Abstract
The family of Helicobacter cysteine-rich proteins (Hcp) constitutes one of the largest protein families that are specific for proteobacteria from the delta/epsilon subgroup. Most of the proteins belonging to this family have so far only been recognized on the genome level. To investigate the expression of Hcp proteins in vivo we analyzed titers of antibody against HcpA (HP0211), HcpB (HP0336), HcpC (HP1098), and HcpE (HP0235) in sera from 30 Helicobacter pylori-positive individuals and in a control group of six H. pylori-negative individuals. Significantly higher titers of antibody were observed for H. pylori-positive individuals (P < 0.00005). The highest and lowest titers were observed for HcpC (Δ mean = 1.06) and HcpB (Δ mean = 0.333), respectively. There is a clear correlation among anti-HcpA, -HcpC, and -HcpE immunoglobulin G titers in H. pylori-positive individuals (correlation > 0.7), but there is only a weak correlation for HcpB (correlation < 0.4). These results confirm that Hcp proteins are expressed by H. pylori under natural environmental conditions and that these proteins are recognized by the immune system of the host. The observed correlations are in agreement with the expected distribution of Hcp proteins among H. pylori strains. HcpA, HcpC, and HcpE are present in the genomes of strains 26695 and J99, whereas HcpB is absent from most strains. Since Hcp proteins are specific for H. pylori, immunological assays including Hcp proteins might be of value to detect H. pylori infection and perhaps to distinguish among different groups of H. pylori-positive patients.
The availability of complete genome data offers a great opportunity to improve our knowledge of pathogenic microorganisms. The genome sequence confirms the presence of a particular open reading frame (ORF), but if the corresponding gene product is expressed and if it has an impact on the pathogenicity of the microorganism, it often remains elusive. The family of Helicobacter cysteine-rich proteins (Hcp) is one of the largest protein families that is specific for proteobacteria from the delta/epsilon subgroup. This family consists of ORFs HP0160, HP0211, HP0235, HP0336, HP0628, HP1098, HP1117, JHP0318, JHP1437, and CJ0413, which share between 22 and 66% sequence identity on the protein level. In this work we follow the nomenclature that was initiated by Cao and coworkers (3) and has later been expanded over the entire protein family (10).
In Helicobacter pylori strains 26695 (14) and J99 (1), all ORFs besides HP0336, JHP1437, and JHP0318 are conserved. The loci for HP0336 and JHP0318 were investigated in nine H. pylori strains that were isolated from individuals who suffered from gastric carcinoma, duodenal ulcers, and chronic gastritis. HP0336 was found to be absent from all strains, whereas JHP0318 was detected in five strains (4).
The recent crystal structure analysis of HcpB (HP0336) (10) confirms the modular architecture of Hcp proteins that was already predicted from the protein sequence (11). Although it was shown that HcpA (HP0211), HcpB, and HcpD (HP0160) have penicillin-binding activities (9, 10, 11), their in vivo functions are unknown. HcpD was previously isolated from H. pylori membrane fractions with an ampicillin affinity resin (9). Antibodies against HcpA were raised by using the supernatant of H. pylori cultures, and the gene was subsequently cloned, confirming that the protein was expressed and secreted (3). It was also shown that HcpA induced IFN-γ expression in a mouse splenocyte system (6). HcpC (HP1098) was found to be associated with H. pylori membranes, but a function was not assigned (2, 13). In a comprehensive immunoproteomics study, the antibody responses against H. pylori strain 26695 proteins were analyzed in sera from patients with different clinical manifestations (7). Increased titers of antibody against HcpC were detected primarily in the sera from patients suffering from gastric cancer (7). HcpE (HP0235) has so far only been identified on the genome level. Data on the gene product have not been reported so far.
Using the recombinant expressed proteins HcpA, HcpB, HcpC, and HcpE, we detected immunoglobulin G (IgG) antibodies against these proteins in the sera of H. pylori-infected individuals, showing that these proteins are expressed under native conditions and that these proteins are recognized by the immune system of the host. Since these proteins are specific for H. pylori, Hcp proteins might be useful targets for the diagnosis of H. pylori infections.
MATERIALS AND METHODS
Recombinant expression of HcpA, HcpB, HcpC, and HcpE.
Hcp proteins were expressed, refolded, and purified under conditions similar to those reported previously for HcpA and HcpB (10, 11). Briefly, the ORFs HP0211, HP0235, HP0336, and HP1098 were amplified by PCR from the genomic DNA of H. pylori strain 26695 (American Tissue and Culture Collection), and the amplification products were inserted into a pTFT74 expression vector. The PCR also introduced a His6 tag at the C-terminal ends of the proteins. Competent Escherichiacoli BL21(DE3) cells were transformed, and the cells were grown at 37°C. Three hours after induction with 1 mM isopropyl-β-d-thiogalactopyranoside, cells were harvested and broken up by passing the resuspended pellet over a French press.
Inclusion bodies were collected by centrifugation (15 min, 20,000 × g, 4°C), and the soluble fraction was discarded. The pellet was washed two times with buffer A (0.1 M Tris-HCl, 20 mM EDTA [pH 6.8]) and subsequently with buffer B (0.5 M GdnCl [guanidine hydrochloride] in buffer A). Inclusion bodies were solubilized in buffer C (5 M GdnCl, 0.2 M Tris-HCl, 0.1 M dithiothreitol, 10 mM EDTA [pH 8.0]), and insoluble material was removed by centrifugation. Solubilized inclusion bodies were dialyzed overnight against buffer D (5 M GdnCl, 0.1 M acetic acid). Hcp proteins were refolded by immobilizing the solubilized inclusion bodies on Ni-nitrilotriacetic acid-agarose (Qiagen). The column (5 to 10 ml) was washed with 50 ml of buffer E (5 M GdnCl, 0.1 M Tris [pH 8.0]), and Hcp proteins were refolded by replacing buffer E immediately with buffer F (50 mM Tris-HCl, 150 mM sodium chloride, 5 mM glutathione [pH 8.0]) and washing the column with 50 ml of buffer F at a flow rate of 1 ml/min. The protein was eluted with buffer G (250 mM imidazole, 50 mM Tris-HCl, 150 mM sodium chloride, 5 mM glutathione [pH 7.0]).
Protein-containing fractions were pooled and dialyzed against 1,000 ml of buffer H (40 mM sodium acetate, 1 mM EDTA [pH 5.5]). Buffer H was also used for gel-permeation chromatography. After concentrating the protein in a Centriprep (Millipore), 1 mg of refolded Hcp proteins was loaded onto a Superdex 75 HR 10/30 column (Amersham Pharmacia) and eluted as single peaks at a flow rate of 0.5 ml/min.
Investigation of patients.
Thirty patients who were serologically positive for H. pylori were selected for this study. Thirteen patients were men (mean age, 49.1 years; range, 19 to 78 years), and 17 patients were women (mean age, 44.5 years; range, 18 to 78 years). The mean age of all patients was 46.8 years (range, 18 to 78 years). Identical serologic analyses were performed on samples from six serologically negative H. pylori patients (3 women and 3 men). The mean age was 50.9 years (range, 15 to 71 years). One sample was taken from each patient.
All serologic assays were performed in the laboratory of Clinical Immunology, University Hospital Zurich, which is accredited by the Swiss Federal Office of Metrology and Accreditation, Swiss Accreditation Service. Anti-H. pylori antibodies were measured with a commercially available enzyme immunoassay (Synelisa H. pylori [IgG], Abs; Pharmacia and Upjohn, Freiburg, Germany) by following the guidelines of the manufacturer.
ELISA conditions.
Anti-HcpA, -HcpB, -HcpC, and -HcpE IgG antibodies were detected by enzyme immunoassay as follows. The antigens (0.2 μg/ml) (HcpA, HcpB, HcpC, and HcpE) in 0.1 M carbonate bicarbonate buffer (pH 9.6) were coated overnight at 4°C onto 96-well microtiter plates (Immulon 2; Dynatech Labs, Chantilly, Va.). The plates were blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS) at room temperature for 60 min. Negative and low- and high-positive controls from Pharmacia as well as serum samples were diluted 1:101 in PBS-0.05% Tween 20. After adding the samples, the plates were incubated at room temperature for 60 min. After four washes, horseradish peroxidase-conjugated rabbit anti-human IgG (DAKO Diagnostics A/S, Copenhagen, Denmark) diluted 1:2,500 in PBS-0.05% Tween 20 was added and incubated at room temperature for 60 min. After four washes, o-phenylendiamine hydrochloride solution was added and the color was allowed to develop at room temperature in the dark. The reaction was stopped with 4 M sulfuric acid, and the optical density (OD) was analyzed at a wavelength of 492 nm with a Tecan SLT enzyme-linked immunosorbent assay (ELISA) reader. All ELISAs were done in duplicate. Results were expressed as OD492 values. To assess the variation among subsequent experiments, the sera of six H. pylori-positive individuals were analyzed against HcpA on all ELISA plates. The variation between subsequent ELISAs was between 3 and 8%. For samples where the absorption was saturated, values were truncated at 4.0 OD492 units. For statistical analysis, Student's t test function, assuming unequal variances, was applied.
RESULTS
Detection of anti-HcpA, -HcpB, -HcpC, and -HcpE IgG antibodies.
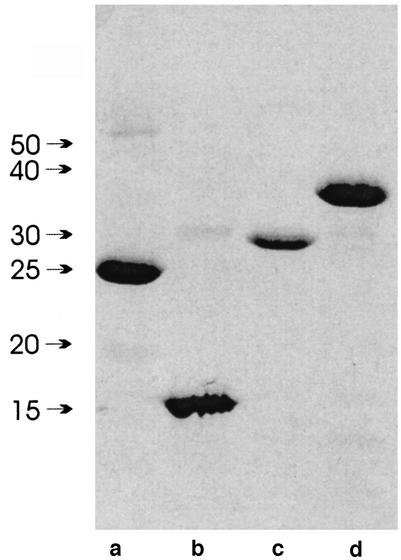
Anti-HcpA, -HcpB, -HcpC, and -HcpE IgG antibodies titers were analyzed by immobilizing the purified proteins to ELISA plates, incubating the plates with sera obtained from 30 H. pylori-infected and 6 uninfected individuals, and detecting bound IgG antibodies by standard ELISA techniques. Expression and refolding of more Hcp family members was attempted, but so far only HcpA, HcpB, HcpC, and HcpE were obtained in sufficient quantities. As shown in Fig. 1, the recombinant Hcp proteins were pure enough to rule out unspecific binding to impurities that might have been present in all Hcp protein preparations. The mean ODs for the anti-Hcp IgG titers in infected and uninfected individuals are given in Table 1. In the group of H. pylori-positive individuals, the average anti-HcpA, -HcpC, and -HcpE IgG titers were 1.11, 1.35, and 1.61, whereas in the control group, they were 0.306, 0.292, and 0.620, respectively. Although high values for the standard deviations indicated significant variations of IgG titers among individuals, the presence of anti-HcpA, -HcpC, and -HcpE IgG antibodies is expected, with probabilities below 5 × 10−4. For HcpB, the difference between the mean titers for infected and uninfected individuals is three times smaller and the P value is 100 times higher than the titers for HcpA, HcpC, and HcpE.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant HcpA (lane a), HcpB (lane b), HcpC (lane c), and HcpE (lane d). Ten micrograms of protein was loaded per lane. Higher-molecular-weight impurities represent Hcp dimers that formed during sample preparation.
TABLE 1.
Mean OD492 values for Hcp proteins in H. pylori-positive and -negative individuals
| Protein (ORF) | OD492 (σOD) for individuals who are H. pylori:
|
P value | |
|---|---|---|---|
| Positive | Negative | ||
| HcpA (HP0211) | 1.108 (1.021) | 0.306 (0.138) | 1.2 × 10−4 |
| HcpB (HP0336) | 1.180 (0.640) | 0.847 (0.362) | 5.2 × 10−2 |
| HcpC (HP1098) | 1.346 (1.083) | 0.292 (0.110) | 5.5 × 10−6 |
| HcpE (HP0235) | 1.612 (0.999) | 0.620 (0.416) | 4.0 × 10−4 |
Correlation between anti-HcpA, -HcpB, -HcpC, and -HcpE IgG titers.
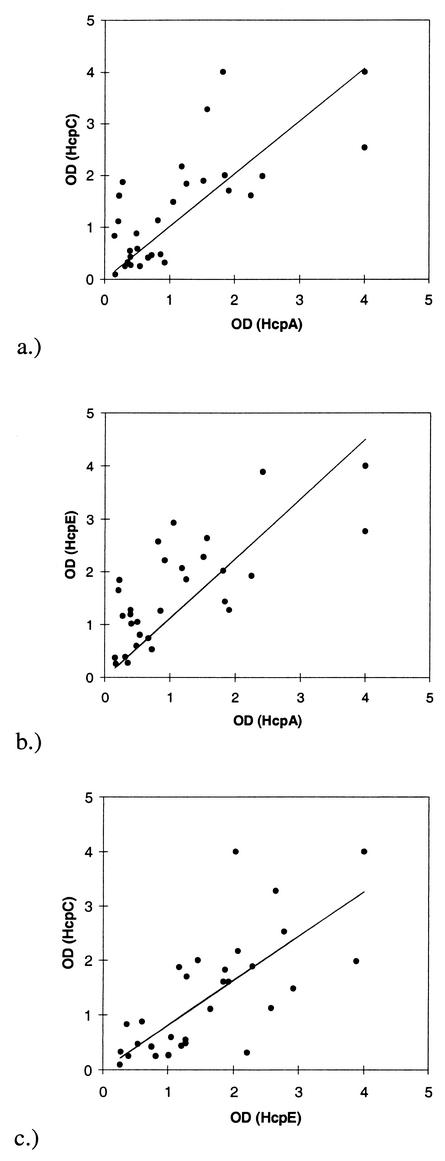
As indicated by the values given in Table 2 and the line-plots shown in Fig. 2, there is a good correlation between anti-HcpA, -HcpC, and -HcpE IgG titers. The correlations for the pairwise comparisons are all above 0.7, indicating a clear trend. For HcpB, this trend is much less significant, as indicated by correlations below 0.4. No correlation was detected between titers of IgG measured by the commercial serological test to validate the infection state and the titers of anti-Hcp IgG (correlation < 0.15 for anti-HcpA, -HcpB, and -HcpC in the group of H. pylori-positive individuals).
TABLE 2.
Correlation between titers of anti-Hcp IgG
| Protein | Correlation witha:
|
||
|---|---|---|---|
| HcpA | HcpB | HcpC | |
| HcpB | 0.293 | ||
| HcpC | 0.734 | 0.357 | |
| HcpE | 0.733 | 0.146 | 0.710 |
Correlations above 0.7 are shown in boldface type.
FIG. 2.
Line plots to indicate the correlations between HcpA and HcpC (a), HcpA and HcpE (b), and HcpC and HcpE (c).
There is substantial variation between titers of anti-Hcp IgG in the group of infected individuals. In some cases the titers of IgG are as low as in the group of H. pylori-negative individuals, but in some cases, the titers are 10 times larger. These fluctuations cannot be addressed as measurement errors but reflect the individual response of the host.
DISCUSSION
The results presented above confirm that anti-HcpA, -HcpC, and -HcpE IgG antibodies exist in the sera of H. pylori-positive individuals, whereas for HcpB, the mean titers of antibody are just slightly increased. The titers and P values for HcpA, HcpC, and HcpE are in the same order of magnitude as other pathogenicity factors, such as the H. pylori vaculating toxin VacA (12). There are two possible reasons for the low P values of HcpB.
HcpA, HcpC, and HcpE possess N-terminal leader peptides that guide these proteins into the periplasmic space, whereas HcpB has no leader peptide and therefore it is assumed that HcpB is localized in the cytosol. The secretion of Hcp proteins into the periplasmic space could increase the antigenicity of HcpA, HcpC, and HcpE because it makes interactions with the host more likely. On the other hand, secretion is not a prerequisite for antigenicity. In the sera of H. pylori-positive individuals, significant titers of anti-UreB IgG were detected although UreB lacks a leader peptide (5).
Another reason for the lower titers of anti-HcpB IgG might be the absence of HcpB from the proteome of many H. pylori strains. The ORFs coding for HcpA, HcpC, and HcpE are conserved in the genome sequences of strains 26695 and J99, whereas HP0336, the ORF coding for HcpB, is absent from the genome of strain J99. HP0336 was also found to be absent from the genomes of nine clinical isolates, as shown by Chanto and coworkers (4). In five of nine cases, HP0336 was replaced by JHP0318. Since HcpB seems to be very specific for strain 26695, the absence of HcpB in H. pylori-positive individuals seems to be the reason for its lower P value. The increased titers of anti-HcpB IgG, particularly in some H. pylori-seronegative patients, could be explained by the presence of a cross-reacting antigen in some H. pylori-positive and -negative individuals.
In addition, the titers of IgG for HcpA, HcpC, and HcpE show a clear correlation (Table 2), indicating that these proteins are expressed and recognized by the immune system at similar disease states. If HcpB would be expressed under the same conditions but the decreased P value for HcpB would be due to cytosolic expression, the titer of anti-HcpB should also correlate with the titers of HcpA, HcpC, and HcpE. From the lack of correlation for anti-HcpB IgG, it can be concluded that HcpB is missing under conditions where HcpA, HcpC, and HcpE are expressed either because the corresponding gene is missing in the genome of the particular H. pylori strain or because expression is dispensable under some conditions.
The correlation of titers of anti-HcpA, -HcpC, and -HcpE IgG could also be explained by cross-reactivity of sera, which is supported by the significant sequence identity levels among these antigens and the modular architectures of the Hcp proteins. HcpA, HcpB, HcpC, and HcpE consist of six, four, seven, and nine repeats of a common α/α motif, respectively (10). If the correlation between titers of anti-HcpA, -HcpC, and -HcpE IgG were caused by the cross-reactivity of the sera, HcpB should also be recognized at the same level. Although HcpB shares the same basic structure and a similar degree of sequence identity, no significant correlations between the titers of anti-HcpB and anti-HcpA, -HcpC, and -HcpE IgG were observed. Therefore, it is unlikely that the correlation between the titers of anti-HcpA, -HcpC, and -HcpE IgG is due to the cross-reactivity of the sera. The same argument contradicts the hypothesis that the sera might recognize the artificial His6 tag rather than the H. pylori proteins.
The high standard deviation for titers of anti-Hcp IgG and the lack of correlation between titers of anti-Hcp IgG and infection state measured by the commercial serological test suggest that Hcp proteins are only expressed under very specific conditions. This hypothesis is in agreement with the observation that at least HcpA is not required for survival in vitro because an HcpA deletion mutant was found to grow normally under standard culture conditions (8). However, the high titers of IgG observed in some H. pylori-positive individuals confirm that Hcp proteins are expressed in vivo.
Anti-HcpC antibodies have recently been detected in a comprehensive immunoproteomics study (7). Although only qualitative data on two-dimensional gel analysis but no quantitative data on antibody titers was given, HcpC was found to be preferentially recognized by the sera of patients suffering from gastric cancer. Since we did not distinguish in our study among different clinical manifestations of H. pylori infection, we cannot comment on this observation. However, if it turns out that elevated titers of anti-HcpC IgG are present in gastric cancer patients, immunological tests including Hcp proteins might be suitable assays to distinguish between different groups of H. pylori-positive individuals.
Acknowledgments
This work was supported by the Hartmann-Müller foundation (Zürich, Switzerland) and by the Swiss National Science Foundation grant no. 3100-063794.001.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed]
- 2.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, P., M. S. McClain, M. H. Forsyth, and T. L. Cover. 1998. Extracellular release of antigenic proteins by Helicobacter pylori. Infect. Immun. 66:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanto, G., A. Occhialini, N. Gras, R. A. Alm, F. Megraud, and A. Marais. 2002. Identification of strain-specific genes located outside the plasticity zone in nine clinical isolates of Helicobacter pylori. Microbiology 148:3671-3680. [DOI] [PubMed] [Google Scholar]
- 5.Dunn, B. E., G. P. Campbell, G. I. Perez-Perez, and M. J. Blaser. 1990. Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265:9464-9469. [PubMed] [Google Scholar]
- 6.Gosciniak, G., A. Przondo-Mordarska, B. Iwanczak, and E. Poniewierka.2001. Neutralisation of cytotoxic vacuolating activity by serum antibodies of Helicobacter pylori-infected patients. Int. J. Med. Microbiol. 291:27-32. [DOI] [PubMed]
- 7.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. R. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 8.Karita, M., M. L. Etterbeek, M. H. Forsyth, M. K. Tummuru, and M. Blaser. 1997. Characterization of Helicobacter pylori dapE and construction of a conditionally lethal dapE mutant. Infect. Immun. 65:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy, P., M. H. Parlow, J. Schneider, S. Burroughs, C. Wickland, N. B. Vakil, B. E. Dunn, and S. H. Phadnis. 1999. Identification of a novel penicillin-binding protein from Helicobacter pylori. J. Bacteriol. 181:5107-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lüthy, L., M. G. Grütter, and P. R. Mittl. 2002. The crystal structure of Helicobacter pylori cysteine-rich protein B reveals a novel fold for a penicillin-binding protein. J. Biol. Chem. 277:10187-10193. [DOI] [PubMed] [Google Scholar]
- 11.Mittl, P. R. E., L. Lüthy, P. Hunziker, and M. G. Grütter. 2000. The cysteine-rich protein A from Helicobacter pylori is a beta-lactamase. J. Biol. Chem. 275:17693-17699. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Perez, G. I., R. M. Peek, Jr., J. C. Atherton, M. J. Blaser, and T. L. Cover. 1999. Detection of anti-VacA antibody responses in serum and gastric juice samples using type s1/m1 and s2/m2 Helicobacter pyloriVacA antigens. Clin. Diagn. Lab. Immunol. 6:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabarth, N., S. Lamer, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and D. Bumann. 2002. Identification of surface proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J. Biol. Chem. 277:27896-27902. [DOI] [PubMed] [Google Scholar]
- 14.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]