Abstract
Anorexia is one of the most common symptoms in advanced cancer and is a frequent cause of discomfort for cancer patients and their families. The pathogenesis of cancer anorexia is multi-factorial and involves most of the hypothalamic neuronal signalling pathways modulating energy homeostasis. It is considered to be the result of a failure of usual appetite and satiety signals. Loss of appetite can arise from decreased taste and smell of food, as well as from dysfunctional hypothalamic signalling pathways and cytokine production. Cytokines in particular, appear to play a key role in energy balance through persistent activation of the melanocortin system and inhibition of the neuropeptide Y pathway. The imbalance between anorexigenic and orexigenic peptides leads to suppression of appetite, and increased satiety and satiation associated with marked weight loss and decline in physical performance. High levels of serotonin also appear to contribute to these effects and recent findings implicate corticotropin-releasing factor in the pathogenesis of cancer anorexia as well. Despite significant advances in our understanding of the regulation of food intake and energy expenditure, few effective therapies are available. A better appreciation of the molecular and neuronal mechanisms that control body weight homeostasis may lead to the development of new therapies for improving the survival and quality of life of these patients.
Keywords: anorexia, cancer, cytokines, neuropeptide, feeding
1. Introduction
Anorexia is a persistent and pathological form of satiety which is characterized by a gradual onset, a profound and persistent loss of appetite resulting in decreased food intake and leading to a progressive depletion of body energy stores. In cancer patients, the development of anorexia is frequently associated with the presence of cachexia, resulting in the cancer anorexia–cachexia syndrome (Tisdale 1997). This syndrome is observed in 80% of patients with advanced-stage cancer, and it is one of the most frequent causes of death (Mantovani et al. 2001). Weight loss has been shown to be a very powerful prognostic indicator of poor outcome and a poor quality of life. The same can be said for anorexia. Thus, a North Central Cancer Treatment Group study of patients with colorectal and lung cancer, showed that loss of appetite and appetite-related variables predicted an early demise (Dewys et al. 1980). Many patients tend to report a complete lack of hunger and amongst those patients who experience hunger, most are relieved with small amounts of oral intake. Reduced food intake may also result from multiple disease processes including dysphagia, chronic nausea, abnormal gut motility, recurrent aspiration, pain, fear and depression. In some cases, careful evaluation of the reversible causes of reduced food intake may lead to relatively straightforward symptom relief and hence improved quality of life (Strasser & Bruera 2002).
The pathogenesis of cancer anorexia is considered multi-factorial. Anorexia can arise from decreased taste and smell of food, and early satiety attributable to alterations in the central and peripheral neurohormonal signals that govern appetite and satiety (Rossi-Fanelli & Laviano 2002). In this review, the mechanisms that govern satiety and appetite in cancer patients are described.
2. Cytokines
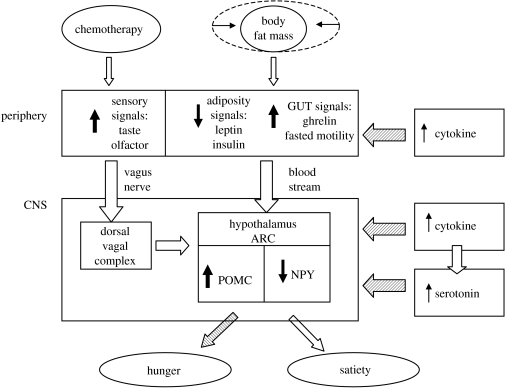
Pro-inflammatory cytokines are thought to play a key role in disturbances of satiety, and an emerging view is that the anorexia–cachexia syndrome is caused predominantly by cytokines either produced by cancer cells or released by the immune system of the host as a response to the presence of the cancer (Inui 2002). The inflammatory cytokines released by immune cells may act either in the central nervous system (CNS) to control food intake and energy homeostasis (Konsman et al. 2002) or on the gastrointestinal tract (see figure 1; Fujimiya & Inui 2000).
Figure 1.
Schematic mechanism of the regulation of the feeding in cancer patients. Reduced fat stores, gut hormones and sensory signals inform CNS about energy balance and food preferences. Pro-inflammatory cytokines and serotonin interact in the CNS inducing anorexia and satiety. In the hypothalamus, increased levels of cytokines play a role activating the anorexigenic pathways and inhibiting the orexigenic pathways. Pro-inflammatory cytokines seem to increase the serotonin concentrations, which amplify the catabolic effects of cytokines and induce satiety. The white arrows show stimulatory input whereas the dashed arrows show anorexigic input. POMC, proopiomelanocortin peptide; NPY, neuropeptide Y; ARC, arcuate nucleus of the hypothalamus; PVN, paraventricular nucleus of the hypothalamus; GUT, gastrointestinal tract; CNS, central nervous system.
(a) Cytokines and gustatory and olfactory sensation
Cytokines are implicated in changes in sensory function in the chorda tympani (involved in the transduction of taste) leading to alterations in taste and in specific food preferences. Patients with end-stage cancer have altered taste thresholds with respect to the bitter modality and these changes are most apparent in those patients with higher concentrations of C-reactive protein, interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α). In these patients, the odour threshold was also lower than in healthy subjects, suggesting that inflammatory mediators may have profound influences on dietary intake as a consequence of alterations in both gustatory and olfactory sensation (Richardson & Davidson 2003). Patients treated with chemotherapy frequently present with a decreased taste and smell of food (Beidler & Smith et al. 1991). It has been hypothesized that chemotherapic agents may interfere with the taste-receptor cell turnover in the tongue, leading to important changes in the coding of taste. These changes may be among the causative factors in the development of food avoidance-inducing anorexia (Holmes 1993). Cancer patients may also experience anorexia secondary to food aversion, which results from the central integration of negative psychological experiences and olfactory and gustatory inputs (Laviano et al. 2002).
(b) Cytokines and gut
Anorectic patients often report early satiety, such that they feel full after ingestion of a small amount of food possibly as a consequence of impaired gastric accommodation or delayed antropyloric transit (Moldawer et al. 1992; Barber et al. 1999). Analogous to the effects described above, cytokines also sensitize vagal afferent nerve fibres projecting to the gastrointestinal tract, resulting in increased activation of the mechanisms that mediate sensations of fullness and that contribute to the process of satiety (Richardson & Davidson 2003). Cytokines also induce the release of cholecystokinin (CCK), peptides derived from the glucagon precursor, insulin and leptin, all of which are hormones acting as satiety signals (Plata-Salaman 2000). Together these effects are likely to contribute to early satiety and to a reduction in the hedonic aspects of food and pleasant sensations associated with social eating (Laviano et al. 2002).
(c) Cytokines in the CNS
In cancer anorexia, cytokines are thought to play a pivotal role in the long-term inhibition of feeding both by stimulating the expression and release of leptin and by mimicking the hypothalamic effect of excessive negative feedback signalling from leptin. These actions lead to alterations in the normal compensatory mechanisms in the face of decreased food intake and body weight (Wigmore et al. 1997), and may involve inhibition of the neuropeptide Y/agouti-related peptide (NPY/AgRP) orexigenic network and persistent stimulation of the proopiomelanocortin (POMC) anorexigenic pathway as well as other neurochemical pathways (Inui 1999a).
(d) Cytokine concentration in serum
Peripheral blood leukocytes of tumour-bearing rats have been shown to secrete greater amounts of IL-1 and TNF activity in vitro than cells from healthy controls. In contrast, intracellular concentrations of IL-1, TNF and IL-6 were not increased in leucocytes of mice implanted with a tumour that did not cause weight loss. However, there is also evidence in favour of a tumour source of anorexigenic cytokines in cancer, since several tumour cell lines that induce the cancer anorexia–cachexia syndrome when implanted in rodents constitutively express IL-1, TNF and IL-6 (McCarthy 1999). Nevertheless, the association of serum cytokines with anorexia is controversial. For example, some studies have found that anorexia in untreated cancer patients did not correlate with circulating cytokines (Maltoni et al. 1997) while others have reported that concentrations of IL-1, IL-6 and TNF-α were significantly elevated in cancer patients and that the concentrations of these cytokines was correlated with tumour progression (Mantovani et al. 2000). It is important to note, however, that production or actions of cytokines within the brain may occur independently of profiles in the periphery. This may explain why some authors reported upregulation of IL-1β mRNA in brain regions (Turrin et al. 2004) while others did not confirm these findings in the development of anorexia in tumour-bearing models (Wang et al. 2003).
(e) Interleukin-1
IL-1 induces satiety and influences meal size, meal duration, and meal frequency in rats as a result of the activation of gluco-sensitive neurons in the ventromedial nucleus (VMN) of the hypothalamus (Laviano et al. 1996, 2000). In a tumour-bearing rat anorexia model, increased IL-1 concentrations in the cerebrospinal fluid were found to correlate inversely with food intake (Opara et al. 1995), while administration of an IL-1β receptor antagonist ameliorated anorexia (Laviano et al. 2000).
(f) Interleukin-6
IL-6 is produced in various cells including adipocytes and has been found to regulate leptin production (Turrin et al. 2004). Evidence for a causative role of IL-6 in the pathogenesis of anorexia and cachexia comes from experiments reporting that treatment with anti-mouse IL-6 antibody was successful in reversing the key parameters of anorexia in mice bearing adenocarcinoma (Strassmann et al. 1992; Tsujinaka et al. 1996). Elevated serum concentrations of IL-6 have been reported in cancer patients, for example, IL-6 is increased in lung cancer patients, where it plays a role in enhancing the acute phase response and is correlated with poor nutritional status, impaired performance status and shorter survival (Martin et al. 1999). Interestingly, it has been reported that IL-6 increases only gradually during the early stages of cachexia but then shows a sudden and steep rise just before death (Iwase et al. 2004). In one study, megestrol acetate reduced anorexia and improved weight, and benefits to appetite were inversely correlated with serum IL-6 concentrations; but in another study, megestrol acetate reduced anorexia independently of serum IL-6 abundance (Mantovani et al. 1998; Jatoi et al. 2002).
(g) Tumour necrosis factor
Episodic TNF administration has been reported to induce anorexia whereas the injection of a TNF-α inhibitor improved food intake in anorectic-tumour bearing rats. Since repetitive administration induced tolerance, TNF-α may play a role in the initiation of the cachectic state, but is unlikely to be solely responsible for cachexia in chronic disease (Argiles et al. 2003). Nevertheless, administration of recombinant human soluble TNF receptor in anorectic-tumour bearing rats led to an improvement in food intake with the amelioration of anorexia (Torelli et al. 1999). In part, the actions of TNF-α may include modulation of the release of leptin (see §3).
(h) Interferon (IFN)
In mice bearing Lewis lung tumours, the development of tumours is associated with IFN-γ production and with progressive weight loss. Moreover, anti-interferon γ antibodies counteract the wasting syndrome seen in cancer cachexia (Matthys et al. 1991). Because IFN-α has an anti-tumour effect, it has been used in clinical trials to increase appetite and prevent weight loss in anorectic patients, although with conflicting results (Cummins & Pruit 1999).
(i) Therapeutic options to counteract cytokines
In order to improve the therapeutic options for anorexia, several drugs are under investigation. The inhibition of the cytokine production through actions directed at transcriptional mechanisms provides a potential target for therapy. For instance, high-mobility group protein-1 (HMGB-1) is a DNA-binding protein that specifically controls the expression of a limited number of genes, including genes that regulate the production of pro-inflammatory cytokines in immune cells (Bianchi & Agresti 2005). It has been found that the intracerebroventricular administration of HMGB-1 induced an increased in TNF and IL-6 in the brain and produced taste aversion and hypophagia in mice. These effects were attenuated by the intracerebroventricular administration of anti-HMGB-1 antibodies (Agnello et al. 2002). Similarly, nuclear factor-κB (NF-κB) is a ubiquitous transcription factor that mediates the cellular response to a diverse array of stimuli, including reactive oxygen species and several cytokines, and represents an upstream component of pathways that produce catabolic cytokines. In tumour-bearing animals, the administration of a low dose of indomethacin has been shown to improve appetite and cachexia, and to reduce the activation of NF-κB and serum concentrations of TNF-α and IL-6 (Zhou et al. 2003). In addition, a new NF-κB inhibitor, dehydroxymethylepoxyquinomicin, prevents the development of cachexia in tumour bearing mice, possibly through inhibition of IL-6 secretion (Kuroda et al. 2005). Clearly, further studies are now required to evaluate the clinical effects and mechanisms of these drugs in cancer patients.
3. Leptin regulation
Leptin is a hormone that reports information on the status of energy reserves of the organism to the brain thereby regulating feeding, substrate utilization, energy balance, and the endocrine and immune systems (Machado et al. 2004). It plays an important role in triggering the adaptive response to starvation since weight loss causes leptin production to fall in proportion to the loss of body fat (Inui 2002). Leptin belongs to the gp 130 family of cytokines, it induces a strong T helper-1 lymphocyte response and is regarded as a pro-inflammatory inducer (Dixit 2004). In advanced cancer patients, the serum leptin concentrations may be depressed as a consequence of reduced body fat mass (Somasundar et al. 2004). In patients with advanced non-small-cell lung cancer, serum leptin concentrations were lower than in controls, and more so in malnourished patients despite an increase in pro-inflammatory cytokines and acute phase reactants (Aleman et al. 2002). Similar observations have been made in patients with cancer in different sites (Simons et al. 1997; Wallace et al. 1998; Mantovani et al. 2000; Brown et al. 2001). In tumour-bearing rats, leptin concentrations decreased in plasma and adipose depots four days after the injection of tumour cells. It has been suggested that leptin secretion in visceral white adipose tissue of tumour-bearing rats might be negatively modulated by TNF-α or prostaglandin E2 produced by macrophages infiltrating adipose tissue in the early stage of cachexia (Machado et al. 2004). In tumour-bearing mice, leptin production is decreased while the hypothalamic leptin-receptor and NPY expression is increased in response to fat depletion suggesting a peripheral as well as a central component in the response (Bing et al. 2001).
4. Ghrelin
Ghrelin is a 28-amino acid acylated polypeptide secreted predominantly from X/A-like enteroendocrine cells of the stomach, and was the first circulating orexigenic hormone to be identified. It exerts antagonistic effects on leptin-induced decreases in food intake through activation of the hypothalamic NPY-Y1 pathway (Inui 2001). The orexigenic effect of ghrelin is thought to be mediated in part by AgRP, and increased fat mass and hypothalamic expression of AgRP have been shown after the injection of a ghrelin agonist in mice lacking NPY (Tschop et al. 2002). Endogenous ghrelin concentrations peak before each meal and fall within 1 h of eating, thus supporting the hypothesis that ghrelin is a hormone that stimulates hunger. Fasting plasma ghrelin concentrations are inversely related to body mass index and they increase with weight loss induced by caloric restriction (Neary et al. 2004). Hanada et al. (2004) demonstrated that both ghrelin biosynthesis and secretion are stimulated in tumour-inoculated cachectic mice; moreover, ghrelin administration increased food intake in both human melanoma cell-bearing and control mice. Peripheral ghrelin administration has recently been shown to stimulate food intake in cancer patients (Neary et al. 2004) as well as in lean, healthy men and women (Laferrere et al. 2005). Since recent studies have shown increased concentrations of active ghrelin in patients with various cancer diagnoses and staging (Shimizu et al. 2003; Garcia et al. 2005), the data are compatible with the view that increases in plasma ghrelin concentrations represent a compensatory mechanism in persistent catabolic state. However, in spite of increased endogenous plasma ghrelin concentrations, cancer patients evidently show resistance to the orexigenic effects of this hormone as indicated by decreased appetite and weight loss (Zigman & Elmquist 2003). Interestingly, though, ghrelin administration to a group of cancer patients induced a 30% increase in appetite, food intake and in pleasantness of meal, suggesting that at appropriately high plasma concentrations ghrelin is able to overcome any resistance to the appetite-stimulating effects of the endogenous peptide (Neary et al. 2004). The ghrelin resistance observed in patients with cancer-induced cachexia may therefore be partial, analogous to the insulin resistance state seen in type 2 diabetes mellitus which is overcome by high doses of insulin (Garcia et al. 2005).
The elevated concentrations of ghrelin that occur in patients with cancer-induced cachexia are accompanied by increased cytokine concentrations and there are presumably complex interactions at work since ghrelin administration antagonizes the effect of cytokines on appetite and body weight (Noguchi et al. 1996; Konsman & Dantzer 2001). Thus, ghrelin suppresses IL-1β, IL-6 and TNF-α production by human T-lymphocytes and monocytes (Dixit 2004), while IL-1β (and leptin) decreased the mRNA expression of ghrelin in the stomach (Asakawa et al. 2001). It seems then, that ghrelin might function as a vital counter-regulatory signal and that the reciprocal regulatory effects of ghrelin and proinflammatory cytokines should be taken into account in developing new treatments for the anorexia-cachexia syndrome (Dixit 2004).
5. Melanocortin system
Several groups have reported that aberrant melanocortin signalling might be a contributing factor in anorexia and cachexia (Marks et al. 2001; Wisse et al. 2001). The melanocortin system is an important member of the family of catabolic central pathways as indicated by both genetic and pharmacological evidence (Inui et al. 2004). This system consists of the endogenous peptide agonists, α-melanocyte stimulating hormone (α-MSH), γ-melanocyte stimulating hormone (γ-MSH) and adrenocorticotropic hormone (ACTH) that are derived from the precursor POMC peptide, an endogenous peptide antagonist (AgRP), and five subtypes of G-protein-coupled receptors. The agonist α-MSH and the antagonist AgRP, interact at hypothalamic type 4 melanocortin receptors (MC4-R) to influence body weight (Foster et al. 2003). AgRP, is mainly expressed in the arcuate nucleus (ARC) of the hypothalamus, where it is co-localized with NPY in neurons that project to adjacent hypothalamic areas such as the paraventricular nucleus (PVN), lateral area (LHA) and dorsomedial nucleus (DMN). These neurons are therefore capable of increasing food intake via two different mechanisms, by increasing NPY signalling and by decreasing melanocortin signalling (Morton & Schwartz 2001). Despite marked loss of body weight, which would normally be expected to downregulate the anorexigenic melanocortin signalling system as a way of conserving energy stores, the melanocortin system remains active during cancer-induced cachexia (Lechan & Tatro 2001). Signalling via α-MSH is increased in tumour-bearing rats and is not decreased by intracerebroventricular ghrelin or NPY infusions. Blockade of MC4-receptors caused a much greater increase of food intake in anorectic rats than the orexigenic agents such as NPY and ghrelin. In addition, administration of a MC4-R antagonist reversed some of the negative metabolic consequences of anorexia as determined by serum leptin, ghrelin, insulin and glucose values (Wisse et al. 2003) and central MC4-R blockade by AgRP or other antagonists reversed anorexia and cachexia in animals bearing prostate carcinoma or sarcoma, strongly suggesting a pathogenic role for this system (Marks et al. 2001; Wisse et al. 2001).
The mechanisms contributing to persistent anorexigenic/cachexic activity of the central melanocortin system during cancer are unknown. Elevations of proinflammatory cytokines might explain these effects, but the sites at which cytokine signalling or tumour products interact with the melanocortin pathway to decrease food intake remain elusive, although they do not appear to involve changes in hypothalamic POMC or AgRP gene expression (Wisse et al. 2003).
6. Serotonin system
Serotonin is a potent anorectic molecule involved in the regulation of food intake and body weight. Food intake leads to increases in serotonin release in the hypothalamus promoting satiation. It has been shown that an increase in the serotonin precursor, tryptophan, occurs in the brains of tumour-bearing rats and in the plasma of cancer patients (Wang et al. 2003). Intra-hypothalamic serotonin concentrations increase in anorectic tumour-bearing rats and reverse to normal after tumour resection, in parallel with improved food intake. Amongst the many receptor subtypes through which serotonin acts, presynaptic serotonin 1A (5-HT1A) receptors, postsynaptic 5-HT1B (1D in humans) receptors and 5-HT2C receptors are all expressed in hypothalamic nuclei (VMN, PVN and ARC) responsible for the satiety-enhancing effects of serotonin (Ramos et al. 2004). Changes of 5-HT1B receptor expression are particularly pronounced in the magnocellular hypothalamic nuclei during the development of cancer anorexia, suggesting that the influence of serotonin on these nuclei may contribute to the decrease in food intake in cancer-related anorexia (Makarenko et al. 2005). Several lines of evidence suggest that serotonin is a critical link between cytokines and CNS neurotransmitters involved in control of food intake. Thus, IL-1 increases brain serotoninergic activity (Shintani et al. 1993; Laviano et al. 2000), resulting in a decrease in food intake. Fenfluramine (a serotonin agonist) raises hypothalamic serotonin concentrations, which activates POMC neurons in the ARC of the hypothalamus, inducing anorexia (Heisler et al. 2002). Moreover, Meguid et al. (2004) have shown an increase in serotonin and a decrease in dopamine concentrations in the VMN, together with a decrease of NPY in the PVN, of the hypothalamus. Taken together these findings support that serotonin plays a relevant role in the onset and maintenance of the cancer-related anorexia.
7. Neuropeptide Y system
Neuropeptide Y is a potent orexigenic neuropeptide. It is synthesized in the neurons of the ARC that project into the PVN, a major integration site for energy homeostasis. NPY evokes feeding, decreases brown adipose tissue thermogenesis and increases energy storage (Williams et al. 2000). Several studies support the hypothesis that alterations of the NPY system play a role in the pathogenesis of cancer anorexia. In the hypothalamus of anorectic-tumour bearing rats, NPY-mRNA is increased, which appears a normal response to the low energy status in this mouse model of cancer (Chance et al. 1995). However, NPY concentrations, release and receptor affinity decreased selectively in rats with anorectic-tumours, supporting the idea that the NPY-induced feeding may be suppressed in these animals (Makarenko et al. 2003; Lee et al. 2004). One hypothesis to explain dysfunctions of the NPY system in cancer-anorexia is based on the observations of a close relationship between NPY-immunoreactive nerve fibres and the hypothalamic neurons that express 5-HT1B receptors, and the fact that NPY and serotonin exert opposing effects on food intake. Recent studies showed that NPY and dopamine concentrations decreased simultaneously while serotonin concentration increased in the PVN at the onset of anorexia in tumour-bearing rats (Makarenko et al. 2005). These changes reverted to normal after tumour resection and corresponded to the reversal of anorexia, suggesting the dynamic interaction between monoamines and NPY in the regulation of food intake in cancer-anorexia as well as in normal conditions (Meguid et al. 2004).
The hypothalamic NPY system is one of the key neural pathways disrupted in anorexia induced by IL-1 and other cytokines. Administration of IL-1 directly into the cerebral ventricles antagonizes NPY-induced feeding in rats at a dose producing concentrations in the cerebrospinal fluid similar to those observed in anorectic-tumour bearing rats (Sonti et al. 1996a,b; Gayle et al. 1997). Since central administration of cytokines increases hypothalamic serotonin concentrations, it has been speculated (Laviano et al. 2005) that during tumour growth, hypothalamic cytokine expression is increased leading to changes in hypothalamic monoamine concentrations, which in turn activate POMC neurons in the ARC. As a consequence, NPY neurons in the ARC are inhibited and NPY concentrations in the PVN depressed. Recent data are consistent with this hypothesis, in so far as they indicate that inhibition of the orexigenic effect of NPY is associated with specific changes in serotonin and dopamine concentrations in the PVN (Meguid et al. 2004).
NPY has been implicated in the appetite-stimulating effects of megestrol acetate, one of the drugs presently used to treat cancer-related anorexia. The mechanisms by which megestrol acetate improves appetite are not well understood. However, one hypothesis is that megestrol acetate acts to inhibit pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α (Lopez et al. 2004). Another hypothesis is that it stimulates NPY synthesis, transport and release in the hypothalamus (McCarthy et al. 1994). Because NPY and AgRP are produced by the same neurons, agents that affect NPY synthesis and might release also influence the synthesis and release of AgRP, so that activation of AgRP-NPY neurons would promote feeding by both activating NPY receptors and antagonizing MC4 receptors. Studies on the interaction of NPY, the melanocortin system and monoamines are now urgently needed to clarify they mechanisms and so provide a basis for the development of new therapeutic approaches to anorexia (Ramos et al. 2004).
8. Corticotropin-releasing factor
Corticotropin-releasing factor (CRF), a 41 amino acid peptide, is a mediator of endocrine, autonomic and immune response in stress, including anorexia and anxiety-like behaviours (Smagin & Dunn 2000). The CRF system includes CRF itself and the related urocortins, at least two different CRF receptors subtypes, and a CRF-binding protein. It has been found that the hypothalamic CRF system is strongly activated in tumour-bearing mice and that it exerts an inhibitory effect on hypothalamic NPY signalling. In the setting of a sustained increase of CRF activity, weight loss therefore progresses without eliciting a normal compensatory response of increased NPY activity (McCarthy et al. 1993; Lee et al. 2004). In addition, it has been suggested that the CRF is involved in the delayed gastric emptying and gastric stasis in cancer patients. Thus CRF acts in the PVN and the DMN of the vagus to inhibit gastric motor function through autonomic pathways (Suto et al. 1994, 1996). These findings together suggest that CRF might play a role in the pathogenesis of cancer anorexia by modulating both central and peripheral mechanisms as part of a response to stress.
Hypothalamic CRF activity is stimulated by pro-inflammatory cytokines such as IL-1 and TNF-α both in the circulation and within the hypothalamus (Inui 1999b). Moreover, leptin exerts its effects on food intake and energy expenditure at least in part via CRF receptor-mediated pathways (Inui 1999a), while the effects of changes of nutritional status on CRF neurons require leptin probably acting directing via leptin receptors on CRF neurons in various nuclei of the hypothalamus, including ARC and PVN (Schulz & Lehnert 2004). It is also known that CRF neurons express the MC4-R and are downstream of arcuate proopiomelanocortin neurons, raising the possibility that the activation of CRF synthesis and release in cachexia is due to increased melanocortin tone (Ramos et al. 2004). Synthetic ligands for the CRF receptors are now emerging that offer new possibilities to target the hypothalamic mechanisms regulating energy balance (Richardson & Davidson 2003).
9. Other neuropeptides
Feeding is powerfully influenced by pleasure and reward. Both melanin-concentrating hormone (MCH) and the orexins are orexigenic peptides expressed in the LHA, a key area involved in feeding, arousal and motivated behaviours. It has been suggested that these neuropeptides play a regulatory role in energy homeostasis (Sakurai 2003) and may contribute to the cancer anorexia–cachexia syndrome. Central administration of orexin-A and MCH stimulate food intake and both MCH and orexins mRNA expression is increased in response to fasting. In tumour-bearing mice, MCH and orexin mRNA in the hypothalamus were said to be increased although no statistical differences were found compared with control mice (Nara-ashizawa et al. 2001a,b). Recent electrophysiological studies have shown that orexin and MCH neurons are regulated by metabolic cues, including leptin, glucose and ghrelin (Williams et al. 2001). Further studies are necessary to elucidate the role of these peptides in the cancer anorexia–cachexia syndrome.
10. Conclusions
The contribution of eating-related disorders to the declining physical, emotional and social functions of patients with cancer and their families is considerable (Strasser 2003). Current therapy with megestrol acetate improves appetite in only 20–30% of patients and does not seem to ameliorate the quality of life of patients with cachexia–anorexia syndrome (Jatoi et al. 2002), so that there is an urgent need for new and effective therapies for the prevention and treatment of this syndrome. The neurochemical mechanisms responsible for cancer anorexia are still a matter of debate. The available data are mostly based on animal studies and a major factor limiting the understanding of anorexia is the lack of knowledge about altered central appetite signals in cancer patients. It is clear, however, that multiple mediators are responsible for tumour-associated anorexia. A better understanding of these mechanisms will help with the development of new therapies to prolong survival and increase quality of life in patients with cancer-induced anorexia.
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Agnello D, Wang H, Yang H, Tracey K.J, Ghezzi P. HMGB-1, a DNA-binding protein with cytokines activity, induces brain TNF and IL-6 production, and mediates anorexia and test aversion. Cytokines. 2002;18:231–236. doi: 10.1006/cyto.2002.0890. doi:10.1006/cyto.2002.0890 [DOI] [PubMed] [Google Scholar]
- Aleman M.R, Santolaria F, Batista N, de La Vega M, Gonzales-Reimers E, Milena A, Llanos M, Gomez-Sirvent J.L. Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine. 2002;19:21–26. doi: 10.1006/cyto.2002.1051. doi:10.1006/cyto.2002.1051 [DOI] [PubMed] [Google Scholar]
- Argiles J.M, Moore-Carrasco R, Busquets S, Lopez-Soriano F.J. Catabolic mediators as targets for cancer cachexia. Drug Disc. Today. 2003;8:838–844. doi: 10.1016/s1359-6446(03)02826-5. doi:10.1016/S1359-6446(03)02826-5 [DOI] [PubMed] [Google Scholar]
- Asakawa A, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. doi:10.1053/gast.2001.22158 [DOI] [PubMed] [Google Scholar]
- Barber M.D, Ross J.A, Fearon K.C. Cancer cachexia. Surg. Oncol. 1999;8:133–141. doi: 10.1016/s0960-7404(99)00045-6. doi:10.1016/S0960-7404(99)00045-6 [DOI] [PubMed] [Google Scholar]
- Beidler L.M, Smith J.C. Effects of radiation therapy and drug on cell turnover and taste. In: Getchell T.V, et al., editors. Smell and taste in health and disease. Raven; New York, NY: 1991. pp. 753–763. [Google Scholar]
- Bianchi M.E, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. doi:10.1016/j.gde.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Bing C, Taylor S, Tisdale M.J, Williams G. Cachexia in MAC16 adenocarcinoma: suppression of hunger despite normal regulation of leptin, insulin and hypothalamic neuropeptide Y. J. Neurochem. 2001;79:1004–1012. doi: 10.1046/j.1471-4159.2001.00639.x. doi:10.1046/j.1471-4159.2001.00639.x [DOI] [PubMed] [Google Scholar]
- Brown D.R, Berkowitz D.E, Breslow M.J. Weight loss is not associated with hyperleptinemia in humans with pancreatic cancer. J. Clin. Endocrinol. Metab. 2001;86:162–166. doi: 10.1210/jcem.86.1.7104. doi:10.1210/jc.86.1.162 [DOI] [PubMed] [Google Scholar]
- Chance W.T, Balasubramaniam A, Fischer J.E. Neuropeptide Y and the development of cancer anorexia. Ann. Surg. 1995;221:579–587. doi: 10.1097/00000658-199505000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins M.J, Pruit B. Low-dose oral use of human interferon alpha in cancer patients. J. Interf. Cytok. Res. 1999;19:937–941. doi: 10.1089/107999099313488. doi:10.1089/107999099313488 [DOI] [PubMed] [Google Scholar]
- Dewys W.D, Begg C, Lavin P.T. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am. J. Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. doi:10.1016/S0149-2918(05)80001-3 [DOI] [PubMed] [Google Scholar]
- Dixit V.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 2004;114:57–66. doi: 10.1172/JCI21134. doi:10.1172/JCI200421134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A.C, et al. Body weight regulation by selective MC4 receptor agonists and antagonists. Ann. N. Y. Acad. Sci. 2003;994:103–110. doi: 10.1111/j.1749-6632.2003.tb03168.x. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2000;21:1565–1582. doi: 10.1016/s0196-9781(00)00313-2. doi:10.1016/S0196-9781(00)00313-2 [DOI] [PubMed] [Google Scholar]
- Garcia J.M, Garcia-Touza M, Hijazi R.A, Taffet G, Epner D, Mann D, Smith R.G, Cunningham G.R, Marcelli M. Active ghrelin levels and active/total ghrelin ratio in cancer-induced cachexia. J. Clin. Endocrinol. Metab. 2005;90:2920–2926. doi: 10.1210/jc.2004-1788. doi:10.1210/jc.2004-1788 [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin S.E, Plata-Salaman C.R. Central nervous system IL-1β system and neuropeptide Y mRNA during IL-1β -induced anorexia in rats. Brain Res. Bull. 1997;44:311–317. doi: 10.1016/s0361-9230(97)00159-7. doi:10.1016/S0361-9230(97)00159-7 [DOI] [PubMed] [Google Scholar]
- Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, Kangawa K, Nakazato M. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53:84–88. doi: 10.1016/j.metabol.2003.06.004. doi:10.1016/j.metabol.2003.06.004 [DOI] [PubMed] [Google Scholar]
- Heisler L.K, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. doi:10.1126/science.1072327 [DOI] [PubMed] [Google Scholar]
- Holmes S. Food avoidance in patients undergoing cancer chemotherapy. Support Care Cancer. 1993;1:326–330. doi: 10.1007/BF00364971. doi:10.1007/BF00364971 [DOI] [PubMed] [Google Scholar]
- Inui A. Cancer anorexia–cachexia syndrome: are neuropeptides the key? Cancer Res. 1999a;59:4493–4501. [PubMed] [Google Scholar]
- Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides-mediation of the actions of leptin. Trends Neurosci. 1999b;22:62–67. doi: 10.1016/s0166-2236(98)01292-2. doi:10.1016/S0166-2236(98)01292-2 [DOI] [PubMed] [Google Scholar]
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2001;2:551–560. doi: 10.1038/35086018. doi:10.1038/35086018 [DOI] [PubMed] [Google Scholar]
- Inui A. Cancer Anorexia–Cachexia Syndrome: current issues in research and management. CA Cancer J. Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- Inui A, Asakawa A, Bowers C.Y, Mantovani G, Laviano A, Meguid M.M, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–456. doi: 10.1096/fj.03-0641rev. doi:10.1096/fj.03-0641rev [DOI] [PubMed] [Google Scholar]
- Iwase S, Murakami T, Satio Y, Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patients. Eur. Cytokine Netw. 2004;15:312–316. [PubMed] [Google Scholar]
- Jatoi A, Yamashita J, Slotan J.A, Novotny P.J, Windschitl H.E, Loprinzi C.L. Does megastrol acetate down-regulate interleukin-6 in patients with cancer-associated anorexia and weight loss? A North Central Cancer Treatment Group Investigation. Support Care Cancer. 2002;10:71–75. doi: 10.1007/s00520-001-0310-7. doi:10.1007/s00520-001-0310-7 [DOI] [PubMed] [Google Scholar]
- Konsman J.P, Dantzer R. How the immune and nervous systems interact during disease-associated anorexia. Nutrition. 2001;17:664–668. doi: 10.1016/s0899-9007(01)00602-5. doi:10.1016/S0899-9007(01)00602-5 [DOI] [PubMed] [Google Scholar]
- Konsman J.P, Parnet P, Dantzer R. Cytokine induced sickness behavior: mechanisms and implications. Trend Neurosci. 2002;143:154–159. doi: 10.1016/s0166-2236(00)02088-9. doi:10.1016/S0166-2236(00)02088-9 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Horiguchi Y, Nakashima J, Kikuchi E, Kanao K, Miyajima A, Ohigashi T, Umezawa K, Murai M. Prevention of cancer cachexia by a novel nuclear factor κB inhibitor in prostate cancer. Clin. Cancer Res. 2005;11:5590–5594. doi: 10.1158/1078-0432.CCR-04-2561. doi:10.1158/1078-0432.CCR-04-2561 [DOI] [PubMed] [Google Scholar]
- Laferrere B, Abraham C, Russell C.D, Bowers C.Y. Growth Hormone Releasing Peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. J. Clin. Endocrinol. Metab. 2005;90:611–614. doi: 10.1210/jc.2004-1719. doi:10.1210/jc.2004-1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviano A, Meguid M.M, Yang Z.J, Gleason J.R, Cangiano C, Rossi-Fanelli F. Cracking the riddle of cancer anorexia. Nutrition. 1996;12:706–710. doi: 10.1016/s0899-9007(96)00164-5. [DOI] [PubMed] [Google Scholar]
- Laviano A, Gleason J.R, Meguid M.M, Yang Z.J, Cangiano C, Rossi-Fanelli F. Effects of intra-VMN mianserin and IL-1ra on meal number in anorectic tumor-bearing rats. J. Invest. Med. 2000;48:40–48. [PubMed] [Google Scholar]
- Laviano A, Russo M, Freda F, Rossi-Fanelli F. Neurochemical mechanisms for cancer anorexia. Nutrition. 2002;18:100–105. doi: 10.1016/s0899-9007(01)00727-4. doi:10.1016/S0899-9007(01)00727-4 [DOI] [PubMed] [Google Scholar]
- Laviano A, Meguid M.M, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome—when all you can eat is yourself. Nature Clin. Pract. Oncol. 2005;2:158–165. doi: 10.1038/ncponc0112. doi:10.1038/ncponc0112 [DOI] [PubMed] [Google Scholar]
- Lechan R.M, Tatro J.B. Hypothalamic melanocortin signaling in cachexia. Endocrinology. 2001;142:3288–3291. doi: 10.1210/endo.142.8.8408. doi:10.1210/en.142.8.3288 [DOI] [PubMed] [Google Scholar]
- Lee J.H, Cha M.J, Choi S.H, Hwang S.J, Kim D.G, Jahng J.W. Neuropeptide Y immunoreactivity and corticotropin-releasing hormone mRNA level are increased in the hypothalamus of mouse bearing a human oral squamous cell carcinoma. Neuropeptides. 2004;38:345–350. doi: 10.1016/j.npep.2004.07.004. doi:10.1016/j.npep.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Lopez A.P, Filgus R.M, Cuchi U.G, Berestein E.G, Pasies A.B, Alegre B.M, Herdman M. Systematic review of megestrol acetate in the treatment of anorexia–cachexia syndrome. J. Pain Symptom Manag. 2004;27:360–369. doi: 10.1016/j.jpainsymman.2003.09.007. doi:10.1016/j.jpainsymman.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Machado A.P, Costa Rosa L.F.P.B, Seelaender M.C.L. Adipose tissue in Walker 256 tumour-induced cachexia: possible association between decreased leptin concentration and mononuclear cell infiltration. Cell Tissue Res. 2004;318:503–514. doi: 10.1007/s00441-004-0987-2. doi:10.1007/s00441-004-0987-2 [DOI] [PubMed] [Google Scholar]
- Makarenko I.G, Meguid M.M, Gatto L, Chen C, Ugrumov M.V. Decreased NPY innervation of the hypothalamic nuclei in rats with cancer anorexia. Brain Res. 2003;961:100–108. doi: 10.1016/s0006-8993(02)03850-7. doi:10.1016/S0006-8993(02)03850-7 [DOI] [PubMed] [Google Scholar]
- Makarenko I.G, Meguid M.M, Gatto L, Goncalves C.G, Ramos E.J, Chen C, Ugrumov M.V. Hypothalamic 5-HT1B-receptor changes in anorectic bearing rats. Neurosci. Lett. 2005;376:71–75. doi: 10.1016/j.neulet.2004.11.026. doi:10.1016/j.neulet.2004.11.026 [DOI] [PubMed] [Google Scholar]
- Maltoni M, et al. Serum levels of tumor necrosis factor alpha and other cytokines do not correlate with weight loss and anorexia in cancer patients. Support Care Cancer. 1997;5:130–135. doi: 10.1007/BF01262570. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona G. Cytokine involvement in cancer-related anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Semin. Oncol. 1998;9:99–106. doi: 10.1615/critrevoncog.v9.i2.10. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Mura L, Massa E, Mudu M.C, Mulas C, Lusso M.R, Madeddu C, Dessi A. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J. Mol. Med. 2000;78:554–561. doi: 10.1007/s001090000137. doi:10.1007/s001090000137 [DOI] [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Massa E, Madeddu C. Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499–514. doi: 10.2165/00003495-200161040-00004. doi:10.2165/00003495-200161040-00004 [DOI] [PubMed] [Google Scholar]
- Marks D.L, Ling N, Cone R.D. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- Martin F, Santolaria F, Battista N. Citokine levels (IL-6 and IFN-gamma) acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11:80–86. doi: 10.1006/cyto.1998.0398. [DOI] [PubMed] [Google Scholar]
- Matthys P, Heremans H, Opdenakker G, Billiau A. Anti-interferon-gamma antibody treatment growth of Lewis lung tumors in mice and tumor-associated cachexia. Eur. J. Cancer. 1991;27:182–187. doi: 10.1016/0277-5379(91)90483-t. [DOI] [PubMed] [Google Scholar]
- McCarthy H.D, McKibbin P.E, Perkins A.V, Linton E.A, Williams G. Alterations in hypothalamic NPY and CRF in anorectic tumor bearing rats. Am. J. Physiol. 1993;264:E638–E643. doi: 10.1152/ajpendo.1993.264.4.E638. [DOI] [PubMed] [Google Scholar]
- McCarthy H.D, Crowder R.E, Dryden S, Williams G. Megestrol acetate stimulates food intake and water intake in the rat: effects on regional hypothalamic neuropeptide Y concentrations. Eur. J. Pharmacol. 1994;256:99–102. doi: 10.1016/0014-2999(94)90229-1. doi:10.1016/0014-2999(94)90229-1 [DOI] [PubMed] [Google Scholar]
- McCarthy D.O. Inhibitors of prostaglandin synthesis do not improve food intake or body weight of tumor-bearing rats. Res. Nurs. Health. 1999;22:380–387. doi: 10.1002/(sici)1098-240x(199910)22:5<380::aid-nur4>3.0.co;2-1. doi:10.1002/(SICI)1098-240X(199910)22:5<380::AID-NUR4>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Meguid M.M, Ramos E.J, Laviano A, Varma M, Sato T, Chen C, Qi Y, Das U.N. Tumor anorexia: effects on neuropeptide Y and monoamines in paraventricular nucleus. Peptides. 2004;25:261–266. doi: 10.1016/j.peptides.2004.01.012. doi:10.1016/j.peptides.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Moldawer L.L, Rogy M.A, Lowry S.F. The role of cytokines in cancer anorexia. J. Paraenter. Enteral. Nutr. 1992;16:43S–49S. doi: 10.1177/014860719201600602. [DOI] [PubMed] [Google Scholar]
- Morton G.J, Schwartz M.W. The NPY/AgRP neuron and energy homeostasis. Int. J. Obes. 2001;25:S56–S62. doi: 10.1038/sj.ijo.0801915. doi:10.1038/sj/ijo/0801915 [DOI] [PubMed] [Google Scholar]
- Nara-ashizawa N, Tsukada T, Maruyama K, Akiyama Y, Kajimura N, Nagasaki K, Iwanaga T, Yamaguchi K. Hypothalamic appetite-regulating neuropeptide mRNA levels in cachectic nude mice bearing human tumor cells. Metabolism. 2001a;50:1213–1219. doi: 10.1053/meta.2001.26706. doi:10.1053/meta.2001.26706 [DOI] [PubMed] [Google Scholar]
- Nara-ashizawa N, Tsukada T, Maruyama K, Akiyama Y, Kajimura N, Yamaguchi K. Response of hypothalamic NPY mRNAs to a negative energy balance is les sensitive in cachectic mice bearing human tumor cells. Nutr. Cancer. 2001b;41:111–118. doi: 10.1080/01635581.2001.9680621. doi:10.1207/S15327914NC41-1&2_16 [DOI] [PubMed] [Google Scholar]
- Neary N.M, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. doi:10.1210/jc.2003-031768 [DOI] [PubMed] [Google Scholar]
- Noguchi Y, Yoshikawa T, Matsumoto A, Svaninger G, Gelin J. Are cytokines possible mediators of cancer cachexia? Surg. Today. 1996;26:467–475. doi: 10.1007/BF00311551. doi:10.1007/BF00311551 [DOI] [PubMed] [Google Scholar]
- Opara E.I, Laviano A, Meguid M.M, Yang Z.J. Correlation between food intake and CSF IL-1 alpha in anorectic tumorbearing rats. Neuroreport. 1995;6:750–752. doi: 10.1097/00001756-199503270-00011. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R. Central nervous system mechanisms contributing to the cachexia–anorexia syndrome. Nutrition. 2000;16:1009–1012. doi: 10.1016/s0899-9007(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Ramos E.J, Suzuki S, Marks D, Inui A, Asakawa A, Meguid M.M. Cancer anorexia–cachexia syndrome: cytokines and neuropeptides. Curr. Opin. Clin. Nutr. Care. 2004;7:427–434. doi: 10.1097/01.mco.0000134363.53782.cb. doi:10.1097/01.mco.0000134363.53782.cb [DOI] [PubMed] [Google Scholar]
- Richardson R.A, Davidson H.I.M. Nutritional demands in acute and chronic illness. Proc. Nutr. Soc. 2003;62:777–781. doi: 10.1079/PNS2003302. doi:10.1079/PNS2003302 [DOI] [PubMed] [Google Scholar]
- Rossi-Fanelli F, Laviano A. Cancer anorexia: a model for the understanding and treatment of secondary anorexia. Int. J. Cardiol. 2002;85:67–72. doi: 10.1016/s0167-5273(02)00234-6. doi:10.1016/S0167-5273(02)00234-6 [DOI] [PubMed] [Google Scholar]
- Sakurai T. Orexin: a link between energy homeostasis and adaptive behaviour. Curr. Opin. Clin. Nutr. Care. 2003;6:353–360. doi: 10.1097/01.mco.0000078995.96795.91. doi:10.1097/00075197-200307000-00001 [DOI] [PubMed] [Google Scholar]
- Schulz C, Lehnert H. Central nervous and metabolic effects of intranasally applied leptin. Endocrinology. 2004;145:2696–2701. doi: 10.1210/en.2003-1431. doi:10.1210/en.2003-1431 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- Shintani F, Kanba S, Nakaki T, Nibuya M, Kinishita N, Suzuki E, Yagi G, Kato R, Asai M. Interleukin-1beta augments release of norepinephrine, dopamine and serotonin in the rat anterior hypothalamus. J. Neurosci. 1993;7:3574–3581. doi: 10.1523/JNEUROSCI.13-08-03574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.P, Schools A.M, Campfield L.A, Wounters E.F, Saris W.H. Plasma concentration of total leptin and human lung-cancer-associated cachexia. Clin. Sci. (Lond.) 1997;93:273–277. doi: 10.1042/cs0930273. [DOI] [PubMed] [Google Scholar]
- Smagin G.N, Dunn A.J. The role of CRF receptor subtypes in stress-induced behaviour responses. Eur. J. Pharmacol. 2000;405:199–206. doi: 10.1016/s0014-2999(00)00553-7. doi:10.1016/S0014-2999(00)00553-7 [DOI] [PubMed] [Google Scholar]
- Somasundar P, McFadden D.W, Hileman S.M, Vons-Davis L. Leptin is a growth factor in cancer. J. Surg. Res. 2004;116:337–349. doi: 10.1016/j.jss.2003.09.004. doi:10.1016/j.jss.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin S.E, Plata-Salaman C.R. Neuropeptide Y blocks and reveres interleukin-1β-induced anorexia in rats. Peptides. 1996a;17:517–520. doi: 10.1016/0196-9781(96)00016-2. doi:10.1016/0196-9781(96)00016-2 [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin S.E, Plata-Salaman C.R. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 1996b;270:1349–1402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- Strasser F. Eating-related disorders in patients with advanced cancer. Support Care Cancer. 2003;11:11–20. doi: 10.1007/s00520-002-0391-y. [DOI] [PubMed] [Google Scholar]
- Strasser F, Bruera E.D. Update on anorexia and cachexia. Hematol. Oncol. Clin. North Am. 2002;16:589–617. doi: 10.1016/s0889-8588(02)00011-4. doi:10.1016/S0889-8588(02)00011-4 [DOI] [PubMed] [Google Scholar]
- Strassmann G, Jacob C.O, Evans R. Mechanisms of experimental cancer cachexia. Interaction between mononuclear phagocytes and colon-26 carcinoma and its relevance to IL-6 mediated cancer cachexia. J. Immunol. 1992;148:3674–3678. [PubMed] [Google Scholar]
- Suto G, Kiraly A, Tache Y. Interleukin-1-beta inhibits gastric emptying in rats: mediation through prostaglandin and corticotropin-releasing factor. Gastroenterology. 1994;106:1568–1575. doi: 10.1016/0016-5085(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Suto G, Kiraly A, Plourde V, Tache Y. Intravenous interleukin-1-beta induced inhibition of gastric emptying: involvement of central corticotropin-releasing factor and prostaglandin pathways in rats. Digestion. 1996;57:135–140. doi: 10.1159/000201326. [DOI] [PubMed] [Google Scholar]
- Tisdale M.J. Biology of cachexia. J. Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. doi:10.1093/jnci/89.23.1763 [DOI] [PubMed] [Google Scholar]
- Torelli G.F, Meguid M.M, Moldawer L.L. Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;277:R850–R855. doi: 10.1152/ajpregu.1999.277.3.R850. [DOI] [PubMed] [Google Scholar]
- Tschop M, Statnick M.A, Suter T.M, Heiman M.L. GH-Releasing Peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143:558–568. doi: 10.1210/endo.143.2.8633. doi:10.1210/en.143.2.558 [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Fujita J, Ebisui C. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J. Clin. Invest. 1996;97:244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin N.P, Ilyin S.E, Gayle D.A, Plata-Salaman C.R, Ramos E.J, Laviano A, Das U.N, Inui A, Meguid M.M. Interleukin-1beta system in anorectic catabolic tumor-bearing rats. Curr. Opin. Clin. Metab. Care. 2004;7:419–426. doi: 10.1097/01.mco.0000134373.16557.92. doi:10.1097/01.mco.0000134373.16557.92 [DOI] [PubMed] [Google Scholar]
- Wallace A.M, Sattar N, McMillan D.C. Effect of weight loss and the inflammatory response on leptin concentrations in gastrointestinal cancer patients. Clin. Cancer Res. 1998;4:2977–2979. [PubMed] [Google Scholar]
- Wang W, Danielsson A, Svanberg E, Lundholm K. Lack of effects by tricyclic antidepressant and serotonin inhibitors on anorexia in MCG 101 tumor-bearing mice with eicosanoid-related cachexia. Nutrition. 2003;19:47–53. doi: 10.1016/s0899-9007(02)00921-8. doi:10.1016/S0899-9007(02)00921-8 [DOI] [PubMed] [Google Scholar]
- Wigmore S.J, Plester C.F, Ross J.A, Featon K.C. Contribution of anorexia and hypermetabolism to weight loss in anicteric patients with pancreatic cancer. Br. J. Surg. 1997;84:196–197. doi:10.1046/j.1365-2168.1997.02525.x [PubMed] [Google Scholar]
- Williams G, Harrold J.A, Cutler D.J. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc. Nutr. Soc. 2000;59:385–396. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- Williams G, Bing C, Cai X.J, Harrold J.A, King P.J, Liu X.H. The hypothalamus and the control of energy homeostasis. Different circuits, different purposes. Physiol. Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. doi:10.1016/S0031-9384(01)00612-6 [DOI] [PubMed] [Google Scholar]
- Wisse B.E, Frayo R.S, Schwartz M.W, Cummings D.E. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. doi:10.1210/en.142.8.3292 [DOI] [PubMed] [Google Scholar]
- Wisse B.E, Schwartz M.W, Cummings D.E. Melanocortin signaling and anorexia in chronic disease states. Ann. N. Y. Acad. Sci. 2003;994:275–281. doi: 10.1111/j.1749-6632.2003.tb03190.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Jiang Z.-W, Tian J, Jiang J, Li N, Li J.-S. Role of NF-kB and cytokine in experimental cancer cachexia. World J. Gastroenterol. 2003;9:1567–1570. doi: 10.3748/wjg.v9.i7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman J.M, Elmquist J.K. Minireview: from anorexia to obesity—the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. doi:10.1210/en.2003-0241 [DOI] [PubMed] [Google Scholar]