Abstract
A cloning and expression system that allows display of proteins on the surface of filamentous phages was exploited to display a 28-kDa glutathione S-transferase (Sm28GST) antigen of the human parasite Schistosoma mansoni. The phage-displayed Sm28GST (pdGST) was immunoreactive and was recognized by immune sera, suggesting that the Sm28GST protein displayed on the surface of phages potentially maintains native conformation. Subsequent immunization studies showed that mice can develop high titers of antibodies against pdGST and do not require any additional adjuvant for immunization. Isotype analysis suggested that the pdGST immunization predominantly induced immunoglobulin G2b (IgG2b), IgG3, and IgM anti-GST antibodies in mice. Furthermore, the pdGST immunization was found to confer about 30% protection after a challenge infection with 100 cercariae of S. mansoni in BALB/c mice. These findings suggest that phage display is a simple, efficient, and promising tool to express candidate vaccine antigens for immunization against infectious agents.
Foreign DNA can be inserted into gene III of filamentous phages to create a fusion protein that is exported to the surface and displayed on the coat of the bacteriophages (27). Such phage-displayed foreign proteins maintain their native three-dimensional conformation and have been shown to be functionally and immunologically active (9, 11, 18). Infectivity of the recombinant fusion virion is not affected by this expression of foreign proteins on the surface (27). Several polypeptides have been displayed on the surface of filamentous phages for a wide variety of applications (26). One of the greatest advantages of phage display over conventional cloning is that in phage display a physical linkage exists between displayed protein and its coding genes (9). In the present study, we have attempted to display the 28-kDa glutathione S-transferase (GST) antigen of the human parasite Schistosoma mansoni (Sm28GST) on the surface of phages.
Despite the fact that phage display is extensively used to express polypeptides, only a few studies have attempted to evaluate the immunogenic potential of phage-displayed proteins. Studies by de la Cruz et al. (11) showed that immunization with the repeat regions of the circumsporozoite protein gene of Plasmodium falciparum displayed on the surface of filamentous phages can induce significant antibody responses in mice and rabbits. Taking advantage of this system, Gram et al. (17) demonstrated that recombinant human interleukin-13 (IL-13) displayed on the surface of phages could be used as an immunogen to generate neutralizing antibodies against this cytokine (17). Frenkel et al. (14) recently reported that immunization of mice with phage-displayed EFRH can reduce the beta-amyloid plaques in the transgenic mouse brain model of Alzheimer's disease. Similarly, immunization of mice with phage-displayed peptide of the human respiratory syncytial virus or herpes simplex virus can confer protection (2, 16). Given that the 28GST of S. mansoni is a potential candidate vaccine antigen (5) and that the phage-displayed proteins could be successfully used to immunize mice, in the present study we evaluated whether immunization with phage-displayed 28GST could confer protection against a challenge infection in the mice.
MATERIALS AND METHODS
The phage display vector, pBJuFo.
Phage display vector pBJuFo was obtained from Chris Gaskins (Invitrogen, Carlsbad, Calif.). The construction and principle of display in pBJuFo has been described previously (26) and is based on the strong association between Jun and Fos leucine zipper domains (8). Multiple cloning sites located downstream to the Fos leucine zipper facilitate cloning of the cDNA of interest to Fos. The Jun leucine zipper is fused to the N terminus of phage surface protein gene III. Following insertion, the Fos-cDNA fusion associates with Jun in the periplasm and the gene product is exported to the surface, displaying the cloned cDNA product. A 14-amino acid V5 epitope incorporated at the N terminus of the multiple cloning site facilitates detection of the recombinant proteins (26). The displayed V5 epitope can then be detected using a mouse anti-V5 monoclonal antibody (Invitrogen).
Cloning of GST in phage display and Escherichia coli expression vectors.
About 1 μg of mRNA was isolated from S. mansoni cercariae by using a MicroPoly(A) Pure kit (Ambion, Austin, Tex.) and was converted into cDNA using Superscript II RNase H− RT (Life Technologies Inc., Gaithersburg, Md.). GST cDNA was PCR amplified using primers designed based on published Sm28GST sequences (GenBank accession no. S71584) and cloned in the phage display vector pBJuFo or in the E. coli expression vector pRSET B (Invitrogen). For cloning Sm28GST in pBJuFo, the forward primer was flanked by a BstX1 restriction site with the sequence 5′-CTGCAGAACCAGTGTGCTGGATCCTTATGCTGGCGAGCATATC-3′, and the reverse primer contained a NotI site, 5′-AAGGAAAAAAGCGGCCGCGAATTCTTAGAAGGGAGTTGCAGG. PCR product was purified and ligated to BstX1/NotI-digested pBJuFo vector. The resultant recombinant phagemid was termed phage-displayed GST (pdGST).
Another set of primers was designed to facilitate cloning of Sm28GST as a histidine-tagged fusion protein in the E. coli expression vector pRSET B. The forward primer contained a flanking BamHI site (5′-CGCGGATCCGATGCTGGCGAGCATATC-3′), and the reverse primer contained a HindIII restriction site (5′-CCCAAGCTTAGAAGGGAGTTGCAGG-3′). The PCR product was ligated to BamHI/HindIII-digested vector, and the resultant recombinant was termed histidine-tagged GST fusion protein (his-GST). Both pdGST and his-GST constructs were sequenced to confirm the orientation and authenticity of the cloned GST gene.
Phage display of pdGST.
Growth of phagemid and purification of recombinant phage were performed as described previously (8). Briefly, cultures of recombinant and control phagemids were grown at 37°C in 50 ml of SOB medium (20 g of tryptone, 5 g of yeast extract, 0.5 g of NaCl per liter, and 10 mM MgCl2 and 10 mM MgSO4) containing 50 μg of ampicillin/ml. When the cultures reached an optical density at 600 nm (OD600) of 0.6, 100 μl of VCSM13 helper phage (Stratagene, LaJolla, Calif.) was added at a concentration of 1011 PFU/ml and further incubated at 37°C with shaking. After 2 h of incubation, kanamycin was added to the cultures to a final concentration of 50 μg/ml and incubated overnight. Following incubation, cultures were centrifuged at 10,000 × g for 5 min to pellet the bacteria. Phage present in the supernatant was concentrated by precipitating with 3% polyethylene glycol 8000 in 4% NaCl for 1 h on ice, followed by centrifugation at 14,000 × g for 20 min. The phage pellet was washed with 2 ml of sterile distilled water and precipitated again using polyethylene glycol-NaCl. The final phage pellet was resuspended in 0.5 ml of phosphate-buffered saline (PBS), filtered through a 0.45-μm-pore-size filter, and stored at −20°C in 15% glycerol.
Detection of Sm28GST displayed on the surface of phage.
Display of Sm28GST on the surface of phage in pdGST was evaluated by an enzyme-linked immunosorbent assay (ELISA) as described previously (15). Briefly, microtiter plates were coated overnight at 4°C with a 1:1,000 dilution of an anti-V5 monoclonal antibody (Invitrogen) that recognizes the V5 epitope present in the Fos-GST fusion protein. After blocking the nonspecific sites with 5% bovine serum albumin, wells were washed and incubated with different dilutions of recombinant phage for 1 h at room temperature. Unbound phage was washed off from the wells, and the anti-V5-captured phage were detected using an anti-M13 monoclonal antibody (at a 1:2,000 dilution) conjugated with horseradish peroxidase (HRP; Amersham Pharmacia, Arlington Heights, Ill.). The color reaction was developed with an o-phenylenediamine substrate (Sigma, St. Louis, Mo.).
Expression of his-GST in E. coli.
The recombinant his-GST construct in pRSET B was transformed into BL21(DE3) cells containing pLysS (Invitrogen). Cells were grown to OD600 = 0.6 and induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h. The presence of recombinant protein was confirmed by immunoblot analysis using an anti-Xpress antibody that recognizes the 8-amino-acid Xpress epitope on the fusion protein. Recombinant his-GST was then purified using metal affinity chromatography under denaturing conditions with the Xpress purification system (Invitrogen).
Immunization.
BALB/c male mice purchased from Charles River Laboratories (Wilmington, Mass.) were immunized with pdGST or his-GST. Mice were treated in accordance with an approved institutional protocol. For immunization, about 109 PFU phage (this corresponded to approximately 40 pg of GST protein) suspended in 50 μl of sterile PBS was injected intraperitoneally or intradermally at multiple sites in the abdomen. Both routes essentially produced a similar pattern of antibody response against GST (data not shown). Combination of both routes gave a higher antibody titer against GST. Therefore, in some studies we used both routes to immunize mice. Mice were boosted at weeks 2 and 4 after immunization with the same amount of phage, and samples of blood were collected from each mouse by ocular bleeding before each immunization and at week 6. For protection studies, groups of mice were immunized similarly with 1 μg of his-GST in Imject alum (Pierce Chemicals, Rockford, Ill.) followed by a booster dose at weeks 2 and 4 with the same amount of his-GST in Imject alum (Pierce Chemicals). Control mice received only the adjuvant plus PBS.
Analysis of antibody responses.
Presence of antibodies against S. mansoni GST in the serum of mice immunized with pdGST or his-GST was detected using an ELISA and by an immunoblot analysis. For the immunoblot analysis, about 50 ng of recombinant his-GST or 5 μg of soluble antigenic extracts of schistosomula of S. mansoni were separated in a sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis (PAGE) gel and transferred to a nitrocellulose membrane. After blocking nonspecific sites with 5% skim milk, membranes were probed with sera (diluted 1:100) collected from immunized mice (sera from three mice each were pooled in these experiments). An HRP-conjugated sheep anti-mouse antibody (Pierce Chemicals) used at a 1:5,000 dilution was the secondary antibody. Color was developed with a diaminobenzamidine substrate (Pierce Chemicals).
The isotype of the anti-pdGST antibody was determined using an antibody isotyping kit (Pierce Chemicals). In brief, wells of a 96-well microtiter plate were coated overnight at 4°C with 100 ng of purified recombinant his-GST/ml. After blocking the nonspecific sites with the blocker solution, different dilutions of mouse sera (pooled from two mice each) were added and incubated for 1 h at 37°C. Plates were then washed and incubated with HRP-conjugated rabbit anti-mouse isotype-specific antibodies for immunoglobulin G1 (IgG1), IgG2a, IgG2b, IgG3, and IgM. Color was developed using 2,2′-azinobis-3-ethylbenzthiazoline-6-sulfonic acid substrate, and absorbance was measured at 405 nm. Preimmune sera from naïve mice and normal rabbit sera served as negative controls. We also determined the levels of anti-phage antibodies in the sera of each group of mice. Briefly, wells of a 96-well plate were coated overnight at 4°C with different dilutions of the serum samples. After adding 109 PFU phage, wells were incubated for 1 h at room temperature. Unbound phage was washed off, and captured phage was detected using an HRP-conjugated anti-M13 monoclonal antibody. The color reaction was developed with an o-phenylenediamine substrate, and absorbance was measured at 405 nm.
Preparation of immune sera and polyclonal rabbit antischistosomular antibodies.
Immune sera were prepared by first immunizing mice with 250 gamma irradiation-attenuated cercariae of S. mansoni and collecting sera on day 30 postimmunization. Polyclonal antibodies against schistosomula of S. mansoni were generated in NZ white rabbits. Briefly, 5,000 schistosomula of S. mansoni were homogenized in NET buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris-Cl [pH 7.4]) in the presence of protease inhibitor cocktail (Sigma), and soluble antigen extracts were separated after centrifugation at 10,000 × g for 15 min at 4°C. The protein concentration in the soluble extracts was determined using a BCA kit (Pierce Chemicals) after filter sterilization. For immunization, about 100 μg of schistosomula protein mixed with 25 μg of Gerbu adjuvant (Gerbu Biotechnik GmbH, Gaiberg, Germany) was injected subcutaneously at multiple sites on two rabbits. A booster dose consisted of the same amount of antigen in Gerbu adjuvant given at 2 and 4 weeks after immunization. The antibody titer was determined every 2 weeks, and serum was collected when the titer reached its peak. Both immune sera and polyclonal antischistosomula sera were used for immunoblot analyses.
Protection studies.
BALB/c mice were immunized with pdGST or his-GST as described above. At 6 weeks after immunization, each mouse was challenged with 100 cercariae of S. mansoni via the abdominal skin by using a coverslip method as described previously (21). On day 42 after challenge infection, adult worms in the portal mesenteric vein were recovered by a perfusion technique described by Duvall and DeWitt (13). Values were compared with those obtained from naïve animals challenged similarly with 100 cercariae. The percent protection was calculated using the following formula: [(number of worms established in naïve animals − number of worms established in immunized animals)/(number of worms established in naïve animals)] × 100. About 7 to 10 mice were used per group, and the experiments were repeated three times.
Statistical analysis.
Statistical analyses to test the significance of variance in the levels of antibodies between control and immune sera were determined by a Mann-Whitney U test using the Sigmastat program (Jandel Scientific, San Rafael, Calif.).
RESULTS
Expression of Sm28GST in phage.
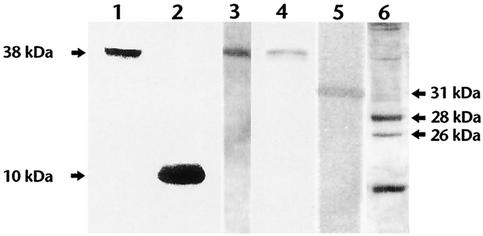
Sm28GST was expressed as a fusion to the Fos. Association of the Fos fusion protein to Jun facilitated display of Sm28GST on the surface of bacteriophage along with the gene III coat protein. To detect pdGST, initially we screened the bacteriophage proteins by a Western blot analysis specifically looking for the V5 epitope in the fusion protein, using anti-V5 monoclonal antibodies. These studies showed that the anti-V5 monoclonal recognized a ∼38-kDa protein in the extracts of recombinant pdGST (Fig. 1). This is in agreement with the expected molecular mass of the GST (28 kDa) plus Fos (10 kDa) fusion protein. In control phage without the GST insert, the anti-V5 antibody recognized only a protein of ∼10 kDa that corresponded with the molecular mass of the Fos fusion protein (Fig. 1, lane 2). Expression of GST in the fusion protein was then confirmed by probing the phage proteins with pooled sera from mice immunized with radiation-attenuated cercariae (Fig. 1, lane 3) and a polyclonal rabbit antischistosomular antibody (Fig. 1, lane 4). The antibodies in these sera also recognized the recombinant his-GST (Fig. 1, lane 5), which has a molecular mass of 31 kDa (28 plus 3 kDa of the histidine tag). Interestingly, the antibodies also recognized two proteins of molecular mass 28 and 26 kDa among several other proteins in the soluble extracts of schistosomula (Fig. 1, lane 6).
FIG. 1.
Expression of Sm28GST in phage. Phage proteins (108 PFU) expressing recombinant 28GST (lanes 1, 3, and 4), control phage protein (lane 2), purified recombinant his-GST (lane 5), or soluble extracts of schistosomula of S. mansoni (lane 6) were separated on an SDS-12% PAGE gel and blotted onto nitrocellulose membranes. Lanes 1 and 2 were probed with anti-V5 antibodies, lane 3 was probed with pooled sera from mice immunized with radiation-attenuated cercariae, and lanes 4, 5, and 6 were probed with a polyclonal rabbit antischistosomula antibody.
Display of S. mansoni GST on the surface of phage.
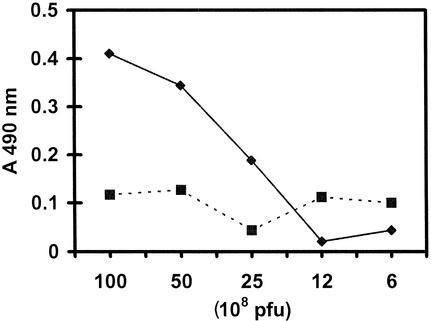
The above studies confirmed that the Sm28GST protein is expressed by the M13 phage. To determine whether the expressed GST is displayed on the surface of phage, a capture ELISA was performed using antibodies against anti-V5 monoclonal antibody (Fig. 2). At the given dilution of V5 monoclonal antibody, about 109 bacteriophage expressing the V5-GST fusion could be detected. These studies demonstrated that GST is expressed on the surface of phages. A similar capture ELISA using immune sera and a rabbit antischistosomular antibody yielded comparable results (data not shown), confirming that GST is indeed expressed on the surface of phages.
FIG. 2.
Sm28GST is displayed on the surface of phage. Phage displaying GST as a V5 fusion protein was captured onto the wells of a microtiter plate using anti-V5 antibodies and detected using an M13 antibody. Solid lines represent recombinant phage displaying SmGST on the surface. Control phage without the V5 epitope are shown as a dotted line.
Immunoreactivity of phage-displayed GST.
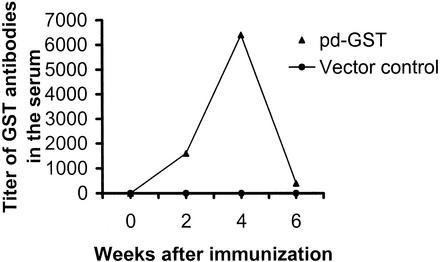
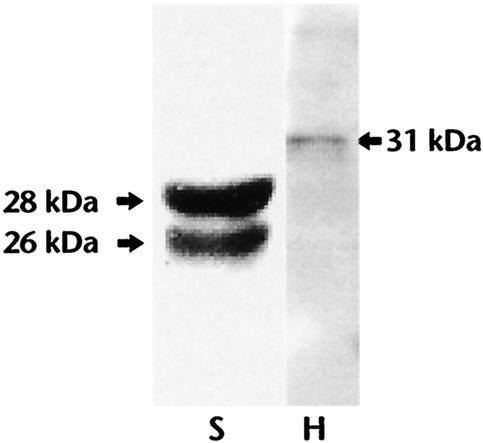
After booster immunization, the titer of antibodies reached 1:6,400 by 4 weeks (Fig. 3). Thereafter, there was a steady decrease in the antibody titer, yet a significant titer of antibodies (1:400) was present in the serum at 6 weeks after immunization. The specificity of these antibodies was then determined by an immunoblot analysis using soluble extracts of schistosomula of S. mansoni (Fig. 4). These studies confirmed that the antibodies in the sera of mice immunized with pdGST strongly recognized a 28-kDa protein in the worm extract and also reacted with his-GST (Fig. 4). Interestingly, in addition to the 28-kDa band, the sera also recognized a 26-kDa protein in the soluble extracts of schistosomula (Fig. 4). Blots probed similarly with the preimmune sera were negative (data not shown).
FIG. 3.
Titer of anti-GST antibodies in mice immunized with pdGST. Mice were immunized with pdGST as described in Materials and Methods. To determine the titer of anti-GST antibodies in the serum, blood was collected on day 0 and at weeks 2, 4, and 6 postimmunization. Serum samples from two to three mice were pooled within each group, and the titer of anti-GST antibodies was determined by an ELISA. Mice immunized similarly with vector alone served as controls. Five mice were used per group, and data are from one of two similar experiments.
FIG. 4.
Immunization of mice with pdGST generates antibodies that specifically recognize the native GST. Soluble extracts of the schistosomula of S. mansoni (S) or purified recombinant his-GST (H) were separated in an SDS-12% PAGE gel, transferred onto a nitrocellulose sheet, and probed with pooled sera from two mice immunized 6 weeks previously with pdGST. The immune sera recognized two protein bands at 26 kDa and a 28-kDa molecular mass in the soluble extracts of schistosomula. The sera also recognized the recombinant his-GST. The data presented are representative of five different Western blot analyses using 10 mice that showed similar results.
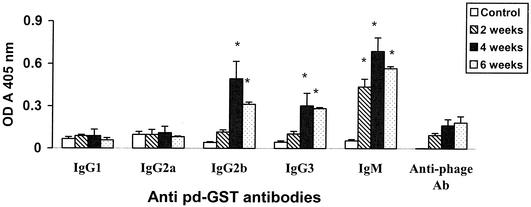
Isotype of pdGST-induced antibodies.
Analysis of the isotype of GST-specific antibodies in the sera of mice immunized with pdGST showed that at 2 weeks after immunization the anti-GST antibodies were predominantly of the IgM isotype (Fig. 5). By 4 and 6 weeks after immunization, significant amounts of IgG2b, IgG3, and IgM anti-GST antibodies were present in the sera of mice immunized with pdGST (Fig. 5). Antigen-specific IgG1 or IgG2a were near background levels. Immunization with his-GST alone without any adjuvant elicited very low levels of IgM antibodies (OD, 0.096 ± 0.008) at 4 weeks after immunization. Control animals injected with blank phage vector did not show any antibodies against GST in their serum (data not shown). However, antibodies against phage were present in all immunized animals from 2 weeks after immunization.
FIG. 5.
Isotype of anti-GST antibodies induced by pdGST immunization. Anti-GST antibodies in the sera of mice immunized with pdGST were analyzed by ELISA using an antibody isotyping kit. Levels of IgG2b, IgG3, and IgM antibodies were significantly higher in the sera of immunized animals than in preimmune sera. However, there was no significant difference in the levels of IgG1 and IgG2a compared with the preimmune values. Levels of anti-phage antibodies (anti-phage Ab) in the serum samples were determined using anti-M13 antibodies. Results presented are representative of one of three similar experiments. Data shown are the mean ± standard deviation of OD values.
Protection conferred by pdGST immunization.
Since immunization with purified his-GST elicited only low levels of antibody responses, in protection studies we used Imject alum adjuvant along with the his-GST proteins to elicit an immune response. However, for pdGST we did not use any adjuvant. The degree of protection was determined by counting the number of worms established in each mouse. These studies showed that fewer worms (41.1 ± 7.40) were recovered from mice immunized with pd-GST than the vector-alone controls (58.7 ± 5.81) (Table 1). A calculation of the difference in worm establishment showed that there was a 29.96% reduction in the number of worms established following vaccination with pdGST. Comparable results were obtained when mice were immunized with purified his-GST plus adjuvant (42.2 ± 3.58 worms, or 24.71% protection), compared to controls (54.30 ± 4.37). The majority of the worms recovered from vector or adjuvant control animals were in pairs (29.0 ± 2.91 and 26.20 ± 2.35, respectively). However, significantly (P > 0.01) fewer worms were found in pairs in mice immunized with pdGST (16 ± 1.763) compared to mice immunized with his-GST (19.0 ± 2.26). Since pairing of male and female worms is essential for egg production, this may have implications in the egg-associated pathology in this infection. Most of the unpaired worms recovered were males. This also potentially suggests that pdGST immunization may have some effect on female worm survival.
TABLE 1.
Effect of pdGST immunization on challenge worm establishment rate
| Treatment | Worm burdena | Individual worm burdens | No. of worm pairs |
|---|---|---|---|
| pdGST | 41.10 ± 7.40** | 37, 39, 38, 58, 39, 38, 39, 38, 34, 51 | 16 ± 1.763* |
| Vector control | 58.70 ± 5.81 | 62, 59, 58, 64, 51, 52, 54, 55, 63, 69 | 29.0 ± 2.91 |
| his-GST | 42.20 ± 3.58** | 40, 38, 46, 39, 41, 46, 49, 41, 42, 40 | 19.0 ± 2.26 |
| Adjuvant control | 54.30 ± 4.37 | 53, 58, 59, 49, 47, 60, 54, 52, 53, 58 | 26.20 ± 2.35 |
Mice were challenged with 100 cercariae of S. mansoni and perfused on day 42 postchallenge. Asterisks indicate significant decreases (**, P < 0.001; *, P < 0.01) compared to controls and his-GST treatment. Data are means ± standard deviations.
DISCUSSION
Results of the present study demonstrate that the 28-kDa GST antigen of S. mansoni can be displayed on the surface of bacteriophages. The phage-displayed GST protein appears to maintain the correct folding and is recognized by immune sera. Subsequent immunization studies using the pdGST in a mouse model showed that a significantly higher titer of anti-GST antibodies could be generated without any additional adjuvant. Challenge studies showed that compared to controls, fewer worms developed in mice immunized with pdGST. In this study, we chose to display Sm28GST primarily because the World Health Organization has recommended this protein as one of the candidate vaccine antigens for immunization against S. mansoni (5, 23). Although GST is known to generate only weaker protective responses in mice, the findings presented in this study do demonstrate that the candidate vaccine antigens could be displayed on the surface of phage and can be potentially used for immunization.
The strategy used in this study for displaying Sm28GST on the surface of phage is based on the strong association of Jun with Fos proteins (8). Sm28GST was cloned downstream of the Fos-V5-encoding gene, where V5 is a reporter gene. Upon expression, the Fos protein associates with Jun that is anchored to the gene III surface coat protein. There are five copies of the gene III per virion, and all can be fused without interfering with phage infectivity (10). Thus, when gene III is expressed by the bacteriophages, the Sm28GST is also displayed as a covalently linked anchor on the surface of the phage. This vector system was initially developed by Crameri et al. (8) and was later modified by Shanmugavelu et al. (26) to isolate juvenile hormone esterase in the fat body tissue and pericardial cells of the tobacco hornworm Manduca sexta. Here we show that this modified vector system could be used efficiently to display selected candidate vaccine antigens of S. mansoni.
Previous reports suggest that proteins displayed by the pJuFo vector system express functionally folded cDNA products (9). Our immunoblot analysis and ELISA results support this notion and show that Sm28GST is displayed on the surface of phage potentially expressing epitopes similar to the native form that allowed the immune sera and antischistosomular antibody to recognize them.
The Sm28GST antigen is expressed by various life cycle stages of S. mansoni and is thought to detoxify electrophilic compounds generated by the host in an attempt to eliminate the parasite (28). Other species of schistosomes (19) and several other parasites also express 28GST (4). Because of its unique detoxifying property, Sm28GST has been a target for vaccine development. Extensive animal studies mainly from Andre Capron's group at the Institute of Pasteur in France show that purified or recombinant Sm28GST can be used successfully to elicit a protective immune response against S. mansoni, especially when combined with adjuvants. However, the maximum protection rate obtained after immunization with 28GST range from 28 to 33.5% (25). In the present study we show that immunization with pd-GST could confer comparable protection (29.96%) without any additional adjuvant.
The pattern of antibody responses to GST differs significantly, depending on the type of adjuvant used (6). For example, when aluminum hydroxide was used as an adjuvant to immunize mice with Sm28GST, a strong IgG1 response was obtained, whereas fusion of Sm28GST with tetanus toxoid resulted in a pronounced IgG2a response (6). Similarly, immunization with Sm28GST in complete Freund's adjuvant resulted in high levels of both IgG1 and IgG2a (7). Nevertheless, rSm28GST by itself is a poor immunogen (7). We could barely detect antibody in the serum after immunization with his-GST without an adjuvant. However, when phage-displayed Sm28GST was used as an immunogen, we observed a strong IgG2b, IgG3, and IgM response in the mice without any additional adjuvant. It is believed that the phage proteins can act as adjuvant and enhance T-cell help (29). This might have promoted the antibody response to pdGST. Interestingly, the type of antibody response elicited after immunization with phage-displayed Sm28GST is comparable to that elicited after a DNA vaccination with Sm28GST-encoding DNA, which is known to confer significant protection (12). Similarly, a recent study in northern Senegal showed that a low level of S. mansoni infection in the male population correlated with a high level of neutralizing IgG3 antibody against Sm28GST in their sera (22). Although we did not check whether the IgG3 antibody elicited by phage-displayed Sm28GST in male mice is a neutralizing antibody, the response seems to be comparable to those in humans.
Immunization with radiation-attenuated cercariae of S. mansoni confers 60 to 90% protection against challenge infection in mice (24). Analysis of sera from these immune mice showed that they contain predominantly the IgM isotype of antibodies against GST and are potentially directed towards the carbohydrate epitopes (24). Similar to the radiation-attenuated vaccine, the phage-displayed Sm28GST immunization also appears to induce a strong IgM anti-Sm28GST antibody response. However, the degree of protection obtained after pdGST vaccination was only about 30%. This may be because Sm28GST is not the only antigen in the irradiated cercariae that is involved in the generation of protective immune responses. Interestingly, the degree of protection conferred by pdGST vaccination is comparable or better than that conferred by recombinant Sm28GST vaccination. Another observation is that the immunization with pdGST appears to reduce the number of female worms, which can potentially reduce the egg-induced pathology in this infection. Clearly, further studies are needed to understand this phenomenon.
Besides 28GST, S. mansoni also contains another isoenzyme of GST that has a molecular mass of 26 kDa (20). Previous studies show that antibodies generated against 28GST do not cross-react with 26GST (20). However, in our studies the antibodies generated against phage-displayed Sm28GST were found to recognize both Sm28 and Sm26GST (Fig. 4). This may be because the phage-displayed GST may resemble more closely the native GST in its folding, conformation, and presentation of the epitopes (9). Emulsification with adjuvants is known to affect the native conformation of antigens (1). This might explain the lack of cross-reactivity observed between 28GST and 26GST in previous studies.
The recombinant phage display approach for generating antibodies has several advantages over conventional systems. These include (i) the relative ease with which the recombinant proteins are generated, (ii) proteins displayed by the phage attain native conformation, (iii) extensive purification steps are not necessary (a simple polyethylene glycol precipitation is sufficient), (iv) phage is an easily renewable source of antigen and, finally, (v) for immunization there is no need of adjuvant and therefore side effects associated with the use of adjuvant can be avoided. Although the titer of antibodies was low, immunization with phage did generate antibodies against the phage (Fig. 5). This may be an unwanted side effect. At present we do not know whether anti-phage antibodies could interfere with multiple vaccination. Nevertheless, we believe that the phage display approach presented in this study may have wider application as a general strategy for eliciting antibody responses to recombinant antigens. Given that GST could be potentially used as a fusion partner, the phage-displayed GST may have broader application in proteomic research as well (3).
Acknowledgments
This study was supported by Public Health Service grant AI 39066 from the National Institute of Allergy and Infectious Diseases (NIAID) awarded to K.R. Life cycle stages of S. mansoni were obtained from Fred Lewis, Biomedical Research Institute, Rockville, Md., through NIH-NIAID contract number N01-A1-55770.
We thank Chris Gaskins and Madasamy Shanmugavelu for their helpful suggestions and comments.
REFERENCES
- 1.Allison, A. C. 1999. Squalene and squalane emulsions as adjuvants. Methods 19:87-93. [DOI] [PubMed] [Google Scholar]
- 2.Bastien, N., M. Trudel, and C. Simard. 1997. Protective immune responses induced by the immunization of mice with a recombinant bacteriophage displaying an epitope of the human respiratory syncytial virus. Virology 234:118-122. [DOI] [PubMed] [Google Scholar]
- 3.Borrebaeck, C. A. 2000. Antibodies in diagnostics—from immunoassays to protein chips. Immunol. Today 21:379-382. [DOI] [PubMed] [Google Scholar]
- 4.Brophy, P. M., and J. Barrett. 1990. Glutathione transferase in helminths. Parasitology 100(Pt. 2):345-349. [DOI] [PubMed] [Google Scholar]
- 5.Capron, A., M. Capron, D. Dombrowicz, and G. Riveau. 2001. Vaccine strategies against schistosomiasis: from concepts to clinical trials. Int. Arch. Allergy Immunol. 124:9-15. [DOI] [PubMed] [Google Scholar]
- 6.Comoy, E. E., A. Capron, and G. Thyphronitis. 1998. Adjuvant is the major parameter influencing the isotype profiles generated during immunization with a protein antigen, the Schistosoma mansoni Sm28-GST. Scand. J. Immunol. 47:444-452. [DOI] [PubMed] [Google Scholar]
- 7.Comoy, E. E., A. Capron, and G. Thyphronitis. 1997. In vivo induction of type 1 and 2 immune responses against protein antigens. Int. Immunol. 9:523-531. [DOI] [PubMed] [Google Scholar]
- 8.Crameri, R., R. Jaussi, G. Menz, and K. Blaser. 1994. Display of expression products of cDNA libraries on phage surfaces. A versatile screening system for selective isolation of genes by specific gene-product/ligand interaction. Eur. J. Biochem. 226:53-58. [DOI] [PubMed] [Google Scholar]
- 9.Crameri, R., and M. Suter. 1993. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for selection of functional gene products linked to the genetic information responsible for their production. Gene 137:69-75. (Erratum, 160:139, 1995.) [DOI] [PubMed]
- 10.Cwirla, S. E., E. A. Peters, R. W. Barrett, and W. J. Dower. 1990. Peptides on phage: a vast library of peptides for identifying ligands. Proc. Natl. Acad. Sci. USA 87:6378-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Cruz, V. F., A. A. Lal, and T. F. McCutchan. 1988. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J. Biol. Chem. 263:4318-4322. [PubMed] [Google Scholar]
- 12.Dupre, L., O. Poulain-Godefroy, E. Ban, N. Ivanoff, M. Mekranfar, A. M. Schacht, A. Capron, and G. Riveau. 1997. Intradermal immunization of rats with plasmid DNA encoding Schistosoma mansoni 28 kDa glutathione S-transferase. Parasite Immunol. 19:505-513. [DOI] [PubMed] [Google Scholar]
- 13.Duvall, R. H., and W. B. DeWitt. 1967. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am. J. Trop. Med. Hyg. 16:483-486. [DOI] [PubMed] [Google Scholar]
- 14.Frenkel, D., I. Dewachter, F. Van Leuven, and B. Solomon. 2003. Reduction of beta-amyloid plaques in brain of transgenic mouse model of Alzheimer's disease by EFRH-phage immunization. Vaccine 7:1060-1065. [DOI] [PubMed] [Google Scholar]
- 15.Fuh, G., and S. S. Sidhu. 2000. Efficient phage display of polypeptides fused to the carboxy-terminus of the M13 gene-3 minor coat protein. FEBS Lett. 480:231-234. [DOI] [PubMed] [Google Scholar]
- 16.Grabowska, A. M., R. Jennings, P. Laing, M. Darsley, C. L. Jameson, L. Swift, and W. L. Irving. 2000. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology 269:47-53. [DOI] [PubMed] [Google Scholar]
- 17.Gram, H., U. Strittmatter, M. Lorenz, D. Gluck, and G. Zenke. 1993. Phage display as a rapid gene expression system: production of bioactive cytokine-phage and generation of neutralizing monoclonal antibodies. J. Immunol. Methods 161:169-176. [DOI] [PubMed] [Google Scholar]
- 18.Hansson, L. O., M. Widersten, and B. Mannervik. 1997. Mechanism-based phage display selection of active-site mutants of human glutathione transferase A1-1 catalyzing SNAr reactions. Biochemistry 36:11252-11260. [DOI] [PubMed] [Google Scholar]
- 19.Henkle, K. J., K. M. Davern, M. D. Wright, A. J. Ramos, and G. F. Mitchell. 1990. Comparison of the cloned genes of the 26- and 28-kilodalton glutathione S-transferases of Schistosoma japonicum and Schistosoma mansoni. Mol. Biochem. Parasitol. 40:23-34. [DOI] [PubMed] [Google Scholar]
- 20.O'Leary, K. A., K. M. Hathaway, and J. W. Tracy. 1992. Schistosoma mansoni: single-step purification and characterization of glutathione S-transferase isoenzyme 4. Exp. Parasitol. 75:47-55. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy, K., Y.-X. He, and B. Salafsky. 1997. ICAM-1 and iNOS expression is increased in the skin of mice after vaccination with γ-irradiated cercariae of Schistosoma mansoni. Exp. Parasitol. 86:118-132. [DOI] [PubMed] [Google Scholar]
- 22.Remoue, F., F. Rogerie, M. C. Gallissot, H. L. Guyatt, J. L. Neyrinck, M. M. Diakkhate, M. Niang, A. E. Butterworth, C. Auriault, A. Capron, and G. Riveau. 2000. Sex-dependent neutralizing humoral response to Schistosoma mansoni 28GST antigen in infected human populations. J. Infect. Dis. 181:1855-1859. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro de Jesus, A., I. Araujo, O. Bacellar, A. Magalhaes, E. Pearce, D. Harn, M. Strand, and E. M. Carvalho. 2000. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect. Immun. 68:2797-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter, D., R. N. Incani, and D. A. Harn. 1993. Isotype responses to candidate vaccine antigens in protective sera obtained from mice vaccinated with irradiated cercariae of Schistosoma mansoni. Infect. Immun. 61:3003-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riveau, G., O. Poulain-Godefroy, L. Dupre, F. Remoue, N. Mielcarek, C. Lochet, and A. Capron. 1998. Glutathione S-transferase of 28 kDa as major vaccine candidates against schistosomiasis. Mem. Inst. Oswaldo Cruz 93:87-94. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugavelu, M., A. R. Baytan, J. D. Chesnut, and B. C. Bonning. 2000. A novel protein that binds juvenile hormone esterase in fat body tissue and pericardial cells of the tobacco hornworm Manduca sexta. L. J. Biol. Chem. 275:1802-1806. [DOI] [PubMed] [Google Scholar]
- 27.Smith, G. P. 1985. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315-1317. [DOI] [PubMed] [Google Scholar]
- 28.Walker, J., P. Crowley, A. D. Moreman, and J. Barrett. 1993. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Mol. Biochem. Parasitol. 61:255-264. [DOI] [PubMed] [Google Scholar]
- 29.Willis, A. E., R. N. Perham, and D. Wraith. 1993. Immunological properties of foreign peptides in multiple displays on a filamentous bacteriophage. Gene 128:79-83. [DOI] [PubMed] [Google Scholar]