Abstract
Cancer patients who are leukopenic due to chemotherapy are susceptible to bacterial infections. Normally, clinical conditions during bacterial infections are caused by pathogen-associated molecular patterns, which are components that bind to Toll-like receptor (TLR) 2 (TLR-2) and TLR-4 on leukocytes, resulting in the production of inflammatory cytokines. The mechanism of this inflammatory response in cancer patients with diminished numbers of leukocytes is not completely clear. The levels of interleukin 1β (IL-1β) and tumor necrosis factor alpha measured in the circulation of leukopenic cancer patients are lower than those measured in that of nonleukopenic patients during bacterial infections, whereas plasma interleukin 8 (IL-8) levels show distinct identical increases during bacterial infections in both leukopenic and nonleukopenic patients. Normally, these cytokines are mainly secreted by leukocytes. In cancer patients with bacterial infections and a diminished number of leukocytes, other sources of IL-8 production, such as endothelial cells, might be expected. Endothelial cells instead of leukocytes become the most important producers of IL-8 during bacterial infections in patients with chemotherapy-induced leukopenia through TLR-2 and TLR-4 signaling. Whole blood samples from six cancer patients were stimulated with lipopolysaccharide (LPS), and then IL-8 concentrations in supernatants were measured. Further, human umbilical vein endothelial cells (HUVECs) were incubated with sera from leukopenic cancer patients with or without bacterial infections, and then IL-8 concentrations in supernatants were measured (n = 6). In addition, the same HUVEC experiment was performed with the addition of neutralizing antibodies against TLR-2 and TLR-4. During leukopenia (<109 cells/liter), LPS stimulation of whole blood did not result in an increase in IL-8 levels. However, when endothelial cells were incubated with sera from leukopenic cancer patients during bacterial infections, a three- to eightfold increase in IL-8 production was found, compared to the IL-8 production found after incubation with sera from patients without signs of infections. This increase did not reflect a higher level of IL-8 already present in the sera. Further, we demonstrated that IL-8 production induced in endothelial cells by sera from patients with documented gram-negative infections could be reduced significantly by up to 40% when the cells were incubated with neutralizing antibodies against TLR-4 (P = 0.028). The addition of TLR-2 antibodies slightly enhanced the reduction of IL-8 production. These results suggest that during bacterial infections in cancer patients with markedly diminished numbers of leukocytes, endothelial cells become important producers of IL-8 through TLR-4 signaling and, to a lesser extent, TLR-2 signaling.
Bacterial infections remain a common and severe problem in cancer patients who are leukopenic (leukocyte count, <109/liter) due to chemotherapy (3, 4). Infections with gram-negative as well as gram-positive microorganisms may lead to septic shock and death. The development of novel strategies to prevent complications of bacterial infections and strategies to diagnose bacterial infections earlier by using inflammatory cytokines, such as interleukin 8 (IL-8), requires further insight into the innate immune response of cancer patients treated with chemotherapy (9, 20).
Clinical responses to bacterial infections are caused by certain components of the bacterial cell wall, called the pathogen-associated molecular patterns (PAMPs). When these components are recognized by receptors of the innate immune system, a complex network of changes in the human body is induced, called the inflammatory response. For example, lipopolysaccharide (LPS), the PAMP from gram-negative bacteria, becomes detached from the bacterial cell membrane and binds to LPS-binding protein, an acute-phase protein (18). LPS-binding protein transports LPS to soluble CD14 or membrane-bound CD14 on monocytes and tissue macrophages, resulting in an effector phase characterized by the production of IL-1β and tumor necrosis factor alpha (TNF-α) and then the production of IL-6 and IL-8 (7, 29).
Recently, the involvement of Toll-like receptors (TLRs) as pattern recognition receptors in the innate immune response was demonstrated. The TLRs are characterized by an extracellular domain containing leucine-rich repeats and an intracellular domain sharing a high degree of similarity with the IL-1 receptor (21). Downstream signaling involves the MyD88/IRAK cascade and the activation of NF-κB-controlled genes involved in the regulation of antimicrobial defenses, such as the IL-1, IL-6, and IL-8 genes (31). LPS-hyporesponsive mice were found to have a mutation in the TLR-4 gene (2, 24, 25). Other studies showed that besides TLR-4, TLR-2 also appears to be a signaling molecule for LPS (32, 33). In addition, TLR-2 was identified as a signal transducer for PAMPs from gram-positive bacteria, such as peptidoglycan and lipoteichoic acid (26). Most effector cells of the innate immune system, such as monocytes and endothelial cells, express TLR-2 and TLR-4 on their membranes (13, 17).
The exact mechanism of the inflammatory response in cancer patients with disturbed innate immunity has not been completely clear. During bacterial infections, the levels of IL-1β and TNF-α measured in the circulation of leukopenic cancer patients are lower than those measured in that of nonleukopenic patients (6, 7, 14, 16). Normally, these cytokines are mainly secreted by leukocytes, especially monocytes. However, bone marrow toxicity as the result of chemotherapy leads to reduced numbers of leukocytes in cancer patients; this factor may be one of the reasons for the low total levels of TNF-α and IL-1β (8). At the same time, leukopenic cancer patients strikingly show distinct increases in plasma IL-8 and IL-6 levels during bacterial infections, suggesting that there may be sources other than leukocytes for IL-8 and IL-6 (5, 9, 11, 12, 15, 20, 22, 23, 30).
Endothelial cells also have the ability to produce inflammatory cytokines, such as IL-8 and IL-6. We hypothesized that endothelial cells instead of leukocytes become the most important producers of IL-8 and IL-6 during bacterial infections in patients with chemotherapy-induced leukopenia. We speculated that in leukopenic cancer patients, TLRs on endothelial cells act as pattern recognition receptors that induce the production of proinflammatory cytokines upon binding of bacterial cell wall components.
In this study, we show that the remaining leukocytes of leukopenic cancer patients do not contribute significantly to IL-8 or TNF-α production upon LPS stimulation. However, sera from leukopenic cancer patients with bacterial infections induce markedly increased production of IL-8 in endothelial cells compared to sera from patients without signs of infections. Moreover, this patient serum-induced IL-8 production in endothelial cells is reduced after the addition of neutralizing antibodies against TLR-2 and/or TLR-4. These results suggest that in cancer patients with markedly diminished numbers of leukocytes, endothelial cells become important producers of IL-8 during the inflammatory response against bacteria through TLR-2 and TLR-4 signaling.
MATERIALS AND METHODS
Patient samples.
Serial blood samples were collected three times weekly from six adult patients who had hematological malignancies (acute myeloid leukemia [n = 5] and Burkitt lymphoma [n = 1]) and who were admitted to the University Hospital Groningen during a leukopenic period following a chemotherapy course. These serial samples were used in the ex vivo whole-blood assay and the human umbilical vein endothelial cell (HUVEC) assay. Leukopenia was defined as a leukocyte count of <109/liter. Clinical sepsis was defined by the following criteria: a systolic blood pressure of <90 mm Hg or both a heart rate of >100/min and a breath rate of >20/min according to systemic inflammatory response syndrome criteria (1).
In addition, blood samples were collected from 10 leukopenic cancer patients at the onset of documented gram-negative or gram-positive bloodstream infections. These were adult and pediatric patients who had hematological malignancies or solid tumors and who had been entered into a prospective study concerning the diagnostic role of IL-8 in a new treatment strategy for ambulatory febrile leukopenia which is currently being performed at the University Hospital Groningen. These blood samples were used for the HUVEC blocking experiments.
Serum and plasma were separated from cells by centrifugation within 30 min after collection and were stored at −80°C.
Reagents and antibodies.
LPS was derived from Pseudomonas aeruginosa serotype 10 and purified by phenol extraction (Sigma, St. Louis, Mo.). The neutralizing anti-human TLR-2 monoclonal antibody (MAb) 2392 was a kind gift from Genentech (South San Francisco, Calif.) and was generated by immunization of BALB/c mice with the purified soluble extracellular domain of human TLR-2 (32). The neutralizing anti-human TLR-4 MAb HTA125 was a generous gift from Kensuke Miyake (Saga Medical School, Nabeshima, Saga, Japan) and was generated by immunization of BALB/c mice with the Ba/F3 cell line expressing TLR-4 (27). Murine isotype antibodies (immunoglobulin G1 [IgG1] and IgG2A) were used as control antibodies in the experiments.
Cell culture.
HUVECs were obtained from the Endothelial Cell Facility at the University Hospital Groningen (H. Moorlag and G. Molema) and cultured in EC medium (RPMI 1640 [BioWhittaker, Walkersville, Md.] supplemented with 2 mM l-glutamine [Gibco, Paisley, United Kingdom], 5 U of heparin [Leo Pharmaceutical Products, Weesp, The Netherlands]/ml, antibiotics, and 50 μg of endothelial cell growth factor [Endothelial Cell Facility]/ml). Third-passage HUVECs were used for all experiments.
Determination of cytokine concentrations.
IL-8 concentrations were measured by using a commercially available enzyme-linked immunosorbent assay (ELISA) (Human IL-8 Flexia; Biosource, Camarillo, Calif.). IL-1β and IL-6 concentrations were also measured by using ELISAs (the respective antibody pairs used were as follows: anti-IL-1β MAb 601 and biotinylated goat anti-human IL-1β antibody 201-NA [R&D, Minneapolis, Minn.]; anti-IL-6 MAb M1595 and biotinylated sheep anti-human IL-6 antibody M1514 [Sanquin, Amsterdam, The Netherlands]). TNF-α concentrations were determined by using a chemiluminescence immunoassay (Immulite; Diagnostic Products Corporation, Los Angeles, Calif.).
Ex vivo whole-blood assay.
Serial whole-blood samples from six patients were incubated ex vivo with and without LPS (500 pg/ml) at 37°C. After 4 h, plasma was separated from the cells by centrifugation, and TNF-α and IL-8 concentrations were measured by following manufacturer instructions for the Milenia ex vivo stimulation kit (Diagnostic Products Corporation). In addition, leukocyte numbers in whole blood were measured with an automated Coulter Counter.
HUVEC assay.
HUVECs were plated on 24-well tissue culture plates (Costar, High Wycombe, United Kingdom) and grown to confluence. Monolayers were washed once with phosphate-buffered saline and incubated at 37°C in EC medium at a 1:1 dilution with serum or plasma from leukopenic cancer patients with or without documented and/or clinically defined bacterial infections. For incubation with patient plasma, calcium chloride was added to neutralize the anticlotting factor. After 8 h, supernatants were removed and IL-8 concentrations were determined by using an ELISA.
HUVEC blocking experiments.
For HUVEC blocking experiments, neutralizing antibodies against TLR-2 and TLR-4 were added to the cells 30 min prior to incubation with patient serum or plasma at concentrations varying from 10 to 30 μg/ml.
Statistical analysis.
To compare patient serum-induced IL-8 production in HUVECs during bacterial infection with patient serum-induced IL-8 production during a period with no signs of infection, the Wilcoxon signed rank test was used. To assess the relationship between plasma IL-8 levels in vivo and patient serum-induced IL-8 production in HUVECs, the Spearman correlation coefficient was determined. To compare patient serum-induced IL-8 production in HUVECs before and after blocking with anti-TLR antibodies, the Wilcoxon signed rank test for paired samples was used. A two-sided P value of <0.05 was considered statistically significant.
RESULTS
The decreased number of leukocytes is related to diminished TNF-α and IL-8 production in leukopenic cancer patients.
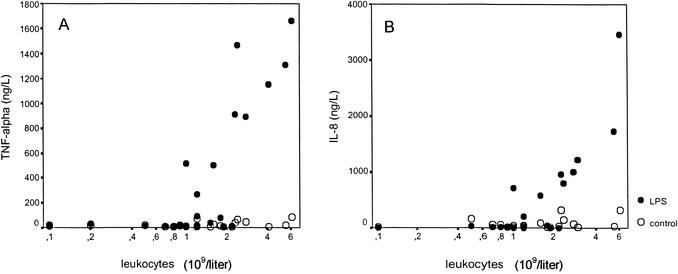
The role of leukocytes in the production of proinflammatory cytokines in leukopenic cancer patients was investigated by stimulating serial whole-blood samples from cancer patients with LPS (500 pg/ml) for 4 h, after which TNF-α and IL-8 levels in supernatants were determined (Fig. 1). TNF-α production and IL-8 production were not higher in LPS-stimulated whole-blood cultures during leukopenia (<109 leukocytes/liter) than in controls without LPS stimulation. When leukopenia was resolved (>2 × 109 leukocytes/liter), LPS stimulation of whole-blood cultures induced TNF-α production and IL-8 production, as expected.
FIG. 1.
Cytokine production. Whole blood obtained from cancer patients during chemotherapy-induced leukopenia and after the recovery of leukocytes was stimulated ex vivo with LPS (500 pg/ml) (filled circles) or not stimulated with LPS (controls) (open circles). TNF-α production (A) and IL-8 production (B) were measured.
These results demonstrate that the decreased number of circulating leukocytes seems to be related to low TNF-α and IL-8 production ex vivo and could well be responsible for the low plasma TNF-α levels in vivo. However, it has been reported that plasma IL-8 levels in vivo show distinct elevations during bacterial infections in cancer patients with low leukocyte counts (9, 20, 30). We questioned whether endothelial cells may play a role in IL-8 production during bacterial infections in cancer patients.
Sera from leukopenic cancer patients during bacterial infections induce increased IL-8 production in HUVECs.
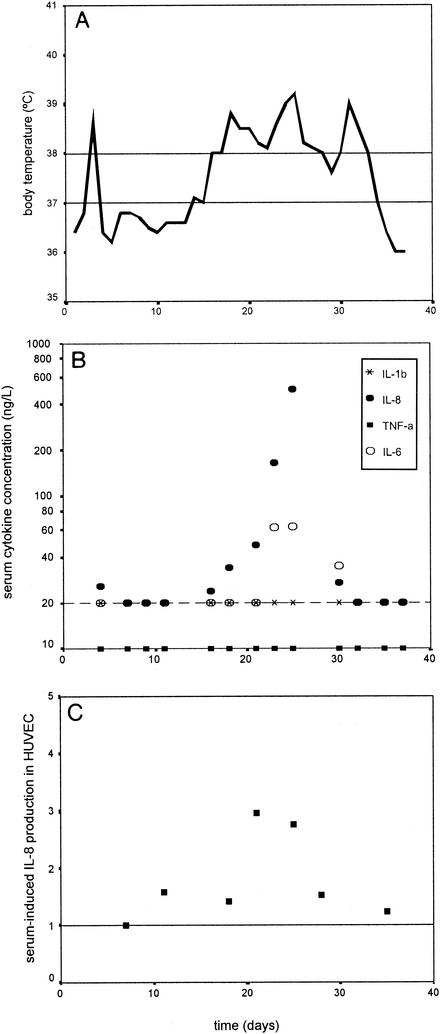
To study the role of endothelial cells in IL-8 production during bacterial infections in cancer patients, HUVEC monolayers were incubated with sera from leukopenic cancer patients with clinical sepsis, documented bacterial infections, or no signs and/or documentation of infections. Sera from leukopenic patients without signs of infections induced IL-8 production in HUVECs (419 ± 168 ng/liter; mean and standard deviation) comparable to the IL-8 production seen after the stimulation of HUVECs with serum from a nonleukopenic control person. However, when HUVECs were incubated with serial serum samples from leukopenic patients (n = 6) at the onset of or during bacterial infections, a significant three- to eightfold increase in IL-8 production (1,515 to 2,889 ng/liter) over that found in the period preceding the infections (P = 0.028) was found (Fig. 2). In addition, a trend correlation was found between serially measured plasma IL-8 levels in vivo and patient serum-induced IL-8 production by HUVECs (r = 0.759; P = 0.08). However, the increase in IL-8 levels in HUVEC supernatants could not be explained by the amount of IL-8 in the added sera, since IL-8 concentrations in HUVEC supernatants were much higher (1,515 to 2,889 ng/liter compared to 20 to 600 ng/liter).
FIG. 2.
HUVEC assay results. Individual body temperature (A), plasma cytokine levels in vivo (B), and IL-8 production by HUVEC stimulated with patient serum (C) were measured during a leukopenic period following chemotherapy in a patient with clinical sepsis from day 17 until day 30. The cytokines measured in panel B were IL-1β (multiplication signs), IL-8 (filled ovals), TNF-α (rectangles), and IL-6 (open ovals). HUVEC-induced IL-8 production in panel C is expressed as the fold increase in IL-8 production in HUVECs compared to production during the period preceding the infection (set as 1). The data are for one patient representative of six patients.
Thus, sera from leukopenic patients stimulate HUVECs to produce IL-8 in much larger amounts during bacterial infections than during periods when they have no signs and/or documentation of infections. These results suggest that in vivo endothelial cells play a role in producing IL-8 during the innate inflammatory response in leukopenic cancer patients.
Neutralizing antibodies against TLR-2 and TLR-4 can reduce patient plasma-induced IL-8 production in HUVECs.
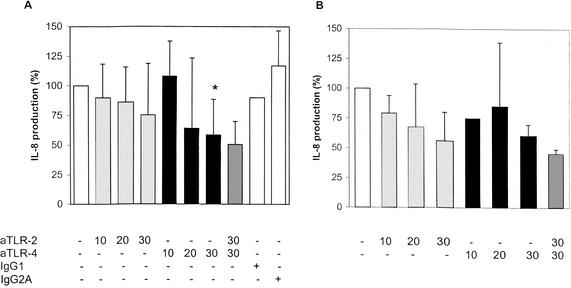
To investigate whether pattern recognition receptors TLR-2 and TLR-4 are involved in increased IL-8 production when HUVECs are stimulated with serum or plasma samples from leukopenic cancer patients with bacterial infections, blocking experiments with antibodies against TLR-2 and/or TLR-4 were performed. HUVEC monolayers in 24-well tissue culture plates were pretreated with anti-TLR-2 and anti-TLR-4 antibodies at various concentrations. Then, plasma from seven leukopenic cancer patients with gram-negative infections (Escherichia coli, n = 2; Klebsiella pneumoniae, n = 1; P. aeruginosa, n = 1; multiorganism, n = 2) and plasma from three patients with gram-positive infections (viridans group streptococci, n = 2; coagulase-negative staphylococci, n = 1) were added. Patient characteristics are shown in Table 1. After 8 h, supernatants of cells were collected, and IL-8 concentrations were measured. Figure 3A shows that for gram-negative infections, anti-TLR-2 antibodies slightly attenuated IL-8 production in HUVECs. However, when anti-TLR-4 antibodies (30 μg/ml) were added to the cultures, a significant reduction in patient plasma-induced IL-8 production to 40% was found (P = 0.028). The effect of anti-TLR-4 antibodies could slightly be enhanced by the addition of anti-TLR-2 antibodies. Figure 3B shows that for gram-positive infections, IL-8 production in HUVECs was inhibited by anti-TLR-2 antibodies by 45% and by anti-TLR-4 antibodies by 40%. The addition of both anti-TLR-2 and anti-TLR-4 antibodies reduced patient plasma-induced IL-8 production in HUVECs to 55%.
TABLE 1.
Characteristics of patients whose samples were used in HUVEC blocking experiments
| Bacteremia (no. of patients) | No. (%) of patients with the indicated characteristic or cancer
|
|||||
|---|---|---|---|---|---|---|
| Female | Male | Children | Acute leukemia
|
|||
| Myeloid | Lympho- blastic | Myeloma | ||||
| Gram-negative (7) | 4 (57) | 3 (43) | 2 (29) | 1 (14) | 3 (43) | 3 (43) |
| Gram-positive (3) | 1 (33) | 2 (67) | 1 (33) | 2 (67) | 0 | 1 (33) |
FIG. 3.
Blocking of IL-8 production. Blocking of IL-8 production by anti-TLR-2 (aTLR-2) and/or anti-TLR-4 (aTLR-4) antibodies at various concentrations (micrograms per milliter) was measured after incubation of HUVECs with plasma from seven leukopenic patients with documented gram-negative infections (A) and three leukopenic patients with documented gram-positive infections (B). The bars represent means and standard deviations. A significant reduction of IL-8 production induced by plasma from patients with gram-negative infections was found after the addition of 30 μg of anti-TLR-4/ml (asterisk) (P = 0.028).
Thus, TLR-2 and TLR-4 seem to be involved in endothelial cell IL-8 production during gram-negative and gram-positive bacterial infections in leukopenic cancer patients.
DISCUSSION
Cancer patients who are treated with chemotherapy are highly susceptible to bacterial infections due to disturbances in their innate immune systems. Reduced numbers of neutrophils are known to be an important risk factor (3). However, the exact pathway of the innate immune response at the time of a bacterial infection during leukopenia still has to be elucidated. In recent decades, new strategies for the early diagnosis of bacterial infections in febrile leukopenic patients have been developed. The plasma IL-8 level seems to be a promising diagnostic parameter in leukopenic cancer patients because of its early increase at the time of an invasive bacterial infection (9, 12).
Leukocytes are normally the main producers of inflammatory cytokines. Previous publications and our own results show that a decreased number of leukocytes is related to diminished IL-8 and TNF-α production in LPS-stimulated whole-blood cultures of samples from cancer patients (10, 28).
Therefore, we investigated whether other effector cells of the innate immune response, such as endothelial cells, may be important producers of IL-8 during bacterial infections in leukopenic cancer patients. When HUVEC monolayers were incubated with sera from leukopenic cancer patients with gram-negative as well as gram-positive bacterial infections, a significant increase in IL-8 production was found compared to IL-8 production after incubation with sera from the same patients when they had no signs of infections. We concluded that endothelial cells may be involved in IL-8 production during bacterial infections in leukopenic cancer patients.
However, the question remains as to which factors in the sera of leukopenic cancer patients with bacterial infections stimulate endothelial cells to produce IL-8. PAMPs on the cell walls of gram-negative bacteria and gram-positive bacteria are known to induce an inflammatory response in the host through binding to pattern recognition receptors on effector cells. Faure et al. analyzed the patterns of expression of pattern recognition receptors TLR-2 and TLR-4 in human vascular endothelial cells and found that endothelial cells express TLR-4 and, to a much smaller extent, TLR-2 (13). To study whether the interaction between PAMPs in sera from leukopenic patients and TLR-2 and TLR-4 on HUVECs was responsible for the increased IL-8 production in HUVECs, blocking experiments with antibodies against TLR-2 and TLR-4 were performed. IL-8 production in HUVECs stimulated with plasma from patients with gram-negative infections could be significantly reduced (up to 40%) by blocking of the TLR-4 pathway. For gram-positive infections, inhibition of patient plasma-induced IL-8 production in HUVECs was most obvious when the TLR-2 pathway was blocked (45%), although anti-TLR-4 antibodies had a similar effect. The observation that IL-8 production induced by plasma from patients with gram-negative infections was reduced by the addition of especially neutralizing antibodies against TLR-4 is in agreement with the current concept that TLR-4 is the long-sought LPS receptor (19).
The partial reduction of IL-8 production in HUVECs after blocking of the TLR-2 and TLR-4 pathways suggests that other serum factors and receptors on HUVECs contributed to IL-8 production in HUVECs as well. It is possible that small amounts of serum TNF-α and IL-1β, the earliest cytokines in the inflammatory cascade, have a role in inducing IL-8 production in HUVECs. However, in the patient sera that we used, TNF-α and IL-1β levels were immeasurably low (below the detection limits of 10 and 20 ng/liter, respectively). In addition, blocking experiments with antibodies against TNF-α and IL-1β did not result in any inhibition of IL-8 production in HUVECs (data not shown).
Our data indicate that in cancer patients with markedly diminished numbers of leukocytes, endothelial cells may have a major role in the production of cytokines important in innate immunity against bacterial microorganisms. As a result of direct and/or indirect interactions between PAMPs of bacteria and TLR-4 and TLR-2 expressed on the cell surface, a signaling route in endothelial cells is started, leading to the production of IL-8 and probably other important mediators of the innate immune system as well.
Knowledge about the exact pathway of the innate immune response in leukopenic cancer patients will help to determine the optimal diagnostic parameter in leukopenic febrile episodes during cancer treatment. In addition, novel treatment possibilities for severe bacterial infections in leukopenic cancer patients, such as blocking of TLR pathways, may be considered in the future.
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine. 1992. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874. [PubMed] [Google Scholar]
- 2.Arbour, N. C., E. Lorenz, B. C. Schutte, J. Zabner, J. N. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 3.Bodey, G. P., M. Buckley, Y. S. Sathe, and E. J. Freireich. 1966. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 64:328-340. [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. E. 1984. Neutropenia, fever, and infection. Am. J. Med. 76:421-428. [DOI] [PubMed] [Google Scholar]
- 5.Bruserud, O., P. E. Akselen, J. Bergheim, and I. Nesthus. 1995. Serum concentrations of E-selectin, P-selectin, ICAM-1 and interleukin 6 in acute leukaemia patients with chemotherapy-induced leucopenia and bacterial infections. Br. J. Haematol. 91:394-402. [DOI] [PubMed] [Google Scholar]
- 6.Bruserud, O., J. Bergheim, F. V. Shammas, and I. Nesthus. 1994. Serum concentrations of tumour necrosis factor-alpha during chemotherapy-induced leukopenia in patients with acute leukaemia and bacterial infections. Leuk. Res. 18:415-421. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, and B. Chernow. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 8.de Bont, E. S., J. L. Kimpen, R. Y. Tamminga, A. E. Niemarkt, L. H. de Leij, and W. A. Kamps. 2000. Intrinsic capacity of monocytes to produce cytokines ex vivo in patients with acute lymphoblastic leukaemia. Cytokine 12:1723-1726. [DOI] [PubMed] [Google Scholar]
- 9.de Bont, E. S., E. Vellenga, J. C. Swaanenburg, V. Fidler, P. J. Visser-van Brummen, and W. A. Kamps. 1999. Plasma IL-8 and IL-6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. Br. J. Haematol. 107:375-380. [DOI] [PubMed] [Google Scholar]
- 10.Denecker, N. E., B. J. Kullberg, J. P. Drenth, J. M. Raemaekers, and J. W. Van der Meer. 1997. Regulation of the production of pro-inflammatory cytokines and antagonists during chemotherapy-induced neutropenia in patients with haematological malignancies. Cytokine 9:702-710. [DOI] [PubMed] [Google Scholar]
- 11.Denizot, Y., P. Fixe, E. Liozon, and V. Praloran. 1996. Serum interleukin-8 (IL-8) and IL-6 concentrations in patients with hematologic malignancies. Blood 87:4016-4017. [PubMed] [Google Scholar]
- 12.Engel, A., E. Mack, P. Kern, and W. V. Kern. 1998. An analysis of interleukin-8, interleukin-6 and C-reactive protein serum concentrations to predict fever, gram-negative bacteremia and complicated infection in neutropenic cancer patients. Infection 26:213-221. [DOI] [PubMed] [Google Scholar]
- 13.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 14.Greendyke, R. M., K. Sharma, and F. R. Gifford. 1994. Serum cytokine levels in patients with neutropenia. Arch. Pathol. Lab. Med. 118:1193-1195. [PubMed] [Google Scholar]
- 15.Heney, D., I. J. Lewis, S. W. Evans, R. Banks, C. C. Bailey, and J. T. Whicher. 1992. Interleukin-6 and its relationship to C-reactive protein and fever in children with febrile neutropenia. J. Infect. Dis. 165:886-890. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann, J. L., H. Blanchard, P. Brunengo, and P. H. Lagrange. 1994. TNF alpha, IL-1 beta and IL-6 plasma levels in neutropenic patients after onset of fever and correlation with the C-reactive protein (CRP) kinetic values. Infection 22:309-315. [DOI] [PubMed] [Google Scholar]
- 17.Hippenstiel, S., S. Soeth, B. Kellas, O. Fuhrmann, J. Seybold, M. Krull, C. Eichel-Streiber, M. Goebeler, S. Ludwig, and N. Suttorp. 2000. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 95:3044-3051. [PubMed] [Google Scholar]
- 18.Jack, R. S., X. Fan, M. Bernheiden, G. Rune, M. Ehlers, A. Weber, G. Kirsch, R. Mentel, B. Furll, M. Freudenberg, G. Schmitz, F. Stelter, and C. Schutt. 1997. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 389:742-745. [DOI] [PubMed] [Google Scholar]
- 19.Kaisho, T., and S. Akira. 2000. Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 20:393-405. [PubMed] [Google Scholar]
- 20.Kern, W. V., M. Heiss, and G. Steinbach. 2001. Prediction of gram-negative bacteremia in patients with cancer and febrile neutropenia by means of interleukin-8 levels in serum: targeting empirical monotherapy versus combination therapy. Clin. Infect. Dis. 32:832-835. [DOI] [PubMed] [Google Scholar]
- 21.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 22.Lehrnbecher, T., D. Venzon, M. de Haas, S. J. Chanock, and J. Kuhl. 1999. Assessment of measuring circulating levels of interleukin-6, interleukin-8, C-reactive protein, soluble Fc gamma receptor type III, and mannose-binding protein in febrile children with cancer and neutropenia. Clin. Infect. Dis. 29:414-419. [DOI] [PubMed] [Google Scholar]
- 23.Ostermann, H., M. Rothenburger, R. M. Mesters, J. van de Loo, and J. Kienast. 1994. Cytokine response to infection in patients with acute myelogenous leukaemia following intensive chemotherapy. Br. J. Haematol. 88:332-337. [DOI] [PubMed] [Google Scholar]
- 24.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 27.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamatsu, Y., K. Akashi, M. Harada, T. Teshima, S. Inaba, K. Shimoda, T. Eto, T. Shibuya, S. Okamura, and Y. Niho. 1993. Cytokine production by peripheral blood monocytes and T cells during haemopoietic recovery after intensive chemotherapy. Br. J. Haematol. 83:21-27. [DOI] [PubMed] [Google Scholar]
- 29.Tracey, K. J., B. Beutler, S. F. Lowry, J. Merryweather, S. Wolpe, I. W. Milsark, R. J. Hariri, T. J. Fahey III, A. Zentella, and J. D. Albert. 1986. Shock and tissue injury induced by recombinant human cachectin. Science 234:470-474. [DOI] [PubMed] [Google Scholar]
- 30.Waage, A., D. Remick, S. Steinshamn, L. Deforge, and J. Lamvik. 1994. Interleukin 8 in serum in granulocytopenic patients with infections. Br. J. Haematol. 86:36-40. [DOI] [PubMed] [Google Scholar]
- 31.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 33.Yang, R. B., M. R. Mark, A. L. Gurney, and P. J. Godowski. 1999. Signaling events induced by lipopolysaccharide-activated Toll-like receptor 2. J. Immunol. 163:639-643. [PubMed] [Google Scholar]