste inputs. The spontaneous firing rate of the neuron (spont) was approximately 5 spikes s−1 (After Rolls et al. 2003b).
In addition, recent findings (Kadohisa et al. 2004) have revealed that some neurons in the orbitofrontal cortex reflect the temperature of substances in the mouth, and that this temperature information is represented independently of other sensory inputs by some neurons, and in combination with taste or texture by other neurons.
7. The mouth feel of fat: orbitofrontal cortex, primary taste cortex and amygdala
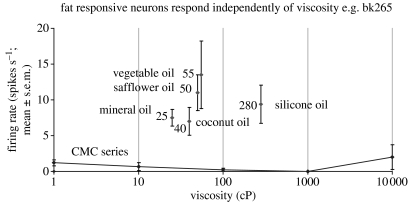
Texture in the mouth is an important indicator of whether fat is present in a food, which is important not only as a high value energy source, but also as a potential source of essential fatty acids. In the orbitofrontal cortex, Rolls et al. (1999) have found a population of neurons that responds when fat is in the mouth. An example of such a neuron is shown in figure 4. The fat-related responses of these neurons are produced at least in part by the texture of the food rather than by chemical receptors sensitive to certain chemicals, in that such neurons typically respond not only to foods such as cream and milk containing fat, but also to paraffin oil (which is a pure hydrocarbon) and to silicone oil (Si(CH3)2O)n). Moreover, the texture channel through which these fat-sensitive neurons are activated are separate from viscosity sensitive channels, in that the responses of these neurons cannot be predicted by the viscosity of the oral stimuli (Verhagen et al. 2003), as illustrated in figure 4. Some of the fat-related neurons do though have convergent inputs from the chemical senses, in that in addition to taste inputs, some of these neurons respond to the odour associated with a fat, such as the odour of cream (Rolls et al. 1999). Feeding to satiety with fat (e.g. cream) decreases the responses of these neurons to zero on the food eaten to satiety, but if the neuron receives a taste input from e.g. glucose taste, that is not decreased by feeding to satiety with cream. Thus there is a representation of the macronutrient fat in this brain area, and the activation produced by fat is reduced by eating fat to satiety.
Figure 4.
A neuron in the primate orbitofrontal cortex responding to the texture of fat in the mouth independently of viscosity. The cell (bk265) increased its firing rate to a range of fats and oils (the viscosity of which is shown in cP). The information that reaches this type of neuron is independent of a viscosity sensing channel, in that the neuron did not respond to the methyl cellulose (CMC) viscosity series. The neuron responded to the texture rather than the chemical structure of the fat in that it also responded to silicone oil (Si(CH3)2O)n) and paraffin (mineral) oil (hydrocarbon). Some of these neurons have taste inputs (After Verhagen et al. 2003).
Fat texture, oral viscosity and temperature, for some neurons in combination with taste, are represented in the macaque primary taste cortex in the rostral insula and adjoining frontal operculum (Verhagen et al. 2004).
These oral sensory properties of food, and also the sight and smell of food, are also represented in the primate amygdala (Rolls 2000c; Rolls & Scott 2003; Kadohisa et al. 2005a,b). Interestingly, the responses of these amygdala neurons do not correlate well with the preferences of the macaques for the oral stimuli (Kadohisa et al. 2005a), and feeding to satiety does not produce the large reduction in the responses of amygdala neurons to food (Rolls 2000c; Rolls & Scott 2003) that is typical of orbitofrontal cortex neurons.
8. Learning about the sight of food: orbitofrontal cortex versus amygdala
Differences between the primate orbitofrontal cortex and amygdala are also found in the way that they learn about stimuli associated with the flavour of food. Neurons in the orbitofrontal cortex reverse their responses very rapidly, often in one trial, to a visual stimulus when it no longer signifies food (Thorpe et al. 1983; Rolls et al. 1996c), whereas rapid visual–taste discrimination reversal in the same task is not a general property of primate amygdala neurons that respond to the sight of food (Sanghera et al. 1979; Rolls 2000c). Thus the primate orbitofrontal cortex appears to be more closely related to hedonic aspects of stimuli relevant to the control of food intake than does the primate amygdala (Rolls 2000c, 2005; Rolls & Scott 2003). Part of the underlying basis for at least the rapid reward reversal learning shown by primate orbitofrontal cortex but not amygdala neurons may be that the orbitofrontal cortex as a cortical structure has well-developed recurrent collateral axon systems that enable the network to operate as a short term memory. A short term memory would then enable a rule to be kept active about which stimulus is currently rewarded, and cortical connectivity would allow this rule network to influence visual neurons in the orbitofrontal cortex using biased competition mechanisms (Deco & Rolls 2005a,b). This provides a computational basis for understanding the special role of the orbitofrontal cortex in the rapid re-evaluation of the responses to be made to food (Rolls 2005; Deco & Rolls 2005a,b). In addition, habituation with a time course of several minutes of the afferent synapses to the orbitofrontal cortex provides a probable neurophysiological basis for sensory-specific satiety (Rolls 2005).
9. Imaging studies in humans
(a) Taste
In humans it has been shown in neuroimaging studies using functional magnetic resonance imaging (fMRI) that taste activates an area of the anterior insula/frontal operculum, which is probably the primary taste cortex, and part of the orbitofrontal cortex, which is probably the secondary taste cortex (Francis et al. 1999; O'Doherty et al. 2001b; de Araujo et al. 2003b).
The orbitofrontal cortex taste area is distinct from areas activated by odours and by pleasant touch (Francis et al. 1999). It has been shown that within individual subjects separate areas of the orbitofrontal cortex are activated by sweet (pleasant) and by salt (unpleasant tastes; O'Doherty et al. 2001b). Francis et al. (1999) also found activation of the human amygdala by the taste of glucose. Extending this study, O'Doherty et al. (2001b) showed that the human amygdala was as much activated by the affectively pleasant taste of glucose as by the affectively negative taste of NaCl, and thus provided evidence that the human amygdala is not especially involved in processing aversive as compared to rewarding stimuli. Zald et al. (1998) had shown earlier that the amygdala, as well as the orbitofrontal cortex, responds to aversive (saline) taste stimuli. The study above (O'Doherty et al. 2001b), however, shows that there is nothing special about aversive taste stimuli in relation to the brain areas activated, for pleasant stimuli also activate the amygdala and orbitofrontal cortex.
Another study has recently shown that umami taste stimuli, of which an exemplar is MSG and which capture what is described as the taste of protein, activate similar cortical regions of the human taste system to those activated by a prototypical taste stimulus, glucose (de Araujo et al. 2003a; see figure 5). A part of the rostral anterior cingulate cortex (ACC) was also activated. When the nucleotide 0.005 M inosine 5′-monophosphate (IMP) was added to MSG (0.05 M), the blood oxygenation-level dependent (BOLD) signal in an anterior part of the orbitofrontal cortex showed supralinear additivity, and this may reflect the subjective enhancement of umami taste that has been described when IMP is added to MSG. Overall, these results illustrate that the responses of the brain can reflect inputs produced by particular combinations of sensory stimuli with supralinear activations, and that the combination of sensory stimuli may be especially represented in particular brain regions.
Figure 5.
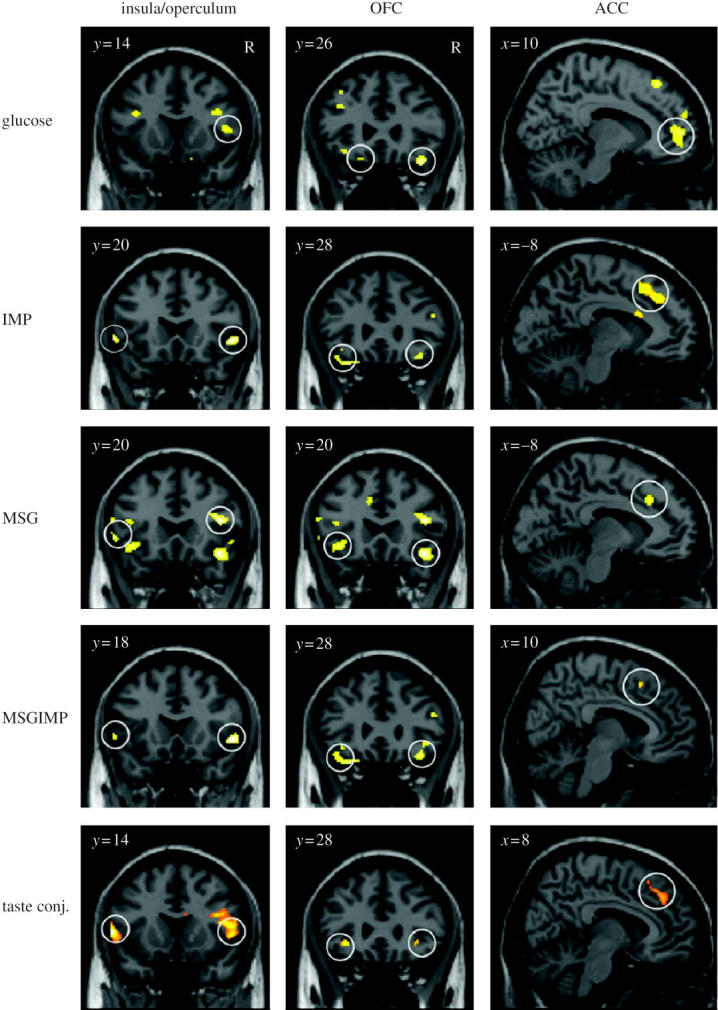
Activation of the human primary taste cortex in the insula/frontal operculum; the orbitofrontal cortex (OFC); and the anterior cingulate cortex (ACC) by taste. The stimuli used included glucose, two umami taste stimuli (monosodium glutamate (MSG) and inosine monophosphate (IMP)), and a mixture of the two umami stimuli. Taste conj. refers to a conjunction analysis over all the taste stimuli (After de Araujo et al. 2003a).
(b) Odour
In humans, in addition to activation of the pyriform (olfactory) cortex (Zald & Pardo 1997; Sobel et al. 2000; Poellinger et al. 2001), there is strong and consistent activation of the orbitofrontal cortex by olfactory stimuli (Zatorre et al. 1992; Francis et al. 1999). In an investigation of where the pleasantness of olfactory stimuli might be represented in humans, O'Doherty et al. (2000) showed that the activation of an area of the orbitofrontal cortex to banana odour was decreased (relative to a control vanilla odour) after bananas were eaten to satiety. Thus activity in a part of the human orbitofrontal cortex olfactory area is related to sensory-specific satiety, and this is one brain region where the pleasantness of odour is represented.
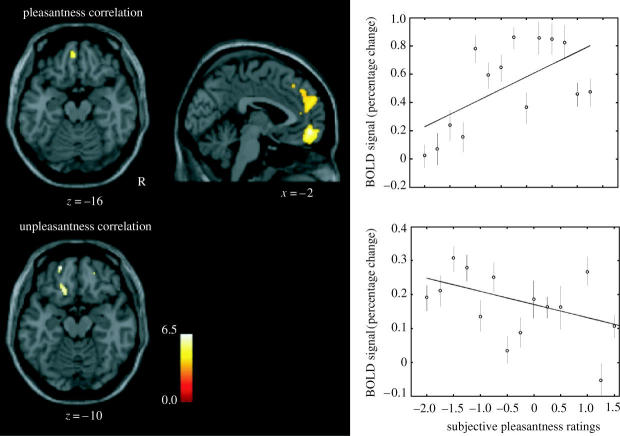
An important issue is whether there are separate regions of the brain discriminable with fMRI that represent pleasant and unpleasant odours. To investigate this, we measured the brain activations produced by three pleasant and three unpleasant odours. The pleasant odours chosen were linalyl acetate (floral, sweet), geranyl acetate (floral) and alpha-ionone (woody, slightly food-related). (Chiral substances were used as racemates.) The unpleasant odours chosen were hexanoic acid, octanol and isovaleric acid. We found that they activated dissociable parts of the human brain (Rolls et al. 2003a). Pleasant but not unpleasant odours were found to activate a medial region of the rostral orbitofrontal cortex (see figure 6). Further, there was a correlation between the subjective pleasantness ratings of the six odours given during the investigation with activation of a medial region of the rostral orbitofrontal cortex (see figure 7). In contrast, a correlation between the subjective unpleasantness ratings of the six odours was found in regions of the left and more lateral orbitofrontal cortex. Activation was also found in the ACC, with a middle part of the anterior cingulate activated by both pleasant and unpleasant odours and a more anterior part of the ACC showing a correlation with the subjective pleasantness ratings of the odours (Rolls et al. 2003a). These results provide evidence that there is a hedonic map of the sense of smell in brain regions such as the orbitofrontal cortex and cingulate cortex.
Figure 6.
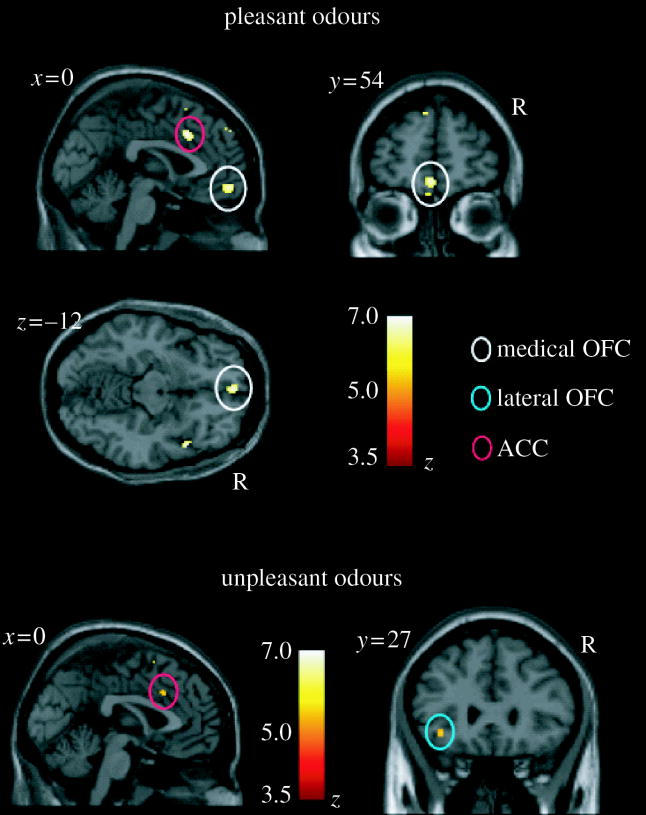
The representation of pleasant and unpleasant odours in the human brain. Above: group conjunction results for the three pleasant odours. Sagittal, horizontal and coronal views are shown at the levels indicated, all including the same activation in the medial orbitofrontal cortex, OFC (X,Y,Z=0,54,−12; z=5.23). Also shown is activation for the three pleasant odours in the anterior cingulate cortex, ACC (X,Y,Z=2,20,32; z=5.44). These activations were significant at p<0.05 fully corrected for multiple comparisons. Below: group conjunction results for the three unpleasant odours. The sagittal view (left) shows an activated region of the anterior cingulate cortex (X,Y,Z=0,18,36; z=4.42, p<0.05, S.V.C.). The coronal view (right) shows an activated region of the lateral orbitofrontal cortex (X,Y,Z=−36,27,−8; z=4.23, p<0.05 S.V.C.). All the activations were thresholded at p<0.00001 to show the extent of the activations (After Rolls et al. 2003a).
Figure 7.
The representation of pleasant and unpleasant odours in the human brain. Random effects group analysis correlation analysis of the BOLD signal with the subjective pleasantness ratings. On the top left is shown the region of the medio-rostral orbitofrontal (peak at [−2, 52, −10]; z=4.28) correlating positively with pleasantness ratings, as well as the region of the ACC in the top middle. On the far top-right of the figure is shown the relation between the subjective pleasantness ratings and the BOLD signal from this cluster (in the medial orbitofrontal cortex at Y=52), together with the regression line. The means and s.e.m. across subjects are shown. At the bottom of the figure is shown the regions of left more lateral orbitofrontal cortex (peaks at [−20, 54, −14]; z=4.26 and [−16, 28, −18]; z=4.08) correlating negatively with pleasantness ratings. On the far bottom-right of the figure is shown the relation between the subjective pleasantness ratings and the BOLD signal from the first cluster (in the lateral orbitofrontal cortex at Y=54), together with the regression line. The means and s.e.m. across subjects are shown. The activations were thresholded at p<0.0001 for extent (After Rolls et al. 2003a).
The topological representation of the hedonic properties of sensory stimuli such as smell in the orbitofrontal cortex can be understood with some of the fundamental principles of computational neuroscience (Rolls & Treves 1998; Rolls & Deco 2002) as follows. Given the evidence described above that the reward-related or affective properties of sensory stimuli, rather than e.g. the intensity of the stimuli, is represented in the orbitofrontal cortex, a topological map of the hedonic value of stimuli is produced in which neurons that have similar hedonic value are placed close together in the map. This self-organizing map results from processes that occur in competitive networks, the building blocks of sensory systems, in which the neurons are coupled by short range (approx. 1 mm) excitation (implemented by the recurrent excitatory connections between cortical pyramidal cells) and longer range inhibition (implemented by inhibitory interneurons; see Rolls & Treves 1998; Rolls & Deco 2002). Such self-organizing maps are a useful feature on brain connectivity, for they help to minimize the length of the connections between neurons that need to exchange information to perform their computations. It is for this reason, it is hypothesized, that neurons with similar hedonic value are placed close together. In the case of olfactory stimuli, this results in an activation region for pleasant olfactory stimuli (in the medial orbitofrontal cortex), and a separate activation region for unpleasant olfactory stimuli (more laterally in the orbitofrontal cortex). Of course, part of the support for such a map (i.e. a factor which helps different types of stimuli to be separated in the map) may arise because unpleasant olfactory stimuli may be more generally associated with other sensory inputs such as trigeminal inputs. Understanding this principle may be very valuable in helping to interpret the results of neuroimaging experiments.
(c) Olfactory–taste convergence to represent flavour, and the influence of satiety
To investigate where in the human brain interactions between taste and odour stimuli may be realized to implement flavour, we performed an event-related fMRI study with sucrose and MSG taste, and strawberry and methional (chicken) odours, delivered unimodally or in different combinations (de Araujo et al. 2003c). The brain regions that were activated by both taste and smell included parts of the caudal orbitofrontal cortex, amygdala, insular cortex and adjoining areas and ACC. It was shown that a small part of the anterior (putatively agranular) insula responds to unimodal taste and to unimodal olfactory stimuli; and that a part of the anterior frontal operculum is a unimodal taste area (putatively primary taste cortex) not activated by olfactory stimuli. Activations to combined olfactory and taste stimuli where there was little or no activation to either alone (providing positive evidence for interactions between the olfactory and taste inputs) were found in a lateral anterior part of the orbitofrontal cortex. Correlations with consonance ratings for the smell and taste combinations, and for their pleasantness, were found in a medial anterior part of the orbitofrontal cortex (see figure 8). Similarly, Small et al. (2004) also found supradditive interactions between congruent taste and smell stimuli in areas including the caudal orbitofrontal cortex, and ACC (see also Small & Prescott 2005). These results provide evidence on the neural substrate for the convergence of taste and olfactory stimuli to produce flavour in humans, and where the pleasantness of flavour is represented in the human brain.
Figure 8.
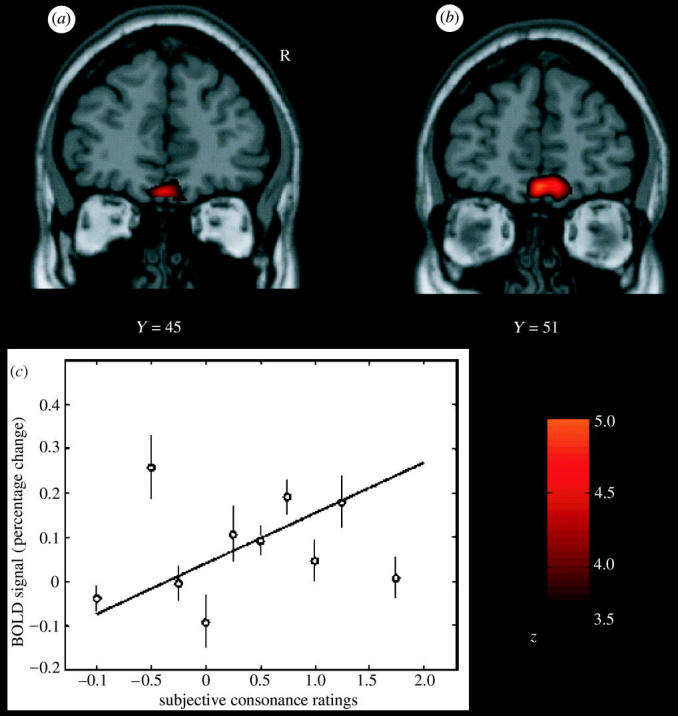
Flavour formation in the human brain, shown by cross-modal olfactory–taste convergence. Brain areas where activations were correlated with the subjective ratings for stimulus (taste–odour) consonance and pleasantness. (a) A second-level, random effects analysis based on individual contrasts (the consonance ratings being the only effect of interest) revealed a significant activation in a medial part of the anterior orbitofrontal cortex. (b) Random effects analysis based on the pleasantness ratings showed a significant cluster of activation located in a (nearby) medial part of the anterior orbitofrontal cortex. The images were thresholded at p<0.0001 for illustration. (c) The relation between the BOLD signal from the cluster of voxels in the medial orbitofrontal cortex shown in (a) and the subjective consonance ratings. The analyses shown included all the stimuli included in this investigation. The means and s.e.m. across subjects are shown, together with the regression line, for which r=0.52 (After de Araujo et al. 2003c).
To assess how satiety influences the brain activations to a whole food which produces taste, olfactory and texture stimulation, we measured brain activation by whole foods before and after the food is eaten to satiety (de Araujo et al. 2003a). The aim was to show using a food that has olfactory, taste and texture components the extent of the region that shows decreases when the food becomes less pleasant, in order to identify the different brain areas where the pleasantness of the odour, taste and texture of food are represented. The foods eaten to satiety were either chocolate milk, or tomato juice. A decrease in activation by the food eaten to satiety relative to the other food was found in the orbitofrontal cortex (Kringelbach et al. 2003) but not in the primary taste cortex (see figure 9). This study provided evidence that the pleasantness of the flavour of food, and sensory-specific satiety, are represented in the orbitofrontal cortex.
Figure 9.
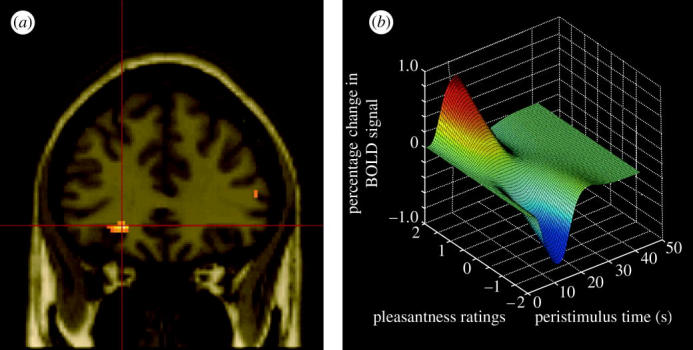
Areas of the human orbitofrontal cortex with activations correlating with pleasantness ratings for food in the mouth. (a) Coronal section through the region of the orbitofrontal cortex from the random effects group analysis showing the peak in the left orbitofrontal cortex (Talairach coordinates X,Y,Z=−22,34,−8, z-score=4.06), in which the BOLD signal in the voxels shown in yellow was significantly correlated with the subjects' subjective pleasantness ratings of the foods throughout an experiment in which the subjects were hungry and found the food pleasant, and were then fed to satiety with the food, after which the pleasantness of the food decreased to neutral or slightly unpleasant. The design was a sensory-specific satiety design, and the pleasantness of the food not eaten in the meal, and the BOLD activation in the orbitofrontal cortex, were not altered by eating the other food to satiety. The two foods were tomato juice and chocolate milk. (b) Plot of the magnitude of the fitted haemodynamic response from a representative single subject against the subjective pleasantness ratings (on a scale from −2 to +2) and peristimulus time in seconds (After Kringelbach et al. 2003).
(d) Oral viscosity and fat texture
The viscosity of food in the mouth is represented in the human primary taste cortex (in the anterior insula), and also in a mid-insular area that is not taste cortex, but which represents oral somatosensory stimuli (de Araujo & Rolls 2004). In these regions, the fMRI BOLD activations are proportional to the log of the viscosity of carboxymethyl cellulose in the mouth. Oral viscosity is also represented in the human orbitofrontal and perigenual cingulate cortices, and it is notable that the perigenual cingulate cortex, an area in which many pleasant stimuli are represented, is strongly activated by the texture of fat in the mouth and also by oral sucrose (de Araujo & Rolls 2004).
(e) The sight of food
O'Doherty et al. (2002) showed that visual stimuli associated with the taste of glucose activated the orbitofrontal cortex and some connected areas, consistent with the primate neurophysiology. Simmons et al. (2005) found that showing pictures of foods, compared to pictures of locations, can also activate the orbitofrontal cortex and some connected areas, though taste stimuli were not used in this study, so that one cannot be sure to what extent the activations to the sight and taste of food overlapped. Consistent with these findings, Pelchat et al. (2004) found that after consuming a monotonous diet, subjects that were instructed to imagine foods that they craved showed more activation in some brain areas, including part of the insula, than subjects who had consumed a normal diet. Similarly, the orbitofrontal cortex and connected areas were also found to be activated after presentation of food stimuli to food-deprived subjects (Wang et al. 2004).
10. Cognitive effects on representations of food
Brie can smell pleasant. However, the same odour taken out of the context of cheese might be unpleasant. There is evidence that the sight (including colour) of a food or wine can influence its flavour. However, what about a more cognitive influence, such as a word? Can this influence the perception and hedonics of food-related stimuli, and if so, how far back down into the sensory system does the cognitive influence reach? To address this, we performed an fMRI investigation in which the delivery of a standard test odour (isovaleric acid combined with cheddar cheese flavour, presented orthonasally using an olfactometer) was paired with a descriptor word on a screen, which on different trials was ‘Cheddar cheese’ or ‘Body odour’. The subjects rated the pleasantness and the intensity of the odour on every trial. Alpha-ionone (pleasant, labelled ‘Flowers’) and octanol (unpleasant, labelled ‘Burned plastic’) were used as reference pleasant and unpleasant stimuli for the psychophysics and neuroimaging. Subjects rated the affective value of the test odour as significantly more unpleasant when labelled ‘Body odour’ than when labelled ‘Cheddar cheese’. We found that the medial orbitofrontal cortex (OFC)/rostral ACC was significantly more activated by the test stimulus labelled ‘Cheddar cheese’ than when labelled ‘Body odour’, and that these activations were correlated with the pleasantness ratings (de Araujo et al. 2005). This cognitive modulation was also found in the medial amygdala olfactory area, and this extended towards the olfactory tubercle. Thus cognitive modulation extends in the olfactory system as far down as the secondary olfactory cortex, in the orbitofrontal cortex, and may even influence some parts of the primary olfactory areas, such as the olfactory tubercle. The implication is that cognitive factors can have profound effects on our responses to the hedonic and sensory properties of food, in that these effects are manifest quite far back into sensory processing, so that at least hedonic representations of odours are affected, and even perceptual representations may be modulated (de Araujo et al. 2005).
11. Conclusions
The reward value of food, and its subjective complement, the rated affective pleasantness of food, is decoded in primates including humans only after several stages of analysis. First, the representation of the taste of the food (its identity and intensity) is made explicit in the primary taste cortex. Only later, in the orbitofrontal cortex, is the reward value made explicit in the representation, for it is here that satiety signals modulate the responses of the taste and flavour neurons. Thus in the control of food intake, the reward value or pleasantness is crucial to the design of how food intake is controlled, and the reward value is represented only in specialized cortical areas. The orbitofrontal cortex is moreover where multimodal representations of food are built, which include taste, texture, olfactory and visual components. The actual satiety signals are complex, and include sensory-specific satiety, computed in the orbitofrontal cortex, gastric distension, gut satiety signals, plasma glucose and hormones such as leptin.
Although representations of the taste and texture of food are found in the primate including human amygdala (O'Doherty et al. 2001b; Rolls & Scott 2003; Wilson & Rolls 2005; Kadohisa et al. 2005a,b), the primate orbitofrontal cortex is more closely related to the changing affective value of food than the amygdala (Sanghera et al. 1979; Rolls 2000c; Rolls & Scott 2003), in that the orbitofrontal cortex shows responses that decrease to zero as the reward decreases to zero with satiety, and in that the orbitofrontal cortex tracks (and probably computes) the changing reward value of stimuli as they are altered by stimulus–reinforcer association learning and reversal.
The brain areas where the pleasantness or affective value of smell and taste are represented are closely related to the brain areas involved in emotion. Emotions can usefully be defined as states elicited by rewards and punishers (Rolls 1999, 2005), and olfactory and taste stimuli can be seen as some of the classes of stimuli that can produce emotional states. Part of the importance of the orbitofrontal cortex in emotion is that it represents some primary (or unlearned) rewards and punishers, such as taste and pleasant touch (Francis et al. 1999; Rolls et al. 2003a,c), and also learns the association between previously neutral stimuli and primary reinforcers. This type of learning is called stimulus–reinforcer association learning, and is the type of learning that is fundamental in learned emotional states. In addition to reinforcers such as taste, odour and touch, quite abstract emotion-producing stimuli are represented in other parts of the orbitofrontal cortex. For example, the medial orbitofrontal cortex is activated in humans according to how much money is won in a probabilistic reward/punishment task, and the lateral orbitofrontal cortex is activated according to how much money is lost in the same task (O'Doherty et al. 2001a).
It is thus becoming possible to start to understand not only where the affective value of smell and taste is represented in the brain, but also how these representations fit into a wider picture of the brain processes underlying emotion. Although functional neuroimaging with its spatial resolution of several millimetres shows that some of these representations are close together in the human medial orbitofrontal cortex, the primate neurophysiology provides clear evidence for the exquisitely rich and separate representations of different types of reward because each neuron in this region is tuned to one of, or to a unique combination of, the range of food-related stimuli described in this paper. The information relevant to the control of food intake about the sensory properties of foods is thus made explicit in the firing rate profiles of single neurons and groups of single neurons to the sensory stimuli. It is at the neuron level that brain computations are performed, and that the information being transmitted between the computing elements of the brain, the neurons, can be measured by the spiking activity (Rolls & Deco 2002; Rolls 2005). By making models at the spiking neuron level of the computations taking place in networks of neurons, it is now possible by integration over the energy required for the activated neurons to predict signals at the much more global level of functional neuroimaging in humans (Deco et al. 2004). By combining information from the neuron and functional imaging level, we are starting to understand the details of the neuronal operations that underlie the affective responses to the sensory properties of food, and how they are modulated by appetite, as described in this article.
The outputs of the orbitofrontal cortex reach brain regions such as the striatum, cingulate cortex and dorsolateral prefrontal cortex where behavioural responses to food may be elicited because these structures produce behaviour which makes the orbitofrontal cortex reward neurons fire, as they represent a goal for behaviour. At the same time, outputs from the orbitofrontal cortex and amygdala, in part via the hypothalamus, may provide for appropriate autonomic and endocrine responses to food to be produced, including the release of hormones such as insulin.
Acknowledgments
This research was supported by the Medical Research Council.
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Baylis L.L, Rolls E.T. Responses of neurons in the primate taste cortex to glutamate. Physiol. Behav. 1991;49:973–979. doi: 10.1016/0031-9384(91)90210-f. doi:10.1016/0031-9384(91)90210-F [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Duclaux R. Specificity of internal signals in producing satiety for taste stimuli. Nature. 1970;227:966–967. doi: 10.1038/227966a0. doi:10.1038/227966a0 [DOI] [PubMed] [Google Scholar]
- Cabanac M, Fantino M. Origin of olfacto-gustatory alliesthesia: intestinal sensitivity to carbohydrate concentration? Physiol. Behav. 1977;10:1039–1045. doi: 10.1016/0031-9384(77)90009-9. doi:10.1016/0031-9384(77)90009-9 [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin A.M, Roper S.D. A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci. 2000;3:113–119. doi: 10.1038/72053. doi:10.1038/72053 [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Rolls E.T. Responses of primate taste cortex neurons to the astringent tastant tannic acid. Chem. Senses. 1996a;21:135–145. doi: 10.1093/chemse/21.2.135. [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Rolls E.T. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J. Neurophysiol. 1996b;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Rolls E.T. Olfactory neuronal responses in the primate orbitofrontal cortex: analysis in an olfactory discrimination task. J. Neurophysiol. 1996c;75:1659–1672. doi: 10.1152/jn.1996.75.4.1659. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T, Rolls E.T. The representation in the human brain of food texture and oral fat. J. Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. doi:10.1523/JNEUROSCI.0130-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo I.E.T, Kringelbach M.L, Rolls E.T, Hobden P. The representation of umami taste in the human brain. J. Neurophysiol. 2003a;90:313–319. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T, Kringelbach M.L, Rolls E.T, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J. Neurophysiol. 2003b;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T, Rolls E.T, Kringelbach M.L, McGlone F, Phillips N. Taste–olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003c;18:2374–2390. doi: 10.1046/j.1460-9568.2003.02915.x. doi:10.1046/j.1460-9568.2003.02915.x [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T, Rolls E.T, Velazco M.I, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. doi:10.1016/j.neuron.2005.04.021 [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls E.T. Attention, short-term memory, and action selection: a unifying theory. Prog. Neurobiol. 2005a;76:236–256. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls E.T. Synaptic and spiking dynamics underlying reward reversal in orbitofrontal cortex. Cereb. Cortex. 2005b;15:15–30. doi: 10.1093/cercor/bhh103. doi:10.1093/cercor/bhh103 [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls E.T, Horwitz B. “What” and “where” in visual working memory: a computational neurodynamical perspective for integrating fMRI and single-neuron data. J. Cog. Neurosci. 2004;16:683–701. doi: 10.1162/089892904323057380. doi:10.1162/089892904323057380 [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls E.T, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E. The representation of the pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls E.T, Verhagen J.V. Orbitofrontal cortex neuronal representation of temperature and capsaicin in the mouth. Neuroscience. 2004;127:207–221. doi: 10.1016/j.neuroscience.2004.04.037. doi:10.1016/j.neuroscience.2004.04.037 [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls E.T, Verhagen J.V. Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex, and amygdala. Chem. Senses. 2005a;30:401–419. doi: 10.1093/chemse/bji036. doi:10.1093/chemse/bji036 [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls E.T, Verhagen J.V. The primate amygdala: neuronal representations of the viscosity, fat texture, grittiness and taste of foods. Neuroscience. 2005;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. doi:10.1016/j.neuroscience.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L, O'Doherty J, Rolls E.T, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb. Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. doi:10.1093/cercor/13.10.1064 [DOI] [PubMed] [Google Scholar]
- Norgren R. Central neural mechanisms of taste. In: Darien-Smith I, editor. Handbook of physiology—The nervous system III. Sensory processes 1. American Physiological Society; Washington, DC: 1984. pp. 1087–1128. [Google Scholar]
- O'Doherty J, Rolls E.T, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety related olfactory activation of the human orbitofrontal cortex. NeuroReport. 2000;11:893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach M.L, Rolls E.T, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001a;4:95–102. doi: 10.1038/82959. doi:10.1038/82959 [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls E.T, Francis S, Bowtell R, McGlone F. The representation of pleasant and aversive taste in the human brain. J. Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P, Deichmann R, Critchley H.D, Dolan R.J. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. doi:10.1016/S0896-6273(02)00603-7 [DOI] [PubMed] [Google Scholar]
- Pelchat M.L, Johnson A, Chan R, Valdez J, Ragland J.D. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. doi:10.1016/j.neuroimage.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen B.R, Kwong K.K. Activation and habituation in olfaction—an fMRI study. NeuroImage. 2001;13:547–560. doi: 10.1006/nimg.2000.0713. doi:10.1006/nimg.2000.0713 [DOI] [PubMed] [Google Scholar]
- Pritchard T.C, Hamilton R.B, Morse J.R, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey Macaca fascicularis. J. Comp. Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. doi:10.1002/cne.902440208 [DOI] [PubMed] [Google Scholar]
- Rolls B.J. The role of sensory-specific satiety in food intake and food selection. In: Capaldi E.D, Powley T.L, editors. Taste, experience, and feeding. American Psychological Association; Washington, DC: 1990. pp. 197–209. [Google Scholar]
- Rolls B.J, Hetherington M. The role of variety in eating and body weight regulation. In: Shepherd R, editor. Handbook of the psychophysiology of human eating. Wiley; Chichester, UK: 1989. pp. 57–84. [Google Scholar]
- Rolls B.J, Rolls E.T, Rowe E.A, Sweeney K. Sensory specific satiety in man. Physiol. Behav. 1981a;27:137–142. doi: 10.1016/0031-9384(81)90310-3. doi:10.1016/0031-9384(81)90310-3 [DOI] [PubMed] [Google Scholar]
- Rolls B.J, Rowe E.A, Rolls E.T, Kingston B, Megson A, Gunary R. Variety in a meal enhances food intake in man. Physiol. Behav. 1981b;26:215–221. doi: 10.1016/0031-9384(81)90014-7. doi:10.1016/0031-9384(81)90014-7 [DOI] [PubMed] [Google Scholar]
- Rolls B.J, Rowe E.A, Rolls E.T. How sensory properties of foods affect human feeding behavior. Physiol. Behav. 1982;29:409–417. doi: 10.1016/0031-9384(82)90259-1. doi:10.1016/0031-9384(82)90259-1 [DOI] [PubMed] [Google Scholar]
- Rolls B.J, Rolls E.T, Rowe E.A. Body fat control and obesity. Behav. Brain Sci. 1983a;4:744–745. [Google Scholar]
- Rolls B.J, Van Duijenvoorde P.M, Rowe E.A. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol. Behav. 1983b;31:21–27. doi: 10.1016/0031-9384(83)90091-4. doi:10.1016/0031-9384(83)90091-4 [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Rolls B.J, Rowe E.A. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol. Behav. 1983c;30:185–192. doi: 10.1016/0031-9384(83)90003-3. doi:10.1016/0031-9384(83)90003-3 [DOI] [PubMed] [Google Scholar]
- Rolls B.J, Van Duijvenvoorde P.M, Rolls E.T. Pleasantness changes and food intake in a varied four-course meal. Appetite. 1984;5:337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Central nervous mechanisms related to feeding and appetite. Br. Med. Bull. 1981;37:131–134. doi: 10.1093/oxfordjournals.bmb.a071689. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The neural control of feeding in primates. In: Booth D.A, editor. Neurophysiology of ingestion. Pergamon; Oxford, UK: 1993. pp. 137–169. [Google Scholar]
- Rolls E.T. Neural processing related to feeding in primates. In: Legg C.R, Booth D.A, editors. Appetite: neural and behavioural bases. Oxford University Press; Oxford, UK: 1994. pp. 11–53. [Google Scholar]
- Rolls E.T. The orbitofrontal cortex. Phil. Trans. R. Soc. B. 1996;351:1433–1444. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Taste and olfactory processing in the brain and its relation to the control of eating. Crit. Rev. Neurobiol. 1997;11:263–287. doi: 10.1615/critrevneurobiol.v11.i4.20. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Taste and olfactory processing in the brain, and its relation to the control of eating. Front. Oral Biol. 1998;9:40–75. doi: 10.1615/critrevneurobiol.v11.i4.20. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Oxford University Press; Oxford, UK: 1999. The brain and emotion. [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cereb. Cortex. 2000a;10:284–294. doi: 10.1093/cercor/10.3.284. doi:10.1093/cercor/10.3.284 [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Taste, olfactory, visual and somatosensory representations of the sensory properties of foods in the brain, and their relation to the control of food intake. In: Berthoud H.R, Seeley R.J, editors. Neural and metabolic control of macronutrient intake. CRC Press; Boca-Raton, FL: 2000b. pp. 247–262. [Google Scholar]
- Rolls E.T. Neurophysiology and functions of the primate amygdala, and the neural basis of emotion. In: Aggleton J.P, editor. The amygdala: a functional analysis. 2nd edn. Oxford University Press; Oxford, UK: 2000c. pp. 447–478. [Google Scholar]
- Rolls E.T. The rules of formation of the olfactory representations found in the orbitofrontal cortex olfactory areas in primates. Chem. Senses. 2001;26:595–604. doi: 10.1093/chemse/26.5.595. doi:10.1093/chemse/26.5.595 [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The cortical representation of taste and smell. In: Rouby G, Schaal B, Dubois D, Gervais R, Holley A, editors. Olfaction, taste and cognition. Cambridge University Press; New York, NY: 2002a. pp. 367–388. [Google Scholar]
- Rolls E.T. The functions of the orbitofrontal cortex. In: Stuss D.T, Knight R.T, editors. Principles of frontal lobe function. ch. 23. Oxford University Press; New York, NY: 2002b. pp. 354–375. [Google Scholar]
- Rolls E.T. Oxford University Press; Oxford, UK: 2005. Emotion explained. [Google Scholar]
- Rolls E.T, Baylis L.L. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J. Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T, Deco G. Oxford University Press; Oxford, UK: 2002. Computational neuroscience of vision. [Google Scholar]
- Rolls E.T, Rolls B.J. Activity of neurones in sensory, hypothalamic and motor areas during feeding in the monkey. In: Katsuki Y, Sato M, Takagi S, Oomura Y, editors. Food intake and chemical senses. University of Tokyo Press; Tokyo, Japan: 1977. pp. 525–549. [Google Scholar]
- Rolls E.T, Rolls B.J. Brain mechanisms involved in feeding. In: Barker L.M, editor. Psychobiology of human food selection. AVI Publishing Company; Westport, CT: 1982. pp. 33–62. [Google Scholar]
- Rolls E.T, Rolls J.H. Olfactory sensory-specific satiety in humans. Physiol. Behav. 1997;61:461–473. doi: 10.1016/s0031-9384(96)00464-7. doi:10.1016/S0031-9384(96)00464-7 [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Scott T.R. Central taste anatomy and neurophysiology. In: Doty R.L, editor. Handbook of olfaction and gustation. 2nd edn. Dekker; New York, NY: 2003. pp. 679–705. [Google Scholar]
- Rolls E.T, Treves A. Oxford University Press; Oxford, UK: 1998. Neural networks and brain function. [Google Scholar]
- Rolls E.T, Murzi E, Yaxley S, Thorpe S.J, Simpson S.J. Sensory-specific satiety: food-specific reduction in responsiveness of ventral forebrain neurons after feeding in the monkey. Brain Res. 1986;368:79–86. doi: 10.1016/0006-8993(86)91044-9. doi:10.1016/0006-8993(86)91044-9 [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Scott T.R, Sienkiewicz Z.J, Yaxley S. The responsiveness of neurones in the frontal opercular gustatory cortex of the macaque monkey is independent of hunger. J. Physiol. 1988;397:1–12. doi: 10.1113/jphysiol.1988.sp016984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T, Sienkiewicz Z.J, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur. J. Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. doi:10.1111/j.1460-9568.1989.tb00774.x [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Yaxley S, Sienkiewicz Z.J. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J. Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Critchley H.D, Treves A. The representation of olfactory information in the primate orbitofrontal cortex. J. Neurophysiol. 1996a;75:1982–1996. doi: 10.1152/jn.1996.75.5.1982. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Critchley H, Wakeman E.A, Mason R. Responses of neurons in the primate taste cortex to the glutamate ion and to inosine 5′-monophosphate. Physiol. Behav. 1996b;59:991–1000. doi: 10.1016/0031-9384(95)02178-7. doi:10.1016/0031-9384(95)02178-7 [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Critchley H.D, Mason R, Wakeman E.A. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J. Neurophysiol. 1996c;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Critchley H.D, Browning A, Hernadi I. The neurophysiology of taste and olfaction in primates, and umami flavor. Ann. NY Acad. Sci. 1998;855:426–437. doi: 10.1111/j.1749-6632.1998.tb10602.x. doi:10.1111/j.1749-6632.1998.tb10602.x [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Critchley H.D, Browning A.S, Hernadi A, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J. Neurosci. 1999;19:1532–1540. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T, Kringelbach M.L, de Araujo I.E.T. Different representations of pleasant and unpleasant odors in the human brain. Eur. J. Neurosci. 2003a;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. doi:10.1046/j.1460-9568.2003.02779.x [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Verhagen J.V, Kadohisa M. Representations of the texture of food in the primate orbitofrontal cortex: neurons responding to viscosity, grittiness and capsaicin. J. Neurophysiol. 2003b;90:3711–3724. doi: 10.1152/jn.00515.2003. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, O'Doherty J, Kringelbach M.L, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex. 2003c;13:308–317. doi: 10.1093/cercor/13.3.308. doi:10.1093/cercor/13.3.308 [DOI] [PubMed] [Google Scholar]
- Sanghera M.K, Rolls E.T, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Exp. Neurol. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. doi:10.1016/0014-4886(79)90175-4 [DOI] [PubMed] [Google Scholar]
- Scott T.R, Yaxley S, Sienkiewicz Z.J, Rolls E.T. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J. Neurophysiol. 1986;56:876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- Scott T.R, Yan J, Rolls E.T. Brain mechanisms of satiety and taste in macaques. Neurobiology. 1995;3:281–292. [PubMed] [Google Scholar]
- Simmons W.K, Martin A, Barsalou L.W. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb. Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. doi:10.1093/cercor/bhi038 [DOI] [PubMed] [Google Scholar]
- Small D.M, Prescott J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. doi:10.1007/s00221-005-2376-9 [DOI] [PubMed] [Google Scholar]
- Small D.M, Voss J, Mak Y.E, Simmons K.B, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J. Neurophysiol. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. doi:10.1152/jn.00050.2004 [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabkakaran V, Zhao Z, Desmond J.E, Glover G.H, Sullivan E.V, Gabrieli J.D.E. Time course of odorant-induced activation in the human primary olfactory cortex. J. Neurophysiol. 2000;83:537–551. doi: 10.1152/jn.2000.83.1.537. [DOI] [PubMed] [Google Scholar]
- Thorpe S.J, Rolls E.T, Maddison S. Neuronal activity in the orbitofrontal cortex of the behaving monkey. Exp. Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. doi:10.1007/BF00235545 [DOI] [PubMed] [Google Scholar]
- Verhagen J.V, Rolls E.T, Kadohisa M. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J. Neurophysiol. 2003;90:1514–1525. doi: 10.1152/jn.00320.2003. [DOI] [PubMed] [Google Scholar]
- Verhagen J.V, Kadohisa M, Rolls E.T. The primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness and taste of foods in the mouth. J. Neurophysiol. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. doi:10.1152/jn.00321.2004 [DOI] [PubMed] [Google Scholar]
- Wang G.J, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. doi:10.1016/j.neuroimage.2003.11.026 [DOI] [PubMed] [Google Scholar]
- Wilson F.A.W, Rolls E.T. The primate amygdala and reinforcement: a dissociation between rule-based and associatively-mediated memory revealed in amygdala neuronal activity. Neuroscience. 2005;133:1061–1072. doi: 10.1016/j.neuroscience.2005.03.022. doi:10.1016/j.neuroscience.2005.03.022 [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls E.T, Sienkiewicz Z.J, Scott T.R. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Res. 1985;347:85–93. doi: 10.1016/0006-8993(85)90891-1. doi:10.1016/0006-8993(85)90891-1 [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls E.T, Sienkiewicz Z.J. The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiol. Behav. 1988;42:223–229. doi: 10.1016/0031-9384(88)90074-1. doi:10.1016/0031-9384(88)90074-1 [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls E.T, Sienkiewicz Z.J. Gustatory responses of single neurons in the insula of the macaque monkey. J. Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- Zald D.H, Pardo J.V. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc. Natl Acad. Sci. USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. doi:10.1073/pnas.94.8.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H, Lee J.T, Fluegel K.W, Pardo J.V. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. doi:10.1093/brain/121.6.1143 [DOI] [PubMed] [Google Scholar]
- Zatorre R.J, Jones-Gotman M, Evans A.C, Meyer E. Functional localization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. doi:10.1038/360339a0 [DOI] [PubMed] [Google Scholar]