Abstract
Interest in the biology of white adipose tissue has risen markedly with the recent surge in obesity and its associated disorders. The tissue is no longer viewed simply as a vehicle for lipid storage; instead, it is recognized as a major endocrine and secretory organ. White adipocytes release a multiplicity of protein hormones, signals and factors, termed adipokines, with an extensive range of physiological actions. Foremost among these various adipokines is the cytokine-like hormone, leptin, which is synthesized predominantly in white fat. Leptin plays a critical role in the control of appetite and energy balance, with mutations in the genes encoding the hormone or its receptor leading to profound obesity in both rodents and man. Leptin regulates appetite primarily through an interaction with hypothalamic neuroendocrine pathways, inhibiting orexigenic peptides such as neuropeptide Y and orexin A, and stimulating anorexigenic peptides such as proopiomelanocortin. White fat also secretes several putative appetite-related adipokines, which include interleukin-6 and adiponectin, but whether these are indeed significant signals in the regulation of food intake has not been established. Through leptin and the other adipokines it is evident that adipose tissue communicates extensively with other organs and plays a pervasive role in metabolic homeostasis.
Keywords: adipocytes, adipokines, appetite, energy balance, leptin, obesity
1. Introduction: energy balance
The control of appetite and energy balance are key biological processes in higher animals, and unravelling the critical mechanisms involved represents a continuing challenge in fundamental physiology. Recently, two practical issues have become major drivers in work on appetite and energy balance. The first relates to animal husbandry and food production, with the demand for the provision of meat animals of low carcass fat to meet nutritional recommendations for a reduction in dietary lipid intake, particularly of saturated fatty acids. The second, and now central, driver reflects the rapid rise in obesity in affluent societies, this being the major nutritional disorder in the developed world.
Obesity is fundamentally a problem of energy balance in that self-evidently it can develop only when energy intake is in excess of energy expenditure. This has led to a major focus on the mechanisms controlling intake and the components and regulatory mechanisms of energy expenditure. Much recent progress has been made in identifying the central neuroendocrine pathways involved both in the control of energy intake and of expenditure (see Trayhurn 2005a). Thus orexigenic pathways involving neuropeptide Y (NPY), melanin concentrating hormone (MCH), orexin A, agouti-related peptide (AgRP) and the endogenous cannabinoid system have each been identified (Ahima et al. 2000; Schwartz et al. 2000; Harrold & Williams 2003; Arch 2005). Similarly, anorexigenic pathways involving proopiomelanocortin (POMC) and the melanocortin system, cocaine and amphetamine regulated transcript (CART) and corticotrophin releasing hormone (CRH) are also recognized (Schwartz et al. 2000; Porte et al. 2005). Much effort is currently being directed towards defining the networks and the hierarchies involved in these neuroendocrine systems.
Several peripheral signals to the central neuroendocrine pathways of appetite and energy balance control have been identified. These signals, which include cholecystokinin, ghrelin, peptide YY and insulin, are predominantly associated with the gastrointestinal tract (Badman & Flier 2005; Otto et al. 2005; Wynne et al. 2005). A source of a further major peripheral signal is white adipose tissue; conceptually, as with the gut, this involves a direct link with an organ central to nutritional status (in the form of ingested nutrients in the case of the gastrointestinal tract, and the size of the lipid stores with adipose tissue). In this article, we specifically consider signals in appetite and energy balance, both established and putative, emanating from white fat.
2. Adipose tissue
The adipose organ consists of two apparently distinct tissue types—brown and white adipose tissue (Cinti 2001). Brown adipose tissue is concerned functionally with thermogenesis, or adaptive heat production, and this is achieved through the regulated uncoupling of mitochondrial oxidative phosphorylation via the presence of the tissue-specific uncoupling protein, UCP-1 (Cannon & Nedergaard 2004). While brown adipose tissue is a net consumer of fatty acids, white fat is the central organ for fuel storage in mammals. The major fuel storage role of white adipose tissue led to lipogenesis and lipolysis long being viewed as the key metabolic processes within the tissue.
Adipose tissue, and white fat in particular, is widely distributed throughout the body and is present in a number of distinct depots, both subcutaneously and around internal organs. In addition, adipocytes may be embedded within other tissues such as around skeletal muscle fibres. This locational diversity is increasingly considered to also reflect a degree of functional heterogeneity, with visceral fat being strongly associated with the metabolic syndrome and other obesity-linked disorders. Adipose tissue is the main variable in overall body composition in mammals, varying by an order of magnitude or more. Even in normal, lean individuals it is a substantial proportion of total tissue mass. For example, in an adult male with a body mass index (BMI) of 22 (close to ideal), some 20% of total tissue mass is white fat. In an obese individual, BMI=30, up to one half of total body tissue mass may be due to white fat.
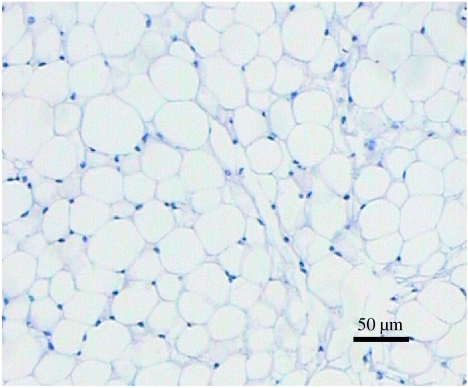
At the histological level, white adipose tissue seems remarkably simple in its structure (figure 1). There appears to be little other than mature adipocytes, filled with a large single (unilocular) lipid droplet, and this is reflected in the fact that the tissue may comprise 85% lipid by weight, enabling fuel to be stored at a high energy density. Despite superficial appearances, there is in practice considerable cellular heterogeneity, with mature adipocytes constituting no more than 50% of the total cell content of the tissue (Hausman 1985). The apparent simplicity of white fat at a histological level is partly why it is only recently that the complexity of adipocytes as secretory cells has become recognized.
Figure 1.
Histological section of white adipose tissue illustrating, at the level of light microscopy, the apparent simplicity of the tissue. A haematoxylin–eosin stained section of epididymal adipose tissue from a NMRI mouse is shown.
3. Adipokines: protein signals released from adipocytes
Fatty acids are quantitatively the most important secretory product from white adipocytes. In addition, certain other lipid moieties, including cholesterol, retinol, prostanoids and steroid hormones, are also released. Not all of these, however, are synthesized de novo within the fat cell; cholesterol and retinol, for example, are taken up from the circulation by adipocytes and stored.
The first protein recognized to be secreted from white adipocytes was lipoprotein lipase, which is responsible for the hydrolysis of circulating tricylglycerols (in the form of lipoproteins) with the subsequent uptake of the fatty acids released (Eckel 1989). A further secreted protein from adipocytes was identified in the late 1980s, namely the complement-related factor adipsin (Cook et al. 1987; Flier et al. 1987). Adipsin was initially thought to be the long sought adipocyte-derived signal in energy balance, but this was subsequently seen not to be the case. A major step forward in the recognition of the endocrine and secretory role of adipose tissue occurred in the early 1990s with the discovery that the pro-inflammatory cytokine tumour necrosis factor-α (TNFα) is synthesized and released by adipocytes (Hotamisligil et al. 1993, 1995). TNFα has subsequently been shown to have extensive metabolic effects in adipose tissue, these effects including the stimulation of both lipolysis and apoptosis (Prins et al. 1997; Gasic et al. 1999; Ryden et al. 2004). In addition, this cytokine plays an important role in the induction of insulin resistance in fat cells (Hotamisligil 2003; Hotamisligil et al. 1995).
Despite the initial expectations with adipsin, it was evident that the early protein factors identified as being secreted from adipocytes did not relate directly to the signalling of appetite or energy balance per se. However, the situation changed radically in 1994 with the discovery of the major adipocyte-derived hormone leptin. Leptin was identified during the characterization of the Ob gene, a mutation in which is responsible for the obesity of the ob/ob mouse (Zhang et al. 1994)—perhaps the most widely used animal model in obesity research. In the decade subsequent to the discovery of leptin, a number of other protein signals and factors secreted from adipocytes have been identified (Frühbeck et al. 2001; Trayhurn & Beattie 2001; Rajala & Scherer 2003; Trayhurn & Wood 2004; Hauner 2005; Trayhurn 2005b). These secretory proteins, which are generally known by the collective term ‘adipokines’, now number in excess of 50 different molecular entities (Trayhurn & Wood 2004; Hauner 2005); thus the adipocyte is a secretory cell of considerable richness and complexity.
4. Leptin
The discovery of leptin resulted in a radical shift in our perspectives on the physiological role of white fat, the tissue now being recognized as a major endocrine organ, which plays a direct—and critical—role in the regulation of energy balance. Indeed, white fat can be viewed as the largest endocrine organ in man, and very particularly in the obese.
Leptin (Greek leptos, meaning thin or small) is synthesized as an 18 000 mol. wt. pro-hormone which is cleaved to yield a 16 000 mol. wt. mature product which is ‘cytokine-like’. White adipose tissue (hereafter referred to simply as adipose tissue) is the main site of Ob gene expression and leptin secretion. Expression and secretion occur exclusively within the differentiated adipocytes. Leptin, however, is also produced in several cell types in other organs. For example, it is produced by gastric cells in the walls of the stomach (Bado et al. 1998; Cinti et al. 2000), in follicular papilla cells of hair follicles (Iguchi et al. 2001), in osteoblasts (Reseland et al. 2001) and in the placenta (Hassink et al. 1997; Hoggard et al. 1997; Masuzaki et al. 1997). In addition, it is produced in certain foetal organs in which synthesis does not occur in the adult (Hoggard et al. 1997). In each of these cases, it is probable that the effect of leptin is essentially local rather than systemic, i.e. a paracrine or autocrine action, rather than endocrine.
Despite the range of specific sites in which leptin is synthesized, white adipose tissue is quantitatively much the most important source of the hormone and the primary determinant of the circulating levels. Indeed, one of the earliest observations following the discovery of leptin was that of a direct relationship between BMI, or percent body fat, and circulating level of the hormone (Considine et al. 1996; Ostlund et al. 1996). In addition to the importance of body fat in determining circulating leptin levels, production of the hormone is subject to acute regulation by other factors. This includes nutritional regulation, as would be expected for a factor involved in the regulation of energy balance, the most potent example of which is the response to fasting; fasting leads to a marked reduction in ob mRNA level in adipose tissue and a rapid fall in circulating leptin levels, changes which are reversed on refeeding (Becker et al. 1995; Trayhurn et al. 1995; Hardie et al. 1996).
A range of hormones and drugs have been shown to directly influence Ob gene expression and leptin secretion from adipocytes, particularly from in vitro studies using adipocyte cell culture systems. The thiazolidinediones, which are PPARγ agonists, strongly inhibit leptin production indicating that this nuclear receptor is involved in the control of leptin gene transcription (Kallen & Lazar 1996; De Vos et al. 1996). Glucocorticoids on the other hand, including dexamethasone, stimulate leptin production (De Vos et al. 1995; Wabitsch et al. 1996). Much emphasis has also been placed on the role of insulin, which like glucocorticoids is stimulatory, and it is suggested that this hormone is particularly important physiologically in regulating circulating leptin levels (Saladin et al. 1995; Leroy et al. 1996; Havel 2000).
Considerable importance has also been given to the regulatory role of catecholamines and the sympathetic nervous system (Trayhurn et al. 1998; Rayner & Trayhurn 2001). Administration of noradrenaline (norepinephrine), or the β-adrenoceptor agonist isoprenaline, potently suppresses Ob gene expression and lowers circulating leptin levels (Trayhurn et al. 1998). In the case of rodents, this suppression operates principally through the β3-adrenoreceptor subtype, since β3-selective agonists are at least as effective as isoprenaline (Giacobino 1996; Mantzoros et al. 1996; Trayhurn et al. 1996). This led to the proposal that the sympathetic nervous system is a key component of the regulation of leptin production in adipocytes and, importantly, provides a negative feedback loop from the brain to fat cells in the control of Ob gene transcription (Trayhurn et al. 1998; Rayner & Trayhurn 2001; Mark et al. 2003). Further evidence for this proposition comes from several sources, including the observation that the blockade of noradrenaline synthesis through the administration of α-methyl-p-tyrosine, a selective tyrosine hydroxylase inhibitor, leads to hyperleptinaemia and an increase in ob mRNA level in white adipose tissue (Rayner et al. 1998; Sivitz et al. 1999).
(a) Targets for leptin: appetite
The initial proposition for the central physiological action of leptin was as a satiety factor, and as such the discovery of the hormone provided a molecular basis for the lipostatic theory of the regulation of energy balance. This theory, developed in the 1950s, proposed that there is a signal from adipose tissue to the hypothalamus in proportion to tissue mass, resulting in a feedback loop to the brain in the control of energy balance (Kennedy 1953). However, until the discovery of leptin no molecular entity had been identified which could meet the criteria for such a signal.
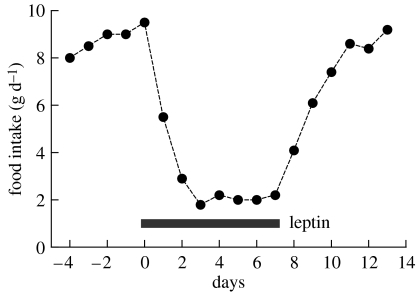
There is no doubt that leptin has a powerful inhibitory effect on food intake, and this is illustrated in figure 2 (adapted from Mercer et al. 1997), which shows the effects of administering recombinant leptin to obese ob/ob mice which lack the functional hormone. Leptin reduces the food intake of these mutant animals by approximately 75%, an effect which ceases once the injection is switched to vehicle. Administering leptin to normal animals also inhibits food intake, but the effect is considerably less dramatic, as would be expected since in this case the endogenously produced hormone is present.
Figure 2.
Appetite suppressing effects of leptin. The figure illustrates the powerful inhibitory effect of leptin on food intake in ob/ob mice, which lack the functional hormone. Mice were injected with vehicle from days −4 to 0 and then with recombinant leptin (1.25 μg g−1 body wt, twice daily) from days 0 to 7; they were then returned to vehicle injections. Adapted from Mercer et al. (1997).
In addition to the effects on food intake, there has been some focus on whether leptin also affects energy expenditure, i.e. the other side of the energy balance relationship. The view has developed that leptin does not stimulate expenditure per se, but may be involved in inhibiting fasting-induced adaptations in expenditure (Doring et al. 1998). One of the strongest pieces of evidence for a key role for leptin in expenditure is that ob/ob mice develop obesity on a normal energy intake, as documented in pair-feeding studies in which young obese mutants were pair-fed to the ad libitum food intake of lean siblings (Thurlby & Trayhurn 1979). This difference in energetic efficiency can only reflect a lower energy expenditure in the leptin-deficient mutants. In addition, the early development of obesity in ob/ob mice takes place on a normal energy intake, hyperphagia only developing some days after weaning at three weeks of age (Trayhurn 1984). These studies were undertaken in the pre-leptin era (i.e. before leptin was discovered), but merit re-interpretation.
A further report from the same period illustrates the importance of leptin in diet-induced thermogenesis. When normal lean mice are fed a cafeteria diet—a mixed diet of highly palatable human-type food items, as pioneered by Rothwell and Stock (Rothwell & Stock 1979)—hyperphagia is induced. In a study on lean and obese ob/ob mice, the lean animals increased their energy intake by approximately 70% on the cafeteria diet. Nevertheless, there was no significant difference in energy deposition, because of the hyperphagia-induced stimulation of energy expenditure in the form of diet-induced thermogenesis (Trayhurn et al. 1982). In this particular experiment the lean animals receiving the cafeteria diet serendipitously had the same energy intake as ob/ob mice consuming the normal diet; in other words, they were ‘pair-fed’ on an energy basis (isocaloric intakes). However, the ob/ob mutants deposited considerably more energy than their lean wild type counterparts, indicating that diet-induced thermogenesis was substantially reduced in the absence of functional leptin (Trayhurn et al. 1982).
Pronounced effects of leptin on food intake have also been observed in studies on humans. Obese children who had been identified as having a mutation in the leptin gene, resulting in a non-functional hormone (Montague et al. 1997), had their food intake substantially reduced by the administration of recombinant leptin, and this was accompanied by a reduction in body weight and body fat (Farooqi et al. 1999, 2002). Thus, as predicted on the basis of rodent studies, leptin is a powerful anorexigenic signal in humans. Again, as with rodents, the effects of leptin administration are not restricted to appetite and energy balance, but include neuroendocrine actions and effects on T-cell responsiveness (Farooqi et al. 2002).
(b) Leptin receptors
The leptin receptor, a member of the cytokine receptor family, was first cloned a year after leptin itself (Tartaglia et al. 1995). It was quickly recognized that the receptor exists as several splice variants: Ob-Ra, Ob-Rb, Ob-Rc, Ob-Rd, Ob-Re and Ob-Rf (Lee et al. 1996; Chua et al. 1997; Tartaglia 1997). Ob-Rb has received most attention since it is the long form of the receptor, with an intracellular signalling domain that contains all the motifs required for the action of leptin on appetite (Lee et al. 1996). The intracellular domain interacts with the Janus kinases (JAK; Lee et al. 1996) and signal transducer and activators of transcription-3 (STAT3) transcription factor (Vaisse et al. 1996), necessary for leptin signal transduction. The JAK/STAT pathway induces expression of the suppressor of cytokine signalling-3, a leptin signalling inhibitor that has been identified as a potential mediator of central leptin resistance (Bjorbaek et al. 1999). Leptin signalling has, however, also been reported with other isoforms of the receptor (Murakami et al. 1997).
Leptin receptors are expressed ubiquitously; indeed, it is difficult to identify a tissue in which they are not present. Following the cloning of the receptor, and the identification of splice variants, strong receptor expression was demonstrated in regions of the hypothalamus, including of the Ob-Rb isoform (Mercer et al. 1996b). Ob-Rb is highly abundant in the hypothalamus, particularly the arcuate nucleus (ARC), ventromedial nucleus, dorsomedial nucleus (DMH) and the lateral hypothalamic area (LHA; Fei et al. 1997; Elmquist et al. 1998). Studies on the co-localization between the leptin receptor and specific neuroendocrine systems have shown that Ob-Rb is expressed by many of the major appetite-regulating neuronal pathways. For example, Ob-Rb mRNA is expressed in the ARC by orexigenic NPY/AgRP neurons (Mercer et al. 1996a) and by anorexigenic POMC/CART neurons (Cheung et al. 1997). This is consistent with the powerful satiety effects of leptin operating through key hypothalamic nuclei.
(c) Leptin entry into the CNS
One of the issues in interpreting the central actions of leptin is the mechanism by which this peripheral signal crosses the blood-brain barrier. Circulating leptin is transported into the brain via a saturable process independent of insulin (Banks et al. 1996). The short form of the receptor, Ob-Ra, is considered to play a key role in the transport of leptin across the blood–brain barrier. Regulation of brain leptin transport appears to be important in mediating the effects of leptin on appetite. Brain transport is abolished during fasting in parallel with the fall in circulating leptin, but transport is increased on refeeding when circulating leptin levels rise (Kastin & Pan 2000). The dynamic changes in brain transport might be also influenced by other nutritional factors, since diet-induced obesity is associated with impaired leptin transport across the blood–brain barrier (Banks et al. 1999).
(d) Leptin–neuroendocrine interactions
Leptin interacts with several central neuroendocrine pathways, both orexigenic and anorexigenic (Ahima et al. 2000; Schwartz et al. 2000; Arch 2005). Orexigenic neurons are inhibited by leptin, and this was initially demonstrated with the NPY pathway. NPY, a powerful stimulator of food intake, is mainly synthesized in the ARC neurons that project to the paraventricular nucleus (PVN), DMH and other areas within the hypothalamus (Bai et al. 1985). Leptin, administered either intracerebroventricularly (ICV) or systemically, reduces the abnormally high NPY mRNA levels in the ARC, and the release of NPY from the PVN in ob/ob mice, but not in leptin resistant db/db mice (Stephens et al. 1995; Schwartz et al. 1996), while ICV injection of leptin decreased NPY protein levels in the ARC and PVN of lean and fa/fa Zucker rats (Cusin et al. 1996). Subsequently, the appetite lowering and thermogenic actions of leptin, via inhibition of the NPY neurons in the ARC, have been demonstrated in normal rats; ICV administration of leptin reduced NPY protein levels in the ARC, PVN and DMH, and this was accompanied by a rapid decline in food intake and increased UCP-1 mRNA levels in brown fat (Wang et al. 1997). Moreover, starvation is normally associated with the fall in leptin and elevated NPY neuronal activity thereby stimulating the drive to eat, whereas leptin blocks the increase in NPY mRNA induced by fasting in normal mice (Ahima et al. 1996). It was, therefore, proposed that regulation of the neuroendocrine system during starvation could be the key physiological role of leptin (Ahima et al. 1996).
In addition to NPY, another orexigenic neuropeptide, AgRP, is a hypothalamic target of leptin. AgRP is co-expressed in 90% of the ARC–NPY neurons (Broberger et al. 1998) and appears to function as an endogenous antagonist to the melanocortin (MC)4-R receptor that mediates the appetite-suppressing action of α-melanocyte-stimulating hormone (α-MSH). AgRP, like NPY, stimulates feeding and is elevated in both leptin-deficient ob/ob and leptin-resistant db/db mice, and its overexpression in transgenic mice leads to obesity (Ollmann et al. 1997). During fasting, AgRP gene expression and peptide release from the hypothalamus increases, but these effects can be reversed by leptin infusion (Mizuno & Mobbs 1999; Korner et al. 2001). Furthermore, an in vitro inhibitory effect of leptin perfusion on AgRP peptide release from hypothalamic slices has been observed in fed rats (Breen et al. 2005).
Other orexigenic peptides, such as MCH and orexin A, synthesized in the LHA, are probably inhibited by leptin. MCH is overexpressed in the hypothalamus of leptin-deficient ob/ob mice (Qu et al. 1996) and in rats where hypoleptinaemia has been induced by food-deprivation (Presse et al. 1996). Leptin replacement blunts increases in MCH mRNA levels in ob/ob mice and fasted mice (Tritos et al. 2001). Interestingly, ablation of the MCH gene in the ob/ob mouse results in a marked reduction in body fat, and the leanness of such combined leptin- and MCH-knockouts is a consequence of a substantial increase in energy expenditure, rather than through an attenuation of hyperphagia. These observations suggest that MCH might integrate energy homeostasis downstream of leptin (Segal-Lieberman et al. 2003). Orexin neurons co-express leptin receptors (Funahashi et al. 2000) and express STAT3, the transcription factor for leptin signal transduction. Orexin A induces feeding (Edwards et al. 1999) but hyperphagia is transient (Haynes et al. 1999), and leptin, when given peripherally, decreases orexin A levels in the LHA (Beck & Richy 1999).
The endocannabinoid system is also considered to be inhibited by leptin since acute treatment with the hormone decreases the hypothalamic endogenous cannabinoids, anandamide and 2-arachidonoyl glycerol, in normal rats as well as in ob/ob mice (Di Marzo et al. 2001).
Anorexigenic systems are, in contrast, stimulated by leptin. POMC is the precursor of α-MSH, an important appetite regulator that acts through binding to the melanocortin receptor family. POMC is synthesized in the ARC and in the nucleus of the solitary tract. In the ARC, approximately 30% of the POMC-expressing neurons carry the Ob-Rb receptor (Cheung et al. 1997). Intraperitoneal leptin administration upregulates hypothalamic POMC mRNA (Schwartz et al. 1997), while situations associated with a fall in leptin, such as fasting or the loss of the leptin signal (ob/ob mouse and fa/fa rat), show decreased POMC mRNA levels (Mizuno et al. 1998). Leptin therefore appears to activate POMC neurons, probably resulting in elevated α-MSH production, thereby inhibiting food intake via its interactions with MC4-R and/or MC3-R.
CART is found to be co-expressed with α-MSH in the lateral ARC (Elias et al. 1998; Kristensen et al. 1998), and neurons expressing CART are also present in the LHA and PVN (Couceyro et al. 1997). Food-deprivation induces a pronounced decrease in CART mRNA within the ARC, while peripheral administration of leptin stimulates CART gene expression in ob/ob mice (Kristensen et al. 1998). Second order neurons, such as those synthesizing CRH in the PVN, are controlled indirectly by leptin targets in the ARC, and mediate the inhibitory effects of leptin on food intake, and the stimulation of thermogenesis and neuroendocrine secretions (Ahima et al. 2000). Thus POMC, CART and CRH are each upregulated by leptin.
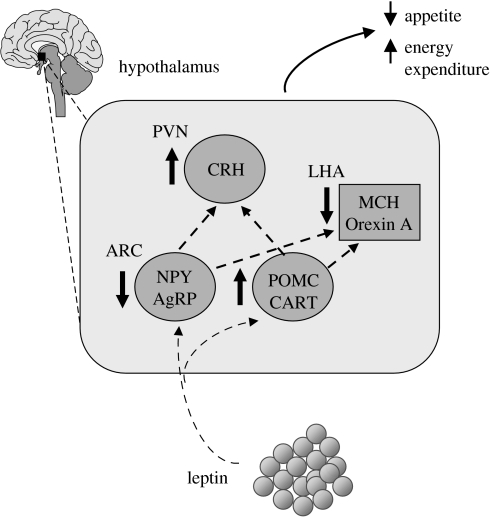
The net effect from these interactions of leptin with central neuroendocrine systems is that there is a powerful, integrated peripheral signal which serves to suppress food intake (figure 3).
Figure 3.
Schematic view of the integrated signalling effect of leptin on appetite through the central hypothalamic neuroendocrine pathways. AgRP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; CRH, corticotrophin releasing hormone; MCH, melanin concentrating hormone; NPY, neuropeptide Y; POMC, pro-opiomelanocortin.
(e) Electrophysiological effects of leptin
The initial studies on the central effects of leptin focused on the expression of neuropeptide genes and on the level of the encoded proteins. Recent studies using electrophysiological techniques have demonstrated effects of the hormone on hypothalamic neurotransmission. Fasting significantly increased the basal spike frequency of ARC NPY/AgRP neurons in mice, whereas treatment with leptin induced a dose-dependent decrease in firing frequency in these fasted-animals (Takahashi & Cone 2005), in agreement with the proposed role of leptin as a starvation signal. In leptin deficient and resistant mice (ob/ob and db/db, respectively), NPY/AgRP neuron spike frequency is increased analogous to fasting, which may underlie their hyperphagic phenotype (Takahashi & Cone 2005).
(f) Leptin and the development of hypothalamic feeding circuits
Recent observations have indicated a novel regulatory role of leptin in neuronal plasticity in hypothalamic neurons that regulate appetite. These studies, using electrophysiological approaches, have revealed leptin-mediated links between nutrition and neuro-development by demonstrating that the lack of leptin in ob/ob mice increases the number of excitatory synapses and decreases the inhibitory inputs on NPY/AgRP neurons while exerting opposite effects on POMC neurons (Pinto et al. 2004). It is likely that leptin can modulate both synapse number and the activity of cells, and this is supported by the observation that leptin repletion in ob/ob mice rapidly reverses both electrophysiological and axosomatic characteristics.
As mentioned earlier, ARC neurons have extensive connections with other hypothalamic nuclei, including the PVN, DMH and LHA, each of which is involved in appetite regulation. A recent study, using a lipophilic tracer that labels axonal projections, has shown that leptin deficiency causes profound and permanent disruption in the development of ARC projections to all three nuclei (Bouret et al. 2004). Interestingly, leptin replacement to neonatal ob/ob mice restored the density of projections, but failed to normalize fibre density in adult ob/ob mice, indicating a critical timing in leptin guidance in the development of hypothalamic neurons.
(g) The leptin system and obesity
It has been widely supposed that the administration of exogenous leptin, thereby increasing the circulating level of the hormone, should inhibit food intake and serve as an approach to obesity therapy. However, little success has been obtained through this approach and the concept of leptin resistance has been introduced. Whether there is resistance in any meaningful sense is open to debate (Arch et al. 1998). Certainly, there is not an unlimited response to leptin and there is no doubt that mutations in the leptin receptor result in a ‘resistance’ to the action of the hormone. This is evident in mutant rodents—the db/db mouse and the fa/fa rat—as indicated earlier, as well as in humans with a leptin receptor mutation (Chua et al. 1996; Lee et al. 1996; Clément et al. 1998). It seems increasingly likely, however, that in terms of energy balance only a small level of leptin is required with the appetite effects plateauing at, or below, normal physiological levels (Leibel 2002).
The increases in leptin that occur in obesity could primarily reflect the fact that there is an expansion of the major tissue in which the hormone is produced, rather than relate to an attempt to overcome a putative resistance. If the levels of circulating leptin physiologically are much higher than is required for the satiety effect, this may reflect the fact that there are many actions of leptin outwith appetite control. In practice, leptin is a pleiotropic hormone and a large number of effects have been described (Trayhurn et al. 1999; Harris 2000). These include as a signal in angiogenesis, immunity, reproduction and in insulin secretion (Barash et al. 1996; Chehab et al. 1997; Pallett et al. 1997; Bouloumie et al. 1998; Lord et al. 1998; Sierra-Honigmann et al. 1998; Morton et al. 1999). A broad range of leptin actions is consistent with the widespread distribution of receptors for the hormone in organs not related directly to appetite and energy balance.
(h) Leptin and the sympathetic nervous system
We have mentioned earlier the link between the sympathetic nervous system and white adipose tissue in terms of the control of leptin production, the sympathetic system providing a feedback loop from the brain to the tissue (Trayhurn et al. 1998; Rayner & Trayhurn 2001; Mark et al. 2003). The interaction between the hormone and the sympathetic system is, however, bi-directional with leptin having been shown to stimulate the sympathetic output to several tissues; these tissues include the kidneys, brown adipose tissue and white fat (Haynes et al. 1997a,b; Mark et al. 2003).
The general view has long been that white adipose tissue, in contrast to brown fat, is sparsely innervated by the sympathetic system (Youngström & Bartness 1998; Bartness et al. 2005). However, the extent and importance of the sympathetic innervation is now recognized, with the sympathetic system playing a key role physiologically in the control of lipolysis (Youngström & Bartness 1998; Bartness et al. 2005). It has also been proposed that adipose tissue contains a parasympathetic innervation (Kreier et al. 2002), but the proposition is somewhat controversial and the evidence has not yet been substantiated (Bartness 2002). The sympathetic origins of white adipose tissue have been elegantly explored by Bartness and colleagues using pseudorabies virus as a retrograde tract tracer (Bartness & Bamshad 1998; Bamshad et al. 1998; Bartness et al. 2005). A number of areas of the brain have been identified from which the sympathetic innervation to adipose tissue (both brown and white) originates, and these include areas within the hypothalamus associated with energy balance regulation. This illustrates the extent to which adipose tissue is under direct central control.
5. Putative appetite signals from adipose tissue
We have commented above on the large number of protein signals and factors which are now recognized to be secreted from white adipocytes. These adipokines include proteins involved in insulin sensitivity (particularly adiponectin), angiogenesis (e.g. vascular endothelial growth factor), lipid metabolism (e.g. cholesteryl ester transfer protein, retinol binding protein), vascular haemostasis (e.g. plasminogen activator inhibitor 1, tissue factor), inflammation (e.g. TNFα, interleukin (IL)-1β, IL-6) and the acute phase response (e.g. haptoglobin, serum amyloid A) (Trayhurn & Beattie 2001; Frühbeck et al. 2001; Rajala & Scherer 2003; Trayhurn & Wood 2004; Hauner 2005; Trayhurn 2005b). The implication of such a diverse range of proteins being secreted from white fat is that the tissue is involved in extensive cross-talk with other tissues and organs and is intimately involved in general metabolic homeostasis—certainly beyond the basic paradigm of fat storage and release.
There continues to be much interest in whether there are signals from adipocytes in appetite and energy balance beyond leptin. However, the evidence for other factors is as yet limited. Fasting induced adipose factor (FIAF, or angiopoietin-like protein 4) appeared a candidate in that its expression in adipose tissue and the circulating levels were reported to dramatically increase in fasting, responses to food deprivation that are reciprocal to that of leptin itself (Kersten et al. 2000). However, it is now evident that FIAF is not always induced by fasting, the response apparently being limited to certain strains of mice. Furthermore, this protein, which like leptin is subject to regulation through the PPARγ nuclear receptor (Kersten et al. 2000; Yoon et al. 2000), is increasingly implicated in the regulation of lipid metabolism, particularly through the inhibition of lipoprotein lipase (Yoshida et al. 2002; Xu et al. 2005).
Adiponectin, a complement-like protein produced exclusively by adipocytes, was discovered by several groups who each proposed differing names, including Arp30 and AdipoQ (Scherer et al. 1995; Hu et al. 1996; Maeda et al. 1996). This hormone has been shown to have a wide range of roles, including in insulin sensitivity, inflammation and vascular function (Scherer et al. 1995; Ouchi et al. 1999; Yokota et al. 2000; Berg et al. 2001; Yamauchi et al. 2001). Adiponectin has strong sequence homology with C1q and types VIII and X collagen (Maeda et al. 1996). In contrast to leptin and a number of other adipokines, the circulating level of adiponectin and the adipose tissue expression of the adiponectin gene are inversely related to adiposity (Arita et al. 1999; Hotta et al. 2000, 2001). Thus adiponectin levels fall with obesity, while increases occur during weight loss induced by a calorie-restricted diet (Arita et al. 1999; Hotta et al. 2000, 2001).
Importantly, adiponectin may be a direct signal in appetite and the control of body weight (Shklyaev et al. 2003; Qi et al. 2004). In addition, peripheral adiponectin administration has been reported to reduce body weight through increased fatty acid combustion and energy dissipation (Berg et al. 2001; Yamauchi et al. 2001; Tomas et al. 2002)—without apparent effects on feeding. However, sustained peripheral expression of transgene adiponectin through a viral vector inhibits food intake and reduces body weight, concomitantly with improved insulin sensitivity and decreased lipid levels, in diet-induced obese rats (Shklyaev et al. 2003).
There is some recent evidence that adiponectin may cross the blood–brain barrier (Qi et al. 2004), and moreover the cloned adiponectin receptors 1 and 2 have been found to be expressed in the CNS (Yamauchi et al. 2003). It is, therefore, proposed that adiponectin could act centrally in the regulation of appetite and energy balance. In this regard, recent work has shown that adiponectin induces c-fos immunoreactivity in the PVN, and stimulates hypothalamic CRH synthesis (Qi et al. 2004). In addition, agouti (Ay/a) mice are not responsive to adiponectin, indicating that the melanocortin system might be involved in a central effect of the adipocyte hormone (Qi et al. 2004).
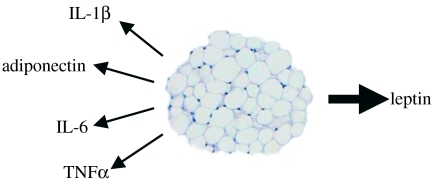
Other putative adipose tissue-derived signals in appetite and energy balance include several cytokines, such as IL-1β, IL-6 and TNFα (figure 4). Interleukin-6 is perhaps the most interesting of these candidates since it induces weight loss, as well as producing insulin resistance in adipocytes (Lagathu et al. 2003; Rotter et al. 2003). IL-6 is expressed together with its receptor in neurons of hypothalamic nuclei that regulate body composition (Shizuya et al. 1998), implying a role in the central modulation of energy homeostasis. This has been demonstrated in IL-6 knockout mice that develop late-onset obesity with abnormal carbohydrate and lipid metabolism (Wallenius et al. 2002b). Chronic ICV administration of IL-6 reduces body fat through upregulation of energy expenditure without causing an acute phase reaction (Wallenius et al. 2002a).
Figure 4.
Leptin and putative appetite signals from white adipose tissue. IL, interleukin; TNFα, tumour necrosis factor-α.
Interleukin-1β and TNFα have been implicated in the anorexia of disease; these cytokines are also produced by adipose tissue. TNFα synthesis in white fat is increased in obesity in rodents and in humans (Hotamisligil et al. 1995; Kern et al. 1995). TNFα inhibits lipoprotein lipase activity, decreases insulin receptor tyrosine kinase activity and stimulates lipolysis in adipose tissue (Kern 1997). ICV infusion of IL-1β or TNFα inhibits feeding (Plata-Salaman et al. 1996; Sonti et al. 1996), while chronic peripheral administration is usually associated with tolerance to their anorectic effects (Weingarten et al. 1992). There is, however, a conceptual difficulty in invoking a specific role in appetite for classical inflammation-related factors such as IL-6 and TNFα; these proteins are released by a range of cells and tissues (including skeletal muscle in severe exercise), and as such it is difficult to attribute to them a specific role as adipocyte-derived signals in food intake and energy balance.
6. Perspectives
Leptin is undoubtedly a powerful peripheral signal from adipose tissue to the brain in the long-term control of appetite and energy balance. It has not, however, so far proved possible to convincingly titrate small or rapid fluctuations in circulating leptin levels with specific changes in appetite or food intake. Leptin is synthesized in brown, as well as white, adipocytes (Deng et al. 1997), but whether this is significant in terms of signalling to appetite is unclear. Given the limited importance of brown adipose tissue quantitatively relative to white fat, particularly in humans, it is unlikely that brown adipocytes are a significant contributor to the circulating levels of the hormone. As with other cells outwith white adipose tissue, it is possible that the role of leptin synthesized and released by brown adipocytes is local rather than endocrine.
The extent to which there are important signals from adipocytes to the brain in the control of intake, additional to leptin, is still uncertain, although indirect effects via peripheral actions on other hormones may occur. An example of a signal indirect to adipocytes is insulin. The circulating levels of insulin, secreted of course not from adipocytes but by pancreatic β-cells, are generally elevated in obesity as a consequence of insulin resistance, and this hormone has long been invoked as a signal to the brain in the control of energy balance and fat stores (Schwartz et al. 1992, 2000; Porte et al. 2005). Indeed, insulin enters the brain and acts so as to reduce food intake (Schwartz et al. 2000).
The discovery of leptin has resulted in a major change in perspectives on the biological role of white adipose tissue—as a key endocrine and secretory organ (Trayhurn & Beattie 2001; Frühbeck et al. 2001; Rajala & Scherer 2003; Trayhurn & Wood 2004). From the wide range of adipokines now recognized to be secreted from white adipocytes it is evident that adipose tissue is tightly integrated into overall metabolic control and communicates extensively with other organs and cell types. Signalling to appetite and energy balance is but one—albeit critically important—component of the physiological role of white adipose tissue. The significance of adipose tissue in homeostatic control is underscored by the sheer size of the organ and its major impact on overall body composition.
A question of growing importance in adipose tissue biology is the extent of cross-talk between mature adipocytes and the other cell types within the organ. The issue has been highlighted by the recent reports that white fat becomes infiltrated by macrophages during the development of obesity, with the macrophages amplifying the inflammatory response within the expanding tissue (Weisberg et al. 2003; Xu et al. 2003). This almost certainly involves the release of inflammatory cytokines and associated factors from macrophages which modulate adipocyte function, as well as the secretion of chemokines such as monocyte chemoattractant protein 1 and migration inhibitory factor from mature fat cells which in turn will act as attractant signals to the macrophages. Conversations between different cell types within adipose tissue will, in all probability, extend beyond mature adipocytes and macrophages, and will involve pre-adipocytes in particular. A key question is the extent to which cellular cross-talk, especially between macrophages and mature adipocytes, is likely to modify the release of appetite-related signals from adipose tissue—and this may be particularly relevant to IL-6 and TNFα in the anorexia of cachexia.
Acknowledgments
Work in our laboratory is supported by grants from the BBSRC, European Union (OB-Age: QLK6-CT-2002-02288), and the Broadgreen and Royal Liverpool University Hospital NHS Trust. We are grateful to our colleagues in the Obesity Biology Unit for their continuing help and support.
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Ahima R.S, Prabakaran D, Mantzoros C, Qu D.Q, Lowell B, Maratos-Flier E, Flier J.S. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. doi:10.1038/382250a0 [DOI] [PubMed] [Google Scholar]
- Ahima R.S, Saper C.B, Flier J.S, Elmquist J.K. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. doi:10.1006/frne.2000.0197 [DOI] [PubMed] [Google Scholar]
- Arch J.R.S. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc. Nutr. Soc. 2005;64:39–46. doi: 10.1079/pns2004407. doi:10.1079/PNS2004407 [DOI] [PubMed] [Google Scholar]
- Arch J.R.S, Stock M.J, Trayhurn P. Leptin resistance in obese humans: does it exist and what does it mean? Int. J. Obes. 1998;22:1159–1163. doi: 10.1038/sj.ijo.0800779. doi:10.1038/sj/ijo/0800779 [DOI] [PubMed] [Google Scholar]
- Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. doi:10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- Badman M.K, Flier J.S. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. doi:10.1126/science.1109951 [DOI] [PubMed] [Google Scholar]
- Bado A, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. doi:10.1038/29547 [DOI] [PubMed] [Google Scholar]
- Bai F.L, Yamano M, Shiotani Y, Emson P.C, Smith A.D, Powell J.F, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. doi:10.1016/0006-8993(85)90730-9 [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki V.T, Adkison M.G, Warren W.S, Bartness T.J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Banks W.A, Kastin A.J, Huang W.T, Jaspan J.B, Maness L.M. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. doi:10.1016/0196-9781(96)00025-3 [DOI] [PubMed] [Google Scholar]
- Banks W.A, Dipalma C.R, Farrell C.L. Impaired transport of leptin across the blood–brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. doi:10.1016/S0196-9781(99)00139-4 [DOI] [PubMed] [Google Scholar]
- Barash I.A, Cheung C.C, Weigle D.S, Ren H.P, Kabigting E.B, Kuijper J.L, Clifton D.K, Steiner R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. doi:10.1210/en.137.7.3144 [DOI] [PubMed] [Google Scholar]
- Bartness T.J. Dual innervation of white adipose tissue: some evidence for parasympathetic nervous system involvement. J. Clin. Invest. 2002;110:1235–1237. doi: 10.1172/JCI17047. doi:10.1172/JCI200217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T.J, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;44:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Bartness T.J, Kay Song C, Shi H, Bowers R.R, Foster M.T. Brain–adipose tissue cross talk. Proc. Nutr. Soc. 2005;64:53–64. doi: 10.1079/pns2004409. doi:10.1079/PNS2004409 [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S. Hypothalamic hypocretin/orexin and neuropeptide Y: divergent interaction with energy depletion and leptin. Biochem. Biophys. Res. Commun. 1999;258:119–122. doi: 10.1006/bbrc.1999.0605. doi:10.1006/bbrc.1999.0605 [DOI] [PubMed] [Google Scholar]
- Becker D.J, Ongemba L.N, Brichard V, Henquin J.C, Brichard S.M. Diet-induced and diabetes-induced changes of ob gene-expression in rat adipose-tissue. FEBS Lett. 1995;371:324–328. doi: 10.1016/0014-5793(95)00943-4. doi:10.1016/0014-5793(95)00943-4 [DOI] [PubMed] [Google Scholar]
- Berg A.H, Combs T.P, Du X, Brownlee M, Scherer P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. doi:10.1038/90992 [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist J.K, Elhaschimi K, Kelly J, Ahima R.S, Hileman S, Flier J.S. Activation of SOCS-3 messenger ribonucleic acid in the hypothalamus by ciliary neurotrophic factor. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. doi:10.1210/en.140.5.2035 [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Drexler H.C.A, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- Bouret S.G, Draper S.J, Simerly R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. doi:10.1126/science.1095004 [DOI] [PubMed] [Google Scholar]
- Breen T.L, Conwell I.M, Wardlaw S.L. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res. 2005;1032:141–148. doi: 10.1016/j.brainres.2004.11.008. doi:10.1016/j.brainres.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl Acad. Sci. USA. 1998;95:15 043–15 048. doi: 10.1073/pnas.95.25.15043. doi:10.1073/pnas.95.25.15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. doi:10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Chehab F.F, Mounzih K, Lu R.H, Lim M.E. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. doi:10.1126/science.275.5296.88 [DOI] [PubMed] [Google Scholar]
- Cheung C.C, Clifton D.K, Steiner R.A. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. doi:10.1210/en.138.10.4489 [DOI] [PubMed] [Google Scholar]
- Chua S.C, Chung W.K, Wupeng X.S, Zhang Y.Y, Liu S.M, Tartaglia L, Leibel R.L. Phenotypes of mouse diabetes and rat fatty due to mutations in the ob (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Chua S.C, Koutras I.K, Han L, Liu S.M, Kay J, Young S.J, Chung W.K, Leibel R.L. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264–270. doi: 10.1006/geno.1997.4962. doi:10.1006/geno.1997.4962 [DOI] [PubMed] [Google Scholar]
- Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- Cinti S, Matteis R.D, Pico C, Ceresi E, Obrador A, Maffeis C, Oliver J, Palou A. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obes. 2000;24:789–793. doi: 10.1038/sj.ijo.0801228. doi:10.1038/sj.ijo.0801228 [DOI] [PubMed] [Google Scholar]
- Clément K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. doi:10.1038/32911 [DOI] [PubMed] [Google Scholar]
- Considine R.V, et al. Serum immunoreactive leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. doi:10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- Cook K.S, Min H.Y, Johnson D, Chaplinsky R.J, Flier J.S, Hunt C.R, Spiegelman B.M. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- Couceyro P.R, Koylu E.O, Kuhar M.J. Further studies on the anatomical distribution of CART by in situ hybridization. J. Chem. Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. doi:10.1016/S0891-0618(97)00212-3 [DOI] [PubMed] [Google Scholar]
- Cusin I, Rohnerjeanrenaud F, Strickerkrongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats: reduced sensitivity compared with lean animals. Diabetes. 1996;45:1446–1451. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene-expression by corticosteroids is accompanied by body-weight loss and reduced food-intake. J. Biol. Chem. 1995;270:15 958–15 961. doi: 10.1074/jbc.270.27.15958. doi:10.1074/jbc.270.27.15958 [DOI] [PubMed] [Google Scholar]
- De Vos P, et al. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma. J. Clin. Invest. 1996;98:1004–1009. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C.J, Moinat M, Curtis L, Nadakal A, Preitner F, Boss O, Assimacopoulos-Jeannet F, Seydoux J, Giacobino J.P. Effects of beta-adrenoceptor subtype stimulation on obese gene mRNA and on leptin secretion in mouse brown adipocytes differentiated in culture. Endocrinology. 1997;138:548–552. doi: 10.1210/endo.138.2.4922. doi:10.1210/en.138.2.548 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. doi:10.1038/35071088 [DOI] [PubMed] [Google Scholar]
- Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int. J. Obes. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. doi:10.1038/sj.ijo.0800547 [DOI] [PubMed] [Google Scholar]
- Eckel R.H. Lipoprotein lipase—a multifunctional enzyme relevant to common metabolic diseases. N. Engl. J. Med. 1989;320:1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- Edwards C.M, Abusnana S, Sunter D, Murphy K.G, Ghatei M.A, Bloom S.R. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. doi:10.1677/joe.0.160R007 [DOI] [PubMed] [Google Scholar]
- Elias C.F, Lee C, Kelly J, Aschkenasi C, Ahima R.S, Couceyro P.R, Kuhar M.J, Saper C.B, Elmquist J.K. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. doi:10.1016/S0896-6273(00)80656-X [DOI] [PubMed] [Google Scholar]
- Elmquist J.K, Bjorbaek C, Ahima R.S, Flier J.S, Saper C.B. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 1998;395:535–547. doi:10.1002/(SICI)1096-9861(19980615)395:4<535::AID-CNE9>3.0.CO;2-2 [PubMed] [Google Scholar]
- Farooqi I.S, Jebb S.A, Langmack G, Lawrence E, Cheetham C.H, Prentice A.M, Hughes I.A, McCamish M.A, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. doi:10.1056/NEJM199909163411204 [DOI] [PubMed] [Google Scholar]
- Farooqi I.S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. doi:10.1172/JCI200215693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Okano H.J, Li C, Lee G.H, Zhao C, Darnell R, Friedman J.M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl Acad. Sci. USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. doi:10.1073/pnas.94.13.7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J.S, Cook K.S, Usher P, Spiegelman B.M. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- Frühbeck G, Gómez-Ambrosi J, Muruzabal F.J, Burrell M.A. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Endocrinol. Metab. 2001;280:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- Funahashi H, Hori T, Shimoda Y, Mizushima H, Ryushi T, Katoh S, Shioda S. Morphological evidence for neural interactions between leptin and orexin in the hypothalamus. Regul. Pept. 2000;92:31–35. doi: 10.1016/s0167-0115(00)00146-4. doi:10.1016/S0167-0115(00)00146-4 [DOI] [PubMed] [Google Scholar]
- Gasic S, Tian B, Green A. Tumor necrosis factor alpha stimulates lipolysis in adipocytes by decreasing G(I) protein concentrations. J. Biol. Chem. 1999;274:6770–6775. doi: 10.1074/jbc.274.10.6770. doi:10.1074/jbc.274.10.6770 [DOI] [PubMed] [Google Scholar]
- Giacobino J.P. Role of the β(3)-adrenoceptor in the control of leptin expression. Horm. Metab. Res. 1996;28:633–637. doi: 10.1055/s-2007-979868. [DOI] [PubMed] [Google Scholar]
- Hardie L.J, Rayner D.V, Holmes S, Trayhurn P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem. Biophys. Res. Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. doi:10.1006/bbrc.1996.0951 [DOI] [PubMed] [Google Scholar]
- Harris R.B. Leptin—much more than a satiety signal. Annu. Rev. Nutr. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. doi:10.1146/annurev.nutr.20.1.45 [DOI] [PubMed] [Google Scholar]
- Harrold J.A, Williams G. The cannabinoid system: a role in both the homeostatic and hedonic control of eating? Br. J. Nutr. 2003;90:729–734. doi: 10.1079/bjn2003942. doi:10.1079/BJN2003942 [DOI] [PubMed] [Google Scholar]
- Hassink S.G, et al. Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics. 1997;100:E11–E16. doi: 10.1542/peds.100.1.e1. doi:10.1542/peds.100.1.e1 [DOI] [PubMed] [Google Scholar]
- Hauner H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005;64:163–169. doi: 10.1079/pns2005428. doi:10.1079/PNS2005428 [DOI] [PubMed] [Google Scholar]
- Hausman G.J. The comparative anatomy of adipose tissue. In: Cryer A, Van R.L.R, editors. New perspectives in adipose tissue: structure, function and development. Butterworths; London, UK: 1985. pp. 1–21. [Google Scholar]
- Havel P.J. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000;59:359–371. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- Haynes W.G, Morgan D.A, Walsh S.A, Mark A.L, Sivitz W.I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 1997a;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes W.G, Sivitz W.I, Morgan D.A, Walsh S.A, Mark A.L. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997b;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- Haynes A.C, Jackson B, Overend P, Buckingham R.E, Wilson S, Tadayyon M, Arch J.R. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. doi:10.1016/S0196-9781(99)00105-9 [DOI] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Duncan J.S, Williams L.M, Trayhurn P, Mercer J.G. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl Acad. Sci. USA. 1997;94:11 073–11 078. doi: 10.1073/pnas.94.20.11073. doi:10.1073/pnas.94.20.11073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammatory pathways and insulin action. Int. J. Obes. 2003;27(Suppl. 3):S53–S55. doi: 10.1038/sj.ijo.0802502. doi:10.1038/sj.ijo.0802502 [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S, Shargill N.S, Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha—direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S, Arner P, Caro J.F, Atkinson R.L, Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscl. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Bodkin N.L, Ortmeyer H.K, Arita Y, Hansen B.C, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10 697–10 703. doi: 10.1074/jbc.271.18.10697. doi:10.1074/jbc.271.18.10697 [DOI] [PubMed] [Google Scholar]
- Iguchi M, Aiba S, Yoshino Y, Tagami H. Human follicular papilla cells carry out nonadipose tissue production of leptin. J. Invest. Dermatol. 2001;117:1349–1356. doi: 10.1046/j.0022-202x.2001.01606.x. doi:10.1046/j.0022-202x.2001.01606.x [DOI] [PubMed] [Google Scholar]
- Kallen C.B, Lazar M.A. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc. Natl Acad. Sci. USA. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. doi:10.1073/pnas.93.12.5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin A.J, Pan W. Dynamic regulation of leptin entry into brain by the blood–brain barrier. Regul. Pept. 2000;92:37–43. doi: 10.1016/s0167-0115(00)00147-6. doi:10.1016/S0167-0115(00)00147-6 [DOI] [PubMed] [Google Scholar]
- Kennedy G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. B. 1953;140:578–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kern P.A. Potential role of TNFα and lipoprotein lipase as candidate genes for obesity. J. Nutr. 1997;127:1917S–1922S. doi: 10.1093/jn/127.9.1917S. [DOI] [PubMed] [Google Scholar]
- Kern P.A, Saghizadeh M, Ong J.M, Bosch R.J, Deem R, Simsolo R.B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J. Clin. Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Tan N.S, Escher P, Metzger D, Chambon P, Gonzalez F.J, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275:28 488–28 493. doi: 10.1074/jbc.M004029200. doi:10.1074/jbc.M004029200 [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua S.C, Jr, Leibel R.L, Wardlaw S.L. Leptin regulation of AGRP and NPY mRNA in the rat hypothalamus. J. Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. doi:10.1046/j.1365-2826.2001.00716.x [DOI] [PubMed] [Google Scholar]
- Kreier F, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—functional implications. J. Clin. Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. doi:10.1172/JCI200215736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen P, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. doi:10.1038/29993 [DOI] [PubMed] [Google Scholar]
- Lagathu C, Bastard J.P, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 2003;311:372–379. doi: 10.1016/j.bbrc.2003.10.013. doi:10.1016/j.bbrc.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Lee G.H, Proenca R, Montez J.M, Carroll K.M, Darvishzadeh J.G, Lee J.I, Friedman J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. doi:10.1038/379632a0 [DOI] [PubMed] [Google Scholar]
- Leibel R.L. The role of leptin in the control of body weight. Nutr. Rev. 2002;60:S15–S19. doi: 10.1301/002966402320634788. doi:10.1301/002966402320634788 [DOI] [PubMed] [Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon B.C, Friedman J.M, Ailhaud G, Dani C. Expression of ob gene in adipose-cells—regulation by insulin. J. Biol. Chem. 1996;271:2365–2368. doi: 10.1074/jbc.271.5.2365. doi:10.1074/jbc.271.5.2365 [DOI] [PubMed] [Google Scholar]
- Lord G.M, Matarese G, Howard L.K, Baker R.J, Bloom S.R, Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. doi:10.1038/29795 [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. doi:10.1006/bbrc.1996.0587 [DOI] [PubMed] [Google Scholar]
- Mantzoros C.S, Qu D.Q, Frederich R.C, Susulic V.S, Lowell B.B, Maratos-Flier E, Flier J.S. Activation of β3 adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- Mark A.L, Rahmouni K, Correia M, Haynes W.G. A leptin–sympathetic–leptin feedback loop: potential implications for regulation of arterial pressure and body fat. Acta Physiol. Scand. 2003;177:345–349. doi: 10.1046/j.1365-201X.2003.01085.x. doi:10.1046/j.1365-201X.2003.01085.x [DOI] [PubMed] [Google Scholar]
- Masuzaki H, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. doi:10.1038/nm0997-1029 [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Hoggard N, Williams L.M, Lawrence C.B, Hannah L.T, Morgan P.J, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J. Neuroendocrinol. 1996a;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. doi:10.1046/j.1365-2826.1996.05161.x [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Hoggard N, Williams L.M, Lawrence C.B, Hannah L.T, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996b;387:113–116. doi: 10.1016/0014-5793(96)00473-5. doi:10.1016/0014-5793(96)00473-5 [DOI] [PubMed] [Google Scholar]
- Mercer J.G, Moar K.M, Rayner D.V, Trayhurn P, Hoggard N. Regulation of leptin receptor and NPY gene expression in hypothalamus of leptin-treated obese (ob/ob) and cold-exposed lean mice. FEBS Lett. 1997;402:185–188. doi: 10.1016/s0014-5793(96)01525-6. doi:10.1016/S0014-5793(96)01525-6 [DOI] [PubMed] [Google Scholar]
- Mizuno T.M, Mobbs C.V. Hypothalamic Agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. doi:10.1210/en.140.2.814 [DOI] [PubMed] [Google Scholar]
- Mizuno T.M, Kleopoulos S.P, Bergen H.T, Roberts J.L, Priest C.A, Mobbs C.V. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Montague C.T, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. doi:10.1038/43185 [DOI] [PubMed] [Google Scholar]
- Morton N.M, Emilsson V, Degroot R.P, Pallett A.L, Cawthorne M.A. Leptin signalling in pancreatic islets and clonal insulin-secreting cells. J. Mol. Endocrinol. 1999;22:173–184. doi: 10.1677/jme.0.0220173. doi:10.1677/jme.0.0220173 [DOI] [PubMed] [Google Scholar]
- Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem. Biophys. Res. Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. doi:10.1006/bbrc.1996.6030 [DOI] [PubMed] [Google Scholar]
- Ollmann M.M, Wilson B.D, Yang Y.K, Kerns J.A, Chen Y.R, Gantz I, Barsh G.S. Antagonism of central melanocortin receptors in vitro and in vivo by Agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. doi:10.1126/science.278.5335.135 [DOI] [PubMed] [Google Scholar]
- Ostlund R.E, Yang J.W, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J. Clin. Endocrinol. Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. doi:10.1210/jc.81.11.3909 [DOI] [PubMed] [Google Scholar]
- Otto B, Spranger J, Benoit S.C, Clegg D.J, Tschop M.H. The many faces of ghrelin: new perspectives for nutrition research? Br. J. Nutr. 2005;93:765–771. doi: 10.1079/bjn20051446. doi:10.1079/BJN20051446 [DOI] [PubMed] [Google Scholar]
- Ouchi N, et al. Novel modulator for endothelial adhesion molecules—adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Pallett A.L, Morton N.M, Cawthorne M.A, Emilsson V. Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic islets. Biochem. Biophys. Res. Commun. 1997;238:267–270. doi: 10.1006/bbrc.1997.7274. doi:10.1006/bbrc.1997.7274 [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry A.G, Liu H, Diano S, Shanabrough M, Cai X, Friedman J.M, Horvath T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. doi:10.1126/science.1089459 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R, Sonti G, Borkoski J.P, Wilson C.D, French-Mullen J.M.B. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol. Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Porte D, Jr, Baskin D.G, Schwartz M.W. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Presse F, Sorokovsky I, Max J.P, Nicolaidis S, Nahon J.L. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. doi:10.1016/0306-4522(95)00481-5 [DOI] [PubMed] [Google Scholar]
- Prins J.B, Niesler C.U, Winterford C.M, Bright N.A, Siddle K, O'Rahilly S, Walker N.I, Cameron D.P. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman S.M, Patel H.R, Berg A.H, Pajvani U.B, Scherer P.E, Ahima R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004;10:524–529. doi: 10.1038/nm1029. doi:10.1038/nm1029 [DOI] [PubMed] [Google Scholar]
- Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. doi:10.1038/380243a0 [DOI] [PubMed] [Google Scholar]
- Rajala M.W, Scherer P.E. Minireview: the adipocyte-at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. doi:10.1210/en.2003-0580 [DOI] [PubMed] [Google Scholar]
- Rayner D.V, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J. Mol. Med. 2001;79:8–20. doi: 10.1007/s001090100198. doi:10.1007/s001090100198 [DOI] [PubMed] [Google Scholar]
- Rayner D.V, Simon E, Duncan J.S, Trayhurn P. Hyperleptinaemia in mice induced by administration of the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine. FEBS Lett. 1998;429:395–398. doi: 10.1016/s0014-5793(98)00642-5. doi:10.1016/S0014-5793(98)00642-5 [DOI] [PubMed] [Google Scholar]
- Reseland J.E, Syversen U, Bakke I, Qvigstad G, Eide L.G, Hjertner O, Gordeladze J.O, Drevon C.A. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J. Bone Miner. Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. doi:10.1359/jbmr.2001.16.8.1426 [DOI] [PubMed] [Google Scholar]
- Rothwell N.J, Stock M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. doi:10.1038/281031a0 [DOI] [PubMed] [Google Scholar]
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278:45 777–45 784. doi: 10.1074/jbc.M301977200. doi:10.1074/jbc.M301977200 [DOI] [PubMed] [Google Scholar]
- Ryden M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF-α-induced lipolysis in human adipocytes. Biochem. Biophys. Res. Commun. 2004;318:168–175. doi: 10.1016/j.bbrc.2004.04.010. doi:10.1016/j.bbrc.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene-expression after food-intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. doi:10.1038/377527a0 [DOI] [PubMed] [Google Scholar]
- Scherer P.E, Williams S, Fogliano M, Baldini G, Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26 746–26 749. doi: 10.1074/jbc.270.45.26746. doi:10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Figlewicz D.P, Baskin D.G, Woods S.C, Porte D. Insulin in the brain—a hormonal regulator of energy balance. Endocrine Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. doi:10.1210/er.13.3.387 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Seeley R.J, Woods S.C, Weigle D.S, Campfield L.A, Burn P, Baskin D.G. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Woods S, Porte D.J, Seeley R.J, Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc. Natl Acad. Sci. USA. 2003;100:10 085–10 090. doi: 10.1073/pnas.1633636100. doi:10.1073/pnas.1633636100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The expressions of mRNAs for interleukin-6 (IL-6) and the IL-6 receptor (IL-6R) in the rat hypothalamus and midbrain during restraint stress. Life Sci. 1998;62:2315–2320. doi: 10.1016/s0024-3205(98)00212-4. doi:10.1016/S0024-3205(98)00212-4 [DOI] [PubMed] [Google Scholar]
- Shklyaev S, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc. Natl Acad. Sci. USA. 2003;100:14 217–14 222. doi: 10.1073/pnas.2333912100. doi:10.1073/pnas.2333912100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigmann M.R, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. doi:10.1126/science.281.5383.1683 [DOI] [PubMed] [Google Scholar]
- Sivitz W.I, Fink B.D, Morgan D.A, Fox J.M, Donohoue P.A, Haynes W.G. Sympathetic inhibition, leptin, and uncoupling protein subtype expression in normal fasting rats. Am. J. Physiol. Endocrinol. Metab. 1999;277:E668–E677. doi: 10.1152/ajpendo.1999.277.4.E668. [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin S.E, Plata-Salaman C.R. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996;270:R1394–R1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- Stephens T.W, et al. The role of neuropeptide-Y in the antiobesity action of the obese gene-product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. doi:10.1038/377530a0 [DOI] [PubMed] [Google Scholar]
- Takahashi K.A, Cone R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. doi:10.1210/en.2004-1397 [DOI] [PubMed] [Google Scholar]
- Tartaglia L.A. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Tartaglia L.A, et al. Identification and expression cloning of a leptin receptor Ob-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. doi:10.1016/0092-8674(95)90151-5 [DOI] [PubMed] [Google Scholar]
- Thurlby P.L, Trayhurn P. The role of thermoregulatory thermogenesis in the development of obesity in genetically obese (ob/ob) mice pair-fed with lean siblings. Br. J. Nutr. 1979;42:377–385. doi: 10.1079/bjn19790127. doi:10.1079/BJN19790127 [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao T.S, Saha A.K, Murrey H.E, Zhang Cc.C, Itani S.I, Lodish H.F, Ruderman N.B. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl Acad. Sci. USA. 2002;99:16 309–16 313. doi: 10.1073/pnas.222657499. doi:10.1073/pnas.222657499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. The development of obesity in animals: the role of genetic susceptibility. Clin. Endocrinol. Metab. 1984;13:451–474. doi: 10.1016/s0300-595x(84)80033-x. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. The biology of obesity. Proc. Nutr. Soc. 2005a;64:31–38. doi: 10.1079/pns2004406. doi:10.1079/PNS2004406 [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol. Scand. 2005b;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. doi:10.1111/j.1365-201X.2005.01468.x [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Beattie J.H. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. doi:10.1079/BJN20041213 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Jones P.M, McGuckin M.M, Goodbody A.E. Effects of overfeeding on energy balance and brown fat thermogenesis in obese (ob/ob) mice. Nature. 1982;295:323–325. doi: 10.1038/295323a0. doi:10.1038/295323a0 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thomas M.E.A, Duncan J.S, Rayner D.V. Effects of fasting and refeeding on ob gene-expression in white adipose-tissue of lean and obese (ob/ob) mice. FEBS Lett. 1995;368:488–490. doi: 10.1016/0014-5793(95)00719-p. doi:10.1016/0014-5793(95)00719-P [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Duncan J.S, Rayner D.V, Hardie L.J. Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the β3-adrenoceptor agonists BRL 35135A and ZD2079. Biochem. Biophys. Res. Commun. 1996;228:605–610. doi: 10.1006/bbrc.1996.1704. doi:10.1006/bbrc.1996.1704 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Duncan J.S, Hoggard N, Rayner D.V. Regulation of leptin production: a dominant role for the sympathetic nervous system? Proc. Nutr. Soc. 1998;57:413–419. doi: 10.1079/pns19980060. doi:10.1079/PNS19980060 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Hoggard N, Mercer J.G, Rayner D.V. Leptin: fundamental aspects. Int. J. Obes. 1999;23:22–28. doi: 10.1038/sj.ijo.0800791. doi:10.1038/sj.ijo.0800791 [DOI] [PubMed] [Google Scholar]
- Tritos N.A, Mastaitis J.W, Kokkotou E, Maratos-Flier E. Characterization of melanin concentrating hormone and preproorexin expression in the murine hypothalamus. Brain Res. 2001;895:160–166. doi: 10.1016/s0006-8993(01)02066-2. doi:10.1016/S0006-8993(01)02066-2 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas J.L, Horvath C.M, Darnell J.E, Stoffel M, Friedman J.M. Leptin activation of STAT3 in the hypothalamus of wildtype and ob/ob mice but not db/db mice. Nat. Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. doi:10.1038/ng0996-95 [DOI] [PubMed] [Google Scholar]
- Wabitsch M, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- Wallenius K, Wallenius V, Sunter D, Dickson S.L, Jansson J.O. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem. Biophys. Res. Commun. 2002a;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. doi:10.1016/S0006-291X(02)00230-9 [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson S.L, Ohlsson C, Jansson J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002;8:75–79. doi: 10.1038/nm0102-75. doi:10.1038/nm0102-75 [DOI] [PubMed] [Google Scholar]
- Wang Q, et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46:335–341. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- Weingarten S, Savoldelli D, Langhans W. Enhancement or loss of the hypophagic effect of interleukin-1 upon chronic administration. Physiol. Behav. 1992;52:831–837. doi: 10.1016/0031-9384(92)90358-9. doi:10.1016/0031-9384(92)90358-9 [DOI] [PubMed] [Google Scholar]
- Weisberg S.P, McCann D, Desai M, Rosenbaum M, Leibel R.L, Ferrante A.W., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. doi:10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J. Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. doi:10.1677/joe.1.05866 [DOI] [PubMed] [Google Scholar]
- Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. doi:10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl Acad. Sci. USA. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. doi:10.1073/pnas.0408452102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. doi:10.1038/90984 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. doi:10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- Yokota T, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- Yoon J.C, Chickering T.W, Rosen E.D, Dussault B, Qin Y, Soukas A, Friedman J.M, Holmes W.E, Spiegelman B.M. Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. doi:10.1128/MCB.20.14.5343-5349.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 2002;43:1770–1772. doi: 10.1194/jlr.c200010-jlr200. doi:10.1194/jlr.C200010-JLR200 [DOI] [PubMed] [Google Scholar]
- Youngström T.G, Bartness T.J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;44:R1488–R1493. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J.M. Positional cloning of the mouse obese gene and its human homolog. Nature. 1994;372:425–432. doi: 10.1038/372425a0. doi:10.1038/372425a0 [DOI] [PubMed] [Google Scholar]