Abstract
Psychophysical comparisons seem to show that obese individuals experience normal sweet and fat sensations, they like sweetness the same or less, but like fat more than the non-obese do. These psychophysical comparisons have been made using scales (visual analogue or category) that assume intensity labels (e.g. extremely) which denote the same absolute perceived intensity to all. In reality, the perceived intensities denoted by labels vary because they depend on experiences with the substances to be judged. This variation makes comparisons invalid. Valid comparisons can be made by asking the subjects to rate their sensory/hedonic experiences in contexts that are not related to the specific experiences of interest. Using this methodology, we present the evidence that the sensory and hedonic properties of sweet and fat vary with body mass index. The obese live in different orosensory and orohedonic worlds than do the non-obese; the obese experience reduced sweetness, which probably intensifies fat sensations, and the obese like both sweet and fat more than the non-obese do. Genetic variation as well as taste pathology contribute to these results. These psychophysical advances will impact experimental as well as clinical studies of obesity and other eating disorders.
Keywords: obesity, psychophysics, preference, 6-n-propylthiouracil, fat, sweet
Pangborn & Simone (1958) summarized the common view about sweetness and obesity: ‘In the mind of the layman, sugar and sweets are ‘fattening’ and most overweight individuals have a ‘sweet tooth’ (p. 24). They tested this using apricots, pears and peaches in syrups of varying sugar content and vanilla ice cream made with varying amounts of sugar. They found no evidence that liking for sweet foods was systematically different across body sizes.
Continued study of sweetness liking showed complexity. The pattern of liking across concentration varied. Some individuals showed a monotonically increasing liking for sweet as concentration was raised (‘the sweeter the better’); in others, liking increased to a maximum and then decreased (Thompson et al. 1977); and in a third group of people, liking for sweet decreased monotonically (Looy et al. 1992). Furthermore, Booth and his colleagues noted that sweetness liking was very personal and varied across foods (Conner et al. 1988). However, most data support the original conclusion of Pangborn and Simone—body weight does not affect the liking for sweet (Wooley et al. 1972; Underwood et al. 1973; Rodin 1975; Thompson et al. 1976; Malcolm et al. 1980; Witherly et al. 1980; Frijters & Rasmussen-Conrad 1982; Drewnowski et al. 1991).
The failure to find the body weight effects on the perception of sweetness completed the picture. There were neither threshold (Grinker et al. 1972; Malcolm et al. 1980; Frijters & Rasmussen-Conrad 1982) nor suprathreshold differences (Witherly 1978; Frijters & Rasmussen-Conrad 1982; Drewnowski et al. 1991 cited in Witherly et al. (1980); Grinker et al. 1972; Wooley et al. 1972; Rodin 1975; Rodin et al. 1976; Thompson et al. 1977; Enns et al. 1979) across body weight.
The view that there are neither sensory nor hedonic differences for sweetness between obese individuals and others has prevailed for nearly 50 years with a few interesting exceptions (e.g. Rodin et al. 1976). Some studies went even farther in debunking the common wisdom (Grinker & Hirsch 1972; Johnson et al. 1979; Cox et al. 1998). For example, Grinker & Hirsch (1972) concluded that, ‘Obese subjects show a pronounced aversion for strong concentrations of sucrose, whereas normal weight subjects prefer moderate concentrations’.
1. Advances in psychophysical measurements
The psychophysical tools with which we compare sensory/hedonic experiences across groups have improved (Bartoshuk et al. 2004b, 2005b; Snyder et al. 2004b). This suggests that it is time to revisit comparisons of the intensity and liking of sweetness between obese and non-obese individuals because the new techniques may detect differences that the old techniques missed.
There is a general point about sensory comparisons: taste thresholds have proved to be poor predictors of real-world taste experience (e.g. Bartoshuk 1978; Duffy et al. 2004c; Snyder et al. 2004b). Thresholds reflect only the lower range of concentrations that can be tasted; as concentration rises above threshold, the functions describing perceived intensity can markedly diverge. Thus, comparisons of taste thresholds across obese and non-obese individuals are essentially irrelevant to understanding food behaviour, and suprathreshold comparisons are required.
Two problems involving suprathreshold comparisons deserve attention. First, we cannot share experiences directly; thus, it is necessary to resort to indirect comparisons. The way in which these comparisons have been made historically is now open to question, which puts in doubt all the studies that used labelled scales (category or visual analogue) to compare sensory or hedonic experiences of the obese and non-obese. Second, preference and sensation are linked. For example, one would hardly be surprised if an individual showed no preference for the taste of sucrose if that individual could not taste sucrose. To compare preferences across obese and non-obese subjects, we assume that the sensations leading to these preferences are the same for both groups. This assumption may have seemed justified given the failure of studies to find sensory differences, but the assumption can now be challenged because new data suggest that there are sensory differences related to body mass index (BMI).
This paper will examine these two problems and illustrate the way in which psychophysical errors can lead to erroneous conclusions. Using new psychophysical techniques, we will show that obese individuals have heightened hedonic responses to sweet for the sweetness they perceive. We will propose explanations based on genetic variation and pathology for reduced sweet taste in the obese and suggest how the decreased perception of sweetness could contribute to increased fat preference in obese individuals.
2. Psychophysical comparisons of sensory and hedonic experiences across groups
(a) The problem
During discussions about foods or beverages, comparisons seem easy. ‘That lemonade tastes extremely sweet to me; does it taste extremely sweet to you?’ The intensity descriptors (e.g. extremely) in ordinary conversation were borrowed to label category and visual analogue scales (VASs) (see Bartoshuk et al. (2002b) for a discussion on the history of those scales). One of the best-known category scales used to study the sensory and hedonic characteristics of foods or beverages was the nine-point scale developed for use by the military in 1949 (Peryam & Girardot 1952; Jones et al. 1955). For example, Drewnowski et al. (1985) assessed the intensities of sensory attributes of sweetened dairy products with nine-point category scales. The scales were labelled such that the top of the scale, ‘9’, referred to ‘extreme’ of whatever attribute was to be measured (e.g. sweet, fat, creamy).
The VAS, developed in the 1960s by Aitken (Aitken et al. 1963), can be used to rate the intensity and liking for sweetness and other food-related stimuli. For example, in a study on eating disorders, subjects answered the question, ‘Do you like this food?’ by placing a rating on a VAS labelled ‘not at all’ at one end and ‘extremely’ at the other end (Stoner et al. 1996).
Labelled scales can provide valid within-subject comparisons and valid group comparisons where the members of each group have been randomly assigned. However, a problem emerges when labelled scales are used to make comparisons across groups when the labels may denote different intensities to the different groups. For example, this problem occurs when pain intensities are compared. Suppose men and women are asked to rate the pain of a headache on a VAS labelled ‘no pain’ at one end and ‘most intense pain ever experienced’ on the other end. The label ‘most intense pain ever experienced’ denotes greater pain to women who select childbirth as their most intense pain than it does to the average man (Bartoshuk et al. 2004a; Dionne et al. 2005). Thus, the pain VAS for these women is expanded compared to that for men. Treating the top of the scale as if it denotes the same pain to both groups obscures differences between them.
We will discuss a new psychophysical methodology that can solve the problem of comparing intensity or hedonic ratings across individuals or groups. Using data collected with the new methodology, we will illustrate how this problem has distorted comparisons of sensory and hedonic experiences between obese and non-obese individuals.
(b) The solution
The solution to the problem of individual differences to orosensory experience is conceptually simple. We ask the subjects to compare the experiences of interest to other unrelated experiences. This allows us to use the unrelated experiences as standards that are the same, on average, across groups. For example, suppose both obese and non-obese subjects were asked to rate the sweetness of a sucrose solution and the loudness of a tone. Since we have no reason to believe that these two groups hear differently, the average rating of loudness should not differ between the two groups. Thus, if the obese group were to rate the sweetness of the sucrose as equal to the loudness of the tone and the non-obese group were to rate the sucrose as twice as sweet as the tone is loud, we could conclude that the sweetness is twice as intense as to the non-obese individuals.
The technique described earlier has been used by psychophysicists for some years (Hall et al. 1975) and was formalized as the method of magnitude matching by Marks & Stevens (Marks & Stevens 1980; Stevens & Marks 1980; Marks et al. 1988). This method works because humans make cross-modality matches with ease (Stevens & Marks 1965).
Remembered sensations can also be used as standards (e.g. ‘most intense pain ever experienced’ is a remembered sensation). We used remembered sensations when we constructed the general labelled magnitude scale (gLMS) (Bartoshuk et al. 2004b). The gLMS is based on the labelled magnitude scale (LMS) devised for oral sensations (Green et al. 1993). The LMS is a labelled scale with the spacing of the labels adjusted to give the scale ratio properties (e.g. a rating of ‘50’ denotes a perceived intensity twice as intense as ‘25’). Comparisons using the LMS are subject to the same problem noted earlier. If ‘strongest imaginable oral sensation’ differs across two groups, the LMS will not provide valid comparisons for taste across those groups. However, the LMS can be generalized to the gLMS by altering the top label to ‘strongest imaginable sensation of any kind’. The ratings run from 0 (no sensation) to 100 (strongest imaginable sensation of any kind). The gLMS produced taste comparisons across nontasters, medium tasters and supertasters (see later for further explanation of these three groups) equivalent to those produced with magnitude estimation using a sound standard (Bartoshuk et al. 2004b). The logic of why this occurs is as follows. The strongest sensation ever experienced is not the same for everyone. For one individual, the pain of childbirth may be the strongest sensation ever experienced; for another, the brightness of the sun may be the strongest. Taste sensations are not usually the strongest sensation (Bartoshuk et al. 2002b). Thus, the ‘strongest imaginable sensation of any kind’ is not related to taste and acts as a standard in the manner described earlier.
Incidentally, the use of ‘imaginable’ on labelled scales does not confer any benefits. Some hoped that ‘imaginable’ might make the tops of labelled scales equivalent to all. To test this, subjects were asked to rate the most intense sensation they could imagine, as well as the most intense sensation they had ever experienced. The two were highly correlated (Bartoshuk et al. 2005b). That is, people imagine the most intense sensation of a given type to be a standard percentage above the most intense sensation of that type they have ever experienced. Given that ‘imagine’ confers no benefit, we suggest deleting the term from scale labels in the future.
A hedonic version of the gLMS was created by placing ‘neutral’ in the centre and having a ‘dislike’ scale to the left and a ‘like’ scale to the right (e.g. Bartoshuk et al. 1999; Duffy et al. 1999; Bartoshuk 2000 and see Lanier et al. (2005b) for a vertical form of the hedonic gLMS). For the horizontal version, the left-most label is ‘strongest imaginable disliking of any kind’ (−100); the right-most label is ‘strongest imaginable liking of any kind’ (100). As with the sensory gLMS, the instructions direct the subjects to rate liking for foods in the context of all hedonic experiences rather than only in the context of food likes and dislikes.
Data collected with the sensory and hedonic forms of the gLMS from attendees at lectures (begun in 1993 with a lecture at the University of Illinois) illustrate the problem discussed earlier. Attendees were asked to provide demographic data (age, sex, height and weight); they then rated the sweetness of a piece of candy (Stop and Shop butterscotch discs) and their preferences for 26 foods and beverages. Since relatively few subjects had BMIs of 50 or over (n=20), these individuals were omitted from the analyses. As would be expected from a dataset drawn from a population with more than average education (Working Group 2000; Zhang & Wang 2000), the prevalence of overweight (24.2%) and obesity (7.9%) is lower in this dataset than in the data from National Health and Examination Survey (NHANES) III (overweight 32%; obesity 22.5%).
Factor analysis (varimax rotated solution) of the liking of these foods and beverages produced two groups which we called ‘sweet foods’ (sugar, oreo cookies, whipped cream, dark chocolate, sweets, honey and milk chocolate) and ‘fat foods’ (cheddar cheese, mayonnaise, whipped cream, whole milk, sour cream, sausage and butter). Both groups had Cronbach's alpha of 0.7, showing they are reliable groups. Foods that did not associate with these groups were: bananas, salt, cooked broccoli, black coffee, margarine, beer, salted pretzels, buttered pop corn, grapefruit juice, jello, marshmallow and strawberries.
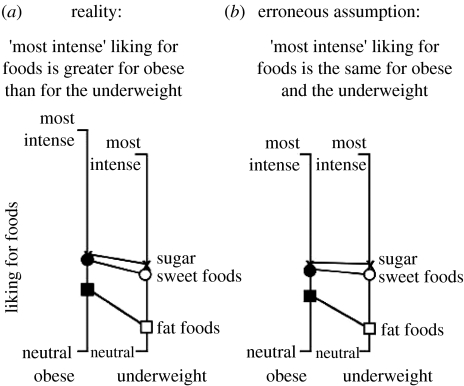
The maximum liking was determined for each subject (only subjects that rated at least 24 out of the 26 items were included). This maximum liking was significantly greater for the obese (BMI=30, n=305) than for the underweight (BMI<18.5, n=144) subjects (t=3.078, p<0.01). Although this is only an approximation and may underestimate the magnitude of the actual difference since the food list was a limited one, the significant difference illustrates the problem of assuming that ‘most intense’ liking for foods is the same for subjects in different BMI groups. Incidentally, the maximum disliking for the food/beverage items also rose with BMI; thus as BMI rises, foods are liked more and disliked less (t=2.760, p<0.01).
Figure 1a shows the preferences for sugar, sweet foods and fat foods as well as the values for ‘most intense’ liking for foods for the obese and underweight. The obese experience greater liking for sugar, the sweet food group and the fat food group, but the difference is greatest for the fat food group. Figure 1b shows what happens if we make the error of assuming that the ‘most intense’ liking for foods is the same for both groups. The obese scale is erroneously compressed. Note that this compression blunts the differences between the obese and underweight. Depending on the difference between the tops of the scales and the difference for the items of interest (e.g. sugar, sweet foods, fat foods), this error may blunt a real difference, make it disappear, or even make it go in the wrong direction (Bartoshuk et al. 2002b). The psychophysical error (figure 1b) abolished the differences for sugar and the sweet foods, but only blunted the difference for the fat food group.
Figure 1.
Differences between obese and underweight individuals for the liking of sugar, sweet foods and fat foods. This figure illustrates one kind of error resulting from the erroneous assumption that the perceived intensities denoted by descriptors like ‘most intense’ do not differ across groups. (a) Shows the data obtained with the hedonic gLMS. Significance of t-tests following ANOVA are: sugar, p<0.05; sweet foods, p<0.01; and fat foods, p<0.0000001. (b) Shows how the data would look if ‘most intense’ liking for foods is erroneously assumed to be equal for both groups (see text for explanation): sugar and sweet foods no longer show significant differences between thin and obese subjects; for fat foods, p<0.0000001.
(c) Magnitude estimation of sweetness in obese and non-obese
In some of the early studies on sensory and hedonic differences across obese and non-obese individuals, magnitude estimation was used rather than labelled scales (Grinker et al. 1972; Thompson et al. 1977; Enns et al. 1979; Frijters & Rasmussen-Conrad 1982). These studies were done without the benefit of the later insights discussed earlier. The investigators gave both the obese and non-obese subjects a particular concentration of sucrose as a standard and assigned it a rating, but once this is done, valid comparisons are impossible. For example, suppose both obese and non-obese subjects are given a 0.3 M sucrose standard and asked to call it ‘10’. When they are subsequently given a series of sucrose solutions, no one should be surprised if they then rate 0.3 M sucrose ‘10’ when it appears. Unfortunately, the obese might actually experience half the sweetness experienced by the normal weight subjects, but the ratings could not reveal that.
Spitzer & Rodin (1981) reviewed studies on human eating behaviour and they warned of this error: ‘It is important, however, to remember that neither anchored ratings nor magnitude estimation procedures allow comparisons of the absolute value of perceived intensity across subjects. This is true for magnitude estimation because there is no common unit across subjects and for category ratings because there is no way to know if the verbal anchors mean the same thing to all subjects’. The warning was ignored until the recent use of magnitude matching and the gLMS.
3. Obesity, tasting sweet and liking sweet and fat
(a) Decreased sweetness in the obese
Multiple regression analyses were used with the data from lecture attendees to evaluate sex, age, sweetness of candy and liking sugar as predictors for BMI across all subjects up to BMI of 50 (n=3740). These variables explained significant variance in BMI (multiple R=0.33; F=111.10; d.f.=4, 3735, p≪0.01). Contributions of sex and age to BMI are well known; semi-partial correlation coefficients (sr) showed that higher BMIs were seen in men (sr=0.18, p≪0.01) and in older individuals (sr=0.25, p≪0.01). However, higher BMIs were also seen in individuals to whom the candy was less sweet (sr=−0.04, p<0.05); this novel result is a consequence of the use of the gLMS.
(b) Increased liking for sweetness in the obese
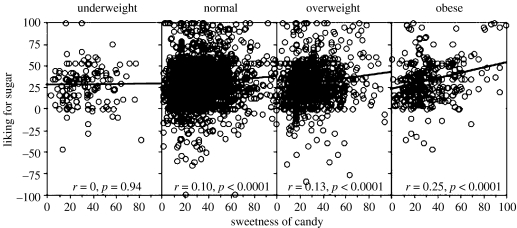
Figure 1 shows that the obese like sugar better than do underweight individuals. Extending this, the above-mentioned multiple regression analysis showed that those with higher BMIs liked sugar better (sr=0.05, p<0.01). However, the increase in sweet liking with BMI seen with these analyses underestimates the size of the effect because sweetness declines with BMI. Moskowitz pioneered a method for displaying hedonic data that takes account of the sensory perception (Moskowitz 1971). Figure 2 plots sugar liking (remembered) as a function of sweetness (tasted) as Moskowitz suggested (Moskowitz et al. 1974). Note that liking increases as a function of sweetness more and more as BMI increases. Thus, for the same perceived sweetness, liking goes up as BMI goes up. Thus, as Moskowitz suggested, analyses of the liking of sweet in terms of the perceived intensity of sweetness is useful for the study of obesity (Moskowitz et al. 1974). We now know that this advice was particularly apt in light of associations between loss of taste and increases in BMI. Plotting affective responses as a function of sensation permits the evaluation of an affective difference per se.
Figure 2.
Sweetness of a piece of candy (tasted) plotted against the liking for sugar (remembered). The subjects are divided by BMI into underweight (below 18.5), normal weight (18.5–24.9), overweight (25.0–29.9) and obese (30.0 and above). Note that as BMI increases, the correlation coefficients increase. The correlation coefficients for the underweight and normal weight subjects was significantly less than that for the obese subjects (p<0.01); the comparisons of the correlations coefficients for the overweight and obese subjects approached significance (p=0.06). r=correlation coefficient.
(c) Decreased sweetness and increased liking for fat
The common view about fat is similar to that for sugar; the obese are presumed to show enhanced liking for high-fat foods. However, contrary to the sugar story, studies on preference and obesity support this common view (Drewnowski et al. 1985, 1991; Pangborn et al. 1985; Mela & Sacchetti 1991). In these studies, mixtures of fat with other dietary components have proved especially interesting. Drewnowski and his colleagues suggested that mixtures of fat and sweet prove particularly palatable. In one study using dairy products (with varied sugar and fat content), obese women preferred a higher ratio of fat to sweet (Drewnowski et al. 1985). Evaluation of food preferences showed that obese women tend to prefer sweet–fat foods (Drewnowski et al. 1992; Macdiarmid et al. 1998) while obese men tend to prefer savory–fat foods (Drewnowski et al. 1992).
Decreased perception of sweetness could contribute to increased preference for fat in the obese because recent work has demonstrated how decreased intensity of one orosensory system can disinhibit another orosensory system. We can demonstrate an association between decreased taste and increased liking for fat foods relative to sweet foods using the data from lecture attendees. For the analysis, we constructed a difference score: liking for fat foods minus liking for sweet foods. Multiple regression showed that sex, age and the sweetness of candy all made significant, independent contributions to explaining the variance of this difference score (Multiple R=0.28; F=98.064; d.f.=3, 3543, p≪0.001). The difference between liking fat foods and liking sweet foods was greater for men (sr=0.08, p≪0.001), older attendees (sr=0.25, p≪0.001) and for those with lower ratings for the sweetness of candy (sr=−0.06, p<0.001). We now review the evidence for dynamic interaction among orosensory systems.
4. Dynamic interactions of orosensory systems
Taste buds are globular clusters of cells (much like the segments of an orange) buried in the tissue of three types of papillae: fungiform (anterior, mobile tongue), foliate (rear edges of the tongue) and circumvallate (arranged in an inverted V across the rear of the tongue) as well as in the tissue where the hard and soft palate meet on the roof of the mouth. The tips of some of the cells in taste buds taper to thin microvilli that contain the sites that interact with taste stimuli. Taste stimuli reach these sites by flowing through the taste pore: the conduit between the taste bud and the surface of the tongue or palate. Taste stimuli must be in solution to permit them to flow through the taste pore and contact taste receptor sites on the microvilli.
Taste buds are innervated by three cranial nerves: VII, IX and X. The chorda tympani branch of CN VII (facial nerve) carries taste from the anterior, mobile part of the tongue; the greater superficial petrosal branch of CN VII, from the roof of the mouth; the glossopharyngeal nerve (CN IX), from the rear of the tongue; and the vagus nerve (CN X), from the throat. These nerves project ipsilaterally to the medulla, thalamus and cortex.
(a) Taste–taste interactions
The first evidence that taste input produces inhibition in the CNS came from Halpern & Nelson (1965). They showed in rat that anaesthesia of the chorda tympani produced increases in responses from the medulla when the posterior tongue (the area innervated by the glossopharyngeal nerve) was stimulated. They concluded that input from the chorda tympani normally sent inhibition to the CNS area receiving input from the glossopharyngeal nerve. Anaesthesia of the chorda tympani released that inhibition leading to intensification of the responses previously inhibited. Other support for that in animal models (Norgren & Pfaffmann 1975; Ninomiya & Funakoshi 1982; Ogawa & Hayama 1984; Sweazey & Smith 1987) was followed by demonstration of similar circuitry in humans. Unilateral dental anaesthesia blocks both taste and somatosensation on the side of the injection (the chorda tympani and lingual branch of the trigeminal nerve travel through the same space as the inferior alveolar nerve, the target of dental anaesthesia); this anaesthesia increased some whole mouth taste intensities (Catalanotto et al. 1993). Otolaryngological anaesthesia of the eardrum provided another technique for anaesthesia of the chorda tympani nerve. When a small amount of lidocaine is injected under the skin near the eardrum, it passes under the skin into the middle ear directly contacting the chorda tympani nerve. Note that with this anaesthesia, only the chorda tympani is blocked leaving somatosensation intact. Unilateral anaesthesia of the chorda tympani intensified sensations resulting from stimulation of the contralateral glossopharyngeal nerve (Lehman et al. 1995; Yanagisawa et al. 1998). The fact that the intensification was contralateral to the anaesthesia shows that the inhibitory interaction had to be central; taste nerves do not communicate with one another across the midline in the periphery. Release of inhibition acts as a constancy mechanism; the intensification of sensations from unanaesthetized nerves compensates for the loss from the anaesthetized nerve. If additional nerves are anaesthetized, whole mouth taste will ultimately be compromised.
Additional evidence for inhibition in the taste system resulted from a remarkable experiment of nature involving Carl Pfaffmann, one of the greats in the history of taste research. Pfaffmann suffered from Ramsey Hunt syndrome near the end of his life. This syndrome involves reactivation of the chicken pox virus with subsequent damage to the auditory nerve (CN VIII). The proximity of this nerve to the cranial nerves subserving taste (VII and IX) permits the virus to damage those nerves as well. Testing near the onset of Pfaffmann's disorder revealed a total loss of taste on his left side with very intense sensations when the right side was stimulated. Taste nerves regenerate; as taste returned to the left side, the sensations on the right side diminished. This suggested that as taste on the left recovered, its ability to inhibit taste on the right also recovered resulting in a return to the normal equilibrium between inputs from the two sides of the tongue (Pfaffmann & Bartoshuk 1989, 1990).
During our anaesthesia experiments, some subjects noted the appearance of taste phantoms: taste sensations in the absence of stimulation (Yanagisawa et al. 1998). This suggested that clinical taste phantoms might be produced by localized taste damage. Indeed, we found taste damage in patients reporting taste phantoms (Bartoshuk et al. 2002a, 2005c).
(b) Taste–trigeminal interactions
Just as with taste–taste interactions, we used anaesthesia to study taste–trigeminal interactions. Unilateral anaesthesia of the chorda tympani produced intensification of the oral burn of capsaicin on the contralateral side of the anterior tongue in supertasters (Tie et al. 1999). If taste normally inhibits one trigeminal sensation, oral burn, might it also inhibit another, oral touch? Some subjects in our anaesthesia experiments gave anecdotal accounts of intensified sensations of creaminess from dairy products. Experiments are currently underway to quantify these anecdotes. Chapo showed that older women (67±7 years) perceived weaker taste sensations from CN VII (chorda tympani) and stronger sensations of creaminess from sampled high-fat foods than did younger women (24±4 years). In addition, the older women liked fat foods more and consumed more calories from fat foods than did the younger women (Chapo et al. 2002).
The possibility that the hedonic properties of fats might increase due to decreased perceived intensity of sweetness raises the question of what produces the defect in sweet taste? We hypothesize that anatomical damage to peripheral nerves mediating sweet taste is likely a major cause.
5. Reduced taste input: damage and genetic variation
(a) Vulnerability of taste
Because of their anatomical paths, taste nerves are vulnerable to damage. The chorda tympani nerve leaves the tongue in the same sheath as the lingual branch of the trigeminal nerve (CN V). As noted earlier, these nerves travel through the pterygomandibular space (the space between the pterygoid muscles and the jaw bone); the inferior alveolar nerve carries pain from the lower, rear teeth and travels through the same space. When the lower, rear teeth are anaesthetized, the anaesthetic needle that enters the pterygomandibular space may damage either the chorda tympani or the trigeminal nerve or both.
The chorda tympani separates from the lingual nerve and passes through the middle ear just behind the eardrum; pathogens associated with ear infections (otitis media) can damage the nerve in the middle ear. In addition, pathogens associated with upper respiratory infections can travel from the oral/nasal cavity through the Eustachian tube into the middle ear. Once the chorda tympani nerve leaves the middle ear, it travels through a long, bony passage, and this makes it vulnerable to head injury. The chorda tympani and greater superficial petrosal nerves join to form the nervous intermedius at the base of the brain; this combined nerve (CN VII) and the glossopharyngeal nerve (CN IX) can both be damaged by acoustic neuromas (tumours on CN VIII) or by the surgery to remove them. The glossopharyngeal nerve is also vulnerable during tonsillectomy because of its proximity to the tonsils. Finally, taste is vulnerable to systemic damage; for example, drugs can damage taste (e.g. Rollin 1978; Schiffman 1983a,b, 1991; Schiffman et al. 2000).
(b) Otitis media and tonsillectomies: damage to taste, intensification of fat sensations, increases in the palatability of high-fat foods and obesity
Otitis media is one of the most common childhood diseases. Given the interactions between taste and the trigeminal system, the damage otitis media does to taste (Bartoshuk & Duffy 1994) can be expected to alter sensory properties of foods and thus affect diet and health. Children with more severe histories of otitis media have significantly higher intakes of sweets and lower intakes of vegetables (Arsenault et al. 2004) and are more likely to be overweight (Tanasescu et al. 2000).
Snyder et al. (2003a,b) found that men and women over 30 years with histories of severe otitis media had significantly higher BMIs than those without that history. In addition, their food preferences (measured with a hedonic version of the gLMS) varied in a sex-specific manner. Normally, women show a decline in their preference for high-sweet foods with age (Snyder et al. 2001). Women with histories of severe otitis media did not show that decline. The men with this history showed an increase in preference for a group of high-fat foods. The increased salience of fat cues may help condition preferences for high-fat foods (Sclafani 2001).
Combining otitis media (damage to CN VII) with tonsillectomy (damage to CN IX) has the potential of producing particularly serious taste loss. Preliminary data showed that adults (40 years and above) with histories of otitis media and tonsillectomy showed reduced taste, liked fat food more and had higher BMIs (Chapo et al. 2005).
Smoking may play a role in obesity through the same release of inhibition mechanism noted earlier. Maternal smoking has been shown to be associated with an elevated risk of obesity in the offspring in a number of studies (Montgomery & Ekbom 2002; Toschke et al. 2002, 2003; Adams et al. 2005). Of special interest, one of those studies found that the risk is still present when the offspring are in their thirties (Power & Jefferis 2002). Snyder has suggested that household smoking may play a role in producing the obesity (Snyder et al. 2004a). Smoking in the household has been associated with an increased incidence of otitis media in several studies (e.g. Lanphear et al. 1997; Paradise et al. 1997; Lieu & Feinstein 2002; Arcavi & Benowitz 2004; DeFranza et al. 2004). Increased otitis media with resulting damage to taste may contribute to the association between smoking and obesity.
Laboratory-based taste testing has revealed pathologies associated with dietary behaviours. For example, loss of taste on the anterior tongue shows damage to the chorda tympani nerve. Such damage has been shown to affect preference for and intake of sweets and alcoholic beverages (Duffy et al. 2003, 2004b; Dinehart et al. 2005, in press). With regard to fat, reduced taste on the anterior tongue has been associated with greater preference for fat in older females (Chapo et al. 2002) and greater waist circumference (central adiposity) among middle-aged females (Lanier et al. 2005a,b).
In addition to pathological damage to peripheral taste nerves, genetic variation may contribute to the defect in sweet taste in obese subjects. The evidence for this is given in §5c.
(c) Genetic variation in taste—sweet, fat and weight
Taste blindness to phenylthiocarbamide (PTC) or 6-n-propylthiouracil (PROP) was first discovered by Fox (1931). Those individuals who could not taste PTC or PROP were called nontasters and those who could as tasters. Subsequent work identified the N–C=S group on the PTC and PROP molecules as responsible for the bitter taste (Fox 1932; Harris & Kalmus 1949). The gene responsible for expressing receptors that bind the N–C=S group was discovered on chromosome 7 (Kim et al. 2003). Magnitude matching permitted comparisons of taste intensities. This method revealed large variation in PROP bitterness among tasters. Those tasters perceiving the most intense bitterness were called supertasters and those perceiving less bitterness were called medium tasters (Bartoshuk et al. 1992). Interestingly, the density of fungiform papillae was correlated with the bitterness of PROP (Bartoshuk et al. 1994). Prior to the discovery of the PTC/PROP gene, family studies had shown that nontasting was recessive (Snyder 1931, 1932). We suspected that supertasters would be homozygous for the dominant allele while medium tasters would be heterozygous. Genotyping for the PTC/PROP gene revealed that this was not the case (Duffy et al. 2004a; Bartoshuk et al. 2005a). Rather, the density of fungiform papillae (the structures that house taste buds) plays a major role in supertasting; to a first approximation, supertasters are tasters with a high density of fungiform papillae. The PTC/PROP gene controls the expression of receptors that bind PTC and PROP. The mechanism responsible for variation in the density of fungiform papillae is not understood.
Given that supertasters have the most taste buds, it is not surprising that they perceive the most intense tastes from NaCl, sucrose, citric acid and quinine (Prutkin et al. 1999b; Bartoshuk 2000; Ko et al. 2000; Prescott et al. 2001b); however, quinine shows the greatest difference (Ko et al. 2000). This suggests that quinine might be able to stimulate PTC/PROP receptors while NaCl, sucrose and citric acid cannot. Cross-adaptation provides some support for this. Cross-adaptation works as follows. If a taste solution is flowed across the tongue (flowing the solutions prevents dilution with saliva) for about 20 s, its taste disappears. This is thought to represent equilibrium with regard to the contact between stimulus molecules and receptor sites. If a second stimulus is applied that stimulates the same receptors, it will not evoke a taste because the equilibrium is not affected. If a second stimulus is applied that does evoke a taste, that second stimulus must have stimulated new receptors not involved in the original equilibrium. Adaptation to quinine resulted in some reduction in the taste of PTC, although the reduction was not statistically significant (McBurney et al. 1972). This result deserves further study.
The taste buds in fungiform papillae are surrounded by basket-like clusters of nerve fibres that carry burn/pain information (Nagy et al. 1982; Whitehead et al. 1985; Silver & Finger 1991; Finger et al. 1994). The number of taste buds is associated with the ability to taste PROP (Bartoshuk et al. 1994) and this presumably underlies the increase in oral irritation with increase in the ability to taste PROP (Prutkin et al. 1999a; Prescott & Swain-Campbell 2000).
Fungiform papillae are also innervated by touch fibres (Zahm & Munger 1985; Toyoshima et al. 1987; Hilliges et al. 1996). Prutkin (Prutkin et al. 2000) showed that the two-point threshold was not only smaller for supertasters but also that it approximated the distance between fungiform papillae. This supports the idea that fungiform papillae act as touch receptors. Fats stimulate the sensations of creaminess, thickness, oiliness, etc., which are types of touch sensations. Just as with oral irritants, increasing perception of the bitterness of PROP is associated with increases in the perceived intensities of fats (Duffy et al. 1996; Tepper & Nurse 1997; Prutkin et al. 1999a; Prescott et al. 2001a) as well as non-fat thickeners like guar gum (Prutkin et al. 1999a).
The first suggestion that ability to taste PROP was related to weight came from Fisher (Fischer et al. 1966). He related PROP status to the body types proposed first by Kretschmer and later by Sheldon. For example, those with the lowest PROP and quinine thresholds (tasters) tended to be Sheldonian ectomorphs (thin), while those with the highest thresholds tended to be endomorphs (heavier).
Similar results for adults have been found in a variety of studies over the last decade (Dabrila et al. 1995 (Supplement); Lucchina 1995; Lucchina et al. 1995; Duffy et al. 1999, 2001; Tepper & Ullrich 1999, 2001; Tepper 2003, 2004). In studies where supertasters were distinguished from medium tasters, supertasters were the thinnest. Lower weights among supertasters appear to result from less liking for high-sweet, high-fat foods (Looy & Weingarten 1992; Duffy et al. 1995, 2003, 2004d; Peterson et al. 1999; Duffy & Bartoshuk 2000; Duffy 2004). An association between PROP and body weight is not always seen (e.g. Duffy & Bartoshuk 2000) and appears to be strongest in individuals who are normal weight to mildly obese (Hutchins et al. 2002a,b; Duffy 2004; Duffy et al. 2004d; Lanier et al. 2005a,b), and may be influenced by other factors including dietary restraint and disinhibition (Tepper & Ullrich 2001).
For children, the results have been less consistent. The earliest study on 425 elementary school children suggested differences between nontasters and tasters in the same direction as those for adults: tasters tended to be taller for the same weight (i.e. to have lower BMIs) than did nontasters (Johnston et al. 1966); however, the differences were very small and the raw data were not presented making modern statistical analysis impossible. A more recent study of preschool children (4–5 years old) failed to find any differences in weight between nontasters and tasters (Keller & Tepper 2002), but another study on 4–5 year olds did find a difference in the expected direction for boys. Of special interest, the taster girls in that study actually weighed more than the nontaster girls (Keller 2004).
6. Discussion
Data collected using the gLMS illustrated three points. (i) The obese experience greater liking for foods in general (including sweet foods and fat foods) than do the non-obese. This means that intensity labels on hedonic scales denote greater liking for the obese; comparisons of sweet liking between obese and non-obese individuals will be blunted if the labels are assumed to denote equivalent perception to both groups (i.e. the point made in figure 1). (ii) Higher BMI is associated with lower perceived sweetness; thus, comparisons of liking for sweet must be corrected for perceived sweetness. Scaling sweet taste and liking with the gLMS and plotting liking as a function of sweetness shows that sweet liking is enhanced among the obese. (iii) Lower perceived sweetness is associated with increased difference between liking fat foods and liking sweet foods.
Comparisons of sensory and affective experiences across individuals or groups are very difficult. Advances in psychophysical methodology now suggest that the history of such comparisons includes errors sufficient to invalidate long-accepted ideas. Those psychophysical advances will continue and it is important to update areas where new methodology challenges old conclusions. We argue here that the obese, on average, experience less sweetness than do the non-obese; further, the obese like sweetness and high-fat foods more. This makes the ‘synergism’ between sweet and fat sensations easy to understand. Mixtures of sweet and fat combine two palatable substances to make an especially palatable combination.
More generally, the use of new methodology allows us to look at liking for foods in the context of all hedonic experience. This larger context shows that the affective experiences produced by food change with BMI. The obese like foods more than do the non-obese. This not only makes intuitive sense but also underlines the error of using conventional labelled scales to compare food preferences across BMI. Intensity labels do not denote the same intensities of liking foods in obese and non-obese individuals.
The demonstration of taste loss in the obese suggests new ways to think about the increased liking for fat in the obese. Our work on interactions among oral sensations suggests that taste damage will increase the perceived intensity of fat sensations. This could facilitate conditioned preferences for high-fat foods. However, the attention to central areas mediating both food pleasure and the pleasure evoked from drugs of abuse (e.g. Volkow & Wise 2005) suggests an additional possibility. The pleasure evoked from taste is believed to be hard-wired (e.g. Steiner 1973). Taste damage reduces the pleasure that can be evoked in this manner. The trigeminal nerve is rarely damaged by damage to the taste system. Thus, most individuals with taste damage would still have an intact trigeminal system and so would still have available a pleasure source from high-fat foods. Dietary shift towards high-fat foods could thus provide compensation for the loss of the hard-wired pleasure provided by taste. In sum, we argue that liking of both fat and sweet rises as BMI rises. In addition, the taste loss associated with BMI produces even greater liking for fat as BMI rises. This suggests an examination of the known clinical disorders that damage taste to see if shifts in food preferences and weight gain accompany these clinical disorders.
Methodological improvements (e.g. magnitude matching, gLMS) in the ability to compare the sensory properties of foods across subjects and groups have led to insights about genetic and pathological variation in sensory experience. Growing understanding about the interactions between the senses that convey information reveals the causes for some of the variation. These insights must now be applied to the clinical psychophysical study of obesity and other eating disorders including those using functional imaging.
We do not yet know why liking for sweet and fat increases as BMI increases; however, we know that the association between orosensory experience and postingestive effects of sweet and fat foods has contributed to that liking (Sclafani 2001), and we are gaining insight into changes in orosensory experience associated with BMI and how those changes may contribute to increased liking.
It is important to remember that much is still to be learned about the association between liking and consuming foods. There is good reason to think that liking and consuming foods are not tightly linked; however, the nature of the linkage requires precise measurement of liking. We argue that much of the literature comparing the liking of foods is compromised by the psychophysical errors noted here.
Acknowledgements
This project was supported by the National Institute on Deafness and Other Communication Disorders grant DC 000283 and by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant no. 2003-35200-12943.
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
On leave for the academic year 2005–2006 at: Department of Clinical and Health Psychology, University of Florida, PO Box 100165, Gainesville, FL 32610-0165, USA.
References
- Adams A.K, Harvey H.E, Prince R.J. Association of maternal smoking with overweight at age 3 y in American Indian children. Am. J. Clin. Nutr. 2005;82:393–398. doi: 10.1093/ajcn.82.2.393. [DOI] [PubMed] [Google Scholar]
- Aitken R.C.B, Ferres H.M, Gedye J.L. Distraction from flashing lights. Aerospace Med. 1963;34:302–306. [PubMed] [Google Scholar]
- Arcavi L, Benowitz N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. doi:10.1001/archinte.164.20.2206 [DOI] [PubMed] [Google Scholar]
- Arsenault, M. A., MacLeod, E., Weinstein, J. L., Phillips, V., Ferris, A. M. & Duffy, V. B. 2004 Reported history of otitis media (OM) in children associates with intake of vegetables and sweets. Poster presented at the meeting of the International Congress of Dietetics, Chicago, IL, May 2004.
- Bartoshuk L.M. The psychophysics of taste. Am. J. Clin. Nutr. 1978;31:1068–1077. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L.M. Comparing sensory experiences across individuals: recent psychophysical advances illuminate genetic variation in taste perception. Chem. Senses. 2000;25:447–460. doi: 10.1093/chemse/25.4.447. doi:10.1093/chemse/25.4.447 [DOI] [PubMed] [Google Scholar]
- Bartoshuk L.M, Duffy V.B. Supertasting and earaches: genetics and pathology alter our taste worlds. Appetite. 1994;23:292–293. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L.M, Fast K, Karrer T.A, Marino S, Price R.A, Reed D.A. PROP supertasters and the perception of sweetness and bitterness. Chem. Senses. 1992;17:594. [Google Scholar]
- Bartoshuk L.M, Duffy V.B, Miller I.J. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol. Behav. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. doi:10.1016/0031-9384(94)90361-1 [DOI] [PubMed] [Google Scholar]
- Bartoshuk L.M, Duffy V.B, Fast K, Green B, Kveton J, Lucchina L.A, Prutkin J.M, Snyder D.J, Tie K. Sensory variability, food preferences and BMI in non-, medium and supertasters. Appetite. 1999;33:228–229. [Google Scholar]
- Bartoshuk L.M, Chapo A.K, Duffy V.B, Grushka M, Norgren R, Kveton J, Pritchard T.C, Snyder D.J. Oral phantoms: evidence for central inhibition produced by taste. Chem. Senses. 2002a;27:A52. [Google Scholar]
- Bartoshuk L.M, Duffy V.B, Fast K, Green B.G, Prutkin J.M, Snyder D.J. Labeled scales (e.g., category Likert, VAS) and invalid across-group comparisons. What we have learned from genetic variation in taste. Food Qual. Prefer. 2002b;14:125–138. doi:10.1016/S0950-3293(02)00077-0 [Google Scholar]
- Bartoshuk L.M, Duffy V.B, Chapo A.K, Fast K, Yiee J.H, Hoffman H.J, Ko C.-W, Snyder D.J. From psychophysics to the clinic: missteps and advances. Food Qual. Prefer. 2004a;15:617–632. doi:10.1016/j.foodqual.2004.05.007 [Google Scholar]
- Bartoshuk L.M, Duffy V.B, Green B.G, Hoffman H.J, Ko C.-W, Lucchina L.A, Marks L.E, Snyder D.J, Weiffenbach J. Valid across-group comparisons with labeled scales: the gLMS vs magnitude matching. Physiol. Behav. 2004b;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. doi:10.1016/j.physbeh.2004.02.033 [DOI] [PubMed] [Google Scholar]
- Bartoshuk L, Davidson A, Kidd J, Kidd K, Speed W, Pakstis A, Reed D, Synder D, Duffy V. Supertasting is not explained by the PTC/PROP gene. Chem. Senses. 2005a;30:A87. [Google Scholar]
- Bartoshuk L.M, Fast K, Snyder D. Differences in our sensory worlds: invalid comparisons with labeled scales. Curr. Dir. Psychol. Sci. 2005b;14:122–125. doi:10.1111/j.0963-7214.2005.00346.x [Google Scholar]
- Bartoshuk L.M, Snyder D.J, Grushka M, Berger A.M, Duffy V.B, Kveton J.F. Taste damage: previously unsuspected consequences. Chem. Senses. 2005c;30(Suppl. 1):i218–i219. doi: 10.1093/chemse/bjh192. doi:10.1093/chemse/bjh192 [DOI] [PubMed] [Google Scholar]
- Catalanotto F.A, Bartoshuk L.M, Östrum K.M, Gent J.F, Fast K. Effects of anesthesia of the facial nerve on taste. Chem. Senses. 1993;18:461–470. [Google Scholar]
- Chapo A.K, Bartoshuk L.M, Ilich J.Z, Duffy V.B. Age-related differences in fat perception and dietary behaviors. Chem. Senses. 2002;27:A19. [Google Scholar]
- Chapo A, Alex J, Coelho D, Duffy V.B, Snyder D, Bartoshuk L. The influence of head trauma, otitis media, and tonsillectomy on oral sensation, fat acceptance, and body mass index (BMI) Chem. Senses. 2005;30:A88. doi:10.1093/chemse/bjh127 [Google Scholar]
- Conner M.T, Haddon A.V, Pickering E.S, Booth D.A. Sweet tooth demonstrated: individual differences in preference for both sweet foods and foods highly sweetened. J. Appl. Psychol. 1988;73:275–280. doi: 10.1037/0021-9010.73.2.275. doi:10.1037/0021-9010.73.2.275 [DOI] [PubMed] [Google Scholar]
- Cox D.N, van Galen M, Hedderley D, Perry L, Moore P.B, Mela D.J. Sensory and hedonic judgements of common foods by lean consumers and consumers with obesity. Obes. Res. 1998;6:438–447. doi: 10.1002/j.1550-8528.1998.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Dabrila G.M, Bartoshuk L.M, Duffy V.B. Preliminary findings of genetic taste status association with fat intake and body mass index in adults. J. Am. Diet. Assoc. 1995;95(Suppl.):A64. doi:10.1016/S0002-8223(95)00502-1 [Google Scholar]
- DeFranza J.R, Aligne C.A, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Dinehart M.E, Bartoshuk L, Kinsley E, Duffy V.B. Bitter taste markers identify sweet and alcohol hedonics and intake. Chem. Senses. 2005;30:A62. doi:10.1093/chemse/bjh114 [Google Scholar]
- Dinehart M.E, Hayes J.E, Bartoshuk L.M, Lanier S.L, Duffy V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness and intake. Physiol. Behav. 2006;83:821–831. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dionne R.A, Bartoshuk L.M, Mogel J, Witter J. Individual responder analyses for pain: does one pain scale fit all? Trends Pharmacol. Sci. 2005;26:125–130. doi: 10.1016/j.tips.2005.01.009. doi:10.1016/j.tips.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Brunzell J.D, Sande K, Iverius P.H, Greenwood M.R.C. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol. Behav. 1985;35:617–622. doi: 10.1016/0031-9384(85)90150-7. doi:10.1016/0031-9384(85)90150-7 [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C.L, Rahaim J.E. Taste preferences in human obesity: environmental and familiar factors. Am. J. Clin. Nutr. 1991;54:635–641. doi: 10.1093/ajcn/54.4.635. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. doi:10.1016/0195-6663(92)90198-F [DOI] [PubMed] [Google Scholar]
- Duffy V.B. Associations between oral sensation, dietary behaviors and risk of cardiovascular disease (CVD) Appetite. 2004;43:5–9. doi: 10.1016/j.appet.2004.02.007. doi:10.1016/j.appet.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Duffy V.B, Bartoshuk L.M. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 2000;100:647–655. doi: 10.1016/S0002-8223(00)00191-7. doi:10.1016/S0002-8223(00)00191-7 [DOI] [PubMed] [Google Scholar]
- Duffy V.B, Weingarten H.P, Bartoshuk L.M. Preference for sweet in young adults associated with PROP (6-n-propylthiouracil) genetic taster status and sex. Chem. Senses. 1995;20:688. [Google Scholar]
- Duffy V.B, Bartoshuk L.M, Lucchina L.A, Snyder D.J, Tym A. Supertasters of PROP (6-n-propylthiouracil) rate the highest creaminess to high-fat milk products. Chem. Senses. 1996;21:598. [Google Scholar]
- Duffy V.B, Fast K, Cohen Z, Chodos E, Bartoshuk L.M. Genetic taste status associates with fat food acceptance and body mass index in adults. Chem. Senses. 1999;24:545–546. [Google Scholar]
- Duffy V.B, Bartoshuk L.M, Peterson J.M, Phillips M.N. Are nontasters at risk for cardiovascular disease (CHD)? Chem. Senses. 2001;26:1115. [Google Scholar]
- Duffy V.B, Peterson J.M, Dinehart M.E, Bartoshuk L.M. Genetic and environmental variation in taste: associations with sweet intensity, preferences and intake. Topics Clin. Nutr. 2003;18:209–220. [Google Scholar]
- Duffy V.B, Davidson A.C, Kidd J.R, Kidd K.K, Speed W.C, Pakstis A.J, Reed D.R, Snyder D.J, Bartoshuk L.M. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol. Clin. Exp. Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy V, Peterson J, Bartoshuk L. Associations between taste genetics, oral sensation and alcohol intake. Physiol. Behav. 2004b;82:435–445. doi: 10.1016/j.physbeh.2004.04.060. doi:10.1016/j.physbeh.2004.04.060 [DOI] [PubMed] [Google Scholar]
- Duffy V.B, Davidson A, Kidd J, Kidd K, Speed W, Pakstis A, Reed D, Snyder D, Bartoshuk L. Associations between PTC/PROP gene, 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol. Clin. Exp. Res. 2004c;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy V.B, Lucchina L.A, Bartoshuk L.M. Genetic variation in taste: potential biomarker for cardiovascular disease risk? In: Prescott J, Tepper B.J, editors. Genetic variations in taste sensitivity: measurement, significance and implications. Dekker; New York: 2004d. pp. 195–228. [Google Scholar]
- Enns M.P, Van Itallie T.B, Grinker J. Contributions of age, sex and degree of fatness on preferences and magnitude estimations for sucrose in humans. Physiol. Behav. 1979;22:999–1003. doi: 10.1016/0031-9384(79)90346-9. doi:10.1016/0031-9384(79)90346-9 [DOI] [PubMed] [Google Scholar]
- Finger T.E, Nelson G.M, Bryant B, Moore P.A. Intragemmal and perigemmal fibers in taste buds: immunocytochemistry and differential sensitivity to capsaicin. Neurosci. Abstr. 1994;402:12. [Google Scholar]
- Fischer R, Griffin F, Rockey M.A. Gustatory chemoreception in man: multidisciplinary aspects and perspectives. Perspect. Biol. Med. 1966;9:549–577. doi: 10.1353/pbm.1966.0050. [DOI] [PubMed] [Google Scholar]
- Fox A.L. Six in ten ‘tasteblind’ to bitter chemical. Sci. News Lett. 1931;9:249. [Google Scholar]
- Fox A.L. The relationship between chemical constitution and taste. Proc. Natl Acad. Sci. USA. 1932;18:115–120. doi: 10.1073/pnas.18.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijters J.E.R, Rasmussen-Conrad E.L. Sensory discrimination, intensity perception, and affective judgment of sucrose-sweetness in the overweight. J. Gen. Psychol. 1982;107:233–247. doi: 10.1080/00221309.1982.9709931. [DOI] [PubMed] [Google Scholar]
- Green B.G, Shaffer G.S, Gilmore M.M. A semantically-labeled magnitude scale of oral sensation with apparent ratio properties. Chem. Senses. 1993;18:683–702. [Google Scholar]
- Grinker J, Hirsch J. Metabolic and behavioral correlates of obesity. In: Knight J, editor. Physiology, emotion, and psychosomatic illness. CIBA Foundation Symposium 8; Amsterdam: 1972. pp. 349–374. [DOI] [PubMed] [Google Scholar]
- Grinker J, Hirsch J, Smith D.V. Taste sensitivity and susceptibility to external influence in obese and normal weight subjects. J. Pers. Soc. Psychol. 1972;22:320–325. doi: 10.1037/h0032924. [DOI] [PubMed] [Google Scholar]
- Hall M.J, Bartoshuk L.M, Cain W.S, Stevens J.C. PTC taste blindness and the taste of caffeine. Nature. 1975;253:442–443. doi: 10.1038/253442a0. doi:10.1038/253442a0 [DOI] [PubMed] [Google Scholar]
- Halpern B.P, Nelson L.M. Bulbar gustatory responses to anterior and to posterior tongue stimulation in the rat. Am. J. Physiol. 1965;209:105–110. doi: 10.1152/ajplegacy.1965.209.1.105. [DOI] [PubMed] [Google Scholar]
- Harris H, Kalmus H. Chemical sensitivity in genetical differences of taste sensitivity. Ann. Eugen. 1949;15:32–45. doi: 10.1111/j.1469-1809.1949.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Astback J, Wang L, Arvidson K, Johansson O. Protein gene product 9.5-immunoreactive nerves and cells in human oral mucosa. Anat. Rec. 1996;245:621–632. doi: 10.1002/(SICI)1097-0185(199608)245:4<621::AID-AR2>3.0.CO;2-R. doi:10.1002/(SICI)1097-0185(199608)245:4<621::AID-AR2>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Hutchins H, Pescatello L.S, Allen G.J, Duffy V.B. Are 6-n-propylthiouracil (PROP) nontasters at risk for high blood pressure? Chem. Senses. 2002a;27:A23. doi:10.1093/chemse/27.1.23 [Google Scholar]
- Hutchins H.L, Pescatello L.S, Allen G.J, Duffy V.B. Associations between genetic variation in taste and cardiovascular disease risk. J. Am. Diet. Assoc. Suppl. 2002b;102:A12. doi:10.1016/S0002-8223(02)90002-7 [Google Scholar]
- Johnson W.G, Keane T.M, Bonar J.R, Downey C. Hedonic ratings of sucrose solutions: effects of body weight, weight loss and dietary restriction. Addict. Behav. 1979;4:231–236. doi: 10.1016/0306-4603(79)90032-7. doi:10.1016/0306-4603(79)90032-7 [DOI] [PubMed] [Google Scholar]
- Johnston F.E, Hertzog K.P, Malina R.M. Phenylthiocarbamide taste sensitivity and its relationship to growth variation. Am. J. Phys. Anthropol. 1966;24:253–255. doi: 10.1002/ajpa.1330240214. doi:10.1002/ajpa.1330240214 [DOI] [PubMed] [Google Scholar]
- Jones L.V, Peryam D.R, Thurstone L.L. Development of a scale for measuring soldier's food preferences. Food Res. 1955;20:512–520. [Google Scholar]
- Keller K.L. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obes. Res. 2004;12:904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- Keller K.L, Tepper B.J. Genetic sensitivity to 6-n-propylthiouracil influences acceptance of certain bitter and spicy foods in preschool children. Chem. Senses. 2002;27:A42–A43. [Google Scholar]
- Kim U.K, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. doi:10.1126/science.1080190 [DOI] [PubMed] [Google Scholar]
- Ko C.W, Hoffman H.J, Lucchina L.A, Snyder D.J, Weiffenbach J.M, Bartoshuk L.M. Differential perceptions of intensity for the four basic taste qualities in PROP supertasters versus nontasters. Chem. Senses. 2000;25:639–640. [Google Scholar]
- Lanier S, Hutchins H, Duffy V.B. Taste and dietary predictors of central adiposity in adult females. Chem. Senses. 2005a;30:A34. [Google Scholar]
- Lanier S.A, Hayes J.E, Duffy V.B. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol. Behav. 2005b;83:821–831. doi: 10.1016/j.physbeh.2004.10.004. doi:10.1016/j.physbeh.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Lanphear B.P, Byrd R.S, Auinger P, Hall C.B. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99:E1–E7. doi: 10.1542/peds.99.3.e1. doi:10.1542/peds.99.3.e1 [DOI] [PubMed] [Google Scholar]
- Lehman C.D, Bartoshuk L.M, Catalanotto F.C, Kveton J.F, Lowlicht R.A. The effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiol. Behav. 1995;57:943–951. doi: 10.1016/0031-9384(95)91121-r. doi:10.1016/0031-9384(95)91121-R [DOI] [PubMed] [Google Scholar]
- Lieu J.E.C, Feinstein A.R. Effect of gestational and passive smoke exposure on ear infections in children. Arch. Pediatr. Adolesc. Med. 2002;156:147–154. doi: 10.1001/archpedi.156.2.147. [DOI] [PubMed] [Google Scholar]
- Looy H, Weingarten H.P. Facial expressions and genetic sensitivity to 6-n-propylthiouracil predict hedonic response to sweet. Physiol. Behav. 1992;52:75–82. doi: 10.1016/0031-9384(92)90435-5. doi:10.1016/0031-9384(92)90435-5 [DOI] [PubMed] [Google Scholar]
- Looy H, Callaghan S, Weingarten H.P. Hedonic response of sucrose likers and dislikers to other gustatory stimuli. Physiol. Behav. 1992;52:219–225. doi: 10.1016/0031-9384(92)90261-y. doi:10.1016/0031-9384(92)90261-Y [DOI] [PubMed] [Google Scholar]
- Lucchina, L. A. 1995 6-n-Propylthiouracil status: genetic determinant of diet-related behaviors and nutritional status in older females. Doctoral dissertation, University of Connecticut.
- Lucchina L, Bartoshuk L.M, Duffy V.B, Marks L.E, Ferris A.M. 6-n-Propylthiouracil perception affects nutritional status of independent-living older females. Chem. Senses. 1995;20:735. [Google Scholar]
- Macdiarmid J.I, Vail A, Cade J.E, Blundell J.E. The sugar–fat relationship revisted: differences in consumption between men and women of varying BMI. Int. J. Obes. 1998;22:1053–1061. doi: 10.1038/sj.ijo.0800724. doi:10.1038/sj.ijo.0800724 [DOI] [PubMed] [Google Scholar]
- Malcolm R, O'Neil P.M, Hirsch A.A, Currey H.S, Moskowitz G. Taste hedonics and thresholds in obesity. Int. J. Obes. 1980;4:203–212. [PubMed] [Google Scholar]
- Marks L.E, Stevens J.C. Measuring sensation in the aged. In: Poon L.W, editor. Aging in the 1980's: psychological issues. American Psychological Association; Washington, DC: 1980. pp. 592–598. [Google Scholar]
- Marks L.E, Stevens J.C, Bartoshuk L.M, Gent J.G, Rifkin B, Stone V.K. Magnitude matching: the measurement of taste and smell. Chem. Senses. 1988;13:63–87. [Google Scholar]
- McBurney D.H, Smith D.V, Shick T.R. Gustatory cross adaptation: sourness and bitterness. Percept. Psychophys. 1972;11:228–232. [Google Scholar]
- Mela D.J, Sacchetti D.A. Sensory preferences for fats: relationships with diet and body composition. Am. J. Clin. Nutr. 1991;53:908–915. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- Montgomery S.M, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. Br. Med. J. 2002;324:26–27. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H.R. The sweetness and pleasantness of sugars. Am. J. Psychol. 1971;84:387–420. doi:10.2307/1420470 [PubMed] [Google Scholar]
- Moskowitz H.R, Kluter R.A, Westerling J, Jacobs H.L. Sugar sweetness and pleasantness: evidence for different psychophysical laws. Science. 1974;184:583–585. doi: 10.1126/science.184.4136.583. [DOI] [PubMed] [Google Scholar]
- Nagy J.I, Goedert M, Hunt S.P, Bond A. The nature of the substance P-containing nerve fibers in taste papillae of the rat tongue. Neuroscience. 1982;7:3137–3151. doi: 10.1016/0306-4522(82)90236-6. doi:10.1016/0306-4522(82)90236-6 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Responsiveness of dog thalamic neurons to taste stimulation of various tongue regions. Physiol. Behav. 1982;29:741–745. doi: 10.1016/0031-9384(82)90249-9. doi:10.1016/0031-9384(82)90249-9 [DOI] [PubMed] [Google Scholar]
- Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. doi:10.1016/0006-8993(75)90469-2 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hayama T. Receptive fields of solitario-parabrachial relay neurons responsive to natural stimulation of the oral cavity in rats. Exp. Brain Res. 1984;54:359–366. doi: 10.1007/BF00236237. [DOI] [PubMed] [Google Scholar]
- Pangborn R.M, Simone M. Body size and sweetness preference. J. Am. Diet. Assoc. 1958;34:924–928. [PubMed] [Google Scholar]
- Pangborn R.M, Bos K.E, Stern J.S. Dietary fat intake and taste responsiveness to fat in milk by under, normal, and overweight women. Appetite. 1985;6:25–40. doi: 10.1016/s0195-6663(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Paradise J.L, Rockette H.E, Colborn D.K, Bernard B.S, Smith C.G, Kurs-Lasky M, Janosky J.E. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–333. doi: 10.1542/peds.99.3.318. doi:10.1542/peds.99.3.318 [DOI] [PubMed] [Google Scholar]
- Peryam D.R, Girardot N.F. Advanced taste test method. Food Eng. 1952;24:58–61. (see also p. 194). [Google Scholar]
- Peterson, J. M., Bartoshuk, L. M. & Duffy, V. B. 1999 Intensity and preference for sweetness influenced by genetic taste variation. J. Am. Diet. Assoc 99(Suppl. 1), A28.
- Pfaffmann C, Bartoshuk L.M. Psychophysical mapping of a human case of left unilateral ageusia (abstract) Chem. Senses. 1989;14:738. [Google Scholar]
- Pfaffmann C, Bartoshuk L.M. Taste loss due to herpes zoster oticus: an update after 19 months (abstract) Chem. Senses. 1990;15:657–658. [Google Scholar]
- Power C, Jefferis B.J.M.H. Fetal environment and subsequent obesity: a study of maternal smoking. Int. J. Epidemiol. 2002;31:413–419. doi:10.1093/ije/31.2.413 [PubMed] [Google Scholar]
- Prescott J, Swain-Campbell N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem. Senses. 2000;25:239–246. doi: 10.1093/chemse/25.3.239. doi:10.1093/chemse/25.3.239 [DOI] [PubMed] [Google Scholar]
- Prescott J, Johnstone V, Munro P. Discriminability of fat content as a function of PROP sensitivity. Chem. Senses. 2001a;26:800. [Google Scholar]
- Prescott J, Ripandelli N, Wakeling I. Binary taste mixture interactions in PROP non-tasters, medium tasters and super-tasters. Chem. Senses. 2001b;26:993–1003. doi: 10.1093/chemse/26.8.993. doi:10.1093/chemse/26.8.993 [DOI] [PubMed] [Google Scholar]
- Prutkin J.M, Fast K, Lucchina L.A, Bartoshuk L.M. PROP (6-n-propylthiouracil) genetics and trigeminal innervation of fungiform papillae. Chem. Senses. 1999a;24:243. doi:10.1093/chemse/24.2.243 [Google Scholar]
- Prutkin J.M, Fast K, Lucchina L.A, Snyder D.J, Bartoshuk L.M. Spatial taste testing and genetic taste variation. Chem. Senses. 1999b;24:604. [Google Scholar]
- Prutkin J.M, et al. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol. Behav. 2000;69:161–173. doi: 10.1016/s0031-9384(00)00199-2. doi:10.1016/S0031-9384(00)00199-2 [DOI] [PubMed] [Google Scholar]
- Rodin J. Effects of obesity and set point on taste responsiveness and ingestion in humans. J. Comp. Physiol. Psychol. 1975;89:1003–1009. doi: 10.1037/h0077193. [DOI] [PubMed] [Google Scholar]
- Rodin J, Moskowitz H.R, Bray G. Relationship between obesity, weight loss, and taste responsiveness. Physiol. Behav. 1976;17:591–597. doi: 10.1016/0031-9384(76)90157-8. doi:10.1016/0031-9384(76)90157-8 [DOI] [PubMed] [Google Scholar]
- Rollin H. Drug-related gustatory disorders. Ann. Otol. 1978;87:37–42. doi: 10.1177/000348947808700108. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S. Taste and smell in disease (first of two parts) N. Engl. J. Med. 1983a;308:1275–1279. doi: 10.1056/NEJM198305263082107. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S. Taste and smell in disease (second of two parts) N. Engl. J. Med. 1983b;308:1337–1343. doi: 10.1056/NEJM198306023082207. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S. Drugs influencing taste and smell perception. In: Getchell T.V, Doty R.L, Bartoshuk L.M, Snow J.B, editors. Smell and taste in health and disease. Raven Press; New York: 1991. pp. 845–850. [Google Scholar]
- Schiffman S.S, Zervakis J, Westall H.L, Graham B.G, Metz A, Bennett J.L, Heald A.E. Effect of antimicrobial and anti-inflammatory medications on the sense of taste. Physiol. Behav. 2000;69:413–424. doi: 10.1016/s0031-9384(99)00262-0. doi:10.1016/S0031-9384(99)00262-0 [DOI] [PubMed] [Google Scholar]
- Sclafani A. Psychobiology of food preferences. Int. J. Obes. 2001;25(Suppl.):S13–S16. doi: 10.1038/sj.ijo.0801905. [DOI] [PubMed] [Google Scholar]
- Silver W.L, Finger T.E. The trigeminal system. In: Getchell T.V, Doty R.L, Bartoshuk L.M, Snow J.B, editors. Smell and taste in health and disease. Raven Press; New York: 1991. pp. 97–108. [Google Scholar]
- Snyder L.H. Inherited taste deficiency. Science. 1931;74:151–152. doi: 10.1126/science.74.1910.151. [DOI] [PubMed] [Google Scholar]
- Snyder L.H. Studies in human inheritance. IX. The inheritance of taste deficiency in man. Ohio J. Sci. 1932;32:436–440. [Google Scholar]
- Snyder D.J, Duffy V.B, Fast K, Weiffenbach J.M, Bartoshuk L.M. PROP genetics interact with age and sex to influence food preferences. Appetite. 2001;37:164. [Google Scholar]
- Snyder D.J, Duffy V.B, Chapo A.K, Cobbett L.E, Bartoshuk L.M. Childhood taste damage modulates obesity risk: effects on fat perception and preference. Obes. Res. 2003a;11(Suppl.):A147. [Google Scholar]
- Snyder D.J, Duffy V.B, Chapo A.K, Cobbett L.E, Bartoshuk L.M. Food preferences mediate relationships between otitis media and body mass index. Appetite. 2003b;40:360. [Google Scholar]
- Snyder, D. J., Bartoshuk, L. M., McKee, S. A. & O'Malley, S. S. 2004a Tobacco exposure during childhood predicts increased obesity risk in adult men. Tenth Annual Meeting of the Society for Research on Nicotine and Tobacco, RP-078.
- Snyder D.J, Fast K, Bartoshuk L.M. Valid comparisons of suprathreshold stimuli. J. Conscious. Stud. 2004b;11:40–57. [Google Scholar]
- Spitzer L, Rodin J. Human eating behavior: a critical review of studies in normal weight and overweight individuals. Appetite. 1981;2:293–329. [Google Scholar]
- Steiner J.E. The gustofacial response: observation on normal and anencephalic newborn infants. In: Bosma J.F, editor. Development in the fetus and infant. US Government Printing Office; Washington, DC: 1973. pp. 254–278. [PubMed] [Google Scholar]
- Stevens J.C, Marks L.E. Cross-modality matching of brightness and loudness. Proc. Natl Acad. Sci. USA. 1965;54:407–411. doi: 10.1073/pnas.54.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.C, Marks L.E. Cross-modality matching functions generated by magnitude estimation. Percept. Psychophys. 1980;27:379–389. doi: 10.3758/bf03204456. [DOI] [PubMed] [Google Scholar]
- Stoner S.A, Fedoroff I.C, Andersen A.E, Rolls B.J. Food preferences and desire to eat in anorexia and bulimia nervosa. Int. J. Eat. Disord. 1996;19:13–22. doi: 10.1002/(SICI)1098-108X(199601)19:1<13::AID-EAT3>3.0.CO;2-Z. doi:10.1002/(SICI)1098-108X(199601)19:1<13::AID-EAT3>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Sweazey R.D, Smith D.V. Convergence onto hamster medullary taste neurons. Brain Res. 1987;408:173–184. doi: 10.1016/0006-8993(87)90369-6. doi:10.1016/0006-8993(87)90369-6 [DOI] [PubMed] [Google Scholar]
- Tanasescu M, Ferris A.M, Himmelgreen D.A, Rodriguez N, Perez-Escamilla R. Biobehavioral factors are associated with obesity in Puerto Rican children. J. Nutr. 2000;130:1734–1742. doi: 10.1093/jn/130.7.1734. [DOI] [PubMed] [Google Scholar]
- Tepper B.J. Taste responsiveness to 6-n-propylthiouracil as a marker for fat intake and obesity: implications for chronic disease risk. Chem. Senses. 2003;28:E60. [Google Scholar]
- Tepper B.J. 6-n-Propylthiouracil as a genetic marker for fat intake, obesity, and chronic disease risk: current evidence and future promise. In: Prescott J, Tepper B.J, editors. Genetic variation in taste sensitivity. Marcel Dekker; New York: 2004. pp. 155–177. [Google Scholar]
- Tepper B.J, Nurse R.J. Fat perception is related to PROP taster status. Physiol. Behav. 1997;61:949–954. doi: 10.1016/s0031-9384(96)00608-7. doi:10.1016/S0031-9384(96)00608-7 [DOI] [PubMed] [Google Scholar]
- Tepper B, Ullrich N. Dietary restraint influences the relationship between PROP taster status and body weight in women. Appetite. 1999;33:234–235. [Google Scholar]
- Tepper B.J, Ullrich N.V. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol. Behav. 2001;75:305–312. doi: 10.1016/s0031-9384(01)00664-3. doi:10.1016/S0031-9384(01)00664-3 [DOI] [PubMed] [Google Scholar]
- Thompson D.A, Moskowitz H.R, Campbell R.C. Effects of body weight and food intake on pleasantness ratings for a sweet stimulus. J. Appl. Physiol. 1976;41:77–83. doi: 10.1152/jappl.1976.41.1.77. [DOI] [PubMed] [Google Scholar]
- Thompson D.A, Moskowitz H.R, Campbell R.G. Taste and olfaction in human obesity. Physiol. Behav. 1977;19:335–337. doi: 10.1016/0031-9384(77)90348-1. doi:10.1016/0031-9384(77)90348-1 [DOI] [PubMed] [Google Scholar]
- Tie K, Fast K, Kveton J, Cohen Z, Duffy V.B, Green B, Prutkin J.M, Bartoshuk L.M. Anesthesia of chorda tympani nerve and effect on oral pain. Chem. Senses. 1999;24:609. [Google Scholar]
- Toschke A.M, Koletzko B, Slikker W, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur. J. Pediatr. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. doi:10.1007/s00431-002-0983-z [DOI] [PubMed] [Google Scholar]
- Toschke A.M, Montgomery S.M, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am. J. Epidemiol. 2003;158:1068–1074. doi: 10.1093/aje/kwg258. doi:10.1093/aje/kwg258 [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Miyamoto K, Itoh A, Shimamura A. Merkel-neurite complexes in the fungiform papillae of two species of monkeys. Cell Tissue Res. 1987;250:237–239. doi: 10.1007/BF00214677. doi:10.1007/BF00214677 [DOI] [PubMed] [Google Scholar]
- Underwood P.J, Belton E, Hulme P. Aversion to sucrose in obesity. Proc. Nutr. Soc. 1973;32:93a–94a. doi: 10.1079/pns19730051. [DOI] [PubMed] [Google Scholar]
- Volkow N.D, Wise R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. doi:10.1038/nn1452 [DOI] [PubMed] [Google Scholar]
- Whitehead M.C, Beeman C.S, Kinsella B.A. Distribution of taste and general sensory nerve endings in fungiform papillae of the hamster. Am. J. Anat. 1985;173:185–201. doi: 10.1002/aja.1001730304. doi:10.1002/aja.1001730304 [DOI] [PubMed] [Google Scholar]
- Witherly, S. A. 1978 Eating rates, taste perception and salivary secretion between obese and non-obese subjects. MS thesis, University of California, Davis.
- Witherly S.A, Pangborn R.M, Stern J. Gustatory responses and eating duration of obese and lean adults. Appetite. 1980;1:53–63. [Google Scholar]
- Wooley O.W, Wooley S.C, Dunham R.B. Calories and sweet taste: effects on sucrose preference in the obese and nonobese. Physiol. Behav. 1972;9:765–768. doi: 10.1016/0031-9384(72)90048-0. doi:10.1016/0031-9384(72)90048-0 [DOI] [PubMed] [Google Scholar]
- Working Group, N.a.N. 2000 The practical guide: identification, evaluation, and treatment of overweight and obesity in adults NIH Publication No. 00-4084, US Department Health and Human Services. Available at http://www.naaso.org/information/practicalguide.asp
- Yanagisawa K, Bartoshuk L.M, Catalanotto F.A, Karrer T.A, Kveton J.F. Anesthesia of the chorda tympani nerve and taste phantoms. Physiol. Behav. 1998;63:329–335. doi: 10.1016/s0031-9384(97)00423-x. doi:10.1016/S0031-9384(97)00423-X [DOI] [PubMed] [Google Scholar]
- Zahm D.S, Munger B.L. The innervation of the primate fungiform papilla—development, distribution and changes following selective ablation. Brain Res. Rev. 1985;9:147–186. doi: 10.1016/0165-0173(85)90011-6. doi:10.1016/0165-0173(85)90011-6 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang Y. Trends in the association between obesity and socioeconomic status in the US adults: 1971–2000. Obes. Res. 2000;12:1622–1632. doi: 10.1038/oby.2004.202. [DOI] [PubMed] [Google Scholar]