Abstract
The brain–gut peptide cholecystokinin (CCK) inhibits food intake following peripheral or site directed central administration. Peripheral exogenous CCK inhibits food intake by reducing the size and duration of a meal. Antagonist studies have demonstrated that the actions of the exogenous peptide mimic those of endogenous CCK. Antagonist administration results in increased meal size and meal duration. The feeding inhibitory actions of CCK are mediated through interactions with CCK-1 receptors. The recent identification of the Otsuka–Long–Evans–Tokushima Fatty (OLETF) rat as a spontaneous CCK-1 receptor knockout model has allowed a more comprehensive evaluation of the feeding actions of CCK. OLETF rats become obese and develop non-insulin dependent diabetes mellitus (NIDDM). Consistent with the absence of CCK-1 receptors, OLETF rats do not respond to exogenous CCK. OLETF rats are hyperphagic and their increased food intake is characterized by a large increase in meal size with a decrease in meal frequency that is not sufficient to compensate for the meal size increase. Deficits in meal size control are evident in OLETF rats as young as 2 days of age. OLETF obesity is secondary to the increased food intake. Pair feeding to amounts consumed by intact control rats normalizes body weight, body fat and elevated insulin and glucose levels. Hypothalamic arcuate nucleus peptide mRNA expression in OLETF rats is appropriate to their obesity and is normalized by pair feeding. In contrast, pair fed and young pre-obese OLETF rats have greatly elevated dorsomedial hypothalamic (DMH) neuropeptide Y (NPY) mRNA expression. Elevated DMH NPY in OLETF rats appears to be a consequence of the absence of CCK-1 receptors. In intact rats NPY and CCK-1 receptors colocalize to neurons within the compact subregion of the DMH and local CCK administration reduces food intake and decreases DMH NPY mRNA expression. We have proposed that the absence of DMH CCK-1 receptors significantly contributes to the OLETF's inability to compensate for their meal size control deficit leading to their overall hyperphagia. Access to a running wheel and the resulting exercise normalizes food intake and body weight in OLETF rats. When given access to running wheels for 6 weeks shortly after weaning, OLETF rats do not gain weight to the same degree as sedentary OLETF rats and do not develop NIDDM. Exercise also prevents elevated levels of DMH NPY mRNA expression, suggesting that exercise exerts an alternative, non-CCK mediated, control on DMH NPY. The OLETF rat is a valuable model for characterizing actions of CCK in energy balance and has provided novel insights into interactions between exercise and food intake.
Keywords: cholecystokinin, satiety, exercise, NPY
1. CCK and satiety
Gibbs et al. (1973) originally demonstrated the ability of exogenously administered cholecystokinin (CCK) to inhibit food intake in rats. Their characterization of the pattern of behavioural changes produced by CCK suggested that CCK administration resulted in the production of satiety. CCK reduced meal size and meal duration and resulted in an earlier appearance of a behavioural sequence of satiety similar to that seen following ingestion of a normal size meal (Antin et al. 1975). Work in multiple laboratories has now demonstrated that exogenous peripheral administration of CCK results in dose-related suppression of short-term food intake in a variety of species and in a variety of experimental situations (see Smith & Gibbs 1998 for review).
The availability of CCK receptor antagonists provided the ability to critically assess the physiological significance of endogenous CCK in the controls of meal size. In a variety of testing paradigms across multiple species, CCK antagonists with specificity to the CCK-1 receptor subtype (see below) have been shown to increase food intake (Reidelberger & O'Rourke 1989; Silver et al. 1989; Moran et al. 1992; Smith & Gibbs 1998 for review). Although results with CCK antagonists in human subjects have been mixed, Beglinger et al. (2001) have demonstrated small but significant increases in caloric intake in response to the CCK-1 antagonist loxiglumide. These increases corresponded to decreased reports of fullness and increased reports of hunger.
Within the periphery, two candidate CCK receptor populations for mediating the satiety actions of exogenously administered or peripherally released endogenous CCK have been identified. CCK receptors are expressed in the nodose ganglion, the site of vagal afferent cell bodies and these receptors are transported to subdiaphragmatic vagal branches (Moran et al. 1987, 1990). CCK receptors are also present in circular muscle fibres of the pyloric sphincter (Smith et al. 1984). Additive roles for these populations in CCK satiety have been proposed from data demonstrating that different aspects of the dose response curve for the inhibition of food intake by exogenous CCK depend upon activation of the two populations (Moran et al. 1988, 1997).
A role for brain CCK in the control of food intake has also been suggested. An original report by Della Fera & Baile (1979) demonstrated feeding inhibitory effects of low doses of intraventricular CCK in sheep. Positive results with intracerebroventricular CCK have also been generated in baboons (Figlewicz et al. 1989). In both cases, feeding inhibitory actions were obtained at doses that were not effective when administered peripherally, providing support for a central site of action. In the rat, a number of investigators have demonstrated feeding inhibitory actions of CCK administered into the cerebral ventricles. However, for the most part, these were obtained with doses that also affected intake when administered peripherally leading to the suggestion that the centrally administered CCK was affecting intake through a peripheral rather than a central site of action (Crawley 1985; Schick et al. 1986). However, specific brain site directed injections have now produced positive results at significantly lower doses. Blevins et al. (2000) have identified multiple brain sites at which CCK inhibits food intake. The magnitude of inhibition was greatest with CCK injections aimed at the dorsomedial hypothalamus (DMH). A role for endogenous central CCK in feeding has also been suggested from the results of studies employing central CCK antagonist injections. Central injections of low doses of CCK antagonists increase feeding in rats at doses that are ineffective when given systemically (Ebenezer 2002). Furthermore, peripheral injections of CCK antagonists that cross the blood–brain barrier increase feeding in vagotomized rats while peptide antagonists that are limited to the periphery do not (Reidelberger et al. 2004). The specific site of action for central CCK antagonists to affect food intake has not been determined. It should be pointed out that, in contrast to actions of peripheral CCK in the control of meal size, a specific action for central CCK in feeding control has yet to be identified.
2. CCK receptor subtypes
We have already made reference to the existence of two CCK receptor subtypes. The possibility of heterogeneity in CCK receptors was first suggested in the original homogenate radioligand studies on pancreas and brain (Innis & Snyder 1980), in which the relative affinity of the binding sites for some CCK analogues differed between these two tissues. Studies that combined autoradiographic localization and analyses of the pharmacological specificity of binding demonstrated receptor heterogeneity at different brain sites (Moran et al. 1986; Hill & Woodruff 1990 see Moran & Ladenheim 1998 for details). Distinct CCK-1 and CCK-2 receptor proteins have been isolated and cloned and the amino acid sequences of these receptors have been identified (Wank et al. 1992; Kopin et al. 1994). Both CCK-1 and CCK-2 receptors consist of seven transmembrane spanning protein domains, and are members of the G-protein coupled superfamily of receptor molecules. The CCK-1 receptors identified in rat brain in autoradiographic studies have now been shown to be the same protein as rat pancreatic CCK-1 receptors. Furthermore, CCK-2 receptors and gastrin receptors represent a single gene product (Kopin et al. 1994). It is important to note that there is significant species variability in the distribution of the two receptor subtypes.
3. OLETF rats
A spontaneously diabetic rat with polyuria, polydipsia and obesity was discovered at the Tokushima Research Institute in 1984 in an out-bred colony of Long–Evans rats that had been purchased from Charles River a few years previously. Through selective breeding, a line of rats characterized by obesity, adult onset hyperglycaemia, chronic diabetes mellitus and late conversion from non-insulin dependent diabetes mellitus (NIDDM) to insulin dependent diabetes mellitus (IDDM) was established by 1991 (Kawano et al. 1992).
These Otsuka–Long–Evans–Tokushima Fatty (OLETF) rats have been extensively studied as a rat model of human NIDDM (Shima et al. 1999). Both male and female OLETF rats develop obesity. Starting from normal weights at the time of weaning, male and female OLETF rats become 30–40% heavier than Long–Evans–Tokushima–Otsuka (LETO) controls by 20 weeks of age (Kawano et al. 1994). Plasma glucose levels following oral glucose loads are elevated as early as eight weeks of age and by 24 weeks of age rats are hyperglycaemic and hyperinsulinaemic. By 65 weeks of age many of the males develop IDDM.
In studying pancreatic exocrine function in OLETF rats through the course of their hyperglycaemia and hyperinsulinaemia, it was discovered that pancreatic acini from OLETF rats were completely insensitive to the amylase stimulatory actions of CCK (Otsuki et al. 1995). This loss of sensitivity to CCK was specific in that OLETF acinar cells retained normal sensitivity to bombesin, carbamylcholine and secretin as measured by amylase production. Subsequent studies demonstrated that 125I-CCK binding to pancreatic acini from OLETF rats was completely absent. Because pancreatic CCK binding and CCK-induced amylase activity depend upon CCK-1 receptors in the rat, these data suggested that OLETF rats lacked CCK-1 receptors.
The possibility that the OLETF rat was a CCK-1 receptor knockout model was examined. Little or no expression of CCK-1 receptor mRNA could be detected in the pancreas of OLETF rats by Northern blot using total RNA (Funakoshi et al. 1994), RT–PCR or southern blot hybridization (Funakoshi et al. 1995). These results were specific to CCK-1 receptor expression, because CCK-2 receptor expression was shown to be normal in OLETF rats. Analysis of the CCK-1 receptor gene structure and base sequence in OLETF rats revealed a 6487 base length deletion flanked by two 3-base pair direct repeats (5′-TGT-3′). This deletion included the promoter region and the first and second exons. The regions both upstream and downstream from the deletion were intact (Takiguchi et al. 1997). This deletion prevented protein expression. These data provided proof that the OLETF rat lacked CCK-1 receptors and represented a naturally occurring CCK-1 receptor knockout. The identification of the OLETF as a CCK-1 receptor knockout provided a tool for broadly characterizing the roles of CCK-1 receptor activation. The present discussion will focus on the characterization of the feeding and obesity in OLETF rats. The data support and amplify earlier work with CCK-1 receptor antagonists, suggest a novel role for central CCK in energy balance and illuminate interactions between exercise and obesity that may have broad implications.
4. Larger meals in OLETF rats
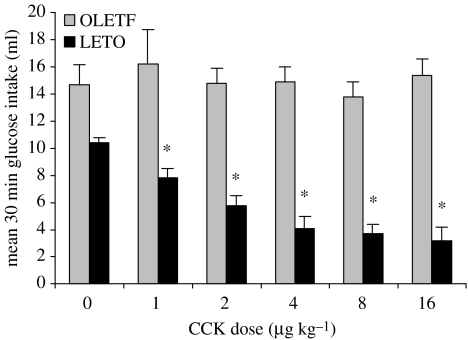
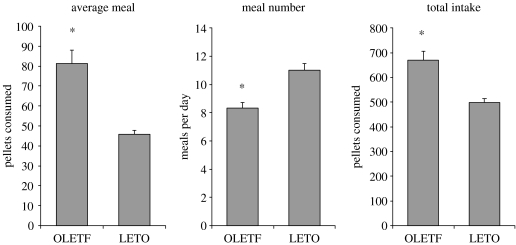
Characterization of the feeding behaviour of OLETF rats provided a validation of the absence of functional CCK-1 receptors and revealed a deficit in their ability to properly respond to within meal feedback. As demonstrated in figure 1, exogenous CCK resulted in a dose-related suppression of intake in LETO rats but, consistent with the absence of CCK-1 receptors, CCK had no effect on glucose consumption in the OLETF rats. These data reinforced earlier conclusions from antagonist studies that exogenous CCK inhibited food intake through interactions with CCK-1 receptors. The results from this experiment also demonstrated a basic difference in intake between OLETF and LETO rats. Even in this scheduled brief access test, OLETF rats consumed significantly more than the LETO controls. Analyses of the pattern of ingestion during consumption of the liquid meal revealed that although the initial rates of licking in OLETF and LETO rats were similar, OLETF rats maintained that initial rate significantly longer than LETO rats. In addition, the rate of decline in lick rate was significantly slower in OLETF than in LETO rats. Such alterations in lick patterns were taken to indicate that OLETF rats lacking CCK-1 receptors have a deficit in their ability to control the size of their meals (Moran et al. 1998). The presence of such a deficit was confirmed in studies examining the patterns of consumption of 45 mg chow pellets in OLETF and LETO rats. As shown in figure 2, the average meal size in OLETF rats was 80% greater than that in the LETO control rats. In response to this increase in meal size, overall meal number was decreased but not to a sufficient degree to compensate for the increase in meal size. Thus, overall food intake in OLETF rats was significantly increased (Moran et al. 1998).
Figure 1.
Effect of peripheral CCK on glucose intake in OLETF and LETO rats. CCK significantly inhibits intake at all doses in LETO rats (* denotes p<0.05) but has no effect on intake in OLETF rats (reprinted with permission from Moran et al. 1998, ©The American Physiological Society).
Figure 2.
Twenty four hour meal patterns in OLETF and LETO rats consuming 45 mg chow pellets. Meal size and overall intake are significantly elevated in OLETF rats. Meal number is significantly decreased. (* denotes p<0.05; adapted from Moran et al. 1998).
The ability of OLETF rats to compensate for the increased caloric density of a high fat diet was also impaired. Consistent with dietary fats being strong CCK secretagogues, the hyperphagia of OLETF rats is exacerbated when they are placed on a high fat diet (Schwartz et al. 1999). In response to access to a high fat, high calorie diet, LETO rats temporarily maintain the weight of food consumed which results in an increase in their daily caloric intake. However, within a few days, the number of grams consumed decreases as LETO rats compensate for the increased caloric density of the high fat diet. The response of OLETF rats differs. OLETF rats actually increase the number of grams consumed when first given access to high fat diet. Although this effect is temporary, their caloric intake remains significantly elevated over time, exacerbating their obesity.
OLETF rats have also been shown to be less sensitive to the feeding inhibitory effects of nutrient gastric or small intestinal nutrient preloads (Schwartz et al. 1999; Covasa & Ritter 2001; De Jonghe et al. 2005). When preloads are given prior to a scheduled period of glucose access, preloads of glucose, fats and proteins all were less effective at reducing intake in OLETF than in LETO rats. When preloads were given prior to chow access following a 17 hr period of food deprivation, reduction of subsequent 4 hour food intake was significantly attenuated in the OLETF relative to the reductions in LETO rats (Covasa & Ritter 2001).
Although theses data are consistent with the increased within-meal intake of OLETF rats arising from a satiety deficit, recent data suggest that OLETF rats may also have altered preference for sweet solutions that could contribute to their increased intake (De Jonghe et al. 2005). In short-access, two bottle preference tests, OLETF rats have an enhanced preference for sucrose solutions. Consistent with this finding, sham feeding OLETF rats also consume more sucrose than LETO rats in the absence of intestinal feedback.
5. Characterization of OLETF obesity
Although OLETF rats are clearly hyperphagic, it was not clear whether the increase in food intake completely accounted for their obesity. Comparisons of growth curves of OLETF rats with ad libitum food access to LETO rats with ad libitum access and to OLETF rats that were pair fed to amounts consumed by the LETO controls addressed this issue. The results clearly demonstrated that the primary deficit in OLETF rats is their hyperphagia. Feeding OLETF rats amounts consumed by LETO controls completely prevented their obesity. Moreover, this experiment demonstrated that pair feeding prevented increases in blood glucose, plasma insulin and leptin, and fat deposition (Bi et al. 2001).
The pair feeding experiment also allowed a determination of whether aspects of arcuate nucleus orexigenic and anorexigenic peptide signalling were appropriate for metabolic status or whether there may be a primary deficit contributing to the apparent inability of OLETF rats to compensate for their increase in meal size. In situ hybridization studies demonstrated that arcuate signalling appeared to be appropriately responding to the obesity in OLETF rats. Neuropeptide Y (NPY) mRNA expression was decreased relative to levels in LETO rats and proopiomelanocortin (POMC) mRNA expression was increased. Pair feeding normalized these levels of mRNA (Bi et al. 2001). Assessment of the responses to exogenous NPY and melanocortin agonist administration were consistent with these gene expression data. In response to decreased NPY mRNA expression, OLETF rats had increased feeding responses to central NPY administration. In contrast, OLETF rats had decreased responsivity to melanocortin agonist administration and increased responsivity to a melanocortin antagonist (Moran et al. 2002).
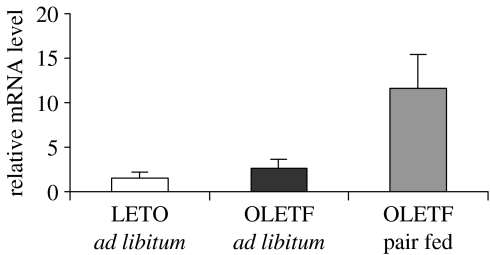
Although arcuate peptide gene expression appeared to be appropriate for the level of body weight, the pair fed group revealed an 8 fold overexpression of NPY in the compact subregion of the DMH (Bi et al. 2001). That is, although normal in ad libitum fed OLETF rats, DMH NPY expression was greatly elevated in OLETF rats pair fed to levels consumed by LETO controls (figure 3). Analyses of patterns of hypothalamic gene expression in pre-obese, recently weaned, OLETF rats revealed a similar elevation in DMH NPY suggesting that increased NPY signalling may play a role in the OLETF rat inability to compensate for a satiety deficit producing over-consumption within individual meals.
Figure 3.
In situ hybridization of NPY mRNA expression in the arcuate (ARC) and dorsomedial hypothalamus (DMH) in LETO, ad libitum fed OLETF rats and OLETF rats pair fed to amounts consumed by LETO rats. ARC NPY is decreased in ad libitum fed OLETF rats and normalized in pair fed OLETF rats. DMH NPY is significantly elevated in pair fed OLETF rats.
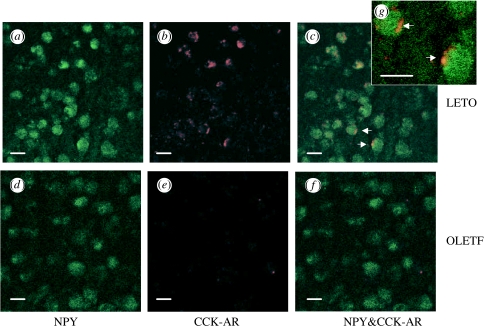
Earlier work in a number of laboratories had identified the DMH as a brain site containing CCK-1 receptor sites (Moran et al. 1986; Hill & Woodruff 1990) and demonstrating CCK-1 mRNA expression (Wank et al. 1992). Experiments aimed at examining the relative distribution of NPY and CCK-1 receptor containing neurons revealed colocalization of CCK-1 receptor and NPY immunoreactivity in a population of neurons in the compact area of the DMH (figure 4). This result suggested that there is normally a functional interaction between CCK and NPY at the level of the DMH. Consistent with this idea, CCK microinjected directly into the DMH inhibits food intake and down regulates DMH NPY mRNA expression (Blevins et al. 2000; Bi et al. 2004).
Figure 4.
Dual immunohistochemistry of anti-NPY and anti-CCK-1 receptor antibodies in the compact subregion of the DMH in LETO and OLETF rats. CCK-1 immunoreactivity colocalizes with NPY immunoreactivity in LETO but not OLETF rats (reprinted with permission from Bi et al. 2004, ©The Endocrine Society).
These data suggest that the hyperphagia and obesity in the OLETF rat is the outcome of two regulatory deficits arising from the absence of CCK-1 receptors. One arises from the absence of vagal CCK-1 receptors, resulting in a satiety deficit leading to large increases in meal size. The second arises from the absence of CCK-1 receptors in the DMH, resulting in a loss of an inhibitory control over DMH NPY expression. The source of CCK to which these receptors respond is not yet clear. It is unlikely to be peripheral, meal released, CCK. The DMH does contain CCK expressing neurons and receives CCK containing terminals from a number of other brain sites.
Further support for a role for this NPY overexpression in OLETF hyperphagia comes from two additional sources. The first is from studies examining patterns of food intake in CCK-1 receptor knockout mice (Kopin et al. 1999). These mice also have increased meal sizes consistent with the absence of peripheral CCK-1 signalling, but they compensate for this deficit, maintaining normal overall food intake and body weight. These mice also do not have increased DMH NPY mRNA expression. Comparisons of the brain distribution of CCK-1 receptor between rats and mice provide a potential basis for these functional differences. In contrast to the rat, the mouse DMH does not contain CCK-1 receptors (Bi et al. 2004). Thus, the lack of obesity in the CCK-1 receptor knockout mouse may be due to their having one deficit rather than two. In the absence of CCK-1 receptors, they have a satiety disruption but no change in the activity of DMH NPY. This pattern of deficits leads to alterations in meal patterns but no overall change in food intake and body weight.
Finally, a recent study has directly addressed whether increased DMH NPY expression can result in increased food intake and obesity. Rats received bilateral DMH injections of an adeno-associated NPY expression vector. In response to these AAV-NPY injections, DMH NPY mRNA expression was increased twofold while arcuate NPY mRNA expression was not affected. Rats receiving such treatment significantly increased their intake of a high fat diet and, over the course of six weeks, became obese (Bi et al. 2005). These data demonstrate the potential for elevated DMH NPY to result in increased overall food intake and body weight.
6. Ingestion in preweanling OLETF pups
Weller and colleagues (Lavi-Avnon et al. 2004; Blumberg et al. 2005) have examined aspects of the development of ingestive behaviours in OLETF rats. OLETF pups consumed more sweetened milk than LETO control pups in an independent ingestion test in which pups lick milk off a filter paper on the floor of the test chamber. Increased intake in OLETF pups was found as early as postnatal day 2 and, consistent with a satiety deficit, the increased intake was attributable to increases in meal size and duration within the test period. Similar satiety deficits were also found in older pups using this paradigm. OLETF pups also weigh more and have a greater degree of fat deposition beginning as early as postnatal day 3 (Zagoory et al. 2004).
7. Exercise-induced attenuation of OLETF obesity
Shortly after the deficit in CCK signalling in OLETF rats was identified, exercise was shown to modulate the degree of obesity in this model. Shima et al. (1996, 1997) demonstrated that access to running wheels significantly reduced the degree of body weight gain and adiposity in OLETF rats. However, the degree of attenuation, the underlying mechanisms and the long-term consequences of such effect had not been documented. The effect of exercise in OLETF rats depends upon the developmental time point that rats are given access to the running wheels. Running wheel access in adult obese OLETF rats results in body weight loss down to levels of comparably aged LETO control rats and, with removal of the running wheels, OLETF rats rapidly regain body weight to levels comparable to those of rats who did not have running wheel access.
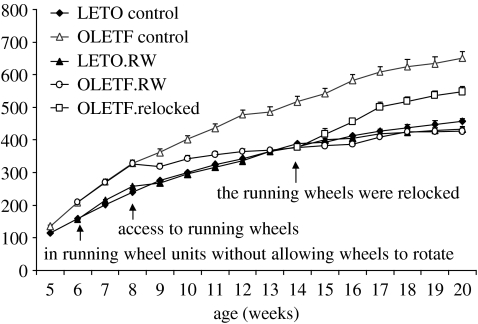
In contrast, when running wheel access is given when OLETF rats are younger and in the stage of developing their obesity, there are long-term consequences. As shown in figure 5, running wheel access begun at eight weeks of age results in an inhibition in the growth curve of OLETF rats such that they track the growth curves of control LETO rats. Running wheel access in LETO rats did not affect their growth even through they were as active in the running wheels as OLETF rats. When running wheels are locked, OLETF rats regain some weight but weight then stabilizes at levels significantly below those of OLETF rats maintained without running wheels. Running wheel induced alterations in food intake explain the body weight effects in OLETF rats. With running wheel access, the hyperphagia of the OLETF rat completely disappears as levels of intake fall to those of LETO control rats. Intake remains similar to that of LETO rats for as long as running wheel access is maintained. When wheels are locked, there is a transient increase in food intake in OLETF rats during the period of weight gain. At the point that weight stabilizes, intake drops back to levels of LETO rats. Analyses of meal patterns during these periods revealed that the increased meal size characteristic of OLETF rats was also affected by running wheel access. Meal sizes diminished with running wheel access to levels comparable to those of LETO rats (Bi et al. 2005).
Figure 5.
Effects of running wheel access on body weight in LETO and OLETF rats. S, sedentary; RW, running wheel access. Running wheel access prevented excess weight gain in OLETF but did not affect LETO rats. Relocking the wheels resulted in temporary weight gain in OLETF rats but running wheel access had a long-term effect on weight (reprinted with permission from Bi et al. 2005, ©The Endocrine Society).
As well as normalizing food intake and body weight, running wheel access normalized plasma glucose and insulin levels in OLETF rats. Within days of running wheel access plasma glucose and insulin levels fell to levels of LETO controls. Even with locking the running wheels, plasma glucose and insulin levels remained low despite the transient period of weight gain in OLETF rats (Bi et al. 2005).
Alterations in patterns of hypothalamic gene expression may underlie some of the feeding and body weight effects of running wheel access in OLETF rats. During the initial exercise period, corticotrophin-releasing factor (CRF) mRNA expression was significantly elevated in the DMH in both OLETF and control LETO rats and DMH CRF remained elevated for as long as running wheel access was maintained. When wheels were locked, a time when food intake and body weight increased in OLETF rats, DMH CRF returned to baseline levels. These patterns of change suggest that alterations in DMH peptide signalling may underlie the activity-induced changes in food intake and body weight. Importantly, even though OLETF rats with exercise experience maintained body weights significantly below that of sedentary OLETF rats, the reduced body weight did not result in increased DMH NPY mRNA expression. Thus, exercise appears to substitute for the missing CCK signalling in controlling DMH NPY in this model.
8. Summary
The OLETF rat lacking CCK-1 receptors validates many of the findings with exogenously administered CCK agonist and antagonist compounds and provides further evidence for an important role for endogenous CCK acting through CCK-1 receptors in satiety. The OLETF model also has identified a potential role for brain CCK signalling in overall energy balance. In the absence of DMH CCK-1 receptors, DMH NPY expression is increased. A role for this increase in the obesity of OLETF rats is suggested by its presence prior to significant body weight gain and its suppression as weight gain occurs. The OLETF rat also provides a model system for identifying how exercise may modulate overall energy balance. Access to running wheels results in reduced food intake, normalization of body weight and amelioration of NIDDM. When running wheel access occurs prior to or at an early stage of obesity, the results are lasting. OLETF rats with running wheel access never regain weight to levels of no-exercised rats and they maintain normal glycaemia. Activity also appears to normalize DMH NPY expression providing a basis for the longer term reductions in food intake and body weight.
A number of issues remain. Although DMH CCK administration reduces DMH NPY expression and inhibits food intake, the source of CCK that would normally interact with DMH CCK-1 receptors and the stimuli governing its release remain to be identified. Little is presently known about how exercise affects hypothalamic gene expression, food intake and body weight and whether exercise effects on insulin sensitivity are direct or whether they are secondary to alterations in hypothalamic signalling. Finally, although data from the OLETF rat identify potentially important roles for the DMH in energy balance, we have little understanding of neural circuitry involving the DMH and how it interacts with other signalling pathways in the controls of energy balance. The OLETF rat provides an excellent model for addressing a number of these issues.
Acknowledgments
This work was supported by NIH grants DK19302, DK57609 and MH67638 and the generous gift of the OLETF and LETO rats from Otsuka Pharmaceutical (Tokushima, Japan).
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Antin J, Gibbs J, Holt J, Young R.C, Smith G.P. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J. Comp. Physiol. Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1149–R1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim E.E, Schwartz G.J, Moran T.H. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott K.A, Kopin A.S, Moran T.H. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. doi:10.1210/en.2004-0284 [DOI] [PubMed] [Google Scholar]
- Bi S, Scott K.A, Hyun J, Ladenheim E.E, Moran T.H. Running wheel activity prevents hyperphagia and obesity in Otsuka–Long–Evans–Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. doi:10.1210/en.2004-1441 [DOI] [PubMed] [Google Scholar]
- Blevins J.E, Stanley B.G, Reidelberger R.D. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res. 2000;860:1–10. doi: 10.1016/s0006-8993(99)02477-4. doi:10.1016/S0006-8993(99)02477-4 [DOI] [PubMed] [Google Scholar]
- Blumberg S, Haba D, Schroeder M, Smith G.P, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;290:R208–R218. doi: 10.1152/ajpregu.00379.2005. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter R.C. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–1348. doi: 10.1016/s0196-9781(01)00461-2. doi:10.1016/S0196-9781(01)00461-2 [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Clarification of the behavioral functions of peripheral and central cholecystokinin: two separate peptide pools. Peptides. 1985;6(Suppl. 2):129–136. doi: 10.1016/0196-9781(85)90145-7. doi:10.1016/0196-9781(85)90145-7 [DOI] [PubMed] [Google Scholar]
- De Jonghe B.C, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- Della Fera M.A, Baile C.A. CCK-octapeptide injected in CSF causes satiety in sheep. Ann. Rech. Vet. 1979;10:234–236. [PubMed] [Google Scholar]
- Ebenezer I.S. Effects of intracerebroventricular administration of the CCK(1) receptor antagonist devazepide on food intake in rats. Eur. J. Pharmacol. 2002;441:79–82. doi: 10.1016/s0014-2999(02)01485-1. doi:10.1016/S0014-2999(02)01485-1 [DOI] [PubMed] [Google Scholar]
- Figlewicz D.P, Sipols A.J, Porte D, Jr, Woods S.C, Liddle R.A. Intraventricular CCK inhibits food intake and gastric emptying in baboons. Am. J. Physiol. 1989;256:R1313–R1317. doi: 10.1152/ajpregu.1989.256.6.R1313. [DOI] [PubMed] [Google Scholar]
- Funakoshi A, Miyasaka K, Jimi A, Kawanai T, Takata Y, Kono A. Little or no expression of the cholecystokinin-A receptor gene in the pancreas of diabetic rats (Otsuka–Long–Evans–Tokushima Fatty = OLETF rats) Biochem. Biophys. Res. Commun. 1994;199:482–488. doi: 10.1006/bbrc.1994.1254. doi:10.1006/bbrc.1994.1254 [DOI] [PubMed] [Google Scholar]
- Funakoshi A, Miyasaka K, Shinozaki H, Masuda M, Kawanami T, Takata Y, Kono A. An animal model of congenital defect of gene expression of cholecystokinin (CCK)-A receptor. Biochem. Biophys. Res. Commun. 1995;210:787–796. doi: 10.1006/bbrc.1995.1728. doi:10.1006/bbrc.1995.1728 [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young R.C, Smith G.P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Hill D.R, Woodruff G.N. Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260. Brain Res. 1990;526:276–283. doi: 10.1016/0006-8993(90)91232-6. doi:10.1016/0006-8993(90)91232-6 [DOI] [PubMed] [Google Scholar]
- Innis R.B, Snyder S.H. Distinct cholecystokinin receptors in brain and pancreas. Proc. Natl Acad. Sci. USA. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka–Long–Evans–Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka–Long–Evans–Tokushima Fatty) rat: a new NIDDM rat strain. Diab. Res. Clin. Pract. 1994;24(Suppl.):S317–S320. doi: 10.1016/0168-8227(94)90269-0. doi:10.1016/0168-8227(94)90269-0 [DOI] [PubMed] [Google Scholar]
- Kopin A.S, Beinborn M, Lee Y.M, McBride E.W, Quinn S.M. The CCK-B/gastrin receptor. Identification of amino acids that determine nonpeptide antagonist affinity. Ann. NY Acad. Sci. 1994;713:67–78. doi: 10.1111/j.1749-6632.1994.tb44053.x. [DOI] [PubMed] [Google Scholar]
- Kopin A.S, Mathes W.F, McBride E.W, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Malkesman O, Hurwitz I, Weller A. Mother–infant interactions in rats lacking CCK(A) receptors. Behav. Neurosci. 2004;118:282–289. doi: 10.1037/0735-7044.118.2.282. doi:10.1037/0735-7044.118.2.282 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Ladenheim E.E. Identification of receptor populations mediating the satiety actions of brain and gut peptides. In: Smith G.P, editor. Satiation: from gut to brain. Oxford University Press; New York, NY: 1998. pp. 126–163. [Google Scholar]
- Moran T.H, Robinson P.H, Goldrich M.S, McHugh P.R. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. doi:10.1016/0006-8993(86)91413-7 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Smith G.P, Hostetler A.M, McHugh P.R. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res. 1987;415:149–152. doi: 10.1016/0006-8993(87)90278-2. doi:10.1016/0006-8993(87)90278-2 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Shnayder L, Hostetler A.M, McHugh P.R. Pylorectomy reduces the satiety action of cholecystokinin. Am. J. Physiol. 1988;255:R1059–R1063. doi: 10.1152/ajpregu.1988.255.6.R1059. [DOI] [PubMed] [Google Scholar]
- Moran T.H, Norgren R, Crosby R.J, McHugh P.R. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res. 1990;526:95–102. doi: 10.1016/0006-8993(90)90253-8. doi:10.1016/0006-8993(90)90253-8 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Ameglio P.J, Schwartz G.J, McHugh P.R. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am. J. Physiol. 1992;262:R46–R50. doi: 10.1152/ajpregu.1992.262.1.R46. [DOI] [PubMed] [Google Scholar]
- Moran T.H, Baldessarini A.R, Salorio C.F, Lowery T, Schwartz G.J. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- Moran T.H, Katz L.F, Plata-Salaman C.R, Schwartz G.J. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am. J. Physiol. 1998;274:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- Moran T.H, Lee P, Ladenheim E.E, Schwartz G.J. Responsivity to NPY and melanocortins in obese OLETF rats lacking CCK-A receptors. Physiol. Behav. 2002;75:397–402. doi: 10.1016/s0031-9384(01)00667-9. doi:10.1016/S0031-9384(01)00667-9 [DOI] [PubMed] [Google Scholar]
- Otsuki M, Akiyama T, Shirohara H, Nakano S, Furumi K, Tachibana I. Loss of sensitivity to cholecystokinin stimulation of isolated pancreatic acini from genetically diabetic rats. Am. J. Physiol. 1995;268:E531–E536. doi: 10.1152/ajpendo.1995.268.3.E531. [DOI] [PubMed] [Google Scholar]
- Reidelberger R.D, O'Rourke M.F. Potent cholecystokinin antagonist L 364718 stimulates food intake in rats. Am. J. Physiol. 1989;257:R1512–R1518. doi: 10.1152/ajpregu.1989.257.6.R1512. [DOI] [PubMed] [Google Scholar]
- Reidelberger R.D, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R1005–R1012. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- Schick R.R, Yaksh T.L, Go V.L. Intracerebroventricular injections of cholecystokinin octapeptide suppress feeding in rats—pharmacological characterization of this action. Regul. Pept. 1986;14:277–291. doi: 10.1016/0167-0115(86)90170-9. doi:10.1016/0167-0115(86)90170-9 [DOI] [PubMed] [Google Scholar]
- Schwartz G.J, Whitney A, Skoglund C, Castonguay T.W, Moran T.H. Decreased responsiveness to dietary fat in Otsuka–Long–Evans–Tokushima Fatty rats lacking CCK-A receptors. Am. J. Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- Shima K, Shi K, Mizuno A, Sano T, Ishida K, Noma Y. Exercise training has a long-lasting effect on prevention of non-insulin-dependent diabetes mellitus in Otsuka–Long–Evans–Tokushima Fatty rats. Metabolism. 1996;45:475–480. doi: 10.1016/s0026-0495(96)90222-x. doi:10.1016/S0026-0495(96)90222-X [DOI] [PubMed] [Google Scholar]
- Shima K, Zhu M, Noma Y, Mizuno A, Murakami T, Sano T, Kuwajima M. Exercise training in Otsuka–Long–Evans–Tokushima Fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus: effects on the B-cell mass, insulin content and fibrosis in the pancreas. Diab. Res. Clin. Pract. 1997;35:11–19. doi: 10.1016/s0168-8227(96)01357-5. doi:10.1016/S0168-8227(96)01357-5 [DOI] [PubMed] [Google Scholar]
- Shima K, Zhu M, Mizuno A. Pathoetiology and prevention of NIDDM lessons from the OLETF rat. J. Med. Invest. 1999;46:121–129. [PubMed] [Google Scholar]
- Silver A.J, Flood J.F, Song A.M, Morley J.E. Evidence for a physiological role for CCK in the regulation of food intake in mice. Am. J. Physiol. 1989;256:R646–R652. doi: 10.1152/ajpregu.1989.256.3.R646. [DOI] [PubMed] [Google Scholar]
- Smith G.P, Gibbs J. The satiating effects of cholecystokinin and bombesin-like peptides. In: Smith G.P, editor. Satiation: from gut to brain. Oxford University Press; New York, NY: 1998. pp. 97–125. [Google Scholar]
- Smith G.T, Moran T.H, Coyle J.T, Kuhar M.J, O'Donahue T.L, McHugh P.R. Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am. J. Physiol. 1984;246:R127–R130. doi: 10.1152/ajpregu.1984.246.1.R127. [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. doi:10.1016/S0378-1119(97)00259-X [DOI] [PubMed] [Google Scholar]
- Wank S.A, Harkins R, Jensen R.T, Shapira H, de Weerth A, Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc. Natl Acad. Sci. USA. 1992;89:3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoory O, Schroeder M, Bi S, Moran T.H, Weller A. Body weight, fat distribution and accumulation in OLETF and LETO rats. Appetite. 2004;42:416. [Google Scholar]