Abstract
The control of food intake and body weight by the brain relies upon the detection and integration of signals reflecting energy stores and fluxes, and their interaction with many different inputs related to food palatability and gastrointestinal handling as well as social, emotional, circadian, habitual and other situational factors. This review focuses upon the role of hormones secreted by the endocrine pancreas: hormones, which individually and collectively influence food intake, with an emphasis upon insulin, glucagon and amylin. Insulin and amylin are co-secreted by B-cells and provide a signal that reflects both circulating energy in the form of glucose and stored energy in the form of visceral adipose tissue. Insulin acts directly at the liver to suppress the synthesis and secretion of glucose, and some plasma insulin is transported into the brain and especially the mediobasal hypothalamus where it elicits a net catabolic response, particularly reduced food intake and loss of body weight. Amylin reduces meal size by stimulating neurons in the hindbrain, and there is evidence that amylin additionally functions as an adiposity signal controlling body weight as well as meal size. Glucagon is secreted from A-cells and increases glucose secretion from the liver. Glucagon acts in the liver to reduce meal size, the signal being relayed to the brain via the vagus nerves. To summarize, hormones of the endocrine pancreas are collectively at the crossroads of many aspects of energy homeostasis. Glucagon and amylin act in the short term to reduce meal size, and insulin sensitizes the brain to short-term meal-generated satiety signals; and insulin and perhaps amylin as well act over longer intervals to modulate the amount of fat maintained and defended by the brain. Hormones of the endocrine pancreas interact with receptors at many points along the gut–brain axis, from the liver to the sensory vagus nerve to the hindbrain to the hypothalamus; and their signals are conveyed both neurally and humorally. Finally, their actions include gastrointestinal and metabolic as well as behavioural effects.
Keywords: glucose, meals, satiety, pancreatic polypeptide, somatostatin
1. Introduction
The normal control of food intake and body weight by the brain relies upon the detection and integration of signals reflecting energy stores and fluxes and their interaction with myriad inputs related to food palatability and gastrointestinal handling as well as social, emotional, circadian, habitual and other situational factors. This review focuses upon the role of hormones secreted by endocrine cells in the islets of Langerhans in the pancreas, hormones which individually and collectively influence food intake, with an emphasis upon insulin, glucagon and amylin.
There are several categories of peripherally arising signals that influence food intake (Woods et al. 1998; Schwartz et al. 2000). One category includes the signals generated during meals that influence satiation (i.e. signals that contribute to the perception of fullness and the termination of an ongoing meal) and satiety (i.e. signals that function to prolong the interval until hunger or the onset of the next meal). These acutely acting signals are collectively called ‘satiety signals’ in this review, and the prototypical example is cholecystonin (CCK). Satiety signals are either relayed to the hindbrain by sensory nerves or else stimulate the hindbrain directly. A second category includes hormones whose secretion is proportional to the amount of fat in the body, and it includes insulin from the pancreatic islets and leptin from adipose tissue, stomach and elsewhere. These adiposity signals are transported from the circulation into the hypothalamus in the forebrain. Satiety signals and adiposity signals arising from the endocrine pancreas, as well as their interactions with other controls of food intake, are the topic of this review. Other peripherally arising signals include steroid hormones from the gonads and adrenal cortex, cytokines and nutrients and they are the topics of other articles in this volume.
2. Overview of the controls over food intake
For body weight (body fat, actually) to remain stable over time, energy intake (food intake) must match energy expenditure (the sum of excreted energy, heat loss and physical work). Any deviation from this equilibrium will result in weight gain or loss. Humans and most mammals take in energy in discrete episodes or meals. For most of us, the impetus to begin a meal is rarely if ever based on a biological deficit or need such as insufficient glucose or other energy source in some critical tissue. Rather, the timing of when specific meals are initiated is based on any of several psychological factors such as habit, time of day, the social situation, convenience and others (Woods 1991; Woods & Strubbe 1994; Strubbe & Woods 2004). Because meal initiation relies on psychological as opposed to more purely physiological factors, if body weight is indeed regulated via a linking of energy intake to energy expenditure, the regulatory process must logically be manifest as a control over how many calories are consumed once a meal begins; i.e. on meal size. Consistent with this, many signals generated during meals are proportional to the number of calories consumed, and some of these secretions function as satiety signals to the central nervous system to help limit meal size (see reviews in (Kaplan & Moran 2004; Moran & Kinzig 2004; Woods 2004; Strader & Woods 2005)). Pancreatic islet hormones in this category include glucagon and amylin and possibly insulin as well.
In contrast to satiety signals that are secreted mainly during meals, adiposity signals are tonically active, providing an ongoing message to the brain proportional to total body fat. When an individual's weight changes, the amount of insulin and leptin secreted into the blood changes in parallel, and this is reflected as an altered adiposity signal reaching the brain. As discussed in detail below in §5a the net effect is a change of the brain's sensitivity to satiety signals. For example, when an individual is food restricted or voluntarily diets sufficiently to lose weight, the consequent reduction of leptin and insulin renders brain circuits that control meal size less sensitive to meal-generated satiety signals. This means that more calories than normal must be consumed before a sufficient signal is generated to stop a meal. This situation persists until body weight returns to normal. Conversely, if an individual gains excess weight, the increased insulin and leptin signal in the brain results in increased sensitivity to satiety signals. Smaller meals are consumed until the excess weight is lost.
3. The endocrine pancreas
The endocrine pancreas comprises of isolated islets containing endocrine cells that are dispersed within the exocrine pancreas. In humans, there are around one million islets, totalling one gram of tissue. Most islets contain at least three types of cells, A-cells that secrete glucagon, B-cells that secrete insulin and amylin and D-cells that secrete somatostatin. Other, distinct, islets contain mainly F-cells that secrete pancreatic polypeptide (PP). Although these individual hormones have numerous functions, the generalization can be made that a major function of the pancreatic islets is the control of glucose homeostasis. Insulin and glucagon are perhaps most important in this regard, with insulin being the primary determinant of glucose removal from the blood and the suppression of glucose secretion by the liver. Glucagon on the other hand is a primary stimulant of glucose production and secretion by the liver. Amylin is co-secreted with insulin, and its best known functions are reducing food intake and gastric emptying, and inhibiting pancreatic glucagon secretion and pancreatic and gastric enzyme secretion.
Like the other endocrine pancreatic hormones, PP exerts a variety of regulatory functions, including modulation of gastric motility, pancreatic exocrine secretion and eating, and many of these effects appear to be related to a modulation of cholinergic output. The primary function of pancreatic somatostatin (which is also made in the gastrointestinal tract and hypothalamus) appears to be to provide a local modulatory influence, mainly inhibition, of the secretion and/or activity of most metabolic processes, including the secretion of insulin, amylin and glucagon (Krantic et al. 2004; Guillemin 2005).
4. Somatostatin, pancreatic polypeptide and ingestive behaviour
Acute administration of somatostatin to animals either systemically (Lotter et al. 1981; Levine & Morley 1982) or directly into the brain (Vijayan & McCann 1977; Lotter et al. 1981) reduces food intake. As is the case with several other satiety signals, the ability of systemic somatostatin to reduce food intake requires an intact vagus nerve (Levine & Morley 1982). Chronic infusion of somatostatin, on the other hand, is without effect on food intake, body weight or other parameters (Lins et al. 1980).
The secretion of PP during meals requires an intact vagus nerve and plasma PP levels are directly proportional to the caloric load consumed. Systemic administration of PP to normal and obese mice reduces food intake (Malaisse-Lagae et al. 1977; Asakawa et al. 2003) and body weight (Malaisse-Lagae et al. 1977), and this is thought to be due to stimulation of Y4 receptors in the dorsal vagal complex, including the area postrema (AP), the nucleus of the solitary tract (NTS) and the dorsal motor nucleus of the vagus (DMV) (Whitcomb et al. 1997). PP presumably modulates gastrointestinal function via the actions of these receptors on vagovagal reflexes because the NTS and DMV are the primary sensory nucleus and the site of vagal lower motor neurons, respectively.
Transgenic mice that over-express PP are hypophagic and lean (Ueno et al. 1999). Their plasma PP levels are 20-fold higher and increase twice as much in response to a meal as in normal wild-type mice. The food intake suppression of PP-overexpressing mice is accompanied by a decrease in gastric emptying and both can be counteracted by systemic administration of PP antibodies. Systemic PP administration also reduces food intake in humans (Berntson et al. 1993; Batterham et al. 2003). In contrast to its peripheral action, when PP is administered directly into the brain, it increases food intake (Clark et al. 1984; Flynn et al. 1999) and stimulates rather than inhibits gastric emptying. It is presently unclear which central Y receptors mediate the orexigenic action of PP. Interestingly, however, Y4-receptor immunoreactivity is abundant on orexin neurons in the lateral hypothalamus (Campbell et al. 2003).
5. Insulin and ingestive behaviour
Historically, insulin was considered to act entirely in the periphery since it is too large a peptide to cross the blood–brain barrier and since neurons do not require insulin in order to take up and oxidize glucose for energy (Seaquist et al. 2003). The best-known action of insulin is to increase glucose uptake in most peripheral tissues, consequently lowering the level of glucose in the blood. Diabetic patients take insulin to reduce their hyperglycemia, and a not uncommon side effect of this routine practice is to lower blood glucose to the point of hypoglycemia. However, because hypoglycemia per se can elicit hunger and induce eating (MacKay et al. 1940; Lotter & Woods 1977), the best-characterized behavioural effect of insulin administration is increased food intake resulting from insufficient glucose reaching the brain. This is not a direct effect of insulin since administering glucose along with insulin does not result in eating (Booth 1968), and since reducing the ability of the brain to utilize glucose for energy by administering non-metabolizable glucose analogues also increases eating (Smith & Epstein 1969; Langhans 1996). The view that the brain per se is insensitive to insulin was dispelled by the findings that insulin does in fact enter the brain, that it reacts with specific insulin receptors on neurons, and that it triggers diverse events including reducing food intake and body weight (see reviews in Plum et al. 2005; Porte et al. 2005).
(a) Insulin decreases feeding behaviour
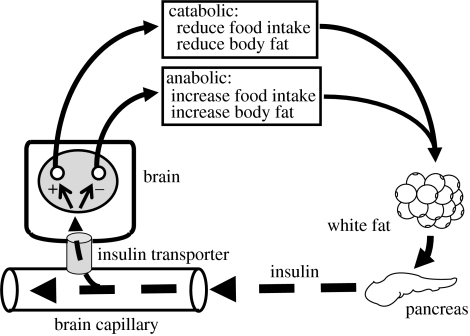
Plasma insulin is low during fasting (basal condition) and increases mainly during and immediately after meals (prandial condition) or glucose administration (stimulated condition). Basal, prandial and stimulated insulin levels are all direct functions of the amount of white adipose tissue in the body, leaner individuals having lower levels and more obese individuals having higher levels (Bagdade et al. 1967; Woods et al. 1974; Polonsky et al. 1988). The amount of insulin in the plasma (along with the amount of leptin in the plasma since its secretion is also directly proportional to white fat (Considine et al. 1996)) may therefore convey an important signal indicating the degree of adiposity to any insulin-sensitive tissue. Indeed, it is now generally accepted that some insulin enters the brain from the circulation, thereby providing a key negative feedback signal in the regulation of body fat (figure 1). When an individual loses weight, less insulin is secreted and less insulin consequently reaches insulin receptors in the hypothalamus and elsewhere in the brain. Because insulin in the brain reduces food intake, food intake consequently increases until body weight (and the insulin signal) is restored. Conversely, when an individual overindulges to the point of gaining body weight, the insulin signal increases, reducing food intake until the weight is lost. Insulin (like leptin) is therefore an important adiposity signal whose activity is integrated with diverse other information to determine food intake; and both hormones fit reasonably well with the concept of a fat-related lipostatic signal to the brain as proposed by Kennedy more than a half a century ago (Kennedy 1953).
Figure 1.
Insulin is secreted into the blood from the pancreas in direct proportion to the amount of fat stored in white adipose tissue. As it circulates through brain capillaries, a small amount of insulin is transported into the brain where it acts on insulin receptors on neurons with either net catabolic or anabolic activity, for example in the arcuate nucleus of the hypothalamus. These neurons in turn influence energy homeostasis (food intake and energy expenditure) and ultimately the amount of fat stored in the body by exerting a net catabolic action.
When exogenous insulin is administered directly into the brain, near or directly within the mediobasal hypothalamus, animals behave as if they have excess fat in the body; i.e. they reduce their food intake and lose weight (see reviews in (Schwartz et al. 1992; Woods et al. 1995; Woods 1996; Woods & Seeley 2001)). The response is dose-dependent (Woods et al. 1979; Riedy et al. 1995), has been documented in numerous species including non-human primates, and is not secondary to illness or incapacitation (Chavez et al. 1995b). Conversely, when insulin antibodies are administered in or near the mediobasal hypothalamus, animals overeat and gain weight (Strubbe & Mein 1977; McGowan et al. 1992). When insulin levels in the brain are held constant for long intervals by means of slow, steady local infusions, body weight is maintained and defended at a level determined by the dose of insulin administered (Woods et al. 1979; Chavez et al. 1995a). Although insulin has not been administered directly into the brains of humans, certain formulations of insulin have been administered intranasally to humans with a consequent increase of cerebrospinal fluid but not plasma insulin (Born et al. 2002). Humans receiving insulin in this way eat less food and lose body fat (Hallschmid et al. 2004a,b).
Although there are suggestions that the brain is able to synthesize insulin (Gerozissis 2004), the bulk of evidence implies the opposite and it is generally acknowledged that most if not all insulin influencing the brain reaches it via the circulation (Schwartz et al. 1994; Woods et al. 2003b; Banks 2004). Insulin enters the brain via insulin receptor-facilitated transport through capillary endothelial cells (Baura et al. 1993; Banks et al. 1997). The process is saturable and selective for insulin. The transport of insulin into the brain is reduced in fasted animals (Strubbe et al. 1988), in animals maintained on a high-fat diet (Woods et al. 2004; Gotoh et al. 2003) and in genetic (Stein et al. 1983) and dietary-induced obesity (Israel et al. 1993; Kaiyala et al. 2000).
Since insulin in the brain ultimately derives from plasma insulin, it should be the case that experimentally induced increases of plasma insulin enter the brain and result in reduced food intake and body weight. The problem, as discussed above, is that experimentally or therapeutically induced increases of plasma insulin typically result in hypoglycemia and a consequent increased tendency to eat. However, when insulin is administered systemically at very low doses that do not elicit hypoglycemia, animals do in fact reduce their food intake (Anika et al. 1980; Vanderweele et al. 1982). Alternatively, when sufficient glucose is administered simultaneously with systemic insulin to circumvent hypoglycemia, animals also eat less (Nicolaidis & Rowland 1976; Woods et al. 1984). In these experiments it cannot be determined whether the insulin is acting in the brain or the periphery to reduce food intake. For whereas increases of plasma insulin are manifest within a few minutes in the brain during meals or following an intravenous insulin infusion (Steffens et al. 1988; Strubbe et al. 1988), centrally administered exogenous insulin usually reduces food intake with a longer latency (Plata-Salaman & Oomura 1986). There is also evidence that insulin acts in the liver to reduce food intake (Surina-Baumgartner et al. 1995), perhaps interacting with glucose (Langhans et al. 2001). Intrameal hepatic portal infusions of insulin antibodies increase meal size, implying that the normal prandial increase in insulinemia is a necessary part of meal termination (Surina-Baumgartner et al. 1995). Although these studies did not disclose the site of origin of the anti-satiating effect of insulin antibodies, the rapid onset of action coupled with the finding that subdiaphragmatic vagotomy eliminates the inhibition of food intake elicited by a low dose of systemic insulin (Vanderweele 1993) is consistent with an abdominal site of action of insulin in satiety. Despite the consistent increase in meal size in response to hepatic portal insulin antagonism, insulin infusions failed to reduce meal size consistently under the same conditions (Surina-Baumgartner et al. 1995). When appropriate doses of insulin and glucose were administered together, however, meal size was reliably reduced (Langhans et al. 2001), consistent with the hypothesis that the acute satiating effect of systemic insulin depends on the presence of glucose.
Insulin receptors are expressed in many areas of the brain (Figlewicz et al. 1985; Corp et al. 1986), with high concentrations in the mediobasal hypothalamus, especially the arcuate nucleus (Plum et al. 2005), which also expresses the most active form of the leptin receptor (Baskin et al. 1999). This region is thought to mediate most of insulin's catabolic action.
Insulin and leptin act in part by increasing the sensitivity of the brain to meal-generated satiety signals. As discussed above, these signals, including amylin and glucagon (figure 2) as well as CCK, are secreted during meals from endocrine cells in both the pancreas and the gastrointestinal tract (Moran 2004; Woods 2004; Strader & Woods 2005). Their cumulated message is integrated within the brain, where it interacts with many other factors (including the signal provided by adiposity signals) to terminate the meal and contribute to the sensation of satiety. When the insulin (Figlewicz et al. 1986; Riedy et al. 1995) or leptin (Matson et al. 1997; Emond et al. 1999, 2001; Matson & Ritter 1999; Ladenheim et al. 2005; Morton et al. 2005) signal is experimentally increased locally within the brain, the ability of CCK and other satiety signals to reduce meal size is increased. Likewise, when the adiposity signal is lowered, the ability of satiety signals to reduce meal size is decreased (McMinn et al. 2000). Thus, adiposity signals contribute to the regulation of body weight by influencing the amount of food eaten during individual meals. When an individual is underweight, the reduced adiposity signal allows larger meals to be consumed until weight is restored and vice versa.
Figure 2.
Insulin, glucagon and amylin are all secreted from the endocrine pancreas, and all participate in the regulation of energy homeostasis. Insulin acts at both the liver and the forebrain to reduce energy intake as well as to suppress hepatic glucose production. Glucagon acts mainly at the liver where it increases glucose production while generating a signal to reduce energy intake that is relayed to the hindbrain. Amylin acts directly at the hindbrain to reduce energy intake. NTS, nucleus of the solitary tract; AP, area postrema; + indicates stimulation.
To summarize, increased insulin in the mediobasal hypothalamus provides a signal that ample or excess energy is available in the body, and one consequence is a reduction of the amount of energy subsequently ingested. Simultaneously, the increased hypothalamic insulin signal also elicits a vagal reflex to the liver to suppress the production of glucose (Obici et al. 2002c). Increased insulin (and probably leptin) is therefore analogous to other signals indicating a surfeit of energy, such as an increase of certain lipids (Davis et al. 1981; Obici et al. 2002b) or glucose (Levin et al. 2004) locally within the hypothalamus; and there is evidence that overlapping intracellular signalling pathways mediate the overall catabolic response to these diverse metabolic signals (Levin et al. 2002; Niswender & Schwartz 2003; Lam et al. 2005a,c; Porte et al. 2005; Seeley & York 2005).
(b) Disruptions of central insulin signalling
Individuals with insulin-dependent diabetes mellitus (IDDM) are hyperphagic. They are not obese because insulin is required for adipocytes to store fat, such that the excess calories consumed are wasted via inefficient utilization as well as via excretion in the urine. When insulin is administered locally into the brain of animals lacking insulin, their hyperphagia is attenuated with no change in other diabetic symptoms (Sipols et al. 1995). Mice lacking insulin receptors selectively in neurons (Brüning et al. 2000), and rats with reduced insulin signalling locally in the hypothalamus (Obici et al. 2002a), have increased food intake and an obese phenotype. Thus, accurate sensing of insulin by hypothalamic receptors is necessary for effective control of food intake, and this is also true of normal control of hepatic glucose output (Lam et al. 2005b). Mice lacking insulin receptors are hyperglycemic and have a short life span (Okamoto & Accili 2003). Genetic rescue of insulin receptors in the pancreas and liver ameliorates but does not normalize the syndrome (Okamoto et al. 2004), and when the insulin receptor is additionally rescued in the brain, the mice are normoglycemic (Okamoto et al. 2004). The important point is that insulin signalling in the hypothalamus is necessary for normal energy homeostasis.
Analogous to insulin receptors in other tissues, hypothalamic insulin receptors are linked to an intracellular signalling cascade utilizing insulin receptor substrate-phosphatidylinositol 3-OH kinase (IRS-PI3K), and when these enzymes are stimulated directly by an insulin mimetic that bypasses the extracellular portion of the insulin receptor, animals eat less and lose weight (Air et al. 2002) and have reduced hepatic glucose production (Obici et al. 2002c). Administering drugs that block the IRS-PI3K pathway locally in the arcuate nucleus prevents insulin from exerting its catabolic effect (Niswender et al. 2003). Although most tissues, including many brain cells, use IRS-1, arcuate neurons and pancreatic B-cells use IRS-2 (Torsoni et al. 2003). Mice lacking IRS-2 have severe obesity and hyperglycemia, and this can be reversed by inserting the IRS-2 gene uniquely into the brain (Burks et al. 2000; Kubota et al. 2004; Lin et al. 2004). The implication from all of these experiments is that the insulin signalling system in the hypothalamus controls many aspects of energy homeostasis and that disruptions of the signalling cascade at any point can lead to overeating, obesity and glucose dysregulation.
As discussed above, plasma insulin is low during weight loss or fasting, and the entry of insulin into the brain is disproportionately decreased even further in such individuals (Owen et al. 1974; Strubbe et al. 1988). This is adaptive since when available energy is low, circulating insulin has less access to key receptors in the hypothalamus thereby allowing larger meals to be consumed once food becomes available; and when available energy is high, insulin more readily enters the brain and limits food intake.
Rats fed a high-fat diet have central insulin resistance (Arase et al. 1988; Chavez et al. 1996; Woods et al. 2004; Gotoh et al. 2003), perhaps contributing to the obesity that often develops. When rats are allowed to select their own mix of macronutrients (protein, carbohydrate and fat), most select a high-fat blend. When insulin is administered into the brain of such rats, food intake and body weight are decreased. However, the intake of dietary carbohydrate and protein is protected as there is a selective reduction of dietary fat (Chavez et al. 1996; van Dijk et al. 1997). This may occur because when insulin is high, carbohydrate is the preferred source of energy by most tissues such that maintaining carbohydrate intake would make teleological sense. Conversely, when insulin is low, fat is the preferred fuel for most tissues. Consistent with this, animals deficient in insulin become hyperphagic on low-fat diets, spilling off the excess consumed carbohydrate as they obtain enough fat for utilizable energy, but normophagic when offered a diet high in fat (Friedman et al. 1983; Chavez et al. 1998).
An important point from this literature is that the ability of insulin to exert its central catabolic action interacts with body fat and its metabolism. It also interacts with the location of fat in the body. Fat distribution differs between males and females. On average, men carry more visceral and less subcutaneous fat, whereas the converse is true of women (Wajchenberg 2000). Body fat distribution in turn is related to the risk for developing the metabolic syndrome, with visceral obesity having a substantially higher risk than subcutaneous obesity. Hence, the metabolic co-morbidities of obesity are more frequent in men (Bjorntorp 1997; Wajchenberg et al. 2002). The relative secretion of leptin and insulin also correlates with fat distribution. Insulin directly correlates with visceral fat and is therefore a risk factor for the metabolic syndrome, whereas leptin correlates better with subcutaneous fat and does not carry the same metabolic risk (Woods et al. 2003a). Insulin is therefore a more relevant adiposity signal in males and leptin a more relevant adiposity signal in females. Consistent with this, the male brain is relatively more sensitive to the catabolic action of insulin than the female brain (Clegg et al. 2003; Hallschmid et al. 2004b), whereas the female brain is relatively more sensitive to leptin (Clegg et al. 2003; Woods et al. 2003a).
(c) Central neural networks influenced by insulin
There are many excellent reviews of the central pathways influenced by insulin and leptin (Woods et al. 1998; Elmquist et al. 1999; Ahima et al. 2000; Schwartz et al. 2000; Cone et al. 2001; Havel 2002; Porte et al. 2002, 2005; Seeley & Woods 2003; Flier 2004; Schwartz & Porte 2005) and they need not be reiterated here. The important features are that the critical receptors are in the mediobasal hypothalamus and especially the arcuate nucleus, and that parallel anabolic and catabolic circuits are both tonically active and both influenced by adiposity as well as many other signals. The presence of opposing hypothalamic circuits for controlling energy homeostasis enables rapid and fine-tuned control over energy homeostasis since the brain can simultaneously turn up one system (e.g. catabolic or anabolic) while turning down the other. Insulin and leptin cause a net reduction of activity in hypothalamic anabolic circuits and a net increase of activity in hypothalamic catabolic circuits.
It is axiomatic that the brain exercises regulatory control over body fat content, and that this is manifest at least partly via the control of food intake. It is just as obvious that the brain influences blood glucose levels as well, in part via direct control of autonomic nervous system projections to the pancreatic islets and liver. Only recently, however, has it become recognized that the same or closely allied neurons in the hypothalamus control both food intake and the impact of islet hormones on hepatic glucose production, and that the same receptors and signals evidently control both responses.
As reviewed above, when insulin is administered near or directly into the arcuate, animals become hypophagic, and when insulin is low or absent in the brain, animals become hyperphagic, and this can be attenuated by local administration of insulin into the brain (Sipols et al. 1995). Increased insulin in the arcuate also reduces hepatic glucose production via the vagus nerve (Obici et al. 2002c; Pocai et al. 2005); likewise, arcuate administration of oleic acid also reduces both food intake and hepatic glucose production (Obici et al. 2002b; Lam et al. 2005b). Thus, molecules that signify ample available energy (insulin or certain fatty acids) initiate a signal to the liver to stop producing so much glucose while simultaneously sending a message to eat less food.
Evidence suggests that normal control of hepatic glucose output relies on an adequate insulin signal both locally in the liver as well as within the hypothalamus, and that an adequate fatty acid signal locally within the hypothalamus is also important. When either signal is compromised in the brain, animals gain excess weight and become systemically insulin resistant (Brüning et al. 2000; Obici et al. 2002c; Okamoto et al. 2004; Lam et al. 2005b), these being two key symptoms of the metabolic syndrome. Leptin elicits comparable actions as insulin in the hypothalamus (Niswender & Schwartz 2003; Munzberg & Myers 2005) although it should be noted that the way the two adiposity signals innervate specific arcuate neurons differs (Xu et al. 2005). As with insulin, leptin in the arcuate reduces food intake and body weight (Campfield et al. 1995; Seeley et al. 1996), and reduced leptin signalling results in hyperphagia and obesity as well as systemic (Coleman 1978; Zhang et al. 1994) and central (Ikeda et al. 1986) insulin resistance. Administration of leptin into the brain reverses insulin-deficiency hyperphagia (Sindelar et al. 1999) and lessens systemic insulin resistance (Haluzik et al. 2004). Hence, convergent signals from insulin and leptin act in the brain to regulate both energy and glucose homeostasis and a defect in either leptin or insulin signalling in the brain can result in overeating, weight gain, insulin resistance and other sequellae of the metabolic syndrome (Porte et al. 2005; Schwartz & Porte 2005).
In sum, plasma insulin is a signal that reflects both circulating energy in the form of glucose and stored energy in the form of visceral adipose tissue. Insulin acts directly at the liver to suppress the synthesis and secretion of glucose, and some plasma insulin is transported into the brain where it provides an important and indeed necessary input for the appropriate regulation of both stored energy and glucose secretion by the liver. As occurs in the brain, the liver is also simultaneously influenced by competing signals with regard to glucose secretion since glucagon both stimulates insulin secretion and increases hepatic glucose output.
6. Amylin and ingestive behaviour
Islet amyloid polypeptide or amylin, is co-secreted with insulin from pancreatic B-cells. Whereas with regard to food intake, insulin functions mainly as an adiposity signal and glucagon functions mainly as a satiety signal, amylin has characteristics of both kinds of signal. Like insulin, plasma amylin levels are low during fasting and increase during meals and following glucose administration, and the levels are all directly proportional to body fat. Amylin and insulin are normally co-secreted in a fixed molecular ratio (insulin to amylin) of between ten and one hundred. Obesity, diabetes mellitus, pancreatic cancer and certain pharmacological interventions all tend to increase the amount of amylin relative to insulin.
(a) Amylin as a satiety signal
Eating results in a rapid increase in plasma amylin that is directly proportional to meal size (Butler et al. 1990; Moore & Cooper 1991; Young & Denaro 1998). Administration of exogenous amylin prior to a meal dose-dependently reduces food intake in rats and mice (Chance et al. 1991; Morley & Flood 1991; Lutz et al. 1994, 1995; Morley et al. 1994; Arnelo et al. 1996a), mainly due to a reduction in meal size (Lutz et al. 1995, 2001b; Reidelberger et al. 2002; Mollet et al. 2004), without producing a conditioned taste aversion (Chance et al. 1992; Lutz et al. 1995; Morley et al. 1997; Asarian et al. 1998; Rushing et al. 2002). The effect of exogenous amylin on meal pattern is therefore similar to that of CCK. As discussed below, plasma amylin is thought to function as a satiety signal by accessing receptors in the AP in the hindbrain, a region with a relatively permeable blood–brain barrier. Nonetheless, some plasma amylin probably does enter the brain via facilitated transport through the blood–brain barrier (Banks et al. 1995), and when amylin is administered directly into the lateral or 3rd-cerebral ventricle, it elicits a potent and long-lasting anorectic effect at very low doses (Rushing et al. 2002). Consistent with this, blockade of central amylin receptors produces a long-lasting increase in food intake, body weight and body adiposity (Rushing et al. 2001).
(b) Disruption of amylin signalling
Several amylin antagonists are available (e.g. amylin 8-37, AC 253 and AC 187), and when administered prior to meals either systemically or directly into the AP, each attenuates the anorectic action of exogenous amylin (Lutz et al. 1996, 1997b, 2000; Mollet et al. 2004; Reidelberger et al. 2004) and increases food intake when administered alone (Grabler & Lutz 2004; Mollet et al. 2004; Reidelberger et al. 2004). These data strongly imply a physiological role of endogenous amylin in the regulation of food intake, specifically in the regulation of meal size. Consistent with this, mice lacking amylin have a significantly increased rate of body weight gain and slightly increased cumulative food intake relative to controls (Gebre-Medhin et al. 1997, 1998; Lutz 2005). Little is known of the post-receptor intracellular signalling mechanisms elicited by amylin, although its anorectic action has been linked to the formation of cGMP in the AP (Riediger et al. 2001; Becskei et al. 2004).
(c) Interactions of amylin with other signals
Amylin and CCK have been reported to interact synergistically to reduce meal size (Bhavsar et al. 1998). While CCK antagonists do not affect the anorectic action of amylin, amylin antagonists attenuate CCK's anorectic action (Morley et al. 1994; Lutz et al. 1996, 1997a, 2000), and the anorectic effect of CCK is almost absent in mice lacking amylin (Mollet et al. 2003). These data have been interpreted to indicate that endogenous amylin has a neuromodulatory function within the AP/NTS region of the hindbrain that facilitates or amplifies other satiety signals such as those elicited by CCK that are conveyed to the NTS via afferent vagal nerves (Ritter & Ladenheim 1985; Moran et al. 1997; Lutz et al. 1998; Mollet et al. 2003). This mechanism would therefore be analogous to that by which the adiposity signals insulin and leptin act in the hypothalamus to influence the brain's sensitivity to meal-generated signals.
Amylin and glucose activate the same neurons in the AP (Riediger et al. 2002), as do amylin and glucagon-like peptide-1 (GLP-1) (Riediger et al. 2002). AP neurons responsive to glucose are also responsive to CCK (Funahashi & Adachi 1993; Wang et al. 2000). Perhaps analogous to what occurs in the arcuate nucleus, AP neurons are therefore able to integrate several metabolic and hormonal signals important in the control of energy homeostasis.
(d) Central neural circuits stimulated by amylin
The AP is the predominant site of action of circulating amylin in the brain. Amylin's anorectic action is completely abolished following lesions in the AP/NTS region (Lutz et al. 1998, 2001b), and amylin dose-dependently stimulates AP neurons in vitro, with a threshold concentration in the range of circulating plasma amylin concentrations (Riediger et al. 2001, 2002). Amylin also induces Fos expression in the AP (Rowland et al. 1997; Rowland & Richmond 1999; Riediger et al. 2001, 2004; Becskei et al. 2004), and amylin antagonists that attenuate amylin's anorectic action also eliminate the Fos response in the AP (Riediger et al. 2001, 2002, 2004). Finally, when rats are refed after being deprived, there is an increase of Fos in the AP/NTS that is subsequently attenuated in the AP when an amylin antagonist is administered (Riediger et al. 2004). The point is that the AP/NTS is able to integrate diverse signals related to meals, and amylin appears to render the region more sensitive to other metabolic signals that reduce food intake.
There is no unique amylin receptor gene. Rather, the functional amylin receptor in the AP (and elsewhere) utilizes a calcitonin receptor (CTR) whose amylin-specificity and affinity are bestowed via the co-expression of one of several receptor activity modifying proteins (RAMPs) (McLatchie et al. 1998; Christopoulos et al. 1999; Foord & Marshall 1999; Muff et al. 1999; Sexton et al. 2001; Fischer et al. 2002). The prototypical amylin receptor results from the interaction of RAMP 1 or RAMP 3 with the CTR. RAMP1 and RAMP3 mRNA have been co-localized with amylin-induced Fos mRNA in the rat AP (Ueda et al. 2001; Barth et al. 2004), and most amylin-sensitive AP neurons also express the CTR (Becskei et al. 2004).
In addition to the AP/NTS, amylin elicits a strong Fos response in the lateral parabrachial nucleus (lPBN), the central nucleus of the amygdala (CeNA) and the bed nucleus of the stria terminalis (Rowland et al. 1997; Rowland & Richmond 1999; Riediger et al. 2004). All of these Fos responses are markedly attenuated in AP-lesioned rats (Rowland & Richmond 1999; Riediger et al. 2004), implying that the other sites are downstream of direct amylin action in the AP. The NTS and the lPBN are critical relay points for satiety and other signals to reach forebrain areas (Berthoud 2002).
There is no conclusive evidence that peripheral amylin has a direct effect on the hypothalamus. However, fasting-induced Fos activation in lateral hypothalamic neurons that is reversed by refeeding can also be reversed by administering systemic amylin to rats without access to food (Becskei et al. 2004). Further, peripheral amylin or its agonist salmon calcitonin down-regulates the expression of both orexin and melanin concentrating hormone (MCH) in the lateral hypothalamus (LHA; Barth et al. 2003). Since the LHA is devoid of amylin binding sites (Beaumont et al. 1993; Sexton et al. 1994; Christopoulos et al. 1995), the presumption is that the LHA receives inhibitory input from amylin-activated neurons in the brainstem. As discussed above, LHA neurons expressing orexin and MCH are also inhibited by signals coming from insulin and leptin, whether directly or indirectly.
(e) Amylin as an adiposity signal
Besides having a strong satiating effect, amylin also shares many characteristics with the adiposity signals insulin and leptin, not the least of which is that its levels are highly correlated with body fat (Cooper 1994; Pieber et al. 1994; Wimalawansa 1997). When amylin is infused chronically via osmotic minipumps placed in the abdominal cavity, there is a sustained reduction in food intake and body weight gain that is abolished in rats with AP/NTS lesions (Arnelo et al. 1996b; Lutz et al. 2001b). Consistent with this, and as discussed above, mice lacking amylin gain weight more rapidly than controls, especially when young (Gebre-Medhin et al. 1997, 1998; Lutz 2005). Obese Zucker fa/fa rats have dysfunctional leptin receptors. Besides being hyperinsulinemic and hyperleptinemic, these rats are also hyperamylinemic. Administering an amylin antagonist to these rats results in increased food intake, presumably by blocking endogenous amylin (Grabler & Lutz 2004). Amylin may therefore function as an important adiposity signal in these animals since their brains are insensitive to insulin's (Ikeda et al. 1986) as well as leptin's catabolic action. Consistent with this, the ability of amylin to reduce meal size is normal in rats on a high-fat diet that develop diet-induced obesity (Eiden et al. 2002).
(f) Pathophysiology of amylin's anorectic action
Few studies have considered amylin's anorectic action under pathophysiological conditions. Amylin has been suggested to contribute to the anorexia occurring during certain pancreatic neoplastic diseases that are characterized by supraphysiological plasma amylin levels (Stridsberg et al. 1995; Permert et al. 1997). Lack of amylin may also contribute to the hyperphagia that occurs in IDDM since these individuals also lack amylin. Consistent with this, long-term treatment of late-stage type-2 diabetics who are overweight and insulin resistant with an amylin analogue in addition to insulin resulted in far greater weight loss than occurred in diabetics receiving insulin only. Thus, co-administration of insulin plus amylin might help to circumvent the increase in body weight that occurs in type-2 diabetics treated with insulin, insulin secretagogues or insulin sensitizers (Weyer et al. 2001; Hollander et al. 2004).
To summarize, blood-borne amylin reduces meal size by stimulating neurons in the AP. Besides enhancing the action of other satiety signals at the level of the hindbrain, the amylin signal interacts with other signals controlling energy homeostasis at the level of the LHA and probably elsewhere. Finally, there is evidence that amylin functions as an adiposity signal in addition to a satiety signal.
7. Glucagon and ingestive behaviour
As mentioned above, pancreatic glucagon's metabolic functions are in many respects opposite to those of insulin. Glucagon's most prominent physiological role is to stimulate glucose production via hepatic glycogenenolysis or gluconeogensis, thereby helping maintain euglycemia during states of rapid glucose utilization or fasts, respectively. Pancreatic glucagon is also secreted as food is ingested, and this is thought to provide a satiety signal leading to termination of the meal (Geary 1990, 1998). In contrast to insulin and amylin, neither basal, prandial, nor stimulated glucagon secretion is related to body adiposity (Holst et al. 1983b; Raben et al. 1994), and repeated administration of glucagon in amounts sufficient to reduce food intake have few metabolic side effects (Geary 1990, 1998). Therefore, unlike insulin and amylin, glucagon appears not to be an adiposity signal.
(a) The glucagon family of peptides
The proglucagon gene is expressed in A-cells of the endocrine pancreas and L-cells of the intestinal wall. It is also expressed in the NTS in the hindbrain (Larsen et al. 1997; Lovshin & Drucker 2000). The control of proglucagon expression and of proglucagon post-translational processing differs among these tissues. Brain and L-cell proglucagon-derived peptides include glicentin (proglucagon 1-69), oxyntomodulin (proglucagon 33-69), glucagon-like peptide-1 (GLP-1; proglucagon 78-107 amide), and GLP-2 (proglucagon 126-158). Pancreatic A-cells synthesize only true pancreatic glucagon (proglucagon 33-61). The hepatocyte glucagon receptor is highly selective for glucagon, with little affinity for any other proglucagon-derived peptides (Hjorth et al. 1994). Other proglucagon-derived molecules that have been implicated in the control of food intake include GLP-1 (Tang-Christensen et al. 1996; Thiele et al. 1998; Rinaman 1999; van Dijk & Thiele 1999; Kinzig et al. 2002; Schick et al. 2003), GLP-2 (Tang-Christensen et al. 2000) and oxyntomodulin (Cohen et al. 2003; Baggio et al. 2004). Only glucagon is considered here.
(b) Prandial glucagon secretion
Eating stimulates an immediate, brief increase in glucagon secretion. It occurs within one minute of the onset of spontaneous meals (de Jong et al. 1977) and also increases during sham feeding (Nilsson & Uvnas-Wallensten 1997). These observations, coupled with the absence of prandial glucagon secretion in patients with transplanted pancreata (Secchi et al. 1995), collectively imply that glucagon secretion can be elicited by cephalic stimuli. Consistent with this, stimulation of the ventromedial hypothalamus, the dorsal vagal complex, or the gastric or hepatic branches of the vagus nerve increase glucagon secretion (Marliss et al. 1973; de Jong et al. 1977; Berthoud et al. 1990). Although most meals, except pure carbohydrate meals, stimulate glucagon secretion, high-protein meals are most effective (Langhans et al. 1984).
The magnitude of the prandial increase in systemic glucagon concentration during most meals is modest (Muller et al. 1971; Holst et al. 1983a; Geary 1996) because first-pass hepatic extraction is very efficient. Portal vein glucagon concentration is considerably higher (Ishida et al. 1983) and can be substantial without any detectable change in systemic plasma glucagon concentration (Dencker et al. 1975; Langhans et al. 1984). For this reason, the primary target of prandial pancreatic glucagon secretion is likely the liver, and, consistent with this, prandial glucagon secretion stimulates hepatic glycogenolysis during meals (Langhans et al. 1982a, 1984). As described below, glucagon's satiety effect also originates in the liver.
(c) Glucagon decreases meal size
Subcutaneous, intramuscular, intraperitoneal, systemic intravenous and intra-hepatic portal administration of glucagon all reduce feeding (Geary 1990, 1998). Hepatic-portal infusions are most reliable, eliciting rapid, dose-dependent decreases in food intake at doses ten times lower than vena caval infusions (Geary et al. 1993), thus providing compelling evidence that glucagon acts in the liver to inhibit eating. Hepatic-portal infusions of glucagon selectively decrease the size of the meal without affecting the duration of the subsequent intermeal interval (Le Sauter et al. 1991), and following intraperitoneal glucagon injections, rats display normal postprandial satiety behaviour (Geary & Smith 1982a), indicating that glucagon contributes to satiation as opposed to postprandial satiety. Intravenous infusion of glucagon also decreases meal size in humans (Geary et al. 1992). The subjects reported feeling equally satiated as during control meals and reported no side effects, and the dose administered produced systemic plasma glucagon concentrations that approximated the range produced by normal meals (Geary 1998).
The signal to reduce meal size generated by glucagon reaches the brain via sensory axons of the vagus nerve since total subdiaphragmatic vagotomy, selective hepatic vagotomy, or lesion of the vagal afferent terminal fields within the NTS all prevent glucagon's feeding-inhibitory effect (Geary & Smith 1983; MacIsaac & Geary 1985; Weatherford & Ritter 1986, 1988; Geary et al. 1993). The transduction mechanism by which glucagon generates a vagal afferent signal is unknown. Glucagon receptors have not been identified on vagal sensory neurons, implying an indirect mechanism. However, glucagon's feeding-inhibitory potency has been dissociated from its glycogenolytic and hyperglycemic effects (Geary & Smith 1982b; Geary et al. 1987) as well as from insulin-mediated effects (De Castro et al. 1978; Geary et al. 1997). Glucagon does have vascular and smooth muscle actions in the liver, and the majority of hepatic vagal afferent fibers innervate the biliary tree and hepatic vasculature (Sawchenko & Friedman 1979; Berthoud et al. 1992); however, the glucagon fragment, glucagon (1-21), which is a full agonist for the vascular and smooth muscle effects, does not inhibit feeding (Geary 1987). Glucagon also inhibits feeding in the absence of any effect on gastric emptying (Stockinger & Geary 1989).
Another possibility is that glucagon affects the hepatocyte in a way that reduces its membrane potential, leading to an electrotonic effect that is transmitted to vagal afferents, as originally proposed by Russek (1981). Glucagon could affect the hepatocyte membrane potential directly (Petersen 1974), as a consequence of increasing intracellular fatty acid oxidation (McGarry & Foster 1980), or as a consequence of decreasing hepatocyte Na+/K+-ATPase activity (Langhans & Scharrer 1987a,b; Rossi et al. 1995). Lutz et al. (2001a) recently established the physiological relevance of glucagon's effect on the hepatic membrane potential by demonstrating that glucagon antibodies depolarize the hepatocyte membrane in vivo. As yet, however, this mechanism has not been convincingly linked to glucagon satiety. Finally, almost nothing is known about the central processing of glucagon satiety, other than the necessity of the hindbrain areas where the signal is received from the vagus nerve (Weatherford & Ritter 1988).
(d) Reducing the glucagon signal increases meal size
Langhans et al. (1982b) demonstrated that pre-meal intraperitoneal administration of a highly specific polyclonal glucagon antibody significantly increased meal size and reduced hepatic glucose secretion, presumably as a result of decreased hepatic glycogenolysis. Hepatic-portal but not vena caval infusions of the same antibody also increased meal size (Le Sauter et al. 1991; Geary et al. 1993). These findings imply that endogenous glucagon, secreted during meals, normally contributes to meal cessation by an action in the liver.
(e) Interactions with other signals
The ability of glucagon to reduce meal size depends upon functional interactions with other meal-related signals because even massive doses of glucagon fail to inhibit sham feeding in rats with open gastric cannulas, in which food does not accumulate in the stomach or enter the intestines in significant amounts (Geary & Smith 1982b). CCK has been implicated as one such signal, since at doses that are insufficient to reduce sham feeding, CCK is able to reinstate glucagon's dose-dependent satiety effect (Le Sauter & Geary 1987). Oestradiol also interacts with glucagon since both the feeding-inhibitory effect of glucagon and the feeding-stimulatory effect of glucagon antibodies are increased by estradiol treatment in ovariectomized rats (Geary & Asarian 2001). Thus, glucagon contributes to the marked sexual differentiation of the control of eating in rats. Whether or not glucagon interacts with the adiposity signals insulin and leptin has not been investigated. Pretreatment with the amylin receptor antagonist, calcitonin gene-related peptide-(8-37), however, blocked the satiety effect of glucagon under some, but not all, test conditions (Lutz et al. 1996). Whether this potential interaction with glucagon signalling relates to amylin's function as a satiety signal, an adiposity signal, or both, is unclear.
(f) Pathophysiology of glucagon satiety
Changes in glucagon secretion and disruptions of food intake have been associated in various diseases, but no causal relationships have been established. For example, glucagonoma produces extremely elevated glucagon levels and severe anorexia (Wynick et al. 1993; Madsen et al. 1995). Whether this involves over-stimulation of normal glucagon satiety or some non-specific feeding-inhibitory effect is unknown (see Geary 1998, for a review). Postprandial glucagon levels are increased in type-2 diabetes mellitus and may contribute to hyperglycemia in both type-1 and type-2 diabetes mellitus (Dinneen et al. 1995), but these changes would seem to predict increased satiety and consequently reduced caloric intake, whereas as discussed above, diabetes is associated with hyperphagia. On the other hand, a missense mutation in the glucagon receptor gene that reduces glucagon signalling has been found in some patients with late-onset type-2 diabetes mellitus (Hager et al. 1995), a phenomenon which potentially could contribute to reduced satiety and overeating.
8. Conclusions
Hormones of the endocrine pancreas are collectively at the crossroads of many aspects of energy homeostasis. Glucagon, amylin and PP act in the short term to reduce meal size, and insulin sensitizes the brain to short-term meal-generated satiety signals; and insulin and perhaps amylin and PP as well act over longer intervals to modulate the amount of fat maintained and defended by the brain. Hormones of the endocrine pancreas interact with receptors at many points along the gut–brain axis, from the liver to the sensory vagus nerve to the hindbrain to the hypothalamus; and their signals are conveyed both neurally and humorally. Finally, their actions include gastrointestinal and metabolic as well as behavioural effects. Thus, the production and release of all of these hormones of the endocrine pancreas (insulin, glucagon, amylin and PP) are influenced by food intake and (at least in part) by body fat (insulin and amylin), and they in turn control caloric intake (insulin, glucagon, amylin, PP) and body weight (insulin, amylin and PP) through peripheral (glucagon, PP and maybe insulin) as well as central (amylin and insulin) mechanisms.
Footnotes
One contribution of 16 to a Theme Issue ‘Appetite’.
References
- Ahima R.S, Saper C.B, Flier J.S, Elmquist J.K. Leptin regulation of neuroendocrine systems. Front. Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. doi:10.1006/frne.2000.0197 [DOI] [PubMed] [Google Scholar]
- Air E.L, Strowski M.Z, Benoit S.C, Conarello S.L, Salituro G.M, Guan X.M, Liu K, Woods S.C, Zhang B.B. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat. Med. 2002;8:179–183. doi: 10.1038/nm0202-179. doi:10.1038/nm0202-179 [DOI] [PubMed] [Google Scholar]
- Anika S.M, Houpt T.L, Houpt K.A. Insulin as a satiety hormone. Physiol. Behav. 1980;25:21–23. doi: 10.1016/0031-9384(80)90175-4. doi:10.1016/0031-9384(80)90175-4 [DOI] [PubMed] [Google Scholar]
- Arase K, Fisler J.S, Shargill N.S, York D.A, Bray G.A. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am. J. Physiol. 1988;255:R974–R981. doi: 10.1152/ajpregu.1988.255.6.R974. [DOI] [PubMed] [Google Scholar]
- Arnelo U, Blevins J.E, Larsson J, Permert J, Westermark P, Reidelberger R.D, Adrian T.E. Effects of acute and chronic infusion of islet amyloid polypeptide on food intake in rats. Scand. J. Gastroenterol. 1996a;31:83–89. doi: 10.3109/00365529609031632. [DOI] [PubMed] [Google Scholar]
- Arnelo U, Permert J, Adrian T.E, Larsson J, Westermark P, Reidelberger R.D. Chronic infusion of islet amyloid polypeptide causes anorexia in rats. Am. J. Physiol. 1996b;271:R1654–R1659. doi: 10.1152/ajpregu.1996.271.6.R1654. [DOI] [PubMed] [Google Scholar]
- Asakawa A, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. doi: 10.1016/s0016-5085(03)00216-6. doi:10.1016/S0016-5085(03)00216-6 [DOI] [PubMed] [Google Scholar]
- Asarian L, Eckel L.A, Geary N. Behaviorally specific inhibition of sham feeding by amylin. Peptides. 1998;19:1711–1718. doi: 10.1016/s0196-9781(98)00127-2. doi:10.1016/S0196-9781(98)00127-2 [DOI] [PubMed] [Google Scholar]
- Bagdade J.D, Bierman E.L, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J. Clin. Invest. 1967;46:1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio L.L, Huang Q, Brown T.J, Drucker D.J. Oxyntomodulin and glucagon-like peptide 1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. doi: 10.1053/j.gastro.2004.04.063. doi:10.1053/j.gastro.2004.04.063 [DOI] [PubMed] [Google Scholar]
- Banks W.A. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. doi:10.1016/j.ejphar.2004.02.040 [DOI] [PubMed] [Google Scholar]
- Banks W.A, Kastin A.J, Maness L.M, Huang W, Jaspan J.B. Permeability of the blood–brain barrier to amylin. Life Sci. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. doi:10.1016/0024-3205(95)02197-Q [DOI] [PubMed] [Google Scholar]
- Banks W.A, Jaspan J.B, Huang W, Kastin A.J. Transport of insulin across the blood–brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. doi:10.1016/S0196-9781(97)00231-3 [DOI] [PubMed] [Google Scholar]
- Barth S.W, Riediger T, Lutz T.A, Rechkemmer G. Differential effects of amylin and salmon calcitonin on neuropeptide gene expression in the lateral hypothalamic area and the arcuate nucleus in the rat. Neurosci. Lett. 2003;341:131–134. doi: 10.1016/s0304-3940(03)00190-3. doi:10.1016/S0304-3940(03)00190-3 [DOI] [PubMed] [Google Scholar]
- Barth S.W, Riediger T, Lutz T.A, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004;997:97–102. doi: 10.1016/j.brainres.2003.10.040. doi:10.1016/j.brainres.2003.10.040 [DOI] [PubMed] [Google Scholar]
- Baskin D.G, Hahn T.M, Schwartz M.W. Leptin sensitive neurons in the hypothalamus. Horm. Metab. Res. 1999;31:345–350. doi: 10.1055/s-2007-978751. [DOI] [PubMed] [Google Scholar]
- Batterham R.L, Le Roux C.W, Cohen M.A, Park A.J, Ellis S.M, Patterson M, Frost G.S, Ghatei M.A, Bloom S.R. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003;88:3989–3992. doi: 10.1210/jc.2003-030630. doi:10.1210/jc.2003-030630 [DOI] [PubMed] [Google Scholar]
- Baura G, Foster D, Porte D, Jr, Kahn S.E, Bergman R.N, Cobelli C, Schwartz M.W. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J. Clin. Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont K, Kenney M.A, Young A.A, Rink T.J. High affinity amylin binding sites in rat brain. Mol. Pharmacol. 1993;44:493–497. [PubMed] [Google Scholar]
- Becskei C, Riediger T, Zünd D, Wookey P, Lutz T.A. Immunohistochemical mapping of calcitonin receptors in the adult brain. Brain Res. 2004;1030:221–233. doi: 10.1016/j.brainres.2004.10.012. doi:10.1016/j.brainres.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Berntson G.G, Zipf W.B, O'Dorisio T.M, Hoffman J.A, Chance R.E. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides. 1993;14:497–503. doi: 10.1016/0196-9781(93)90138-7. doi:10.1016/0196-9781(93)90138-7 [DOI] [PubMed] [Google Scholar]
- Berthoud H.R. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. doi:10.1016/S0149-7634(02)00014-3 [DOI] [PubMed] [Google Scholar]
- Berthoud H.R, Fox E.A, Powley T.L. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am. J. Physiol. 1990;258:R160–R168. doi: 10.1152/ajpregu.1990.258.1.R160. [DOI] [PubMed] [Google Scholar]
- Berthoud H.R, Kressel M, Neuhuber W.L. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat. Embryol. 1992;186:431–442. doi: 10.1007/BF00185458. doi:10.1007/BF00185458 [DOI] [PubMed] [Google Scholar]
- Bhavsar S, Watkins J, Young A. Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol. Behav. 1998;64:557–561. doi: 10.1016/s0031-9384(98)00110-3. doi:10.1016/S0031-9384(98)00110-3 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Body fat distribution, insulin reistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. doi:10.1016/S0899-9007(97)00191-3 [DOI] [PubMed] [Google Scholar]
- Booth D.A. Effects of intrahypothalamic glucose injection on eating and drinking elicited by insulin. J. Comp. Physiol. Psychol. 1968;65:13–16. doi: 10.1037/h0025396. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor G.P, Bickel U, Fehm H.L. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. doi:10.1038/nn849 [DOI] [PubMed] [Google Scholar]
- Brüning J.C, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. doi:10.1126/science.289.5487.2122 [DOI] [PubMed] [Google Scholar]
- Burks D.J, de Mora J.F, Schubert M, Withers D.J, Myers M.G, Towery H.H, Altamuro S.L, Flint C.L, White M.F. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. doi:10.1038/35030105 [DOI] [PubMed] [Google Scholar]
- Butler P.C, Chou J, Carter W.B, Wang Y.N, Bu B.H, Chang D, Chang J.K, Rizza R.A. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- Campbell R.E, Smith M.S, Allen S.E, Grayson B.E, Ffrench-Mullen J.M, Grove K.L. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J. Neurosci. Methods. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield L.A, Smith F.J, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chance W.T, Balasubramaniam A, Zhang F.S, Wimalawansa S.J, Fischer J.E. Anorexia following the intrahypothalamic administration of amylin. Brain Res. 1991;539:352–354. doi: 10.1016/0006-8993(91)91644-g. doi:10.1016/0006-8993(91)91644-G [DOI] [PubMed] [Google Scholar]
- Chance W.T, Balasubramaniam A, Chen X, Fischer J.E. Tests of adipsia and conditioned taste aversion following the intrahypothalamic injection of amylin. Peptides. 1992;13:961–964. doi: 10.1016/0196-9781(92)90057-a. doi:10.1016/0196-9781(92)90057-A [DOI] [PubMed] [Google Scholar]
- Chavez M, Kaiyala K, Madden L.J, Schwartz M.W, Woods S.C. Intraventricular insulin and the level of maintained body weight in rats. Behav. Neurosci. 1995a;109:528–531. doi: 10.1037//0735-7044.109.3.528. doi:10.1037/0735-7044.109.3.528 [DOI] [PubMed] [Google Scholar]
- Chavez M, Seeley R.J, Woods S.C. A comparison between the effects of intraventricular insulin and intraperitoneal LiCl on three measures sensitive to emetic agents. Behav. Neurosci. 1995b;109:547–550. doi:10.1037/0735-7044.109.3.547 [PubMed] [Google Scholar]
- Chavez M, Riedy C.A, van Dijk G, Woods S.C. Central insulin and macronutrient intake in the rat. Am. J. Physiol. 1996;271:R727–R731. doi: 10.1152/ajpregu.1996.271.3.R727. [DOI] [PubMed] [Google Scholar]
- Chavez M, Seeley R.J, Havel P.J, Friedman M.I, Matson C.A, Woods S.C, Schwartz M.W. Effect of a high-fat diet on food intake and hypothalamic neuropeptide gene expression in streptozotocin diabetes. J. Clin. Invest. 1998;102:340–346. doi: 10.1172/JCI603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos G, Paxinos G, Huang X.F, Beaumont K, Toga A.W, Sexton P.M. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can. J. Physiol. Pharmacol. 1995;73:1037–1041. doi: 10.1139/y95-146. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry K.J, Morfis M, Tilakaratne N, Gao Y, Fraser N.J, Main M.J, Foord S.M, Sexton P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Clark J.T, Kalra P.S, Crowley W.R, Kalra S.P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Clegg D.J, Riedy C.A, Smith K.A, Benoit S.C, Woods S.C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Cohen M.A, Ellis S.M, Le Roux C.W, Batterham R.L, Park A, Patterson M, Frost G.S, Ghatei M.A, Bloom S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. doi:10.1210/jc.2003-030421 [DOI] [PubMed] [Google Scholar]
- Coleman D.L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. doi:10.1007/BF00429772 [DOI] [PubMed] [Google Scholar]
- Cone R.D, Cowley M.A, Butler A.A, Fan W, Marks D.L, Low M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Considine R.V, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. doi:10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- Cooper G.J. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr. Rev. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. doi:10.1210/er.15.2.163 [DOI] [PubMed] [Google Scholar]
- Corp E.S, Woods S.C, Porte D, Jr, Dorsa D.M, Figlewicz D.P, Baskin D.G. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci. Lett. 1986;70:17–22. doi: 10.1016/0304-3940(86)90430-1. doi:10.1016/0304-3940(86)90430-1 [DOI] [PubMed] [Google Scholar]
- Davis D.D, Wirtshafter D, Asin K.E, Brief D. Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science. 1981;212:81–83. doi: 10.1126/science.7193909. [DOI] [PubMed] [Google Scholar]
- De Castro J.M, Paullin S.K, DeLugas J.M. Insulin and glucagon as determinants of body weight setpoint and microregulation in rats. J. Comp. Physiol. Psychol. 1978;92:571–579. doi: 10.1037/h0077485. [DOI] [PubMed] [Google Scholar]
- de Jong A, Strubbe J.H, Steffens A.B. Hypothalamic influence on insulin and glucagon release in the rat. Am. J. Physiol. 1977;233:E380–E388. doi: 10.1152/ajpendo.1977.233.5.E380. [DOI] [PubMed] [Google Scholar]
- Dencker H, Hedner P, Holst J, Tranberg K.G. Pancreatic glucagon response to an ordinary meal. Scand. J. Gastroenterol. 1975;10:471–474. [PubMed] [Google Scholar]
- Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycemia in IDDM. Diabetologia. 1995;38:337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- Eiden S, Daniel C, Steinbrueck A, Schmidt I, Simon E. Salmon calcitonin—a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J. Physiol. 2002;541:1041–1048. doi: 10.1113/jphysiol.2002.018671. doi:10.1113/jphysiol.2002.018671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist J.K, Elias C.F, Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. doi:10.1016/S0896-6273(00)81084-3 [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz G.J, Ladenheim E.E, Moran T.H. Central leptin modulates behavioral and neural responsivity to CCK. Am. J. Physiol. 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- Emond M, Ladenheim E.E, Schwartz G.J, Moran T.H. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol. Behav. 2001;72:123–128. doi: 10.1016/s0031-9384(00)00393-0. doi:10.1016/S0031-9384(00)00393-0 [DOI] [PubMed] [Google Scholar]
- Figlewicz D.P, Dorsa D.M, Stein L.J, Baskin D.G, Paquette T, Greenwood M.R.C, Woods S.C, Porte D., Jr Brain and liver insulin binding is decreased in Zucker rats carrying the “fa” gene. Endocrinology. 1985;117:1537–1543. doi: 10.1210/endo-117-4-1537. [DOI] [PubMed] [Google Scholar]
- Figlewicz D.P, Stein L.J, West D, Porte D, Jr, Woods S.C. Intracisternal insulin alters sensitivity to CCK-induced meal suppression in baboons. Am. J. Physiol. 1986;250:R856–R860. doi: 10.1152/ajpregu.1986.250.5.R856. [DOI] [PubMed] [Google Scholar]
- Fischer J.A, Muff R, Born W. Functional relevance of G-protein-coupled-receptor-associated proteins, exemplified by receptor-activity-modifying proteins (RAMPS) Biochem. Soc. Trans. 2002;30:455–460. doi: 10.1042/bst0300455. doi:10.1042/BST0300455 [DOI] [PubMed] [Google Scholar]
- Flier J.S. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. doi:10.1016/S0092-8674(03)01081-X [DOI] [PubMed] [Google Scholar]
- Flynn M.C, Turrin N.P, Plata-Salaman C.R, Ffrench-Mullen J.M. Feeding response to neuropeptide Y-related compounds in rats treated with Y5 receptor antisense or sense phosphothio-oligodeoxynucleotide. Physiol. Behav. 1999;66:881–884. doi: 10.1016/s0031-9384(99)00031-1. doi:10.1016/S0031-9384(99)00031-1 [DOI] [PubMed] [Google Scholar]
- Foord S.M, Marshall F.H. RAMPs: accessory proteins for seven transmembrane domain receptors. Trends Pharmacol. Sci. 1999;20:184–187. doi: 10.1016/s0165-6147(99)01347-4. doi:10.1016/S0165-6147(99)01347-4 [DOI] [PubMed] [Google Scholar]
- Friedman M.I, Edens N.K, Ramirez I, Granneman J. Food intake in diabetic rats: isolation of primary metabolic effects of fat feeding. Am. J. Physiol. 1983;249:R44–R51. doi: 10.1152/ajpregu.1985.249.1.R44. [DOI] [PubMed] [Google Scholar]
- Funahashi N, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain Res. Bull. 1993;32:531–535. doi: 10.1016/0361-9230(93)90303-s. doi:10.1016/0361-9230(93)90303-S [DOI] [PubMed] [Google Scholar]
- Geary N. Glucagon-(1-21) fails to inhibit meal size in rats. Peptides. 1987;8:943–945. doi: 10.1016/0196-9781(87)90085-4. doi:10.1016/0196-9781(87)90085-4 [DOI] [PubMed] [Google Scholar]
- Geary N. Pancreatic glucagon signals postprandial satiety. Neurosci. Biobehav. Rev. 1990;14:323–338. doi: 10.1016/s0149-7634(05)80042-9. doi:10.1016/S0149-7634(05)80042-9 [DOI] [PubMed] [Google Scholar]
- Geary N. Failure of pulsatile infusion to increase glucagon's satiating potency. Physiol. Behav. 1996;59:613–616. doi: 10.1016/0031-9384(95)02121-3. doi:10.1016/0031-9384(95)02121-3 [DOI] [PubMed] [Google Scholar]
- Geary N. Glucagon and the control of meal size. In: Smith G.P, editor. Satiation. From gut to brain. Oxford University press; New York, NY: 1998. pp. 164–197. [Google Scholar]
- Geary N, Smith G.P. Pancreatic glucagon and postprandial satiety in the rat. Physiol. Behav. 1982a;28:313–322. doi: 10.1016/0031-9384(82)90081-6. doi:10.1016/0031-9384(82)90081-6 [DOI] [PubMed] [Google Scholar]
- Geary N, Smith G.P. Pancreatic glucagon fails to inhibit sham feeding in the rat. Peptides. 1982b;3:163–166. doi: 10.1016/0196-9781(82)90046-8. doi:10.1016/0196-9781(82)90046-8 [DOI] [PubMed] [Google Scholar]
- Geary N, Smith G.P. Selective hepatic vagotomy blocks pancreatric glucagon's satiety effect. Physiol. Behav. 1983;31:391–394. doi: 10.1016/0031-9384(83)90207-x. doi:10.1016/0031-9384(83)90207-X [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L. Estradiol increases glucagon's satiating potency in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1290–R1294. doi: 10.1152/ajpregu.2001.281.4.R1290. [DOI] [PubMed] [Google Scholar]
- Geary N, Farhoody N, Gersony A. Food deprivation dissociates pancreatic glucagon's effects on satiety and hepatic glucose production at dark onset. Physiol. Behav. 1987;39:507–511. doi: 10.1016/0031-9384(87)90381-7. doi:10.1016/0031-9384(87)90381-7 [DOI] [PubMed] [Google Scholar]
- Geary N, Kissileff H.R, Pi-Sunyer F.X, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am. J. Physiol. 1992;262:R975–R980. doi: 10.1152/ajpregu.1992.262.6.R975. [DOI] [PubMed] [Google Scholar]
- Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am. J. Physiol. 1993;264:R116–R122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Langhans W. The satiating potency of hepatic portal glucagon in rats is not affected by insulin or insulin antibodies. Physiol. Behav. 1997;61:199–208. doi: 10.1016/s0031-9384(96)00361-7. doi:10.1016/S0031-9384(96)00361-7 [DOI] [PubMed] [Google Scholar]
- Gebre-Medhin S, Mulder H, Pekney M, Zhang Y, Tornell J, Westermark P, Sundler F, Ahren B, Betsholtz C. Altered glucose homeostasis, body weight and nociception in IAPP (amylin) null mutant mice. Diabetes. 1997;46:29A. [Google Scholar]
- Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, Sundler F, Ahren B, Betsholtz C. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochem. Biophys. Res. Commun. 1998;250:271–277. doi: 10.1006/bbrc.1998.9308. doi:10.1006/bbrc.1998.9308 [DOI] [PubMed] [Google Scholar]
- Gerozissis K. Brain insulin and feeding: a bi-directional communication. Eur. J. Pharmacol. 2004;490:59–70. doi: 10.1016/j.ejphar.2004.02.044. doi:10.1016/j.ejphar.2004.02.044 [DOI] [PubMed] [Google Scholar]
- Gotoh K, Wortman M.D, Benoit S.C, Clegg D.J, D'Alessio D, Tso P, Seeley R.J, Woods S.C. High fat diet prevents the anorectic effect of central insulin. In: Schwartz M.W, Barsh G.S, editors. Keystone Symposium: Obesity. Keystone Publishing; Keystone, CO: 2003. p. 136. [Google Scholar]
- Grabler V, Lutz T.A. Chronic infusion of the amylin antagonist AC 187 increases feeding in Zucker fa/fa rats but not in lean controls. Physiol. Behav. 2004;81:481–488. doi: 10.1016/j.physbeh.2004.02.002. doi:10.1016/j.physbeh.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Guillemin R. Hypothalamic hormones a.k.a hypothalamic releasing factors. J. Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. doi:10.1677/joe.1.05883 [DOI] [PubMed] [Google Scholar]
- Hager J, et al. A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat. Genet. 1995;9:299–304. doi: 10.1038/ng0395-299. doi:10.1038/ng0395-299 [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Born J, Fehm H.L, Kern W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 2004;83:55–64. doi: 10.1016/j.physbeh.2004.07.023. doi:10.1016/j.physbeh.2004.07.023 [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Fehm H.L, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004b;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- Haluzik M, Parizkova J, Haluzik M.M. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol. Res. 2004;53:123–129. [PubMed] [Google Scholar]
- Havel P.J. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 2002;13:51–59. doi: 10.1097/00041433-200202000-00008. doi:10.1097/00041433-200202000-00008 [DOI] [PubMed] [Google Scholar]
- Hjorth S.A, Adelhorst K, Pedersen B.B, Kirk O, Schwartz T.W. Glucagon and glucagon-like peptide-1: selective receptor receognition via distinct peptide epitopes. J. Biol. Chem. 1994;269:30 121–30 124. [PubMed] [Google Scholar]
- Hollander P, Maggs D.G, Ruggles J.A, Fineman M, Shen L, Koltermann O.G, Weyer C. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obes. Res. 2004;12:661–668. doi: 10.1038/oby.2004.76. [DOI] [PubMed] [Google Scholar]
- Holst J.J, Pedersen J.H, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia. 1983a;25:396–399. doi: 10.1007/BF00282517. doi:10.1007/BF00282517 [DOI] [PubMed] [Google Scholar]
- Holst J.J, Schwartz T.W, Lovgreen N.A, Pedersen O, Beck-Nielsen H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int. J. Obes. 1983b;7:529–538. [PubMed] [Google Scholar]
- Ikeda H, West D.B, Pustek J.J, Figlewicz D.P, Greenwood M.R.C, Porte D, Jr, Woods S.C. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- Ishida T, Chap Z, Chou J, Lewis R, Hartley C, Entman M, Field J.B. Differential effects of oral, peripheral intravenous and intraportal glucose on hepatic glucose uptake and insulin and glucagon extraction in conscious dogs. J. Clin. Invest. 1983;72:590–601. doi: 10.1172/JCI111007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel P.A, Park C.R, Schwartz M.W, Green P.K, Sipols A.J, Woods S.C, Porte D, Jr, Figlewicz D.P. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res. Bull. 1993;30:571–575. doi: 10.1016/0361-9230(93)90084-o. doi:10.1016/0361-9230(93)90084-O [DOI] [PubMed] [Google Scholar]
- Kaiyala K, Prigeon R.L, Kahn S.E, Woods S.C, Schwartz M.W. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kaplan J.M, Moran T.H. Gastrointestinal signaling in the control of food intake. In: Stricker E.M, Woods S.C, editors. Handbook of behavioral neurobiology. Neurobiology of food and fluid intake. vol. 4. Kluwer Academic/Plenum Publishing; New York, NY: 2004. pp. 273–303. [Google Scholar]
- Kennedy G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. B. 1953;140:579–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kinzig K.P, D'Alessio D.A, Seeley R.J. The diverse roles of CNS GLP-1 in the control of food intake and the mediation of visceral illness. J. Neurosci. 2002;22:10 470–10 476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantic S, Goddard I, Saveanu A, Giannetti N, Fombonne J, Cardoso A, Jaquet P, Enjalbert A. Novel modalities of somatostatin actions. Eur. J. Endocrinol. 2004;151:643–655. doi: 10.1530/eje.0.1510643. doi:10.1530/eje.0.1510643 [DOI] [PubMed] [Google Scholar]
- Kubota N, et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J. Clin. Invest. 2004;114:917–927. doi: 10.1172/JCI21484. doi:10.1172/JCI200421484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim E.E, Emond M, Moran T.H. Leptin enhances feeding suppression and neural activation produced by systemically administered bombesin. Am. J. Physiol. 2005;289:E473–E477. doi: 10.1152/ajpregu.00835.2004. [DOI] [PubMed] [Google Scholar]
- Lam T.K, Guttierez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. doi:10.1126/science.1112085 [DOI] [PubMed] [Google Scholar]
- Lam T.K, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz G.J, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 2005b;11:320–327. doi: 10.1038/nm1201. doi:10.1038/nm1201 [DOI] [PubMed] [Google Scholar]
- Lam T.K, Schwartz G.J, Rossetti L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 2005c;8:579–584. doi: 10.1038/nn1456. doi:10.1038/nn1456 [DOI] [PubMed] [Google Scholar]
- Langhans W. Metabolic and glucostatic control of feeding. Proc. Nutr. Soc. 1996;55:497–515. doi: 10.1079/pns19960044. [DOI] [PubMed] [Google Scholar]
- Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic acid oxidation. J. Auton. Nerv. Syst. 1987a;18:13–18. doi: 10.1016/0165-1838(87)90129-9. doi:10.1016/0165-1838(87)90129-9 [DOI] [PubMed] [Google Scholar]
- Langhans W, Scharrer E. Role of fatty acid oxidation in control of meal pattern. Behav. Neural Biol. 1987b;47:7–16. doi: 10.1016/s0163-1047(87)90112-9. doi:10.1016/S0163-1047(87)90112-9 [DOI] [PubMed] [Google Scholar]
- Langhans W, Geary N, Scharrer E. Liver glycogen content decreases during meals in rats. Am. J. Physiol. 1982a;243:R450–R453. doi: 10.1152/ajpregu.1982.243.3.R450. [DOI] [PubMed] [Google Scholar]
- Langhans W, Zieger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science. 1982b;218:894–896. doi: 10.1126/science.7134979. [DOI] [PubMed] [Google Scholar]
- Langhans W, Pantel K, Muller-Schell W, Eggenberger E, Scharrer E. Hepatic handling of pancreatic glucagon and glucose during meals in rats. Am. J. Physiol. 1984;247:R827–R832. doi: 10.1152/ajpregu.1984.247.5.R827. [DOI] [PubMed] [Google Scholar]
- Langhans W, Grossmann F, Geary N. Intrameal hepatic-portal infusion of glucose reduces spontaneous meal size in rats. Physiol. Behav. 2001;73:499–507. doi: 10.1016/s0031-9384(01)00479-6. doi:10.1016/S0031-9384(01)00479-6 [DOI] [PubMed] [Google Scholar]
- Larsen P.J, Tang-Christensen M, Holst J.J, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. doi:10.1016/S0306-4522(96)00434-4 [DOI] [PubMed] [Google Scholar]
- Le Sauter J, Geary N. Pancreatic glucagon and cholecystokinin synergistically inhibit sham feeding in rats. Am. J. Physiol. 1987;253:R719–R725. doi: 10.1152/ajpregu.1987.253.5.R719. [DOI] [PubMed] [Google Scholar]
- Le Sauter J, Noh U, Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Am. J. Physiol. 1991;261:R162–R165. doi: 10.1152/ajpregu.1991.261.1.R162. [DOI] [PubMed] [Google Scholar]
- Levine A.S, Morley J.E. Peripherally administered somatostatin reduces feeding by a vagal mediated mechanism. Pharmacol. Biochem. Behav. 1982;16:897–902. doi: 10.1016/0091-3057(82)90041-7. doi:10.1016/0091-3057(82)90041-7 [DOI] [PubMed] [Google Scholar]
- Levin B.E, Dunn-Meynell A.A, Routh V.H. CNS sensing and regulation of peripheral glucose levels. Int. Rev. Neurobiol. 2002;51:219–258. doi: 10.1016/s0074-7742(02)51007-2. [DOI] [PubMed] [Google Scholar]
- Levin B.E, Routh V.H, Kang L, Sanders N.M, Dunn-Meynell A.A. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Lin X, Tagauchi A, Park S, Kushner J.A, Li F, Li Y, White M.F. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J. Clin. Invest. 2004;114:908–916. doi: 10.1172/JCI22217. doi:10.1172/JCI200422217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lins P.E, Petersson B, Efendic S. Effects of short-term and prolonged infusions of somatostatin on endocrine pancreas, body weight and food intake in rats. Acta Physiol. Scand. 1980;110:267–275. doi: 10.1111/j.1748-1716.1980.tb06663.x. [DOI] [PubMed] [Google Scholar]
- Lotter E.C, Woods S.C. Injections of insulin and changes of body weight. Physiol. Behav. 1977;18:293–297. doi: 10.1016/0031-9384(77)90136-6. doi:10.1016/0031-9384(77)90136-6 [DOI] [PubMed] [Google Scholar]
- Lotter E.C, Krinsky R, McKay J.M, Treneer C.M, Porte D, Jr, Woods S.C. Somatostatin decreases food intake of rats and baboons. J. Comp. Physiol. Psychol. 1981;95:278–287. doi: 10.1037/h0077777. [DOI] [PubMed] [Google Scholar]
- Lovshin J, Drucker D.J. Synthesis, secretion and biological actions of the glucagon-like peptides. Pediatr. Diab. 2000;1:49–57. doi: 10.1034/j.1399-5448.2000.010108.x. doi:10.1034/j.1399-5448.2000.010108.x [DOI] [PubMed] [Google Scholar]
- Lutz T.A. The pancreatic hormone amylin as a centrally acting satiating hormone. Curr. Drug Targets. 2005;6:181–189. doi: 10.2174/1389450053174596. [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Del Prete E, Scharrer E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol. Behav. 1994;55:891–895. doi: 10.1016/0031-9384(94)90076-0. doi:10.1016/0031-9384(94)90076-0 [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Geary N, Szabady M.M, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol. Behav. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. doi:10.1016/0031-9384(95)02067-5 [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Del Prete E, Szabady M.M, Scharrer E. Attenuation of the anorectic effects of glucagon, cholecystokinin, and bombesin by the amylin receptor antagonist CGRP(8-37) Peptides. 1996;17:119–124. doi: 10.1016/0196-9781(95)02046-2. doi:10.1016/0196-9781(95)02046-2 [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Pieber T.R, Walzer B, Del Prete E, Scharrer E. Different influence of CGRP (8-37), an amylin and CGRP antagonist, on the anorectic effects of cholecystokinin and bombesin in diabetic and normal rats. Peptides. 1997a;18:643–649. doi: 10.1016/s0196-9781(97)00124-1. doi:10.1016/S0196-9781(97)00124-1 [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Rossi R, Althaus J, Del Prete E, Scharrer E. Evidence for a physiological role of central calcitonin gene-related peptide (CGRP) receptors in the control of food intake in rats. Neurosci. Lett. 1997b;230:159–162. doi: 10.1016/s0304-3940(97)00503-x. doi:10.1016/S0304-3940(97)00503-X [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides. 1998;19:309–317. doi: 10.1016/s0196-9781(97)00292-1. doi:10.1016/S0196-9781(97)00292-1 [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Tschudy S, Rushing P.A, Scharrer E. Attenuation of the anorectic effects of cholecystokinin and bombesin by the specific amylin antagonist AC 253. Physiol. Behav. 2000;70:533–536. doi: 10.1016/s0031-9384(00)00302-4. doi:10.1016/S0031-9384(00)00302-4 [DOI] [PubMed] [Google Scholar]
- Lutz T, Estermann A, Geary N, Scharrer E. Physiological effect of circulating glucagon on the hepatic membrane potential. Am. J. Physiol. 2001a;281:1540–1544. doi: 10.1152/ajpregu.2001.281.5.R1540. [DOI] [PubMed] [Google Scholar]
- Lutz T.A, Mollet A, Rushing P.A, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS)-lesioned rats. Int. J. Obes. Relat. Metab. Disord. 2001b;25:1005–1011. doi: 10.1038/sj.ijo.0801664. doi:10.1038/sj.ijo.0801664 [DOI] [PubMed] [Google Scholar]
- MacIsaac L, Geary N. Partial liver denervations dissociate the inhibitory effects of pancreatic glucagon and epinephrine on feeding. Physiol. Behav. 1985;35:233–237. doi: 10.1016/0031-9384(85)90342-7. doi:10.1016/0031-9384(85)90342-7 [DOI] [PubMed] [Google Scholar]
- MacKay E.M, Calloway J.W, Barnes R.H. Hyperalimentation in normal animals produced by protamine insulin. J. Nutr. 1940;20:59–66. [Google Scholar]
- Madsen O.D, Karlsen C, Blume N, Jensen H.I, Larsson L.I, Holst J.J. Transplantable glucagonomas derived from pluripotent rat islet tumor tissue cause severe anorexia and adipsia. Scand. J. Clin. Lab Invest. Suppl. 1995;220:27–35. [PubMed] [Google Scholar]
- Malaisse-Lagae F, Carpentier J.L, Patel Y.C, Malaisse W.J, Orci L. Pancreatic polypeptide: a possible role in the regulation of food intake in the mouse. Hypothesis. Experientia. 1977;33:915–917. doi: 10.1007/BF01951279. doi:10.1007/BF01951279 [DOI] [PubMed] [Google Scholar]
- Marliss E.B, Girardier L, Seydoux J, Wollheim C.B, Kanazawa Y, Orci L, Renold A.E, Porte D.J. Glucagon release induced by pancreatic nerve stimulation in the dog. J. Clin. Invest. 1973;52:1246–1259. doi: 10.1172/JCI107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C.A, Ritter R.C. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am. J. Physiol. 1999;276:R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- Matson C.A, Wiater M.F, Kuijper J.L, Weigle D.S. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides. 1997;18:1275–1278. doi: 10.1016/s0196-9781(97)00138-1. doi:10.1016/S0196-9781(97)00138-1 [DOI] [PubMed] [Google Scholar]
- McGarry J.D, Foster D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. doi:10.1146/annurev.bi.49.070180.002143 [DOI] [PubMed] [Google Scholar]
- McGowan M.K, Andrews K.M, Grossman S.P. Chronic intrahyphothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol. Behav. 1992;51:753–766. doi: 10.1016/0031-9384(92)90112-f. doi:10.1016/0031-9384(92)90112-F [DOI] [PubMed] [Google Scholar]
- McLatchie L.M, Fraser N.J, Main M.J, Wise A, Brown J, Thompson N, Solari R, Lee M.G, Foord S.M. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. doi:10.1038/30666 [DOI] [PubMed] [Google Scholar]
- McMinn J.E, Sindelar D.K, Havel P.J, Schwartz M.W. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141:4442–4448. doi: 10.1210/endo.141.12.7815. doi:10.1210/en.141.12.4442 [DOI] [PubMed] [Google Scholar]
- Mollet A, Meier S, Grabler V, Gilg S, Scharrer E, Lutz T.A. Endogenous amylin contributes to the anorectic effects of cholecystokinin and bombesin. Peptides. 2003;24:91–98. doi: 10.1016/s0196-9781(02)00280-2. doi:10.1016/S0196-9781(02)00280-2 [DOI] [PubMed] [Google Scholar]
- Mollet A, Gilg S, Riediger T, Lutz T.A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol. Behav. 2004;81:149–155. doi: 10.1016/j.physbeh.2004.01.006. doi:10.1016/j.physbeh.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Moore C.X, Cooper G.J. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem. Biophys. Res. Commun. 1991;179:1–9. doi: 10.1016/0006-291x(91)91325-7. doi:10.1016/0006-291X(91)91325-7 [DOI] [PubMed] [Google Scholar]
- Moran T.H. Gut peptides in the control of food intake: 30 years of ideas. Physiol. Behav. 2004;82:175–180. doi: 10.1016/j.physbeh.2004.04.048. doi:10.1016/j.physbeh.2004.04.048 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Kinzig K.P. Gastrointestinal satiety signals II. Cholecystokinin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. doi:10.1152/ajpgi.00434.2003 [DOI] [PubMed] [Google Scholar]
- Moran T.H, Baldessarini A.R, Salorio C.F, Lowery T, Schwartz G.J. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- Morley J.E, Flood J.F. Amylin decreases food intake in mice. Peptides. 1991;12:865–869. doi: 10.1016/0196-9781(91)90148-i. doi:10.1016/0196-9781(91)90148-I [DOI] [PubMed] [Google Scholar]
- Morley J.E, Flood J.F, Horowitz M, Morley P.M, Walter M.J. Modulation of food intake by peripherally administered amylin. Am. J. Physiol. 1994;267:R178–R184. doi: 10.1152/ajpregu.1994.267.1.R178. [DOI] [PubMed] [Google Scholar]
- Morley J.E, Suarez M.D, Mattamal M, Flood J.F. Amylin and food intake in mice: effects on motivation to eat and mechanism of action. Pharmacol. Biochem. Behav. 1997;56:123–129. doi: 10.1016/S0091-3057(96)00168-2. doi:10.1016/S0091-3057(96)00168-2 [DOI] [PubMed] [Google Scholar]
- Morton G.J, Blevins J.E, Williams D.L, Niswender K.D, Gelling R.W, Rhodes C.J, Baskin D.G, Schwartz M.W. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. doi:10.1172/JCI200522081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff R, Buhlmann N, Fischer J.A, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. doi:10.1210/en.140.6.2924 [DOI] [PubMed] [Google Scholar]
- Muller W.A, Faloona G.R, Unger R.H. The influence of the antecedant diet upon glucagon and insulin secretion. N. Engl. J. Med. 1971;285:1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers M.G.J. Molecular and anatomical determinants of central insulin resistance. Nat. Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. doi:10.1038/nn1454 [DOI] [PubMed] [Google Scholar]
- Nicolaidis S, Rowland N. Metering of intravenous versus oral nutrients and regulation of energy balance. Am. J. Physiol. 1976;231:661–668. doi: 10.1152/ajplegacy.1976.231.3.661. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Uvnas-Wallensten K. Effect of teasing and sham feeding on plasma glucagon concentration in dogs. Acta Physiol. Scand. 1997;100:298–302. doi: 10.1111/j.1748-1716.1977.tb05953.x. [DOI] [PubMed] [Google Scholar]
- Niswender K.D, Schwartz M.W. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. doi:10.1016/S0091-3022(02)00105-X [DOI] [PubMed] [Google Scholar]
- Niswender K.D, Morrison C.D, Clegg D.J, Olson R, Baskin D.G, Myers M.G, Jr, Seeley R.J, Schwartz M.W. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin D.G, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002a;5:566–572. doi: 10.1038/nn0602-861. doi:10.1038/nn861 [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002b;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang B.B, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002c;8:1376–1382. doi: 10.1038/nm1202-798. doi:10.1038/nm798 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Accili D. In vivo mutagenesis of the insulin reeptor. J. Biol. Chem. 2003;278:28 359–28 362. doi: 10.1074/jbc.R300009200. doi:10.1074/jbc.R300009200 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nakae J, Kitamura T, Park B.C, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J. Clin. Invest. 2004;114:214–223. doi: 10.1172/JCI21645. doi:10.1172/JCI200421645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O.E, Reichard G.A.J, Boden G, Shuman C. Comparative measurements of glucose, beta-hydroxybutyrate, acetoacetate, and insulin in blood and cerebrospinal fluid during starvation. Metabolism. 1974;23:7–14. doi: 10.1016/0026-0495(74)90098-5. doi:10.1016/0026-0495(74)90098-5 [DOI] [PubMed] [Google Scholar]
- Permert J, Larsson J, Fruin A.B, Tatemoto K, Herrington M.K, von Schenck H, Adrian T.E. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–68. doi: 10.1097/00006676-199707000-00009. doi:10.1097/00006676-199707000-00009 [DOI] [PubMed] [Google Scholar]
- Petersen O.H. The effect of glucagon on the liver cell membrane potential. J. Physiol. 1974;239:647–656. doi: 10.1113/jphysiol.1974.sp010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieber T.R, Roitelman J, Lee Y, Luskey K.L, Stein D.T. Direct plasma radioimmunoassay for rat amylin-(1-37): concentrations with acquired and genetic obesity. Am. J. Physiol. 1994;267:E156–E164. doi: 10.1152/ajpendo.1994.267.1.E156. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R, Oomura Y. Effect of intra-third ventricular administration of insulin on food intake after food deprivation. Physiol. Behav. 1986;37:735–739. doi: 10.1016/0031-9384(86)90178-2. doi:10.1016/0031-9384(86)90178-2 [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. doi:10.1016/j.tem.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam T.K, Gutierrez-Juarez R, Obici S, Schwartz G.J, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. doi:10.1038/nature03439 [DOI] [PubMed] [Google Scholar]
- Polonsky K.S, Given E, Carter V. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Invest. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D.J, Baskin D.G, Schwartz M.W. Leptin and insulin action in the central nervous system. Nutr. Rev. 2002;60:S20–S29. doi: 10.1301/002966402320634797. doi:10.1301/002966402320634797 [DOI] [PubMed] [Google Scholar]
- Porte D.J, Baskin D.G, Schwartz M.W. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- Raben A, Andersen H.B, Christensen N.J, Madsen J, Holst J.J, Astrup A. Evidence for an abnormal postprandial response to a high-fat meal in women predisposed to obesity. Am. J. Physiol. 1994;267:E549–E559. doi: 10.1152/ajpendo.1994.267.4.E549. [DOI] [PubMed] [Google Scholar]
- Reidelberger R.D, Kelsey L, Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. Am. J. Physiol. 2002;282:R1395–R1404. doi: 10.1152/ajpregu.00597.2001. [DOI] [PubMed] [Google Scholar]
- Reidelberger R.D, Haver A.C, Arnelo U, Smith D.D, Schaffert C.S, Permert J. Amylin receptor blockade stimulates food intake in rats. Am. J. Physiol. 2004;287:R568–R574. doi: 10.1152/ajpregu.00213.2004. [DOI] [PubMed] [Google Scholar]
- Riediger T, Schmid H.A, Lutz T, Simon E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am. J. Physiol. 2001;281:R1833–R1843. doi: 10.1152/ajpregu.2001.281.6.R1833. [DOI] [PubMed] [Google Scholar]
- Riediger T, Schmid H.A, Lutz T, Simon E. Amylin and glucose co-activate area postrema neurons of the rat. Neurosci. Lett. 2002;328:121–124. doi: 10.1016/s0304-3940(02)00482-2. doi:10.1016/S0304-3940(02)00482-2 [DOI] [PubMed] [Google Scholar]
- Riediger T, Zünd D, Becskei C, Lutz T.A. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut–brain axis. Am. J. Physiol. 2004;286:R114–R122. doi: 10.1152/ajpregu.00333.2003. [DOI] [PubMed] [Google Scholar]
- Riedy C.A, Chavez M, Figlewicz D.P, Woods S.C. Central insulin enhances sensitivity to cholecystokinin. Physiol. Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. doi:10.1016/0031-9384(95)00108-U [DOI] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am. J. Physiol. 1999;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- Ritter R.C, Ladenheim E.E. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am. J. Physiol. 1985;248:R501–R504. doi: 10.1152/ajpregu.1985.248.4.R501. [DOI] [PubMed] [Google Scholar]
- Rossi R, Geronimi M, Gloor P, Seebacher M.C, Scharrer E. Hyperpolarization of the cell membrane of mouse hepatocytes by fatty acid oxidation. Physiol. Behav. 1995;57:509–514. doi: 10.1016/0031-9384(94)00292-d. doi:10.1016/0031-9384(94)00292-D [DOI] [PubMed] [Google Scholar]
- Rowland N.E, Richmond R.M. Area postrema and the anorectic actions of dexfenfluramine and amylin. Brain Res. 1999;820:86–91. doi: 10.1016/s0006-8993(98)01348-1. doi:10.1016/S0006-8993(98)01348-1 [DOI] [PubMed] [Google Scholar]
- Rowland N.E, Crews E.C, Gentry R.M. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul. Pept. 1997;71:171–174. doi: 10.1016/s0167-0115(97)01034-3. doi:10.1016/S0167-0115(97)01034-3 [DOI] [PubMed] [Google Scholar]
- Rushing P.A, Hagan M.M, Seeley R.J, Lutz T.A, D'Alessio D.A, Air E.L, Woods S.C. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142:5035–5038. doi: 10.1210/endo.142.11.8593. doi:10.1210/en.142.11.5035 [DOI] [PubMed] [Google Scholar]
- Rushing P.A, Seeley R.J, Air E.L, Lutz T.A, Woods S.C. Acute 3rd-ventricular amylin infusion potently reduces food intake but does not produce aversive consequences. Peptides. 2002;23:985–988. doi: 10.1016/s0196-9781(02)00022-0. doi:10.1016/S0196-9781(02)00022-0 [DOI] [PubMed] [Google Scholar]
- Russek M. Current status of the hepatostatic theory of food intake control. Appetite. 1981;2:137–143. doi: 10.1016/s0195-6663(81)80007-4. [DOI] [PubMed] [Google Scholar]
- Sawchenko P.E, Friedman M.I. Sensory functions of the liver—a review. Am. J. Physiol. 1979;236:R5–R20. doi: 10.1152/ajpregu.1979.236.1.R5. [DOI] [PubMed] [Google Scholar]
- Schick R.R, Zimmermann J.P, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothgalamic sites to suppress feeding in rats. Am. J. Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Porte D.J. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. doi:10.1126/science.1104344 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Figlewicz D.P, Baskin D.G, Woods S.C, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr. Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. doi:10.1210/er.13.3.387 [DOI] [PubMed] [Google Scholar]
- Schwartz M.W, Figlewicz D.P, Baskin D.G, Woods S.C, Porte D., Jr Insulin and the central regulaton of energy balance: update 1994. Endocr. Monogr. Rev. 1994;2:109–113. [Google Scholar]
- Schwartz M.W, Woods S.C, Porte D.J, Seeley R.J, Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seaquist E.R, Damberg G.S, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2003;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- Secchi A, Caldara R, Caumo A, Monti L.D, Bonfatti D, Di Carlo V, Pozza G. Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism. 1995;44:1153–1158. doi: 10.1016/0026-0495(95)90008-x. doi:10.1016/0026-0495(95)90008-X [DOI] [PubMed] [Google Scholar]
- Seeley R.J, Woods S.C. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat. Rev. Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. doi:10.1038/nrn1245 [DOI] [PubMed] [Google Scholar]
- Seeley R.J, York D.A. Fuel sensing and the central nervous system (CNS): implications for the regulation of energy balance and the treatment for obesity. Obes. Rev. 2005;6:259–265. doi: 10.1111/j.1467-789X.2005.00193.x. doi:10.1111/j.1467-789X.2005.00193.x [DOI] [PubMed] [Google Scholar]
- Seeley R.J, van Dijk G, Campfield L.A, Smith F.J, Nelligan J.A, Bell S.M, Baskin D.G, Woods S.C, Schwartz M.W. The effect of intraventricular administration of leptin on food intake and body weight in the rat. Horm. Metab. Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- Sexton P.M, Paxinos G, Kenney M.A, Wookey P.J, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. doi:10.1016/0306-4522(94)90388-3 [DOI] [PubMed] [Google Scholar]
- Sexton P.M, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell. Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. doi:10.1016/S0898-6568(00)00143-1 [DOI] [PubMed] [Google Scholar]
- Sindelar D.K, Havel P.J, Seeley R.J, Wilkinson C.W, Woods S.C, Schwartz M.W. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999;48:1275–1280. doi: 10.2337/diabetes.48.6.1275. [DOI] [PubMed] [Google Scholar]
- Sipols A.J, Baskin D.G, Schwartz M.W. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44:147–151. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- Smith G.P, Epstein A.N. Increased feeding in response to decreased glucose utilization in rat and monkey. Am. J. Physiol. 1969;217:1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- Steffens A.B, Scheurink A.J, Porte D, Jr, Woods S.C. Penetration of peripheral glucose and insulin into cerebrospinal fluid in the rat. Am. J. Physiol. 1988;255:R200–R204. doi: 10.1152/ajpregu.1988.255.2.R200. [DOI] [PubMed] [Google Scholar]
- Stein L.J, Dorsa D.M, Baskin D.G, Figlewicz D.P, Ikeda H, Frankmann S.P, Greenwood M.R, Porte D, Jr, Woods S.C. Immunoreactive insulin levels are elevated in the cerebrospinal fluid of genetically obese Zucker rats. Endocrinology. 1983;113:2299–2301. doi: 10.1210/endo-113-6-2299. [DOI] [PubMed] [Google Scholar]
- Stockinger Z, Geary N. Pancreatic glucagon does not alter intrameal gastric emptying of milk in the rat. Physiol. Behav. 1989;45:1259–1261. doi: 10.1016/0031-9384(89)90118-2. doi:10.1016/0031-9384(89)90118-2 [DOI] [PubMed] [Google Scholar]
- Strader A.D, Woods S.C. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. doi:10.1053/j.gastro.2004.10.043 [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Lundqvist G, Skogseid B, Wilander E, Oeberg K. Islet amyloid polypeptide (IAPP) in patients with neuroendocrine tumours. Regul. Pept. 1995;26:119–131. doi: 10.1016/0167-0115(94)00097-h. doi:10.1016/0167-0115(94)00097-H [DOI] [PubMed] [Google Scholar]
- Strubbe J.H, Mein C.G. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol. Behav. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. doi:10.1016/0031-9384(77)90343-2 [DOI] [PubMed] [Google Scholar]
- Strubbe J.H, Woods S.C. The timing of meals. Psychol. Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. doi:10.1037/0033-295X.111.1.128 [DOI] [PubMed] [Google Scholar]
- Strubbe J.H, Porte D.J, Woods S.C. Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol. Behav. 1988;44:205–208. doi: 10.1016/0031-9384(88)90139-4. doi:10.1016/0031-9384(88)90139-4 [DOI] [PubMed] [Google Scholar]
- Surina-Baumgartner D.M, Langhans W, Geary N. Hepatic portal insulin antibody infusion increases, but insulin does not alter, spontaneous meal size in rats. Am. J. Physiol. 1995;269:R978–R982. doi: 10.1152/ajpregu.1995.269.5.R978. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen P.J, Goke R, Fink-Jensen A, Jessop D.S, Moller M, Sheikh S.P. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am. J. Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen P.J, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat. Med. 2000;6:802–807. doi: 10.1038/77535. doi:10.1038/77535 [DOI] [PubMed] [Google Scholar]
- Thiele T.E, Seeley R.J, D'Alessio D, Eng J, Bernstein I.L, Woods S.C, van Dijk G. Central infusion of glucagon-like peptide-1-(7-36) amide (GLP-1) receptor antagonist attenuates lithium chloride-induced c-Fos induction in rat brainstem. Brain Res. 1998;801:164–170. doi: 10.1016/s0006-8993(98)00584-8. doi:10.1016/S0006-8993(98)00584-8 [DOI] [PubMed] [Google Scholar]
- Torsoni M.A, Carvalheira J.B, de Carvalho-Filho M.P.-d.-S.M.A, Saad M.J, Velloso M.A. Molecular and functional resistance to insulin in hypothalamus of rats exposed to cold. Am. J. Physiol. 2003;285:E216–E223. doi: 10.1152/ajpendo.00031.2003. [DOI] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res. Mol. Brain Res. 2001;10:36–45. doi: 10.1016/s0169-328x(01)00179-6. doi:10.1016/S0169-328X(01)00179-6 [DOI] [PubMed] [Google Scholar]
- Ueno N, et al. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. doi:10.1016/S0016-5085(99)70293-3 [DOI] [PubMed] [Google Scholar]
- van Dijk G, Thiele T.E. Glucagon-like peptide-1 (7-36) amide: a central regulator of satiety and interoceptive stress. Neuropeptides. 1999;33:406–414. doi: 10.1054/npep.1999.0053. doi:10.1054/npep.1999.0053 [DOI] [PubMed] [Google Scholar]
- van Dijk G, de Groote C, Chavez M, van der Werf Y, Steffens A.B, Strubbe J.H. Insulin in the arcuate nucleus reduces fat consumption in rats. Brain Res. 1997;777:147–152. doi: 10.1016/s0006-8993(97)01103-7. doi:10.1016/S0006-8993(97)01103-7 [DOI] [PubMed] [Google Scholar]
- Vanderweele D.A. Insulin and satiety from feeding in pancreatic-normal and diabetic rats. Physiol. Behav. 1993;54:477–485. doi: 10.1016/0031-9384(93)90239-c. doi:10.1016/0031-9384(93)90239-C [DOI] [PubMed] [Google Scholar]
- Vanderweele D.A, Haraczkiewicz E, Van Itallie T.B. Elevated insulin and satiety in obese and normal weight rats. Appetite. 1982;3:99–109. doi: 10.1016/s0195-6663(82)80003-2. [DOI] [PubMed] [Google Scholar]
- Vijayan E, McCann S.M. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH) Endocrinology. 1977;100:1727–1730. doi: 10.1210/endo-100-6-1727. [DOI] [PubMed] [Google Scholar]
- Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. doi:10.1210/er.21.6.697 [DOI] [PubMed] [Google Scholar]
- Wajchenberg B.L, Giannella-Neto D, da Silva M.E, Santos R.F. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm. Metab. Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. doi:10.1055/s-2002-38256 [DOI] [PubMed] [Google Scholar]
- Wang L, Barachina M.D, Martinez V, Wei J.Y, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul. Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. doi:10.1016/S0167-0115(00)00153-1 [DOI] [PubMed] [Google Scholar]
- Weatherford S.C, Ritter S. Glucagon satiety: diurnal variation after hepatic branch vagotomy or intraportal alloxan. Brain Res. Bull. 1986;17:545–549. doi: 10.1016/0361-9230(86)90224-8. doi:10.1016/0361-9230(86)90224-8 [DOI] [PubMed] [Google Scholar]
- Weatherford S.C, Ritter S. Lesion of vagal afferent terminals im pairs glucagon-induced suppression of food intake. Physiol. Behav. 1988;43:645–650. doi: 10.1016/0031-9384(88)90220-x. doi:10.1016/0031-9384(88)90220-X [DOI] [PubMed] [Google Scholar]
- Weyer C, Maggs D.G, Young A.A, Kolterman O.G. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiologic approach toward improved metabolic control. Curr. Pharm. Des. 2001;7:1353–1373. doi: 10.2174/1381612013397357. doi:10.2174/1381612013397357 [DOI] [PubMed] [Google Scholar]
- Whitcomb D.C, Puccio A.M, Vigna S.R, Taylor I.L, Hoffman G.E. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res. 1997;760:137–149. doi: 10.1016/s0006-8993(97)00295-3. doi:10.1016/S0006-8993(97)00295-3 [DOI] [PubMed] [Google Scholar]
- Wimalawansa S.J. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit. Rev. Neurobiol. 1997;11:167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Woods S.C. The eating paradox: How we tolerate food. Psychol. Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. doi:10.1037/0033-295X.98.4.488 [DOI] [PubMed] [Google Scholar]
- Woods S.C. Insulin and the brain: A mutual dependency. Prog. Psychobiol. Physiol. Psychol. 1996;16:53–81. [Google Scholar]
- Woods S.C. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. doi:10.1152/ajpgi.00448.2003 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Strubbe J.H. The psychobiology of meals. Psychon. Bull. Rev. 1994;1:141–155. doi: 10.3758/BF03200770. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Seeley R.J. Insulin as an adiposity signal. Int. J. Obes. Relat. Metab. Disord. 2001;25:S35–S38. doi: 10.1038/sj.ijo.0801909. doi:10.1038/sj/ijo/0801909 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Decke E, Vasselli J.R. Metabolic hormones and regulation of body weight. Psychol. Rev. 1974;81:26–43. doi: 10.1037/h0035927. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Lotter E.C, McKay L.D, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. doi:10.1038/282503a0 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Stein L.J, McKay L.D, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am. J. Physiol. 1984;247:R393–R401. doi: 10.1152/ajpregu.1984.247.2.R393. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Chavez M, Park C.R, Riedy C, Kaiyala K, Richardson R.D, Figlewicz D.P, Schwartz M.W, Porte D, Seeley R.J. The evaluation of insulin as a metabolic signal controlling behavior via the brain. Neurosci. Biobehav. Rev. 1995;20:139–144. doi: 10.1016/0149-7634(95)00044-f. doi:10.1016/0149-7634(95)00044-F [DOI] [PubMed] [Google Scholar]
- Woods S.C, Seeley R.J, Porte D.J, Schwartz M.W. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. doi:10.1126/science.280.5368.1378 [DOI] [PubMed] [Google Scholar]
- Woods S.C, Gotoh K, Clegg D.J. Gender differences in the control of energy homeostasis. Exp. Biol. Med. 2003a;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- Woods S.C, Seeley R.J, Baskin D.G, Schwartz M.W. Insulin and the blood–brain barrier. Curr. Pharm. Des. 2003b;9:795–800. doi: 10.2174/1381612033455323. doi:10.2174/1381612033455323 [DOI] [PubMed] [Google Scholar]
- Woods S.C, D'Alessio D.A, Tso P, Rushing P.A, Clegg D.J, Benoit S.C, Gotoh K, Liu M, Seeley R.J. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol. Behav. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. doi:10.1016/j.physbeh.2004.07.026 [DOI] [PubMed] [Google Scholar]
- Wynick D, Hammond P.J, Bloom S.R. The glucagonoma syndrome. Clin. Dermatol. 1993;11:93–97. doi: 10.1016/0738-081x(93)90103-j. doi:10.1016/0738-081X(93)90103-J [DOI] [PubMed] [Google Scholar]
- Xu A.W, Kaelin C.B, Takeda K, Akira S, Schwartz M.W, Barsh G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 2005;115:951–958. doi: 10.1172/JCI24301. doi:10.1172/JCI200524301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Denaro M. Roles of amylin in diabetes and in regulation of nutrient load. Nutrition. 1998;14:524–527. doi: 10.1016/s0899-9007(98)00044-6. doi:10.1016/S0899-9007(98)00044-6 editorial. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. doi:10.1038/372425a0 [DOI] [PubMed] [Google Scholar]