Abstract
Several sex differences in eating, their control by gonadal steroid hormones and their peripheral and central mediating mechanisms are reviewed. Adult female rats and mice as well as women eat less during the peri-ovulatory phase of the ovarian cycle (estrus in rats and mice) than other phases, an effect under the control of cyclic changes in estradiol secretion. Women also appear to eat more sweets during the luteal phase of the cycle than other phases, possibly due to simultaneous increases in estradiol and progesterone. In rats and mice, gonadectomy reveals further sex differences: orchiectomy decreases food intake by decreasing meal frequency and ovariectomy increases food intake by increasing meal size. These changes are reversed by testosterone and estradiol treatment, respectively. A variety of peripheral feedback controls of eating, including ghrelin, cholecystokinin (CCK), glucagon, hepatic fatty acid oxidation, insulin and leptin, has been shown to be estradiol-sensitive under at least some conditions and may mediate the estrogenic inhibition of eating. Of these, most progress has been made in the case of CCK. Neurons expressing estrogen receptor-α in the nucleus tractus solitarius of the brainstem appear to increase their sensitivity to CCK-induced vagal afferent input so as to lead to an increase in the satiating potency of CCK, and consequently decreased food intake, during the peri-ovulatory period in rats. Central serotonergic mechanisms also appear to be part of the effect of estradiol on eating. The physiological roles of other peripheral feedback controls of eating and their central mediators remain to be established.
Keywords: food intake, obesity, eating disorders, bulimia nervosa, 5-hydroxytryptamine, estrogen
1. Introduction
Gonadal steroid hormones (GSH; abbreviations are listed in the glossary), i.e. the androgens, estrogens and progestins, are pluripotent signalling molecules with a diverse variety of biological actions, many of which bear no clear relationship to their primary reproductive functions within the HPG axis (Pfaff et al. 2002). This article reviews one such ancillary action, the modulation of eating by GSH. As is the case for most GSH functions, the effects of GSH on eating are species specific. Therefore, we focus our review on rats and mice, the animals for which the most data are available, and discuss human data wherever possible.
Especially in rodents, GSH have many actions that may affect body weight and adiposity independent of eating, including effects on energy expenditure, gastrointestinal function, metabolism, growth and body composition (Chen et al. 1995; Tarnopolsky 1999; Cortwright & Koves 2000; Wajchenberg 2000; Blaak 2001; Heymsfeld et al. 2002; Clegg et al. 2003; Bruns & Kemnitz 2004; Williams 2004). The mechanisms underlying these effects and their relations to eating are not well understood (Woods et al. 2003; Geary 2004a,b). Therefore, we limit our review to GSH effects on eating per se.
The review has three sections. First, we describe the major sex differences in eating in adults and their dependence on secretion of GSH. This section begins with a brief overview of some relevant aspects of HPG axis function. Second, we review progress in understanding how E2 interacts with the controls of normal eating behaviour to produce sex differences in eating. We do not consider the mechanisms through which T increases eating because almost nothing is known about them. Finally, we describe some developmental aspects of sex differences in eating.
2. The hypothalamic–pituitary–gonadal axis and eating
(a) Hypothalamic–pituitary–gonadal axis function
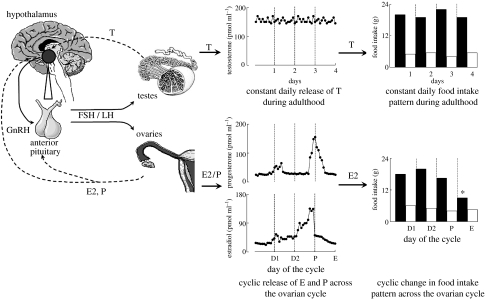
The HPG axis exerts its effects through a three-level hormonal hierarchy consisting of (i) the hypothalamic releasing hormone GnRH (or LHRH), which is synthesized in neuronal cell bodies located primarily in the arcuate nucleus and preoptic area, (ii) the anterior pituitary hormones FSH and LH and (iii) the gonadal hormones, which include both steroid (such as T, E2 and P) and peptide hormones (such as inhibin). T, E2 and P act on numerous target tissues. Each also provides positive and negative feedback controls of HPG function via receptors in the hypothalamus and pituitary (Ross et al. 1970; Freeman 1994; Hotchkiss & Knobil 1994; Griffin & Ojeda 2000; Bulun & Adashi 2003; Becker et al. 2005). The left-hand side of figure 1 schematizes this system.
Figure 1.
Schematic depicting HPG axis control of food intake in adult male and female rats and mice. In both sexes, GnRH is synthesized in the medial preoptic area and the arcuate nucleus of the hypothalamus and transported via the portal system to the anterior pituitary where it acts on the gonadotrophs to release FSH and LH, which, in turn, control the secretion of E2, P from the ovaries and T from the testes. Positive and negative feedbacks (dashed lines) between gonads, pituitary and the hypothalamus maintain normal HPG axis function. In adult males (upper panels), constant daily T release results in a constant level of daily food intake (the circadian rhythm is controlled separately). In females (lower panels), E and P are released cyclically. E, but not P, produces the cyclic variation in food intake, with a nadir during the peri-ovulatory phase of the cycle, the night of estrus. See text for details. Dashed vertical lines in middle and right panels denote 24 hour periods; open and filled columns in right panels denote light and dark phases of the 24 hour period, respectively. D1, diestrus 1; D2, diestrus 2; P, proestrus; E, estrus. *Nocturnal food intake typically less during E than other cycle days.
In adult females, GnRH is secreted in a pulsatile pattern into the hypophyseal portal circulation, stimulating FSH and LH secretion directly and E2 secretion indirectly. During the pre-ovulatory phase of the ovarian cycle (diestrus and proestrus in rats and mice, the follicular phase in women), E2 exerts mainly positive feedback effects, causing progressive increases in the frequency and magnitude of GnRH and LH pulses. This culminates in a surge of FSH and LH that initiates ovulation, which occurs after about 10–12 h after the peak of the LH surge in both rats and women. The pre-ovulatory increases in E2 as well as the increase in P secretion that begin at the time of the LH surge are necessary for the estrous increase in sexual receptivity in rats and mice.
Many aspects of ovarian cycles are species specific, which complicates rat and mouse models of human function. In addition to the very different durations of the rodent and human cycles, the immediate post-ovulatory phase of the cycle is very different in women versus rats and mice. In women, both E and P secretion are increased during most of the luteal phase, which last about 10 days, and, as described below, eating may change during the luteal phase. There is no close counterpart to this in rats and mice. Rather, the corpora lutea are formed at various points in the cycle and last across cycles. The final phase of the cycle, metestrus in rats and mice and the menstrual phase in women, are relatively similar from the perspective of decreased GSH secretion, although rodents do not menstruate. Furthermore, metestrus lasts only 6–8 h in rats and mice and occurs diurnally, when these animals eat very little. Thus, rats and mice are poor models for eating during the post-ovulatory phases of the human cycle.
In the male, T secretion is under the same hierarchical HPG axis control as in the female, including homologous positive and negative feedback mechanisms. GnRH and LH pulses remain constant in males, however, resulting in almost constant plasma levels of T in adults (see figure 1).
(b) Sex differences in eating
There are few overt sex differences in eating. Meal size and meal frequency are similar between males and females and in both humans and rats, and total food intake appears to vary roughly in proportion to body size. Population estimates of body size suggest both sexes tend to match total intake to energy expenditure similarly, but imprecisely, so that body adiposity increases with age in both sexes. Women display higher rates of morbid obesity (BMI>40) than men (Flegal et al. 2002; Freedman et al. 2002), but whether this has a physiological cause is unknown.
(i) Peri-ovulatory decrease in eating
The one clear phenotypic sex difference in eating in humans, rats and mice is that cycling females eat less during the peri-ovulatory phase of the ovarian cycle (figure 1; reviewed in Geary 2004a,b). Rats and mice have 4–5 days ovarian cycles, and food intake is up to 25% less during the night following the LH surge (the night of ovulation and estrus) in comparison to the other nights of the cycle (about 80–90% of food intake occurs nocturnally in rats and mice). The plasma concentration of E2 is highest just before the LH surge and is very low during estrus. Nevertheless, because activated ER stimulates transcription factors, so that most physiological effects of E2 have a latency of 12 h or more, it is entirely plausible that the estrous decrease in food intake in rats and mice is caused by the preceding increase in E2 secretion. The cyclic decrease in food intake is due to a decrease in the size of spontaneous meals; meal number actually increases (Drewett 1974; Blaustein & Wade 1976; Asarian & Geary 2002).
Eating also varies during the menstrual cycle in women. Daily food intake in women is lowest during the peri-ovulatory period, which is usually defined as the 4 days surrounding the LH surge (Lissner et al. 1988; Gong et al. 1989; Lyons et al. 1989; Buffenstein et al. 1995). Plasma E2 levels are maximal during this phase of the menstrual cycle. Some studies also demonstrate that average daily food intake is lower during the follicular phase, during which E2 secretion increases, than during the luteal phase (Lissner et al. 1988; Lyons et al. 1989; Buffenstein et al. 1995; Pelkman et al. 2001). Both E2 and P are elevated through much of the luteal phase. Taken together, the data suggest that women may eat about 10% less during one-third to one-half of the cycle, an amount more than sufficient to affect energy balance. The cyclic change in eating apparently does not occur during anovulatory cycles (Barr et al. 1995; Rock et al. 1996). It can also be suppressed in women whose eating behaviour is under strong cognitive restraint (Dye & Blundell 1997). Whether meal size or number is affected is unknown.
(ii) Gonadectomy and eating
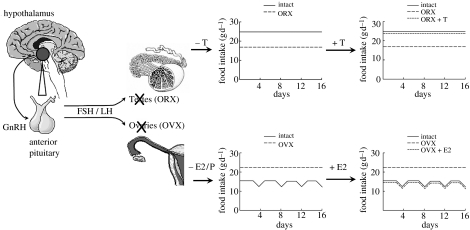
Gonadectomy unveils further sex differences in the control of eating in rats and mice: gonadectomized females eat more, whereas gonadectomized males eat less (Drewett 1973; Gentry & Wade 1976; Chai et al. 1999; Wallen et al. 2001; figure 2). Interestingly, the two changes in food intake are expressed differently—as decreased meal frequency in males and increased meal size in females (Blaustein & Wade 1976; Chai et al. 1999; Asarian & Geary 2002). Gonadectomy also eliminates the cyclic decrease in eating in females. Drewett (1973) pointed out that this suggest that the normal HPG influence on eating involves two functional components, a tonic inhibition revealed by the increased basal level of eating after ovariectomy and a phasic or cyclic inhibition revealed by the absence of the peri-ovulatory decrease in eating. As reviewed below, it appears that at least partially independent physiological mechanisms control the tonic and phasic effects, in that cholecystokinin (CCK) appears to contribute to the latter but not to the former. There are no data on the effects of gonadectomy on eating in women, and menopause does not provide a natural experiment for several reasons (see Geary 2004a,b).
Figure 2.
Schematic depicting the effects of gonadectomy and E2 or T treatment on food intake in adult male and female rats and mice. In males (upper panels), orchidectomy (ORX; medium-dash lines in centre and right panels) results in a decrease in daily food intake, and T treatment (ORX+T; short-dashed line) reverses this effect. In females (lower panels), ovariectomy (OVX; medium-dashed lines in centre and right panels) eliminates cycling and increases daily food intake, and cyclic E2 treatment (OVX+E2; short dashed line) reverses these effects. P treatment has no effect. See text for details.
The effects of gonadectomy on eating in rats and mice are apparently due to T in males (Chai et al. 1999; Wallen et al. 2001) and E2 in females (Wade 1972; Blaustein et al. 1976; Geary 2004a,b; figure 2, lower). Asarian & Geary (2002) demonstrated that a 4-day cyclic regimen of E2 was sufficient to produce apparently normal cyclic patterns of spontaneous meal size and number and daily food intake in rats. P has no effect on eating except in pharmacological doses in rats (Wade 1975), and even a pharmacological dose had no effect on eating in women (Pelkman et al. 2001). Pharmacological doses of P can reverse the inhibitory effect of E2 on eating in rats (Wade 1975). If the same is true of women, it may explain the lack of effect on eating of mixed hormonal contraceptive treatments (Eck et al. 1997; Rosenberg 1998). Levels of GnRH, LH, FSH and prolactin do not correlate with eating in female rats; rather, they increase both when eating decreases in the peri-ovulatory period and when eating increases after ovariectomy. Furthermore, elimination of LH, FSH and prolactin by hypophysectomy did not increase eating in female rats (and did not eliminate the inhibitory effect of E2 on eating; Wade & Zucker 1970a,b).
(iii) Nutrient selection
Cyclic changes in food intake do not appear attributable to differing food selection. One report that macronutrient selection varied during the estrous cycle in rats (Bartness & Waldbillig 1984) has not been confirmed in other studies (Geiselman et al. 1981; Liebowitz et al. 1998; Heisler et al. 1999). In women, there is little evidence for physiologically-based cyclic variations in consumption of particular foods or in macronutrient selection (Buffenstein et al. 1995; Drewnowski 1997; Dye & Blundell 1997; Rogers & Smit 2000; Bowen et al. 2003; Geary 2004a,b; Zellner et al. 2004).
3. Mechanisms mediating hypothalamic–pituitary–gonadal axis effects on eating
(a) Estrogen receptor mechanisms
(i) Estrogen receptor type
Two nuclear ER have been identified, ERα and ERβ, and mice null for each gene (knockouts) have been produced (Lubahn et al. 1993; Krege et al. 1998). E2 treatment decreased food intake and weight gain in ovariectomized wild type and ERβ knockout mice similarly, but had no effect in ERα knockout mice, suggesting that E2's eating effects are mediated by ERα (Geary et al. 2001; Geary 2004a,b). Similarly, increased adiposity has been reported to occur in premenopausal women who have a polymorphism of the ERα gene, but not in postmenopausal women or in men (Speer et al. 2001; Okura et al. 2003).
(ii) Brain site
E2 appears to affect eating via stimulation of brain ER (Geary 2004a,b; Rivera et al. 2005), although indirect effects mediated by peripheral GSH receptors have not been fully excluded. The specific site(s) of the critical ER are unknown. One group (Butera & Beikirch 1989; Butera et al. 1992, 1996) reported that implants of dilute E2 into the paraventricular nucleus of the hypothalamus was sufficient to decrease eating in ovariectomized rats and that peripherally administered E2 did not inhibit eating after paraventricular lesions, but others were not able to replicate these effects (Dagnault & Richard 1994; Hrupka et al. 2002). We (Thammacharoen et al. in press; see §3b(iii)) have found that administration of dilute E2 to the caudal brainstem is sufficient to decrease eating. Other reports of decreased eating after E2 administration directly into the brain used doses that were too large to have localizing value (reviewed in Geary 2004a,b).
(b) Hypothalamic–pituitary–gonadal function and interactions with peripheral feedback signals controlling eating
The most fruitful investigations of the mechanisms of E2's inhibitory effect on eating are based on the hypothesis that E2 acts in the brain to alter neural processing of peripheral feedback signals that control eating. There are many hypothesized, although few physiologically proven, peripheral feedback controls of eating that, like E2, affect meal size (Smith 1998; Geary 2004a,b; Geary & Schwartz 2005). These include (i) orosensory signals that increase or decrease food reward and control meal size by affecting the maintenance of eating during the meal, (ii) ‘satiation signals’ arising from postingestive actions of food in the stomach, intestines or postabsorptive sites (e.g. CCK and glucagon), that are initiated during the meal and act to decrease meal size, and (iii) ‘adiposity signals’ (e.g. insulin and leptin) that are generated independent of ongoing eating and provide tonic restraints limiting meal size. Information about sexual differentiation available for these and other signals is reviewed here. These data indicate that E2 affects the potency of several, but not all, peripheral feedback controls of meal size. The mechanisms underlying these functional interactions with E2 have been examined only in the case of one such signal, CCK. Finally, the possibility that these feedback controls operate differently in men and women has received very little attention (Kissileff et al. 2003), and the possibility that they contribute to the peri-ovulatory decrease in eating in women has received none.
How T interacts with peripheral feedback controls of eating in rats or mice has been much less extensively examined than the effects of E2. Part of the reason for this may be that, as described above, T selectively increases meal number, not meal size, in rats and very few physiological controls of spontaneous meal frequency are known.
(i) Orosensory stimuli
Orosensory, or flavour, stimuli provide potent positive feedback signals that maintain eating once it has begun and thereby increase meal size. But E2 does not appear to inhibit eating by reducing the potency of orosensory stimuli in either rats or women. The data from rats have been recently reviewed (Geary 2004a,b) and are not treated here. Although men and women certainly differ in their food preferences and food choices in developed Western societies (Rolls et al. 1991; Drewnowski 1997; Dye & Blundell 1997; Westenhoefer 2005), these differences appear to result mainly from learned or cognitive responses based on education, familial, cultural and other individuated influences. For example, American and European women display greater interest in healthy eating and in weight control than do men (Bowen et al. 2003; Westenhoefer 2005), and American but not Spanish women who are chocolate cravers reported more cravings peri-menstrually (Zellner et al. 2004). Some innate flavour preferences, however, may be of relevance. In particular, increased intake of sweet foods has been detected during the luteal phase (Bowen & Grunberg 1990; Fong & Kretsch 1993). It is possible that this increase in sweet intake is due to the simultaneous increases in E2 and P during the luteal phase. This is because female rats display a marked increase in consumption of 3% glucose and 0.25–0.75% saccharine mixtures that is reduced by ovariectomy and reinstated by treatment with E2 and P, but not by either hormone alone (Valenstein et al. 1967; Wade & Zucker 1969; Zucker 1969). It would be useful to investigate this in animals in which the post-ovulatory phase of the ovarian cycle is more similar to that of women.
(ii) Ghrelin
Ghrelin is a recently discovered peptide hormone that is synthesized and released by gastric endocrine cells. It is unique among gastrointestinal hormones in that it is secreted in response to emptying, rather than filling, of the gut, and it is unique among peripheral feedback controls of eating in that it stimulates, rather than inhibits, eating (Kojima et al. 1999; Geary 2004a,b; van der Lely et al. 2004; Kojima & Kangawa 2005; Ueno et al. 2005; Williams & Cummings 2005). Peripheral ghrelin administration decreases the latency to the next meal and increases meal size (Azzara et al. 2005). Clegg et al. (submitted) reported that exogenous ghrelin increases eating, less during estrus than during other phases of the ovarian cycle in females, less in intact females than in ovariectomized females, and less in E2-treated ovariectomized females than untreated females. These data suggest that changes in the eating-stimulatory potency of endogenous ghrelin may contribute to both the tonic and the cyclic inhibitory effects of E2 on eating. One aspect of the data appeared inconsistent with this conclusion, however. That is, E2 treatment reduced the eating-stimulatory effect of intraperitoneal injection of ghrelin in ovariectomized rats by decreasing the latency to eat without affecting meal size, whereas E2 normally selectively reduces meal size. Therefore, it will be important to determine whether E2 and ghrelin interact to control meal size under more physiological conditions.
(iii) Cholecystokinin
CCK released from the small intestine during meals acts on abdominal CCK-1 (or CCK-A) receptors to produce a vagally mediated satiating effect in animals and humans (Cox 1998; Smith 1998; Beglinger et al. 2001; Geary 2001, 2004a,b). Three results following manipulation of endogenous CCK indicate convincingly that CCK plays a major role in the peri-ovulatory decrease in eating in rats: (i) in intact rats, the de-satiating effect of CCK-1 receptor antagonism was much larger during estrus than during diestrus; (ii) in ovariectomized rats, the de-satiating effect of CCK-1 receptor antagonism was larger on the day that modelled estrus than on the day that modelled diestrus; and (iii) in ovariectomized rats, the CCK-dependent satiating potency of intraduodenal lipid infusions was increased on the day that modelled estrus (Huang et al. 1993; Asarian & Geary 1999, submitted; Eckel & Geary 1999). These effects appear to involve an increase in the potency of CCK rather than increased CCK secretion, because E2 also increased the satiating potency of exogenous CCK in ovariectomized rats (Lindén et al. 1990; Butera et al. 1993; Geary et al. 1994). The increase in CCK's satiating potency appears not to involve changes in vagal CCK-1 receptor function, because E2 failed to affect the number or affinity of CCK-1 receptors in the NTS, which includes the terminal fields of vagal afferent neurons (Geary et al. 1996). Finally, the effect of E2 on the satiating potency of intestinal food stimuli is apparently selective. This is because the satiating potency of intraintestinal infusions of l-phenylalanine, which do not elicit CCK secretion in the rat (Brenner et al. 1993), was not increased by E2 treatment in ovariectomized rats (Asarian & Geary submitted).
Experiments mapping brain activity with c-fos immunocytochemistry indicate that E2 can affect eating-induced neuronal activation in a widespread neuronal network. Thus, E2 increased c-fos expression in the NTS, the paraventricular nucleus and the central nucleus of the amygdala after either eating or CCK injection (Eckel & Geary 2001; Eckel et al. 2002). The effect of CCK on c-fos in the NTS was shown to depend on ERα in mice (Geary et al. 2001). More recently, we have obtained results indicating that E2 acts specifically in the NTS to increase CCK's satiating potency: (i) the c-fos expression that was associated with CCK- and E2-dependent increases in the satiating potency of intraduodenal lipid infusions occurred only in a circumscribed part of the NTS, just caudal to the area postrema; (ii) many of these c-fos positive cells also expressed ERα; and (iii) local administration of E2 to the surface of the brainstem, just caudal to the area postrema, was sufficient both to decrease eating and to increase CCK-induced c-fos in the caudal NTS of ovariectomized rats (Asarian & Geary submitted; Thammacharoen et al. in press). The neurotransmitter phenotype of these neurons is not known, although they did not express either GLP-1 or tyrosine hydroxylase, each of which is expressed by many neurons in this area (Asarian & Geary submitted). The lack of co-localization with tyrosine hydroxylase is surprising, given previous indications that NTS catecholaminergic neurons might be involved in the effect of CCK on eating (Rinaman et al. 1995).
In summary, one mechanism through which E2 decreases eating, at least in rats, is by increasing the satiating action of CCK. The interaction of E2 and CCK is the only mechanism discovered so far that can be linked to both the endogenous feedback control signal and endogenous E2. Furthermore, its likely site, the caudal NTS, is known. It would seem important to determine the significance of this interaction in normal and disordered eating in women.
(iv) Pancreatic glucagon, amylin and hepatic fatty acid oxidation
Glucagon and amylin are released from the pancreas during meals, apparently largely due to cephalic phase reflexes, and each appears to act as a physiological negative feedback control of meal size in male animals (Geary 1998; Lutz 2005; Woods et al. submitted). We (Geary & Asarian 2001) demonstrated that in ovariectomized rats E2 (i) increased the satiating potency of intrameal hepatic portal infusions of glucagon and (ii) increased the de-satiating potency of antagonism of endogenous glucagon by hepatic portal infusion of glucagon antibodies. The design left open the question of whether this effect was related to the cyclic or tonic actions of E2 on meal size; whether a similar phenomenon occurs in intact, cycling female rats is also not clear. Interestingly, E2 had no effect on amylin's satiating potency when hepatic portal infusions of amylin were tested under the same conditions in the same rats that were used for the glucagon tests (L. Asarian, T. A. Lutz, N. Geary 2003, unpublished data). We also found that E2 had no effect on the satiating potency of intraperitoneal injections of amylin (unpublished data). This appears to be another example of the selective action of E2 on satiation. The reasons for this selectivity are unknown, but it is interesting to note that both CCK and glucagon satiation signals are transmitted from the periphery to the brain via vagal afferents, whereas amylin satiation is independent of the vagus.
When animals or people are adapted to diets with moderate amounts of fat (i.e. more than 20% energy from fat), hepatic FAO appears to provide a physiological control of meal size, although it remains uncertain whether intrameal changes in FAO affect the sizes of ongoing meals or whether this signal acts only over longer periods (Leonhardt & Langhans 2004; Jambor de Sousa et al. 2006). We therefore tested the effect of E2 on the de-satiating effect of antagonism of FAO in ovariectomized rats using pre-test treatment with mercaptoacetate, which blocks hepatic mitochondrial β-oxidation of long-chain fatty acids (Mangiarancina et al. 2003). E2 significantly reduced the eating-stimulatory effect of mercaptoacetate. Again, the design left open the question of whether this effect was directed at cyclic or tonic actions of E2; whether a similar phenomenon occurs in intact, cycling female rats also remains to be tested. Further, whether this E2-dependent effect on satiation resulted from alterations of hepatic metabolism, vagal afferent signalling or central information processing requires investigation.
(v) Leptin and insulin
Increased adiposity appears to produce a number of negative feedback control signals that inhibit eating, potentially including leptin, insulin, amylin and ghrelin (Cummings et al. 2002; Niswender & Schwartz 2003; Benoit et al. 2004; van der Lely et al. 2004; Geary 2004a,b; Lutz 2005; Woods et al. submitted). The two most extensively characterized of these hormones are insulin and leptin. Like the signals reviewed previously, leptin and insulin both selectively decrease meal size in rats, although this has been demonstrated in female rats only in the case of leptin (Eckel et al. 1998). Leptin also has other effects that make it an attractive candidate for a sexually differentiated control of eating: it plays crucial physiological roles in the control of pubertal development in both sexes (Chan & Mantzoros 2001; Chehab et al. 2002) and in the control of GnRH secretion and ovulation in women (Welt et al. 2004).
Exogenous leptin and insulin have markedly different eating-inhibitory potencies in male and female rats (Clegg et al. 2003). Using age- and weight-matched male and female rats, these investigators demonstrated that acute intracerebroventricular administration of leptin reduced eating more potently in females, whereas insulin reduced eating more potently in males. Futhermore, the eating-inhibitory effect of insulin in ovariectomized rats was decreased by E2. In contrast, in the case of leptin, the eating-inhibitory potency of centrally administered leptin appears to be increased by E2. This is because intracerebroventricular injections of leptin that inhibited eating in intact rats failed to do so in ovariectomized rats (Ainslie et al. 2001) and E2 treatment increased the eating-inhibitory effect of intracerebroventricular leptin in ovariectomized rats (Clegg et al. in press). Nevertheless, other data suggest that E2 may not affect the eating-inhibitory effect of leptin. In both intact and ovariectomized mice, chronic subcutaneous infusions of E2 via constant-release pellets did not affect the effect of chronic subcutaneous infusions of leptin via osmotic minipumps to decrease body fat mass (Pelleymounter et al. 1999). Similar results have been reported in rats (Chen & Heiman 2001). Because body fat usually responds more sensitively to leptin than does food intake (i.e. Ahima et al. 1999) and because the chronic subcutaneous administration better mimics a tonic hormonal action, these data cast doubt on the physiological relevance of the effects of acute, intracerebroventricular leptin.
Another interesting question regarding sex differences in the eating-inhibitory effect of leptin regards hypothalamic α-MSH. Leptin is thought to inhibit eating in large part by stimulating α-MSH release which acts, in turn, on downstream neurons expressing MC-3 or 4 receptors. However, (i) intracerebroventricular administration of the MC-3 and 4 receptor agonist melanotan II (MTII) did not differentially affect eating in male and female rats (Clegg et al. 2003); (ii) MTII did not differentially affect eating in E2-treated and untreated ovariectomized rats (Polidori & Geary 2002); (iii) intracerebroventricular administration of the MC-3 and 4 receptor antagonist SHU9119 did not differentially affect eating in E2-treated and untreated ovariectomized rats (Polidori & Geary 2002); and (iv) intracerebroventricular administration of the functional α-MSH antagonist agouti-related peptide did not differentially affect eating in E2-treated and untreated ovariectomized rats (Polidori & Geary 2002).
In summary, the potential sex differences in the eating effects of insulin and leptin and the interaction of E2 and these two adiposity signals requires further work. A full understanding of their physiological significance in the control of eating must also await studies in which hormonal manipulations are convincingly within the physiological range. In this regard, it is important to note that several lines of evidence suggest that adiposity signals normally function to detect and correct deficits in body adiposity rather than surfeits of adiposity (Schwartz et al. 2003), so that tests of exogenous insulin or leptin in normal or overweight animals may be uninformative.
(c) Hypothalamic–pituitary–gonadal function and interactions with central neurotransmitter mechanisms controlling eating
Progress in identifying the interneuronal signalling molecules that mediate the eating effects of GSH has been modest (Mystkowski & Schwartz 2000; Geary 2004a,b). Unfortunately, many studies in this literature are of dubious value, because the designs deviate so far from the physiology of normal eating or HPG axis function. In addition, the analysis of the effects on sex differences in the function of many interneuronal signalling molecules that control eating is complicated by the fact that the same molecules acting in the same regions of the brain are also implicated in the control of ovarian cycling (Freeman 1994; Hotchkiss & Knobil 1994; Griffin & Ojeda 2000; Bulun & Adashi 2003). This, for example, is true of α-MSH and neuropeptide Y.
(d) 5-Hydroxytryptamine
There are interesting data available concerning sex differences in the role of serotonin, or 5-HT, in eating. Central 5-HT inhibits eating by reducing meal size (Blundell 1992; Simansky 1998). Evidence of sex differences in this effect includes: (i) the 5-HT agonist fenfluramine inhibited eating more in female rats than in male rats (Eckel et al. 2005); (ii) fenfluramine inhibited eating more during estrus than during diestrus (Eckel et al. 2005); (iii) 8-OH-DPAT, which decreases 5-HT function by stimulating 5-HT1A autoreceptors, stimulated eating less during proestrus and estrus than during diestrus in cycling rats (Uphouse et al. 1991); (iv) the eating-inhibitory effect of fenfluramine was increased by E2 treatment in ovariectomized rats (Rivera & Eckel 2005); (v) the eating-stimulatory effect of 8-OH-DPAT was decreased by E2 treatment, and unaffected by P treatment, in ovariectomized rats (Salamanca & Uphouse 1992); (vi) the eating-inhibitory effect of chronic fenfluramine administration was not affected by chronic E2 treatment in ovariectomized rats (Souquet & Rowland 1990). Taken together, these studies suggest that the phasic inhibitory effect of E2 on eating during estrus, but not the tonic inhibitory effect of E2 on eating, may be mediated by increased 5-HT synaptic activity. Furthermore, these data are consistent with a critical role for CCK in the peri-ovulatory decrease in eating (discussed above) because hypothalamic 5-HT appears to be involved in the central processing of the CCK satiation signal (Poeschla et al. 1992, 1993).
The amygdala is another site mediating sex differences in the eating action of 5-HT, although the data are not entirely consistent. On the one hand, (i) E2 increased eating- and CCK-induced expression of c-fos in the central nucleus of the amygdala (Eckel & Geary 2001; Eckel et al. 2002), (ii) infusions of the 5-HT receptor antagonist metergoline directly into the posterior basolateral amygdala increased diurnal eating less during diestrus than during the day after estrus (Parker et al. 2002) and (iii) female rats were more hyperphagic following amygdala lesions than were male rats (King et al. 1999, 2003). On the other hand, the effects of amygdala lesions and of ovariectomy appeared additive and, therefore, independent (King et al. 1999, 2003).
It is also interesting to note that when rats were fed more palatable foods or were fed different macronutrients separately, there were no cyclic variations in the effect of 5-HT on eating (Heisler et al. 1999; Parker et al. 2002). These data may be relevant to the hypothesis that 5-HT controls food selection during the luteal or menstrual phases of the human cycle (Dye & Blundell 1997; Heisler et al. 1999).
4. Development of effects of 17-β-estradiol and testosterone on eating
(a) Early development
GSH's mechanisms of action are classically divided into organizational actions, i.e. actions in early development that permanently affect differentiation of the neural tissues destined to affect eating in the adult, and activational actions, i.e. transient effects dependent on changing patterns of GSH secretion (Phoenix et al. 1959; Gorski 2000). The effects reviewed above are activational. In this section, we review some organizational effects of GSH on eating.
The mammalian brain, like the HPG axis, is inherently female. Masculine structures and functions result from actions of GSH during critical periods of early development. In males, estrogens that are derived from aromatization of testosterone produce masculinization; in females, the hepatic protein α-fetoprotein neutralizes estrogens and prevents masculinization. In rats, the critical period for this process of sexual differentiation extends until about post-natal day 10. This permits feminization of genetic males by neonatal castration and masculinization of genetic females by treatment with doses of T or of estrogens that overwhelms the binding capacity of α-fetoprotein.
The limited data available suggest that sex differences in adult eating partially depend on organizational effects of GSH. First, beginning around puberty, neonatally masculinized (but gonadally intact) females ate about 10% more than non-masculinized females; unfortunately, however, the design was not sufficiently powerful for this difference to be statistically reliable (Bell & Zucker 1971). Second, neonatally masculinized, ovariectomized females and intact males were less sensitive to the tonic feeding-inhibitory effect of exogenous E2 as adults than were non-masculinized, ovariectomized females (Gentry & Wade 1976). Furthermore, this behavioural effect was relatively specific, in that neonatal masculinization did not influence the effect of E2 on running wheel activity. In contrast to these effects, neonatal feminization of male rats did not affect the eating-stimulatory effect of T in adulthood (Bell & Zucker 1971). Taken together, these data suggest that the masculinization of the brain in early development selectively decreases the sensitivity of the adult to the eating-inhibitory effects of E2, but that feminization of the brain does not affect the sensitivity of the adult to the eating-stimulatory effects of T. That is, organizational effects of GSH affect the substrate necessary for later activational effects of E2, but not of T. Finally, given the variety of specific activational sex differences in eating control signals reviewed here, it would seem important to determine if the organizational requirements for activational controls are uniform or vary by control. For example, the sensitivity of the eating-stimulatory effect of ghrelin to E2 reviewed above may not require a feminine brain because E2 also markedly decreased the eating-stimulatory effect of ghrelin in adult male rats (Clegg et al. submitted).
The specific neural bases for organization of sex-sensitive eating effects are unknown, and will probably remain so until after the neural bases for the activational effects of GSH are better characterized. Nevertheless, the availability of novel tools for analysis of the molecular genetic mechanisms of sexual differentiation (DeVriess et al. 2002; Forger et al. 2004) as well as the discovery that leptin, and likely other signalling molecules important in the control of eating (Bouret & Simerly 2004), have potent trophic effects on the development of some hypothalamic neural networks during early development encourage work on this problem.
(b) Puberty
Puberty can be considered an organizational event. Although prepubertal increases in secretion of GSH are necessary for pubertal development, they are not sufficient for it, at least in females. Rather, an increase in the responsivity of the HPG axis to E2 is also required to produce sufficient LH secretion for normal puberty (Ojeda & Urbanski 1994; Sisk & Foster 2004). Similarly, maturation of the substrate may be required to the estrogenic inhibition of eating, as suggested by the observation that exogenous E2 inhibits eating only in post-pubertal rats (Wade & Zucker 1970a,b; Wade 1974). Part of this maturation may be related to the circuitry mediating CCK's contribution to the cyclic control of eating in female rats because CCK-R1 antagonism did not increase eating in pre-pubertal rats (Mangiarancina et al. 2004).
5. Comment
In rats and mice, as well as many other animals, eating decreases cyclically during the peri-ovulatory phase of the ovarian cycle. Eating also changes tonically in response to gonadectomy and GSH treatment, especially to T in males and E2 in females. Several potential physiological controllers of these eating responses have been identified in recent years. A variety of data indicate that the peri-ovulatory decrease in eating is caused by a change in the satiating potency of peripheral CCK that is due to an action of E2 on ERα in the neurons of the caudal NTS. In contrast, CCK appears not to contribute to the tonic inhibitory effect of E2 on eating. There is also evidence that a variety of other peripheral negative feedback controls of eating contribute to these effects of E2 on eating, although, as summarized in table 1, evidence for their physiological action is not yet as complete as is the case for CCK. These signals include meal-related signals, such as pancreatic glucagon, adiposity signals, such as leptin, and signals that may function in both fashions, such as insulin and ghrelin. In contrast to these promising indicators of the mechanisms by which E2 inhibits eating, almost nothing is known about T's stimulatory effect on eating. That T selectively affects meal frequency in contrast to E2's selective effect on meal size, however, suggests that T may act on eating in a fundamentally different way than E2.
Table 1.
Interactions between E2 and peripheral feedback signals in the control of eating. (Meal and adiposity signals are explained in the text; signals are listed in the order treated in the text. Symbols not defined in text: MS, meal size; IMI, intermeal interval; C, cyclic; ↓, decrease; ↑, increase; –, none; ?, unknown; **, highly likely physiological status (evidence of modulation of endogenous signal by endogenous E2); *, some evidence of physiological status (i.e. modulation of endogenous signal by exogenous E2); —, uncertain physiological relevance (i.e. evidence only of modulation of exogenous signal by exogenous E2).)
| feedback mechanism | feeding effect | effect of E2 on potency of signal | cyclic/tonic effect | physiological status of interaction |
|---|---|---|---|---|
| meal signals | ||||
| flavour | ↑ or ↓ MS | 0 | – | — |
| ghrelina | ↑ MS, ↓ IMI | ↓ | ? | — |
| CCK | ↓ MS | ↑ | C | ** |
| glucagon | ↓ MS | ↑ | ? | * |
| amylina | ↓ MS | 0 | – | — |
| FAO | ↓ MS | ↑ | ? | * |
| adiposity signals | ||||
| insulin | ↓ MS | ↑ | ? | — |
| leptin | ↓ MS | ↓ | ? | — |
Amylin and ghrelin are listed as meal signals, but may function as both meal signals and adiposity signals.
E2's effect to increase the satiating potency of peripheral meal-related negative feedback controls of meal size suggests that E2 qualifies as an indirect control of meal size, as defined in Smith's (1996, 2000) theory of the direct and indirect control of meal size. According to Smith's hypothesis, negative feedback signals activated by preabsorptive food stimuli act in the brainstem to provide the direct control of meal size, and other, indirect controls modulate meal size by affecting the neural processing of the direct controls. E2's action in the NTS affecting the satiating potency of endogenous CCK seems to fit this hypothesis precisely. Some of the other interactions of E2 and peripheral negative feedback signals controlling eating do not appear to correspond to this hypothesis as closely. First, E2 may also act on at least one negative feedback control of meal size, hepatic FAO, that unlike Smith's (1996, 2000) direct controls, arises from a postabsorptive site. Second, E2 may interact with some adiposity signals, which would suggest interactions between two indirect controls. In this case, the original theory may still be correct if the final neural effect is a triple interaction, i.e. among E2, an adiposity signal, and a direct control of eating like CCK. This possibility could be investigated with experiments that combine the designs of tests revealing E2–CCK interactions (reviewed above) and leptin–CCK interactions (e.g. Matson et al. 2002; Morton et al. 2005). Although this might seem a cumbersome approach, the results of our efforts to phenotype the neurons mediating the interactive effect of E2 and CCK in the NTS suggest that analyses of interactions can provide sensitive probes into physiological function.
At least one of the effects of GSH on eating in animals, the peri-ovulatory decrease in eating, is clearly expressed in humans. Thus, the effects of GSH on eating are not merely of comparative interest, but relate directly to human physiology. The marked increase in vulnerability to eating disorders in women as well as the increased frequency of morbid obesity in women (Flegal et al. 2002; Freedman et al. 2002) indicate that the effects of GSH on human eating may also have pathophysiological importance. Better understanding of sex differences in the physiology of human eating might reveal (i) aetiological factors in disordered eating, (ii) mechanisms that exacerbate the course of or hinder recovery from disordered eating once it has begun, or (iii) potential avenues for novel physiologically based therapies. While the progress made in animal models of the effects of GSH on eating is modest, it seems already sufficient to suggest rational translational approaches to address these possibilities. CCK again provides a case in point. Women with bulimia nervosa have abnormally low CCK secretion and a reduced perception of satiation during meals (Gericottti & Liddle 1988; Devlin et al. 1998). The CCK effect is apparently secondary to the eating disorder, because it resolved when binge frequency improved; whether reduced CCK satiation might exacerbate bulimia nervosa, whether it might provide a useful therapeutic intervention and whether an interaction with E2 plays any role remain to be determined. The translational strategy can also be applied in the human to animal direction. For example, owing to differences in cycle physiology, rats and mice are not adequate models for analyses of the apparent increase in selection of sweet food displayed by women in the luteal phase.
In conclusion, the study of sex differences in eating has emerged as an interesting and important part of the physiology of eating. Two general points require attention if there is to be continued progress in this area. First, many new discoveries, and the correction of some older errors in this area, have depended on the application of methods that closely mimic normal physiology to the greatest extent possible (Geary 2001, 2004a,b). This effort should be continued and increased. Second, as mentioned in §1, GSH appear to have actions that can affect body weight and adiposity independent of eating, including effects on energy expenditure, gastrointestinal function, metabolism, growth and body composition (Chen et al. 1995; Tarnopolsky 1999; Cortwright & Koves 2000; Wajchenberg 2000; Blaak 2001; Heymsfeld et al. 2002; Clegg et al. 2003; Bruns & Kemnitz 2004; Williams 2004). Current evidence suggests that coordinated brain mechanisms orchestrate all these controls (e.g. Schwartz et al. 2003; Woods et al. submitted). Therefore, in order to build an accurate picture of the role of sex in eating, energy expenditure and energy balance, it is increasingly important to study the various GHS-sensitive mechanisms together.
Acknowledgments
The authors are supported by grants DK 54523 and MH 65024 from the National Institutes of Health of the USA and by the Swiss Federal Institute of Technology (ETH) Zürich.
Glossary
- 5-HT
5-hydroxytryptamine, serotonin
- 8-OH-DPAT
8-hydroxy-2-9(di-n-propylamino)tetralin
- BMI
body mass index, weight (kg)/height (m)2
- CCK
cholecystokinin
- E2
17-β-estradiol, the primary estrogen
- ER
estrogen receptor, with α and β subtypes
- FAO
fatty acid oxidation
- FSH
follicle-stimulating hormone
- GnRH
gonadatropin-releasing hormone, also known as LHRH
- GSH
gonadal steroid hormones
- HPG
hypothalamic–pituitary–gonadal
- LH
luteinizing hormone
- LHRH
luteinizing hormone-releasing hormone, also known as GnRH
- α-MSH
α-melanocortin stimulating hormone
- MC
melanocortin
- NTS
nucleus tractus solitarius
- P
progesterone, the primary progestin
- T
testosterone, the primary androgen
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Ahima R.S, Kelly J, Elmquist J.K, Flier J.S. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. doi:10.1210/en.140.11.4923 [DOI] [PubMed] [Google Scholar]
- Ainslie D.A, Morris M.J, Wittert G, Turnbull H, Proietto J, Thorburn A.W. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptides Y. Int. J. Obes. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. doi:10.1038/sj.ijo.0801806 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides. 1999;20:445–450. doi: 10.1016/s0196-9781(99)00024-8. doi:10.1016/S0196-9781(99)00024-8 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. doi:10.1006/hbeh.2002.1835 [DOI] [PubMed] [Google Scholar]
- Asarian, L. & Geary, N. Submitted. Site and phenotypic characterization of ERα neurons mediating the increased satiating potency of CCK by estradiol in ovariectomized rats.
- Azzara A.V, Schuss B, Hong S, Schwartz G.J. Peripheral ghrelin administration increases food intake, meal size, and progressive-ratio responding for food. Appetite. 2005;44:332. [Google Scholar]
- Barr S.I, Janelle K.C, Prior J.C. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am. J. Clin. Nutr. 1995;61:39–43. doi: 10.1093/ajcn/61.1.39. [DOI] [PubMed] [Google Scholar]
- Bartness T.J, Waldbillig R.J. Dietary self-selection in intact, ovariectomized, and estradiol-treated female rats. Behav. Neurosci. 1984;98:125–137. doi: 10.1037//0735-7044.98.1.125. doi:10.1037//0735-7044.98.1.125 [DOI] [PubMed] [Google Scholar]
- Becker J.B, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. doi:10.1210/en.2004-1142 [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. 2001;280:R1149–R1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- Bell D.D, Zucker I. Sex differences in body weight and eating: organization and activation by gonadal hormones in the rat. Physiol. Behav. 1971;7:27–34. doi: 10.1016/0031-9384(71)90231-9. doi:10.1016/0031-9384(71)90231-9 [DOI] [PubMed] [Google Scholar]
- Benoit S.C, Clegg D.J, Seeley R.J, Woods S.C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. doi:10.1210/rp.59.1.267 [DOI] [PubMed] [Google Scholar]
- Blaak E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. doi:10.1097/00075197-200111000-00006 [DOI] [PubMed] [Google Scholar]
- Blaustein J.D, Wade G.N. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. doi:10.1016/0031-9384(76)90064-0 [DOI] [PubMed] [Google Scholar]
- Blaustein J.D, Gentry R.T, Roy E.J, Wade G.N. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol. Behav. 1976;17:1027–1030. doi: 10.1016/0031-9384(76)90028-7. doi:10.1016/0031-9384(76)90028-7 [DOI] [PubMed] [Google Scholar]
- Blundell J.E. Serotonin and the biology of feeding. Am. J. Clin. Nutr. 1992;55:155S–159S. doi: 10.1093/ajcn/55.1.155s. [DOI] [PubMed] [Google Scholar]
- Bouret S.G, Simerly R.B. Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–2626. doi: 10.1210/en.2004-0231. doi:10.1210/en.2004-0231 [DOI] [PubMed] [Google Scholar]
- Bowen D.J, Grunberg N.E. Variations in food preference and consumption across the menstrual cycle. Physiol. Behav. 1990;47:287–291. doi: 10.1016/0031-9384(90)90144-s. doi:10.1016/0031-9384(90)90144-S [DOI] [PubMed] [Google Scholar]
- Bowen D, Green P, Vizenor N, Vu C, Kreuter P, Rolls B. Effects of fat content on fat hedonics: cognition or taste? Physiol. Behav. 2003;78:247–253. doi: 10.1016/s0031-9384(02)00973-3. doi:10.1016/S0031-9384(02)00973-3 [DOI] [PubMed] [Google Scholar]
- Brenner L, Yox D.P, Ritter R.C. Suppression of sham feeding by intraintestinal nutrients is not correlated with plasma cholecytokinin elevation. Am. J. Physiol. 1993;33:R972–R976. doi: 10.1152/ajpregu.1993.264.5.R972. [DOI] [PubMed] [Google Scholar]
- Bruns C.M, Kemnitz J.W. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 2004;45:160–169. doi: 10.1093/ilar.45.2.160. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt S.D, McDevitt R.M, Prentice A.M. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol. Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. doi:10.1016/0031-9384(95)02003-9 [DOI] [PubMed] [Google Scholar]
- Bulun S.E, Adashi E.Y. The physiology and pathology of the female reproductive axis. In: Larsen P.R, Kronenberg H.M, Melmed S, Polonsky K.S, editors. Williams textbook of endocrinology. Saunders; Philadelphia, PA: 2003. pp. 587–664. [Google Scholar]
- Butera P.C, Beikirch R.J. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. doi:10.1016/0006-8993(89)90062-0 [DOI] [PubMed] [Google Scholar]
- Butera P.C, Willard D.M, Raymond S.A. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. doi:10.1016/0006-8993(92)90694-5 [DOI] [PubMed] [Google Scholar]
- Butera P.C, Bradway D.M, Cataldo N.J. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol. Behav. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-u. doi:10.1016/0031-9384(93)90387-U [DOI] [PubMed] [Google Scholar]
- Butera P.C, Xiong M, Davis R.J, Platania S.P. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav. Neurosci. 1996;110:823–830. doi: 10.1037//0735-7044.110.4.823. doi:10.1037/0735-7044.110.4.823 [DOI] [PubMed] [Google Scholar]
- Chai J.K, Blaha V, Meguid M.M, Laviano A, Yang Z.J, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am. J. Physiol. 1999;276:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Chan J.L, Mantzoros C.S. Leptin and the hypothalamic–pituitary regulation of the gonadotropin–gonadal axis. Pituitary. 2001;4:87–92. doi: 10.1023/a:1012947113197. doi:10.1023/A:1012947113197 [DOI] [PubMed] [Google Scholar]
- Chehab F.F, Qiu J, Mounzih K, Ewart-Toland A, Ogus S. Leptin and reproduction. Nutr. Rev. 2002;60:S39–S46. doi: 10.1301/002966402320634823. doi:10.1301/002966402320634823 [DOI] [PubMed] [Google Scholar]
- Chen Y, Heiman M.L. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am. J. Physiol. 2001;280:E315–E322. doi: 10.1152/ajpendo.2001.280.2.E315. [DOI] [PubMed] [Google Scholar]
- Chen T.S, Doong M.L, Chang F.Y, Lee S.D, Wang P.S. Effects of sex steroid hormones on gastric emptying and gastrointestional transit in rats. Am. J. Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- Clegg D.J, Riedy C.A, Blake Smith K.A, Benoit S.C, Woods S.C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Clegg, D. J., Brown, L. M., Woods, S. C., Benoit, S. C. In press. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes [DOI] [PubMed]
- Clegg, D. J., Brown, L. M, Benoit, S. C., Woods, S. C. & Geary, N. Submitted. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Endocrinology [DOI] [PubMed]
- Cortright R.N, Koves T.R. Sex differences in substrate metabolism and energy homeostasis. Can. J. Appl. Physiol. 2000;25:288–311. doi: 10.1139/h00-023. [DOI] [PubMed] [Google Scholar]
- Cox J.E. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am. J. Physiol. 1998;274:R1390–R1396. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]
- Cummings D.E, Weigle D.S, Frayo R.S, Breen P.A, Ma M.K, Dellinger E.P, Purnell J.Q. Plasma ghrelin levels after diet-induced weight loss of gastric bypass surgery. N. Engl. J. Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. doi:10.1056/NEJMoa012908 [DOI] [PubMed] [Google Scholar]
- Dagnault A, Richard D. Lesions of hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am. J. Physiol. 1994;267:E32–E38. doi: 10.1152/ajpendo.1994.267.1.E32. [DOI] [PubMed] [Google Scholar]
- Devlin M.J, Walsh B.T, Guss J.L, Kissilleff H.R, Liddle R.A, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am. J. Clin. Nutr. 1998;65:114–120. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- De Vries G.J, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett R.F. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim. Behav. 1973;21:772–780. doi: 10.1016/s0003-3472(73)80103-4. doi:10.1016/S0003-3472(73)80103-4 [DOI] [PubMed] [Google Scholar]
- Drewett R.F. The meal patterns of the oestrous cycle and their motivational significance. Q. J. Exp. Psychol. 1974;26:489–494. doi: 10.1080/14640747408400438. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Taste preferences and food intake. Annu. Rev. Nutr. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. doi:10.1146/annurev.nutr.17.1.237 [DOI] [PubMed] [Google Scholar]
- Dye L, Blundell J.E. Menstrual cycle and appetite control: implications for weight regulation. Hum. Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. doi:10.1093/humrep/12.6.1142 [DOI] [PubMed] [Google Scholar]
- Eck L.H, Bennett A.G, Egan B.M, Ray J.W, Mitchell C.O, Smith M.A, Klesges R.C. Differences in macronutrient selections in users and nonusers of an oral contraceptive. Am. J. Clin. Nutr. 1997;65:419–424. doi: 10.1093/ajcn/65.2.419. [DOI] [PubMed] [Google Scholar]
- Eckel L.A, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. doi:10.1016/S0196-9781(99)00025-X [DOI] [PubMed] [Google Scholar]
- Eckel L.A, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. 2001;281:R738–R746. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- Eckel L.A, Langhans W, Kahler A, Campfield L.A, Smith F.J, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am. J. Physiol. 1998;275:R186–R193. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- Eckel L.A, Houpt T.A, Geary N. Estradiol replacement increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. 2002;283:R1378–R1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- Eckel L.A, Rivera H.M, Atchley D.P. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am. J. Physiol. 2005;288:R1486–R1491. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- Flegal K.M, Carroll M.D, Ogden C.L, Johnson C.L. Prevalence and trends in obesity among US adults, 1999–2000. J. Am. Med. Assoc. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. doi:10.1001/jama.288.14.1723 [DOI] [PubMed] [Google Scholar]
- Fong A.K, Kretsch M.J. Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle. Am. J. Clin. Nutr. 1993;57:43–46. doi: 10.1093/ajcn/57.1.43. [DOI] [PubMed] [Google Scholar]
- Forger N.G, Rosen G.J, Waters E.M, Jacob D, Simerly R.B, de Vries G.J. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc. Natl Acad. Sci. USA. 2004;14:13 666–13 671. doi: 10.1073/pnas.0404644101. doi:10.1073/pnas.0404644101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D.S, Khan L.K, Serdula M.K, Galuska D.A, Dietz W.H. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. J. Am. Med. Assoc. 2002;288:1758–1761. doi: 10.1001/jama.288.14.1758. doi:10.1001/jama.288.14.1758 [DOI] [PubMed] [Google Scholar]
- Freeman M.E. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill J.D, editors. The physiology of reproduction. 2nd edn. Raven Press; New York, NY: 1994. pp. 613–658. [Google Scholar]
- Geary N. Glucagon and the control of meal size. In: Smith G.P, editor. Satiation: from gut to brain. Oxford University Press; New York, NY: 1998. pp. 164–197. [Google Scholar]
- Geary N. Estradiol, CCK, and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. doi:10.1016/S0196-9781(01)00449-1 [DOI] [PubMed] [Google Scholar]
- Geary N. The estrogenic inhibition of eating. In: Stricker E.M, Woods S.C, editors. Handbook of behavioral neurobiology, vol. 14. Neurobiology of food and fluid intake. 2nd edn. Kluwer; New York, NY: 2004a. pp. 307–345. [Google Scholar]
- Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol. Behav. 2004b;81:719–733. doi: 10.1016/j.physbeh.2004.04.013. doi:10.1016/j.physbeh.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L. Estradiol increases glucagon's satiating potency in ovariectomized rats. Am. J. Physiol. 2001;281:R1290–R1294. doi: 10.1152/ajpregu.2001.281.4.R1290. [DOI] [PubMed] [Google Scholar]
- Geary N, Schwartz G.J. Appetite. In: Sadock B.J, Sadock V.A, editors. Comprehensive textbook of psychiatry. 8th edn. Lippincott; Philadelphia, PA: 2005. pp. 295–308. [Google Scholar]
- Geary N, Trace D, McEwen B, Smith G.P. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol. Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. doi:10.1016/0031-9384(94)90196-1 [DOI] [PubMed] [Google Scholar]
- Geary N, Smith G.P, Corp E.S. The increased satiating potency of CCK-8 by estradiol is not mediated by upregulation of NTS CCK receptors. Brain Res. 1996;179:179–186. doi: 10.1016/0006-8993(96)00099-6. doi:10.1016/0006-8993(96)00099-6 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach K.S, Pfaff D.W, Ogawa S. Deficits in E2-dependent control of feeding weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. doi:10.1210/en.142.11.4751 [DOI] [PubMed] [Google Scholar]
- Geiselman P.J, Martin J.R, VanderWeele D.A, Novin D. Dietary self-selection in cycling and neonatally ovariectomized rats. Appetite. 1981;2:87–101. doi: 10.1016/s0195-6663(81)80002-5. [DOI] [PubMed] [Google Scholar]
- Gentry R.T, Wade G.N. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J. Comp. Physiol. Psychol. 1976;90:747–754. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Geriacotti T.D, Liddle R.A. Impaired cholecystokinin release in bulimia nervosa. N. Engl. J. Med. 1988;319:683–688. doi: 10.1056/NEJM198809153191105. [DOI] [PubMed] [Google Scholar]
- Gong E.J, Garrel D, Calloway D.H. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989;49:252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- Gorski R.A. Sexual differentiation of the nervous system. In: Kandel E.R, Schwartz J.H, Jessell T.M, editors. Principles of neural science. 4th edn. McGraw-Hill; New York, NY: 2000. pp. 1131–1148. [Google Scholar]
- Griffin J.E, Ojeda A.R. 4th edn. Oxford; New York, NY: 2000. Textbook of endocrine physiology. [Google Scholar]
- Heisler L.K, Kanarek R.B, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol. Biochem. Behav. 1999;63:377–385. doi: 10.1016/s0091-3057(99)00021-0. doi:10.1016/S0091-3057(99)00021-0 [DOI] [PubMed] [Google Scholar]
- Heymsfield S.B, Gallagher D, Kotler D.P, Wang Z, Allison D.B, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am. J. Physiol. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J, Knobil E. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill J.D, editors. The physiology of reproduction. 2nd edn. Raven Press; New York, NY: 1994. pp. 711–745. [Google Scholar]
- Hrupka B.J, Smith G.P, Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol. Behav. 2002;77:233–241. doi: 10.1016/s0031-9384(02)00857-0. doi:10.1016/S0031-9384(02)00857-0 [DOI] [PubMed] [Google Scholar]
- Huang Y.S, Doi R, Chowdhury P, Pasley J.N, Nishikawa M, Huang T.J, Rayford P.L. Effect of cholecystokinin on food intake at different stages of the estrous cycle in female rats. J. Assoc. Acad. Minor. Phys. 1993;4:56–58. [PubMed] [Google Scholar]
- Jambor de Sousa U.L, Arnold M, Langhans W, Geary N, Leonhardt M. Caprylic acid infusion acts in the liver to decrease food intake in rats. Physiol. Behav. 2006;87:388–395. doi: 10.1016/j.physbeh.2005.11.004. doi:10.1016/j.physbeh.2005.11.004 [DOI] [PubMed] [Google Scholar]
- King B.M, Rollins B.L, Stines S.G, Cassis S.A, McGuire H.B, Lagarde M.L. Sex differences in body weight gains following amygdaloid lesions in rats. Am. J. Physiol. 1999;277:R975–R980. doi: 10.1152/ajpregu.1999.277.4.R975. [DOI] [PubMed] [Google Scholar]
- King B.M, Rollins B.L, Grundmann S.J, Olivier L.G. Excessive weight gains in female rats with transections of the stria terminalis. Physiol. Behav. 2003;78:563–568. doi: 10.1016/s0031-9384(03)00042-8. doi:10.1016/S0031-9384(03)00042-8 [DOI] [PubMed] [Google Scholar]
- Kissileff H.R, Carretta J.C, Geliebter A, Pi-Sunyer F.X. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am. J. Physiol. 2003;285:R992–R998. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Krege J.H, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl Acad. Sci. USA. 1998;95:15 677–15 682. doi: 10.1073/pnas.95.26.15677. doi:10.1073/pnas.95.26.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz S.F, Akabayashi A, Alexander J.T, Wang J. Gonadal steroids and hypothalamic galanin and neuropeptide Y: role in eating behavior and body weight control in female rats. Endocrinology. 1998;139:1771–1780. doi: 10.1210/endo.139.4.5867. doi:10.1210/en.139.4.1771 [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol. Behav. 2004;83:645–651. doi: 10.1016/j.physbeh.2004.07.033. doi:10.1016/j.physbeh.2004.07.033 [DOI] [PubMed] [Google Scholar]
- Lindén A, Uvnäs-Moberg K, Forsberg G, Bednar I, Södersten P. Involvement of cholecystokinin in food intake: III. Oestradiol potentiates the inhibitory effect of cholecystokinin octapeptide on food intake in ovariectomized rats. J. Neuroendocrinol. 1990;2:797–801. doi: 10.1111/j.1365-2826.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Lissner L, Stevens J, Levitsky D.A, Rasmussen K.M, Strupp B.J. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am. J. Clin. Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- Lubahn D.B, Moyer J.S, Golding T.S, Couse J.F, Korach K.S, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl Acad. Sci. USA. 1993;90:11 162–11 166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz T.A. Pancreatic amylin as a centrally acting satiating hormone. Curr. Drug Targets. 2005;6:81–89. doi: 10.2174/1389450053174596. doi:10.2174/1389450053344885 [DOI] [PubMed] [Google Scholar]
- Lyons P.M, Truswell A.S, Mira M, Vizzard J, Abraham S.F. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am. J. Clin. Nutr. 1989;49:1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- Mangiaracina M, Langhans W, Geary N. Mercaptoacetate stimulates eating in untreated ovariectomized rats, but not in estradiol-treated rats. Appetite. 2003;40:347. [Google Scholar]
- Mangiarancina M, Wolfe A, Azzara A, Schwartz G.J, Walsh B.T, Geary N. The satiating potency of endogenous CCK increases after puberty in female rats. Appetite. 2004;42:382. [Google Scholar]
- Matson C.A, Reid D.F, Ritter R.C. Daily CCK injection enhances reduction of body weight by chronic intracerebroventricular leptin infusion. Am. J. Physiol. 2002;282:R1368–R1373. doi: 10.1152/ajpregu.00080.2001. [DOI] [PubMed] [Google Scholar]
- Morton G.J, Blevins J.E, Williams D.E, Niswender K.D, Gelling R.W, Rhodes C.J, Baskin D.G, Schwartz M.W. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. doi:10.1172/JCI200522081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystkowski P, Schwartz M.W. Gonadal steroids and energy homeostasis in the leptin era. Nutrition. 2000;16:937–946. doi: 10.1016/s0899-9007(00)00458-5. doi:10.1016/S0899-9007(00)00458-5 [DOI] [PubMed] [Google Scholar]
- Niswender K.D, Schwartz M.W. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. doi:10.1016/S0091-3022(02)00105-X [DOI] [PubMed] [Google Scholar]
- Ojeda S.R, Urbanski H.F. Puberty in the rat. In: Knobil E, Neill J.D, editors. The physiology of reproduction. 2nd edn. Raven Press; New York, NY: 1994. pp. 363–409. [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor α gene with body fat distribution. Int. J. Obes. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. doi:10.1038/sj.ijo.0802378 [DOI] [PubMed] [Google Scholar]
- Parker G.C, Bishop C, Coscina D.V. Estrous cycle and food availability affect feeding induced by amygdala 5-HT receptor blockade. Pharmacol. Biochem. Behav. 2002;71:701–707. doi: 10.1016/s0091-3057(01)00668-2. doi:10.1016/S0091-3057(01)00668-2 [DOI] [PubMed] [Google Scholar]
- Pelkman C.L, Chow M, Heinbach R.A, Rolls B.J. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am. J. Clin. Nutr. 2001;73:19–26. doi: 10.1093/ajcn/73.1.19. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M.A, Baker M.B, McCaleb M. Does estradiol mediate leptin's effects on adiposity and body weight? Am. J. Physiol. 1999;276:E955–E963. doi: 10.1152/ajpendo.1999.276.5.E955. [DOI] [PubMed] [Google Scholar]
- Pfaff D.W, Arnold A.A, Etgen A.M, Fahrback S.E, Rubin R.T, editors. Hormones, brain and behavior. Elsevier; Amsterdam: 2002. [Google Scholar]
- Phoenix C.H, Goy R.W, Gerall A.A, Young W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Poeschla B, Gibbs J, Simansky K.J, Smith G.P. The 5-HT1A agonist 8-OH-DPAT attenuates the satiating action of cholecystokinin. Pharmacol. Biochem. Behav. 1992;42:541–543. doi: 10.1016/0091-3057(92)90152-6. doi:10.1016/0091-3057(92)90152-6 [DOI] [PubMed] [Google Scholar]
- Poeschla B, Gibbs J, Simansky K.J, Greenberg D, Smith G.P. Cholecystokinin-induced satiety depends on activation of 5-HT1C receptors. Am. J. Physiol. 1993;264:R62–R64. doi: 10.1152/ajpregu.1993.264.1.R62. [DOI] [PubMed] [Google Scholar]
- Polidori C, Geary N. Estradiol treatment fails to affect the feeding response to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–1700. doi: 10.1016/s0196-9781(02)00112-2. doi:10.1016/S0196-9781(02)00112-2 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Hoffman G.E, Dohanics J, Le W.W, Stricker E.M, Verbalis J.G. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J. Comp. Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. doi:10.1002/cne.903600204 [DOI] [PubMed] [Google Scholar]
- Rivera H.M, Eckel L.A. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol. Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. doi:10.1016/j.physbeh.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Rivera H.M, Boersma G, Eckel L.A. Estradiol's inhibitory effect on food intake is attenuated by antagonism of central, but not peripheral, estrogen receptors. Appetite. 2005;44:374. [Google Scholar]
- Rock C.L, Gorenflow D.W, Drewnowski A, Demitrack M.A. Nutritional characteristics, eating pathology, and hormonal status in young women. Am. J. Clin. Nutr. 1996;64:566–571. doi: 10.1093/ajcn/64.4.566. [DOI] [PubMed] [Google Scholar]
- Rogers P.J, Smit H.J. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol. Biochem. Behav. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. doi:10.1016/S0091-3057(00)00197-0 [DOI] [PubMed] [Google Scholar]
- Rolls B.J, Fedoroff I.C, Guthrie J.F. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10:133–142. doi: 10.1037//0278-6133.10.2.133. doi:10.1037/0278-6133.10.2.133 [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Weight change with oral contraceptive use and during the menstrual cycle. Results of daily measurements. Contraception. 1998;58:345–349. doi: 10.1016/s0010-7824(98)00127-9. doi:10.1016/S0010-7824(98)00127-9 [DOI] [PubMed] [Google Scholar]
- Ross G.T, Cargille C.M, Lipsett M.B, Rayford P.L, Marshall J.R, Strott C.A, Rodbard D. Pituitary and gonadal hormones in woman during spontaneous and induced ovulatory cycles. Recent Prog. Horm. Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Salamanca S, Uphouse L. Estradiol modulation of the hyperphagia induced by the 5-HT1A agonist, 8-OH-DPAT. Pharmacol. Biochem. Behav. 1992;43:953–955. doi: 10.1016/0091-3057(92)90431-e. doi:10.1016/0091-3057(92)90431-E [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Woods S.C, Seeley R.J, Barsh G.S, Baskin D.G, Leibel R.L. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Simanksy K.J. Serotonin and the structure of satiation. In: Smith G.P, editor. Satiation: from gut to brain. Oxford University Press; New York, NY: 1998. pp. 217–262. [Google Scholar]
- Sisk C.L, Foster D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. doi:10.1038/nn1326 [DOI] [PubMed] [Google Scholar]
- Smith G.P. The direct and indirect controls of meal size. Neurosci. Biobehav. Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. doi:10.1016/0149-7634(95)00038-G [DOI] [PubMed] [Google Scholar]
- Smith G.P, editor. Satiation: from gut to brain. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Smith G.P. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;10:814–820. doi: 10.1016/s0899-9007(00)00457-3. doi:10.1016/S0899-9007(00)00457-3 [DOI] [PubMed] [Google Scholar]
- Souquet A.-M, Rowland N.E. Dexfenfluramine: action with estradiol on food intake and body weight in ovariectomized rats. Am. J. Physiol. 1990;258:R211–R215. doi: 10.1152/ajpregu.1990.258.1.R211. [DOI] [PubMed] [Google Scholar]
- Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takacs I, Lakatos P. Vitamin D and estrogen receptor gene polymorphism in type 2 diabetes mellitus and in android type obesity. Eur. J. Endocrinol. 2001;144:385–389. doi: 10.1530/eje.0.1440385. doi:10.1530/eje.0.1440385 [DOI] [PubMed] [Google Scholar]
- Tarnopolsky M. CRC Press; Boca Raton, FL: 1999. Gender differences in metabolism. Practical and nutritional implications. [Google Scholar]
- Thammacharoen, S., Lutz, T. A., Geary, N. & Asarian, L. In press. Hindbrain estradiol implants inhibit feeding and increase NTS c-Fos immunoreactivity in female rats. Appetite
- Ueno H, Yamaguchi H, Kangawa K, Nakazato M. Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regul. Pept. 2005;126:11–19. doi: 10.1016/j.regpep.2004.08.007. doi:10.1016/j.regpep.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Uphouse L, Salamanca S, Caldarola-Pastuszka M. Gender and estrous cycle differences in the response to the 5-HT1A agonist 8-OH-DPAT. Pharmacol. Biochem. Behav. 1991;40:901–906. doi: 10.1016/0091-3057(91)90104-a. doi:10.1016/0091-3057(91)90104-A [DOI] [PubMed] [Google Scholar]
- Valenstein E.S, Kakolewski J.W, Cox V.C. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- van der Lely A.J, Tschop M, Heiman M.L, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. doi:10.1210/er.2002-0029 [DOI] [PubMed] [Google Scholar]
- Wade G.N. Gonadal hormones and behavioral regulation of body weight. Physiol. Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. doi:10.1016/0031-9384(72)90340-X [DOI] [PubMed] [Google Scholar]
- Wade G.N. Interaction between estradiol-17 beta and growth hormone in control of food intake in weanling rats. J. Comp. Physiol. Psychol. 1974;86:359–362. doi: 10.1037/h0035945. [DOI] [PubMed] [Google Scholar]
- Wade G.N. Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Wade G.N, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J. Comp. Physiol. Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Wade G.N, Zucker I. Development of hormonal control over food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1970a;70:213–220. doi: 10.1037/h0028713. [DOI] [PubMed] [Google Scholar]
- Wade G.N, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J. Comp. Physiol. Psychol. 1970b;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. doi:10.1210/er.21.6.697 [DOI] [PubMed] [Google Scholar]
- Wallen W.J, Belanger M.P, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J. Nutr. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- Welt C.K, Chan J.L, Bullen J, Murphy R, Smith P, DePaoli A.M, Karalis A, Mantzoros C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. doi:10.1056/NEJMoa040388 [DOI] [PubMed] [Google Scholar]
- Westenhoefer J. Age and gender dependent profile of food choice. Forum Nutr. 2005;57:44–51. doi: 10.1159/000083753. [DOI] [PubMed] [Google Scholar]
- Williams C.M. Lipid metabolism in women. Proc. Nutr. Soc. 2004;63:153–160. doi: 10.1079/PNS2003314. doi:10.1079/PNS2003314 [DOI] [PubMed] [Google Scholar]
- Williams D.L, Cummings D.E. Regulation of ghrelin in physiologic and pathophysiologic states. J. Nutr. 2005;135:1320–1325. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Gotoh K, Clegg D.J. Gender differences in the control of energy homeostasis. Exp. Biol. Med. 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Lutz T, Geary N, Langhans W. Pancreatic signals controlling food intake—insulin, glucagon, and amylin. Phil. Trans. R. Soc. B. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. doi:10.1098/rstb.2006.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner D.A, Garriga-Trillo A, Centeno S, Wadsworth E. Chocolate cravings and the menstrual cycle. Appetite. 2004;42:119–121. doi: 10.1016/j.appet.2003.11.004. doi:10.1016/j.appet.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Zucker I. Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiol. Behav. 1969;4:595–602. doi:10.1016/0031-9384(69)90160-7 [Google Scholar]