Abstract
We have previously shown that young adults living in a rural area of northern Malawi showed greater gamma interferon (IFN-γ) responses to purified protein derivatives (PPD) prepared from environmental mycobacteria than to PPD from Mycobacterium tuberculosis. In order to define the mycobacterial species to which individuals living in a rural African population have been exposed and sensitized, we tested T-cell recognition of recombinant and purified antigens from M. tuberculosis (38 kDa, MPT64, and ESAT-6), M. bovis (MPB70), M. bovis BCG (Ag85), and M. leprae (65 kDa, 35 kDa, and 18 kDa) in >600 non-M. bovis BCG-vaccinated young adults in the Karonga District of northern Malawi. IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA) in day 6 supernatants of diluted whole-blood cultures. The recombinant M. leprae 35-kDa and 18-kDa and purified native M. bovis BCG Ag85 antigens induced the highest percentages of responders, though both leprosy and bovine tuberculosis are now rare in this population. The M. tuberculosis antigens ESAT-6 and MPT64 and the M. bovis antigen MPB70 induced the lowest percentages of responders. One of the subjects subsequently developed extrapulmonary tuberculosis; this individual had a 15-mm-diameter reaction to the Mantoux test and responded to M. tuberculosis PPD, Ag85, MPT64, and ESAT-6 but not to any of the leprosy antigens. We conclude that in this rural African population, exposure to M. tuberculosis or M. bovis is much less frequent than exposure to environmental mycobacteria such as M. avium, which have antigens homologous to the M. leprae 35-kDa and 18-kDa antigens. M. tuberculosis ESAT-6 showed the strongest association with the size of the Mantoux skin test induration, suggesting that among the three M. tuberculosis antigens tested it provided the best indication of exposure to, or infection with, M. tuberculosis.
There is great variation in the protection that Mycobacterium bovis BCG vaccines can provide against tuberculosis in different locations (17). In two large randomized controlled studies of BCG vaccination in Malawi and the United Kingdom, we have shown that prior to the vaccination of previously unvaccinated young adults in Malawi, over 60% of individuals show gamma interferon (IFN-γ) responses to purified protein derivative (PPD) from M. tuberculosis and that BCG vaccination provides only a moderate increase in this T-cell response (8). When PPDs from a number of nontuberculous environmental (atypical) mycobacteria were used to stimulate whole-blood cultures from the same subjects, PPDs from members of the M. avium-M. intracellulare-M. scrofulaceum complex induced stronger IFN-γ responses than PPD from M. tuberculosis (6). To further define the mycobacterial species to which these subjects had been exposed, we have now used a panel of recombinant and purified antigens, with known species distributions, to test IFN-γ responses, as a measure of the type 1 T-cell response induced by mycobacterial antigens, in day 6 supernatants from diluted whole-blood cultures. We have also assessed the potential of these recombinant antigens for identifying infection with M. tuberculosis and M. leprae, by studying the association between the IFN-γ response to these antigens and the skin test response to M. tuberculosis PPD.
Most studies of T-cell recognition of individual mycobacterial antigens have used patients infected with pathogenic mycobacteria or the contacts of these patients. Our study is unusual in that non-BCG-vaccinated, human immunodeficiency virus-negative, healthy individuals living in a rural area of northern Malawi have been tested for their ability to make a type 1 cytokine response to a panel of seven recombinant mycobacterial antigens and one purified native mycobacterial antigen. These subjects were clinically well at the time of testing and were recruited in their villages and homes.
T-cell responses to several recombinant mycobacterial antigens have been proposed as markers of infection with M. leprae or M. tuberculosis (56, 73). The 45-, 35-, and 18-kDa antigens have been described as potentially specific to M. leprae, in terms of greater T-cell recognition by tuberculoid leprosy patients, healthy contacts of leprosy patients, or M. leprae-vaccinated volunteers than by lepromatous leprosy patients or control subjects (37, 42, 57). For M. tuberculosis, the existence of genes within regions of the genome that are deleted in attenuated M. bovis BCG vaccine strains (38) has led to the identification of antigens whose recognition can discriminate between BCG vaccination and infection with M. tuberculosis (2). For example, the M. tuberculosis antigen ESAT-6 (short for “early-secreted antigen target 6-kDa protein”) has been shown to have potential as a specific antigen for the diagnosis of infection with M. tuberculosis (3, 30, 45, 59, 61). The current incidence of tuberculosis in the Karonga District of northern Malawi is higher than that of leprosy (tuberculosis incidence is 0.1% per annum and rising, while leprosy incidence is <0.005% per annum and falling), although the incidence of leprosy was much greater 20 years ago. As most of the study population recruited here were <28 years old, it is unlikely that they would have been exposed to many infectious patients with leprosy. We thus hypothesized that if exposure to M. tuberculosis were responsible for the induction of T-cell memory to the recombinant antigens, we would observe greater recognition of the M. tuberculosis antigens than of the M. leprae antigens. Alternatively, if sensitization had been induced by exposure to environmental mycobacteria, M. leprae antigens with homologues in species such as M. avium would be more strongly recognized.
We show that the patterns of recombinant antigen recognition support the hypothesis that individuals living in this area are more likely to be exposed to mycobacteria such as M. avium rather than to M. tuberculosis, M. bovis, or M. leprae. One of the tested subjects subsequently developed tuberculosis; at the time of testing this subject responded both to MPT64 and to M. tuberculosis ESAT-6. Overall, the ESAT-6 antigen appeared to be more M. tuberculosis specific than either MPT64 or the 38-kDa antigen, the other M. tuberculosis antigens tested, as it was more strongly associated with the Mantoux skin test reaction.
MATERIALS AND METHODS
Subjects.
Recruitment of subjects took place between February and November 1998 (7). Subjects were selected from the project database from two areas in the district, one in the north (wetter environment, higher leprosy prevalence) and one in the south (drier environment, lower leprosy prevalence), separated by approximately 50 km. They were visited in their homes, where an interviewer confirmed identifications, explained the study, and obtained written consent. Candidates were examined for evidence of a BCG scar; generalized rashes; and signs of tuberculosis, leprosy, or other severe illness. Women were tested for pregnancy. Any of these conditions was a criterion for exclusion from the study. All candidates were precounseled for human immunodeficiency virus antibody testing, which was performed using a particle agglutination test (Edgware modification of Serodia; Mast Diagnostics Ltd., Bootle, Merseyside, United Kingdom) and confirmed by enzyme-linked immunosorbent assay (ELISA) (Vironostika HIV Uni-form II plus 0; Organon Teknika Ltd., Cambridge, United Kingdom); any subjects that tested positive were excluded from the study. Data from 613 subjects, 309 males and 304 females, mean age 19 years (range, 10 to 28 years), are presented here. Approval for these studies was given by the Health Sciences Research Committee of Malawi and by the Ethics Committee of the London School of Hygiene & Tropical Medicine.
Skin testing.
Skin testing was carried out with M. tuberculosis PPD batch RT23 (2 TU; Statens Serum institut, Copenhagen, Denmark) using the Mantoux technique. Tests were administered on the volar surface of the forearm and read after 48 to 72 h. Indurations were measured across and along the arm, and the mean induration was employed for analyses. Individuals with an induration greater than 10 mm in diameter were referred to a project medical officer for investigation. No cases of tuberculosis were identified at recruitment.
Whole-blood assay.
The whole-blood assays and ELISAs were performed in the project's laboratory in Chilumba, Malawi (7). A 5-ml intravenous blood sample, collected before skin testing was performed, was transferred into 50 U of preservative-free sodium heparin (Monoparin; CP Pharmaceuticals Ltd., Wrexham, United Kingdom) and diluted in RPMI tissue culture medium containing penicillin (100 IU/ml), streptomycin (100 μg/ml),and 2 mM l-glutamine (Gibco BRL, Paisley, United Kingdom) to give a final dilution of 1 in 10 in the well. Cell cultures were incubated with antigens at a final concentration of 10 μg/ml for the recombinant and purified antigens or 5 μg/ml for the PPDs at 37°C with 5% CO2. Supernatants discussed in this work were harvested on day 6 and stored at −20 or −70°C prior to ELISA.
Antigens.
The M. leprae 65-kDa antigen (alternative names: hsp65, CIE Ag82, 65-kDa antigen, and GroEL homologue) (73), batches Ml65-5B and Ml65-6, was supplied through the Immunology of Mycobacteria antigen bank, supported by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. Purification was performed by J. van Embden, Bilthoven, The Netherlands (production date, 24 May 1991). The protein was obtained from heat-induced (42°C) Escherichia coli K-12 strain M1518, which carries plasmid pZW1003, derived from pIL161 (31). Cells were lysed by lysozyme, EDTA treatment, and sonication. After centrifugation the pellet containing the M. leprae 65-kDa antigen in inclusion bodies was dissolved in 6 M urea. The protein was precipitated with ammonium sulfate (20 to 55% cut). After desalting the protein was further purified by DEAE column chromatography, desalted by gel permeation chromatography, and lyophilized. The protein preparation, quantitated using the Bio-Rad protein assay (11), was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
The M. leprae 35-kDa antigen was provided by Warwick Britton, Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia. The pWL19 plasmid which expressed the gene encoding the M. leprae 35-kDa protein was used to transfect M. smegmatis (57). Single M. smegmatis pWL19 colonies were inoculated into Middlebrook 7H9 broth plus albumin dextrose catalase supplement and incubated for 3 days at 37°C with shaking. Cells were sonicated, and the sonicate was applied to an affinity column of anti-35-kDa protein monoclonal immunoglobulin G antibody ML-03 coupled to activated Sepharose 4B. The bound protein was eluted with 0.1 M diethylamine (pH 11) and dialyzed against 0.1× phosphate-buffered saline (PBS) before freeze-drying (57). The protein concentration was measured using the Pierce reagent. The freeze-dried protein was dissolved to 1 mg/ml in endotoxin-free Limulus amebocyte lysate (LAL) water and stored in aliquots at −20°C.
The M. leprae 18-kDa antigen was provided by Ross Prestidge at Genesis, Auckland, New Zealand. The protein was obtained from E. coli host strain DH5α, into which the plasmid pmL3 was introduced (9). The bacteria were grown at 37°C and following harvesting cells were suspended in 10 M urea. After centrifugation, the recombinant protein was precipitated from the supernatant with ammonium sulfate (361 g/liter). The precipitate was dissolved in 10 mM Tris-150 mM NaCl, pH 8.0, and the pH was adjusted to pH 4.0 with HCl. The precipitate was dissolved in 1 M Tris-HCl, pH 8.0, and further purified by reversed-phase high-performance liquid chromatography and lyophilized. The purified antigen contained <100 U of endotoxin/mg of protein. The M. leprae 18-kDa antigen used here consisted of the truncated 15-kDa form released following enzymatic cleavage of the whole protein; previous work has shown that the full-length 18-kDa antigen and the truncated protein gave equivalent lymphocyte proliferation responses, with most T- and B-cell epitopes being located between amino acids 38 and 148 (22).
The M. tuberculosis 38-kDa antigen was provided by M. Singh and J. Paulsen at Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany. The protein was obtained from heat-induced (42°C) E. coli K-12 strain CAG629, which carried plasmid pMA9-2 (50). Cells were disrupted in a high-pressure homogenizer and centrifuged. The pellet containing M. tuberculosis 38-kDa antigen in inclusion bodies was washed twice with a buffer containing 2% Triton X-100 and dissolved in 6 M guanidine HCl. The solution was centrifuged, and the supernatant was applied to a Sephadex G-25 gel filtration column to allow renaturation of the 38-kDa protein. The protein was further purified by additional steps of ion-exchange chromatography and lyophilized. The protein preparation was analyzed by SDS-PAGE and Western blotting. E. coli endotoxin concentration, determined according to the LAL assay (Whittaker Bioproducts, Walkersville, Md.), was 408 U/mg of protein.
The M. tuberculosis ESAT-6 and MPT64 antigens were provided by P. Andersen of the Statens Serum institut. The esat-6 gene was inserted into the plasmid pMCT6, and the recombinant ESAT-6 antigen, produced in E. coli XL1-Blue, was purified by metal ion-exchange chromatography on a Ni+ column using phosphate buffers containing 8 M urea, which was removed after the purification (19). The MPT64 antigen was prepared in the Lactococcus lactis system. L. lactis containing the plasmid encoding MPT64 was grown in synthetic medium, recombinant MPT64 (rMPT64) secreted into the medium was separated from the bacteria by cross-flow filtration, and the permeate was concentrated and dialyzed by cross-flow filtration. Following ultracentrifugation the rMPT64 was purified by anion exchange, eluted with salt steps, and ultrafiltrated into sterile PBS. The final protein solution was filtered through a 0.2-μm-pore-size filter. The purity of the rMPT64 was evaluated by SDS-PAGE followed by silver staining and Western blotting. The preparation of MPT64 tested here, P54, contained less than 0.05 ng of endotoxin/μg of protein, as determined using the LAL test.
The M. bovis MPB70 antigen was provided by A. Whelan, Central Veterinary Laboratories, United Kingdom. mpb70 was expressed in E. coli using the plasmid vector pBluescript KS II(+) (Stratagene, La Jolla, Calif.). The protein was expressed in a soluble form and purified from the culture supernatant. Purification was achieved in three steps by sequential application of anion exchange fluidized-bed adsorption, anion exchange chromatography, and finally hydrophobic interaction chromatography (64). The final diluent buffer was PBS.
The M. bovis BCG Ag85 complex, composed of Ag85A, Ag85B, and Ag85C, was provided by K. Huygen at the Pasteur Institute of Brussels, Belgium. The antigen (batch 97/15) was purified from BCG culture filtrate (strain GL2; derived from Paris strain 1173P2) by sequential chromatography on phenylsepharose and DEAE Sephacell and concentrated through Amicon 10,000 before gel filtration on Sephadex G-75 (13). The material was provided in 0.5% glycerol.
All the recombinant and purified antigens were diluted with serum-free medium (RPMI supplemented with penicillin [100 IU/ml] and streptomycin [100 μg/ml]) plus 2 mM glutamine (Gibco BRL, Paisley, United Kingdom) for use at a final concentration of 10 μg/ml.
The 12 PPD “new tuberculin” antigen preparations, prepared for in vitro use, were obtained from the Statens Serum institut (M. tuberculosis RT48, lot 191; M. avium batch RS10/2, lots 37 and 39; M. intracellulare batch RS23, lot 27; M. scrofulaceum batch RS95, lots 17 and 18; M. kansasii batch RS30, lots 18 and 19; M. fortuitum batch RS20, lots 16 and 17; and M. marinum batch RS170, lots 10 and 11); Central Veterinary Laboratories, Weybridge, United Kingdom (M. bovis and M. avium); M. Brennan, U.S. Public Health Service (M. intracellulare PPD-B); and J. Stanford, University College London (new tuberculins: M. kansasii batch 14-4-77 and M. vaccae batch R877R) (6). Following initial experiments to determine the optimal concentration, all PPD preparations were used at a final concentration of 5 μg/ml.
Positive controls included the mitogen phytohemagglutinin (final concentration, 5 μg/ml; Difco Laboratories/Becton Dickinson, Oxford, United Kingdom) and a nonmycobacterial antigen, streptokinase-streptodornase (Varidase [final concentration, 250 U/ml]; Wyeth Laboratories, Maidenhead, Berks, United Kingdom), and culture medium alone served as the negative control (NC).
Endotoxin determination.
A QCL1000 LAL kit (Biowhittaker) was used to determine the endotoxin levels of recombinant and PPD mycobacterial antigens. The lipopolysaccharide contamination was 26 endotoxin units (EU)/ml for the M. leprae 65-kDa antigen, 5.85 EU/ml for the M. leprae 35-kDa antigen, 0.68 EU/ml for the M. leprae 18-kDa antigen, 2.2 EU/ml for the M. tuberculosis 38-kDa antigen, 1.37 EU/ml for M. bovis MPB70, and 38 EU/ml for M. bovis BCG Ag85. Samples of the batches of ESAT-6 were not available for testing. There was no association between the percentage of IFN-γ responders to the antigens and the endotoxin concentration in the antigen preparations (Pearson r = −0.17; P = 0.72).
Measurement of IFN-γ.
Quantitative IFN-γ ELISAs were carried out in single wells in 100-μl volumes using commercially available mouse anti-human IFN-γ antibody pairs (clones NIB42 and 4S.B3; Pharmingen, San Diego, Calif.) as previously described (7). Recombinant cytokine (2,000 to 31 pg/ml; Pharmingen) was used for the standard curve; the lower detection limit of the ELISA was 31 pg/ml. NC values were subtracted from all results. Samples from only 4% of subjects produced >62 pg/ml of IFN-γ in unstimulated cultures. The distributions of IFN-γ responses to the positive-control stimuli phytohemagglutinin and streptokinase-streptodornase have been reported elsewhere (7). To control for interplate and intraplate variation, a positive-control supernatant was used in duplicate on each ELISA plate. The mean variability of these duplicate measurements was 3.7% (intraplate variation). The coefficient of variation between plates (interplate variation) was 12.3%. Supernatants from a subset of 62 subjects were also tested on two separate occasions approximately 1 year apart to assess the reproducibility of the IFN-γ ELISA over time. Positive IFN-γ responses to the recombinant antigen ESAT-6 were detected on both occasions in supernatants from 9 of the 62 individuals tested (Spearman rank r = 0.95), while the supernatants from the remaining 53 subjects were negative on both occasions.
Statistical analysis.
Associations between pairs of antigens, between the tuberculin skin test response and IFN-γ response, and between geographical area and IFN-γ response, were assessed through cross-tabulations and chi-square tests. Associations between pairs of antigens were also quantified using the Spearman rank correlation coefficient. McNemar's test was used to compare pairs of antigens for prevalence of responsiveness.
RESULTS
IFN-γ responses to recombinant and native purified mycobacterial antigens in Malawi.
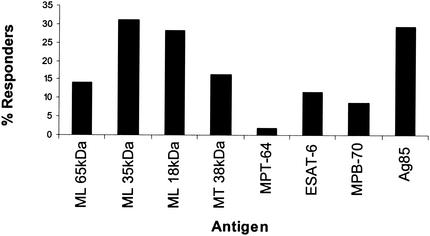
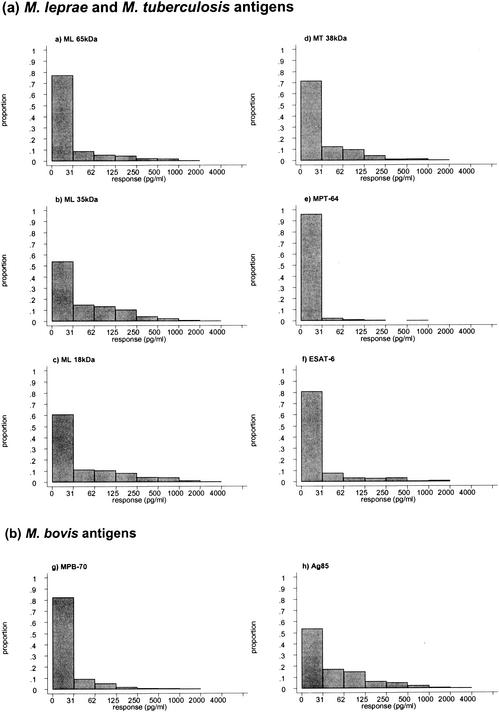
Diluted whole-blood cultures from healthy young adults in Malawi, who had not previously received BCG vaccination, were stimulated for 6 days with the panel of antigens. Using a response cutoff of 62 pg IFN-γ/ml to define a responder, the proportion of subjects making a positive response to each of the recombinant and native antigens was calculated. The highest percentage responders was observed in reaction to the M. leprae 35-kDa (31%), M. bovis BCG Ag85 (29%), and M. leprae 18-kDa (28%) antigens (Fig. 1). The highest response frequency to an M. tuberculosis antigen was seen for the 38-kDa antigen (16%). Overall the lowest frequencies of responses were observed in response to ESAT-6, MPB70, and MPT64. The frequency of recognition of ESAT-6 (11% responders) was greater than that of MPT64 (2% responders) or MPB70 (9% responders) (P < 0.001 and P = 0.007, respectively). The distributions of the IFN-γ responses to each of the antigens is shown in Fig. 2.
FIG. 1.
Frequency of IFN-γ responders to the panel of mycobacterial antigens. Whole-blood assays were stimulated with the recombinant and purified antigens at final concentrations of 10 μg/ml, and IFN-γ was measured in day 6 supernatants by ELISA. A responder was defined as a subject making >62 pg of IFN-γ/ml. The numbers of subjects tested with each antigen was 601 for the M. leprae (ML) 65-kDa antigen, 604 for the M. leprae 35-kDa antigen, 604 for the M. leprae 18-kDa antigen, 607 for the M. tuberculosis (MT) 38-kDa antigen, 458 for M. tuberculosis MPT64, 376 for M. tuberculosis ESAT-6, 607 for M. bovis MPB70, and 535 for M. bovis BCG Ag85.
FIG. 2.
Frequency distributions of IFN-γ responses to the panel of mycobacterial antigens. Whole-blood assays were stimulated with the recombinant and purified antigens at final concentrations of 10 μg/ml, and IFN-γ was measured in day 6 supernatants by ELISA. The numbers of subjects tested with each antigen was 601 for the M. leprae 65-kDa antigen, 604 for the M. leprae 35-kDa antigen, 604 for the M. leprae 18-kDa antigen, 607 for the M. tuberculosis 38-kDa antigen, 458 for M. tuberculosis MPT64, and 376 for M. tuberculosis ESAT-6 (a) and 607 for M. bovis MPB70 and 535 for M. bovis BCG Ag85 (b).
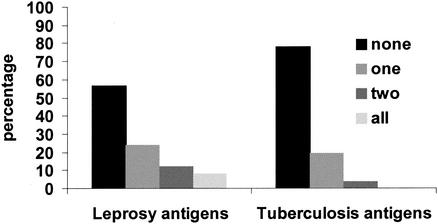
Further analysis was undertaken to assess whether individuals were responding to none, one, two, or three of the M. leprae (65-, 35-, and 18-kDa) or M. tuberculosis (38-kDa, MPT64, and ESAT-6) antigens. We postulated that a response to all three antigens might reflect specific recognition of leprosy or tuberculosis bacilli. Figure 3 shows the percentage of individuals who showed IFN-γ responses (defined as >62 pg/ml) to none, one, two, or three of the antigens. The concurrence of response to the three M. leprae antigens is higher than would be expected by chance (P < 0.001), but it is not strong. Using the Spearman rank test, pairwise correlations among the three leprosy or three tuberculosis antigens were only moderately strong, with all r values being ≤0.55, although they were statistically significant given the large sample size. These results suggest that recognition of at least some of the three M. leprae or M. tuberculosis antigens may be due not to M. leprae or M. tuberculosis exposure but to sensitization by other environmental mycobacteria expressing some but not all of these antigens.
FIG. 3.
Association between responses to M. leprae or M. tuberculosis recombinant antigens within individuals. The percentage of individuals with IFN-γ responses >62 pg/ml to none, one, two, or all three of the leprosy recombinant antigens tested (65-, 35-, and 18-kDa antigens) and to none, one, two, or three of the tuberculosis recombinant antigens tested (38 kDa, MPT64, and ESAT-6) is shown. A total of 599 individuals were tested with the three leprosy antigens, and 284 were tested with the three tuberculosis antigens.
Association of responses to M. leprae antigens with incidence of leprosy.
We have previously shown that there is a higher incidence of leprosy in the northern part of Karonga District than in the southern part but that the proportion of individuals who show recognition of an M. leprae soluble antigen upon skin test is higher in the south (15, 53). The IFN-γ responses to the three recombinant leprosy antigens were therefore analyzed by geographical location of the village in which the recruited subjects were living. In each case the prevalence of responders was lower in the north than in the south: 87 of 336 (26%) versus 82 of 268 (31%) for the 18-kDa antigen (P = 0.2), 30 of 334 (9%) versus 47 of 214 (22%) for the 65-kDa antigen (P < 0.001), and 91 of 336 (27%) versus 97 of 268 (36%) for the 35-kDa antigen (P = 0.016).
These results indicate that exposure to M. leprae was unlikely to have induced T-cell responses to the M. leprae 65-, 35-, and 18-kDa antigens. There was no significant difference in the responses to Ag85 between the north and the south (P = 0.131).
Associations between responses to recombinant antigens and PPDs from environmental and tuberculous mycobacteria.
The IFN-γ responses to the recombinant and purified antigens were compared with those to PPDs derived from species with or without known homologues of the antigens. Most associations were statistically significant (partly due to the large sample size) but it is the magnitude of the correlation coefficients that is important in assessing the strength of the associations. Out of 96 pairwise comparisons tested by Spearman rank correlation (Table 1), the highest correlations were between the M. leprae 35-kDa antigen and PPD-B (M. intracellulare) (r = 0.62) or M. kansasii PPD (UCL) (r = 0.51) and between the M. leprae 65-kDa antigen and M. kansasii PPD (SSI) (r = 0.52). Both M. intracellulare and M. kansasii have been tested for the presence of the 35kDa gene by gene hybridization without a homologue being identified (70). The next-highest correlations were obtained between Ag85 and PPD-B (M. intracellulare) (r = 0.48), Ag85 and M. kansasii PPD (UCL) (r = 0.47), and the 35-kDa antigen and M. avium PPD (SSI) (r = 0.47). All mycobacteria tested so far have been shown to express Ag85 genes and a homologue of the M. leprae 35-kDa gene has also been identified in M. avium by gene hybridization (70). The correlation coefficient between ESAT-6 and M. tuberculosis PPD was of similar magnitude to those between ESAT-6 and several of the environmental mycobacterial PPDs. Also, the correlation coefficient between M. tuberculosis PPD and ESAT-6 was not as high as that between M. tuberculosis PPD and each of Ag85, M. leprae 35 kDa, and M. leprae 65 kDa.
TABLE 1.
Correlation coefficients of IFN-γ responses to recombinant and purified antigens with IFN-γ responses to PPDs from different mycobacterial species
| Antigen | Correlation coefficient between pairs of antigensa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tub | Avium 1 | Avium 2 | Intracell | PPD-B | Scrof | Marinum | Kans 1 | Kans 2 | Fort | Bovis | Vaccae | |
| Ag85 | 0.37 | 0.43 | 0.39 | 0.45 | 0.48 | 0.41 | 0.44 | 0.30* | 0.47 | 0.37 | 0.38 | 0.35 |
| ESAT-6 | 0.23 | 0.19* | 0.31 | 0.29 | 0.06** | 0.25 | 0.24 | 0.18** | 0.13* | 0.21* | 0.25 | 0.09** |
| MPT64 | 0.19 | 0.21 | 0.14* | 0.17 | 0.18* | 0.16* | 0.20 | NT | 0.19 | 0.21 | 0.21 | 0.33 |
| M. tuberculosis 38 kDa | 0.12* | 0.29 | 0.16* | 0.24 | 0.31 | 0.20 | 0.26 | 0.05** | 0.38 | 0.26 | 0.10* | 0.31 |
| MPB70 | 0.19 | 0.26 | 0.15* | 0.24 | 0.32 | 0.20 | 0.24 | 0.17** | 0.31 | 0.20 | 0.06** | 0.23 |
| M. leprae 65 kDa | 0.29 | 0.29 | 0.27 | 0.28 | 0.36 | 0.23 | 0.24 | 0.52 | 0.35 | 0.33 | 0.26 | 0.33 |
| M. leprae 35 kDa | 0.33 | 0.47 | 0.42 | 0.45 | 0.62 | 0.45 | 0.46 | 0.25* | 0.51 | 0.42 | 0.30 | 0.50 |
| M. leprae 18 kDa | 0.21 | 0.23 | 0.22 | 0.23 | 0.31 | 0.23 | 0.22 | 0.12** | 0.31 | 0.26 | 0.19* | 0.28 |
Abbreviations: Tub, M. tuberculosis RT48; Avium 1, M. avium (SSI); Avium 2, M. avium (CVL); Intracell, M. intracellulare (SSI); PPD-B, M. intracellulare PPB-B; Scrof, M. scrofulaceum (SSI); Marinum, M. marinum (SSI); Kans 1, M. kansasii (SSI); Kans 2, M. kansasii (UCL); Fort, M. fortuitum (SSI); Bovis, M. bovis (CVL); Vaccae, M. vaccae (UCL); NT, not tested. All correlations are highly significant (P < 0.0001) unless indicated otherwise: *, P < 0.05; **, nonsignificant.
The association between the response to M. tuberculosis PPD and the recombinant antigens was also assessed by calculating the percentage of individuals making a positive (>62 pg/ml) IFN-γ response to the recombinant antigens according to whether they made a high (>500 pg/ml) or lower (≤500 pg/ml) response to M. tuberculosis PPD. The strongest association was with ESAT-6: 34% (21 of 62) of individuals with a response to M. tuberculosis PPD >500 pg/ml made a positive response to ESAT-6 compared to 7% (21 of 308) among individuals with a response to M. tuberculosis ≤500 pg/ml (P < 0.001).
Association of IFN-γ responses with skin test responsiveness to M. tuberculosis PPD.
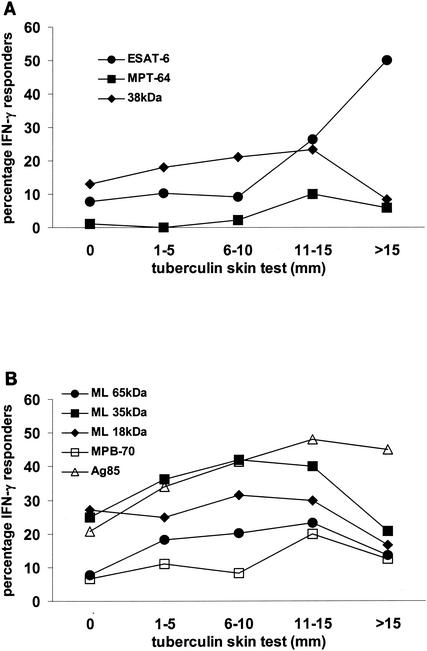
Large indurations in the Mantoux skin test are associated with an increased risk of developing tuberculosis (15). We therefore tested whether subjects who made an IFN-γ response to the M. tuberculosis recombinant antigens also showed large skin test indurations in response to PPD. IFN-γ responses to ESAT-6 (P < 0.001), and to a lesser extent MPT64 (P = 0.022, test for trend) showed a strong association with the diameter of Mantoux skin test induration to M. tuberculosis PPD RT23 (Fig. 4A). In subjects with a skin test induration of >10 mm in diameter, 38% (14 of 37) gave an IFN-γ response of >62 pg/ml to ESAT-6. Half of those subjects (9 of 18) with a skin test induration of >15 mm in diameter gave an IFN-γ response to ESAT-6 of >62 pg/ml. The association between Mantoux induration and IFN-γ production to ESAT-6 was highly significant at all cutoffs for IFN-γ production, including the most stringent criterion of >250 pg of IFN-γ/ml. This supports the hypothesis that T-cell responses to ESAT-6 can help identify individuals who have been exposed to, or infected with, M. tuberculosis. In contrast the M. tuberculosis 38-kDa antigen was recognized by a far higher proportion of subjects overall (16%), but these responses were not associated significantly with the skin test response (P = 0.15) (Fig. 4A).
FIG. 4.
Association of IFN-γ responses to recombinant antigens with Mantoux skin test induration to M. tuberculosis PPD. Subjects were tested for IFN-γ production in response to the M. tuberculosis ESAT-6, MPT64, and 38-kDa antigens (A) and to the M. leprae (ML) 65-, 35-, and 18-kDa antigens; MPB70; and M. bovis BCG Ag85 (B), and for Mantoux skin test induration in response to M. tuberculosis PPD. The proportion of subjects making >62 pg of IFN-γ/ml in response to each antigen in each DTH category is indicated. A total of 339 subjects who had data on DTH response were tested for responses to ESAT-6, 349 were tested for responses to MPT64, 549 were tested for responses to the 38-kDa antigen, 491 were tested for responses to the 65-kDa antigen, 546 were tested for responses to the 35- and 18-kDa antigens, 549 were tested for responses to MPB70, and 482 were tested for responses to Ag85.
We also assessed the association between the Mantoux skin test response to M. tuberculosis PPD and the IFN-γ response to the M. leprae and M. bovis or M. bovis BCG antigens used. There were associations between the Mantoux induration and the IFN-γ response to the M. leprae 65-kDa (P = 0.005) and M. leprae 35-kDa (P = 0.003) antigens, but the associations with the responses to MPB70 and the M. leprae 18-kDa antigen were not statistically significant (P = 0.13 and P = 0.56). There was a significant association between the Mantoux response and IFN-γ responses to M. bovis BCG Ag85 (P < 0.001); this may indicate that individuals with larger skin test indurations are being exposed to Ag85 secreted by living mycobacteria.
Incidence of tuberculosis.
One of the subjects recruited into this study developed tuberculosis of the bones and joints over the subsequent 3 years. This subject had an initial Mantoux induration of 15 mm and IFN-γ results of 2,285 pg/ml to M. tuberculosis PPD, 482 pg/ml to Ag85, 173 pg/ml to MPT64, and 71 pg/ml to ESAT-6. All other responses were below 31 pg/ml.
DISCUSSION
We have shown previously that the IFN-γ response to PPD of M. tuberculosis in this rural Malawian population correlates strongly with skin test responses to M. tuberculosis PPD RT23 (7). There is also a high prevalence of T-cell recognition of PPDs, measured in terms of IFN-γ production, from those strains of environmental mycobacteria that are thought to be relatively closely related to M. tuberculosis, such as those of the M. avium-M. intracellulare-M. scrofulaceum complex (6, 16). Exposure to nontuberculous mycobacteria such as M. avium, M. scrofulaceum, and M. intracellulare thus appears to induce T-cell reactivity to cross-reactive antigens present in M. leprae or M. tuberculosis. We have now tested IFN-γ responses in day 6 diluted whole-blood cultures to a panel of eight recombinant and purified antigens, in order to assess if the patterns of T-cell recognition indicate which mycobacterial species are most common in this environment and whether these in vitro T-cell responses were associated with skin test responses to antigens from M. tuberculosis. We selected IFN-γ production as a measure of type 1 immunity; IFN-γ production is widely used as representative of the activation of Th1 and Tc1 T cells and can be readily measured using diluted-whole-blood assays on large numbers of samples (6-8). Strong IFN-γ responses have been detected to the antigens used here in subjects infected with or exposed to mycobacteria (e.g., see references 14, 26, and 59). Other measures of T-cell activation such as lymphocyte proliferation could not be used in our field laboratory, and we have identified few subjects who made positive type 2 responses (interleukin-5) to these recombinant antigens (Black et al., unpublished data).
The recombinant and purified antigens used in this study were all provided by laboratories specializing in their production. The methods of production and purification varied, as did the final concentrations of contaminating endotoxin. The response rates overall were low, in terms of the proportion of subjects making IFN-γ responses of >62 pg/ml in stimulated cultures, whereas higher responses might be expected to contaminating proteins from E. coli, to which all subjects would have been exposed. There was no correlation between the frequency of responders to individual antigens and the concentration of contaminating endotoxin detected in each antigen preparation.
Of all the antigens tested, the M. leprae 35-kDa antigen induced the highest frequency of responders in this population. The M. leprae 35-kDa antigen was first identified as one of the major native proteins of M. leprae and termed major membrane protein 1 (21). Following the cloning of the 35-kDa gene (70), a homologous gene was identified in M. avium, with 90% identity at the amino acid level to the M. leprae protein, but no homologue was detected in M. intracellulare, M. tuberculosis, or M. bovis BCG (58). More recently, a homologue has also been identified in M. avium subsp. paratuberculosis, the causative agent of Johne's disease (4). The M. leprae 35-kDa antigen can induce protection against M. leprae infection in mice when used as a DNA vaccine (40); similar results using a DNA vaccine expressing the M. avium 35-kDa antigen, which induced protection against M. avium infection, suggest that it is also an immunodominant antigen in M. avium (39). The strong recognition detected in this group of healthy young adults who have not been BCG vaccinated might have been induced by exposure to M. leprae, but (i) as leprosy is now rare in the district, (ii) as our subjects were ≤28 years old, and, most importantly, (iii) as the prevalence of IFN-γ responses was highest in that part of the district which has consistently had the least leprosy, it is more likely to result from exposure to an environmental mycobacterium such as M. avium. It is possible that other confounding factors correlate with geographic differences and the decline in clinical leprosy and that exposure to M. leprae is not related to disease prevalence, but we consider this to be unlikely. Synthetic peptides from the M. leprae and M. avium 35-kDa sequences might be used to confirm such environmental cross-sensitization (68).
The M. leprae 18-kDa antigen was also recognized by samples from many of the subjects tested here. The M. leprae 18-kDa antigen (10, 74) was originally described as leprosy specific, and T-cell responses were detected in M. leprae-vaccinated volunteers (42) and tuberculoid leprosy patients and healthy leprosy contacts (14). Subsequent studies identified a homologue of the 18-kDa gene in M. avium and M. intracellulare (10) and in M. habana (32). DNA hybridization with a probe encoding the whole M. leprae 18-kDa sequence also demonstrated hybridization with additional species such as M. kansasii and M. scrofulaceum (41). Functional cross-reactivity with M. tuberculosis and mycobacteria other than M. leprae has been demonstrated by reactivity of the M. leprae 18-kDa protein with sera from tuberculosis patients (47, 63) and by detecting responses by T cells of non-leprosy-exposed subjects (1, 43; Menz and Dockrell, unpublished results). The higher prevalence of IFN-γ responses to the M. leprae 18-kDa antigen detected in the south of the district suggest that it is not specific for M. leprae, which is now rare in Karonga District, in particular in the south, but more probably reflects exposure to an environmental mycobacterium, perhaps a strain or relative of M. avium.
We detected a lower frequency of IFN-γ responders to the M. leprae 65-kDa antigen than to the 18- or 35-kDa antigens, but the prevalence was significantly higher in the region with a low incidence of leprosy than in that with the high leprosy incidence (P < 0.001). The 65-kDa GroEL protein is a heat shock protein (72) that is expressed in all the mycobacterial species tested to date, with homologues in all other bacterial species and in humans (23, 33). The hsp65 proteins in M. leprae, M. tuberculosis, and M. bovis BCG show over 95% homology (49), and thus any detected T-cell responses to the M. leprae 65-kDa are not likely to be M. leprae specific.
Though leprosy has always been more prevalent in the north than in the south of Karonga District (53), there was a higher prevalence of response to the M. leprae 18-, 35-, and 65-kDa antigens in individuals from the south of the district compared to individuals from the north, the differences being statistically significant for the 35- and 65-kDa antigens. This is in keeping with previous findings that the skin test response to a soluble M. leprae antigen preparation, Rees M. leprae soluble antigen, is higher in southern than northern residents, an observation that has been associated with environmental mycobacterial induced protection against, rather than infection with, the leprosy bacillus (53). In this context, the IFN-γ responses that we have measured against the M. leprae 65- and 35-kDa antigens, appear to be correlates of protection against M. leprae, rather than indicators of exposure to M. leprae (15).
We detected a higher proportion of subjects making a positive IFN-γ response to the M. tuberculosis 38-kDa antigen than to the other M. tuberculosis antigens tested, but these responses were not strongly associated with the Mantoux skin test induration. This antigen, a putative phosphate transport receptor (34), showed initial promise for the serological diagnosis of tuberculosis (71), with specificities of 86 to 92% reported in patients with sputum positive tuberculosis (25, 76). However, it is now evident that T-cell responses to this antigen are not specific, as T-cell proliferation was detected in 60% of both tuberculosis patients and healthy BCG-vaccinated subjects (71), and delayed-type hypersensitivity (DTH) responses to the 38-kDa antigen were elicited in guinea pigs sensitized with M. bovis, M. bovis BCG, M. kansasii, or M. intracellulare (27, 69, 71). The 38-kDa antigen gene is duplicated in M. intracellulare but absent from M. avium (54). A PCR assay designed to amplify the M. tuberculosis 38-kDa antigen gene gave positive results in M. bovis and M. africanum species, as well as M. tuberculosis, but not in other atypical mycobacteria (75).
Several other antigens with some specificity for M. tuberculosis infection have now been described, of which ESAT-6, a component of short-term culture filtrates, is probably the most promising. The esat-6 gene is present in M. tuberculosis, M. bovis, M. kansasii, M. szulgai, and M. marinum but absent from M. bovis BCG (2, 20). ESAT-6 has consistently been shown to be a promising candidate for early tuberculosis diagnosis due to the high degree of sensitivity and specificity found in T-cell assays (3, 30, 51, 59, 61). Positive responses to ESAT-6 have not been detected in PPD skin test negative controls (3, 30), BCG vaccinees (26), or patients infected with mycobacteria of the M. avium complex (35). When ESAT-6 has been used as a T-cell antigen in Africa or India, the proportion of healthy subjects responding to ESAT-6 has been much higher than that seen in developed-country settings (29, 45, 62), which may be a result of greater exposure to M. tuberculosis (or to other mycobacteria expressing ESAT-6) in these settings. Recent studies of the M. leprae homologue of ESAT-6 have shown that although there is only 36% amino acid homology with M. tuberculosis ESAT-6 (52), there may be T-cell cross-reactivity in IFN-γ responses to the two antigens in humans (18). In Malawi, the responses we detected were much lower than those detected in healthy controls in The Gambia (30% positive for IFN-γ production by ELISA [62]) or in urban Mumbai, India (60% positive for IFN-γ production by enzyme-linked immunospot assay [29]). In addition to variation in the sensitivities of the different methods used to detect IFN-γ responses to ESAT-6, the higher prevalence of tuberculosis and leprosy in urban Mumbai (29) compared to rural Karonga, and the older age of the Mumbai subjects (mean, 33 years, compared to 19 years for the Karonga subjects) probably explain the differences between these results.
The correlation analysis of IFN-γ responses did not provide evidence that the ESAT-6 antigen was specific for M. tuberculosis and M. kansasii (2, 20). This is probably due to the high level of cross-reactivity among the PPD preparations and the fact that most ESAT-6 responses were below the detection limit of the assay. However, three lines of evidence do suggest that ESAT-6 shows functional specificity for exposure to M. tuberculosis. First, the association between the skin test response and each of the recombinant antigens was strongest for ESAT-6. Second, when we studied the association between the IFN-γ response to M. tuberculosis PPD and the response to the recombinant antigens by calculating the percentage of individuals making a positive response to each recombinant antigen according to whether or not they made a high IFN-γ response to M. tuberculosis PPD, the association was strongest for ESAT-6. Third, the one subject who subsequently developed tuberculosis responded to ESAT-6.
Responses to the MPT64 antigen of M. tuberculosis were lower than those to ESAT-6. The mpt64 gene encodes a secreted protein of 23 kDa, MPT64; the mpb64 gene of M. bovis is identical except for one silent mutation (44). MPT64 is not expressed by environmental mycobacteria such as M. kansasii or M. marinum that express ESAT-6 (P. Andersen, personal communication). MPT64 might therefore be expected to be a better antigen for diagnosing M. tuberculosis infection than ESAT-6. However, results from skin testing in humans, and of lymphocyte responses in tuberculosis patients and controls have given variable results (2). MPT64 is also less efficient in inducing protection against M. tuberculosis than either Ag85 or ESAT-6 in animal models (28). In Malawi, we found that the MPT64 antigen gave weaker IFN-γ responses than ESAT-6; this might indicate greater specificity for M. tuberculosis infection, but MPT64 showed a weaker association with the skin test response to M. tuberculosis PPD than ESAT-6. Another interpretation is that MPT64 is less immunogenic than ESAT-6, despite the presence of sequences recognized by multiple HLA-DR phenotypes (46).
One of the subjects described in this study subsequently developed extrapulmonary tuberculosis. Although without symptoms at the time of recruitment, this individual had a 15-mm-diameter Mantoux response; made a strong IFN-γ response to M. tuberculosis PPD; and also responded to ESAT-6, MPT64, and Ag85. Although we had detected fewer responders to MPT64 than to ESAT-6 overall, this subject responded more strongly to MPT64 than to ESAT-6.
We also tested the M. bovis antigen, MPB70. The gene for MPB70 is present in M. bovis BCG but expressed to greater or lesser extents in different strains (i.e., lower expression in Glaxo and Pasteur strains than in Tokyo or virulent M. bovis) (64, 67). Evidence for a smaller gene of 4.6 kb in M. kansasii that hybridizes to a MPB83 gene probe has also been obtained using Southern blotting (65). The mpt70 gene is also present in M. tuberculosis; although expression is not observed in bacterial cultures, it may be up-regulated during infection in vivo, based on increased T-cell responses in tuberculosis patients compared to BCG vaccinees (48). The frequency of responders to MPB70 was low in this study. This is consistent with the fact that bovine tuberculosis is rarely identified in the Karonga District of Malawi, despite there being routine meat inspection, and M. bovis has only been identified twice in more than 2,000 positive cultures from human tuberculosis patients over 15 years in Karonga District (one of whom was an immigrant from Tanzania). Responses to the PPD of M. bovis were among the lowest we have observed to any mycobacterial PPD, with >50% of individuals tested producing an IFN-γ response of ≤62 pg/ml (6). All these data indicate that our subjects have had very little natural exposure to M. bovis.
In contrast to MPB70, the M. bovis BCG native antigen Ag85 (85A, -B, and -C), which forms a major component of the secreted proteins found in culture filtrates of M. tuberculosis (66), induced a much higher prevalence of positive IFN-γ responses. Members of the Ag85 complex, which function as fibronectin-binding proteins (55) and as mycolyl transferases (5), are highly conserved in M. bovis BCG, M. leprae, and M. tuberculosis (55). Homologues of Ag85 have been found in all the mycobacterial species from which the PPD preparations tested here were derived (56). T-cell recognition of Ag85 can be induced by infection with M. scrofulaceum and T-cell lines raised against the 91-108 peptide from Ag85B responded to PPDs from environmental mycobacteria (36, 60). The high frequency of IFN-γ responders to M. bovis BCG Ag85 detected here confirms the immunogenicity of this antigen (24). It was interesting that although not M. tuberculosis specific, the M. bovis BCG Ag85 antigen induced IFN-γ responses that were strongly associated with the skin test response to PPD RT23 from M. tuberculosis, implying that individuals showing large skin test responses have been exposed to live mycobacteria.
We have studied healthy young adults living in a rural area of northern Malawi who had not previously received BCG vaccination. This has provided a unique opportunity to assess T-cell responses to a panel of mycobacterial antigens in the absence of prior sensitization by BCG vaccination and in the absence of overt mycobacterial disease. The large number of subjects tested has allowed us to investigate the relationships between the T-cell recognition of individual antigens and different PPDs and Mantoux skin test responses. We find that the M. tuberculosis ESAT-6 antigen is more “M. tuberculosis specific” than MPT64 in this setting and that only a minority of our study subjects had been exposed to, or infected with, M. tuberculosis. The high frequency of responders to the M. leprae antigens, even in the area with the least leprosy, indicates that they are not specific for M. leprae but that they reveal immunological responses induced by environmental mycobacteria, the exposure to which provides protection against leprosy. The completion of the genome sequence of M. leprae (12) may now allow more specific leprosy antigens to be identified that would reveal M. leprae infection, an achievement that has remained elusive to date. It may also reveal additional M. leprae antigens shared by other mycobacteria, the responses to which are associated with protection against this disease.
Acknowledgments
We thank the people of Karonga for participating in these studies and the Health Sciences Research Committee of the Government of Malawi for permission to conduct this study. We thank all those who contributed to the production and purification of the antigens used, including Adam Whelan, Central Veterinary Laboratories, Weybridge, United Kingdom (MPB70), and Jan van Embden, National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands (ML65 kDa). We also thank Mike Brennan for providing the M. intracellulare PPD-B, John Stanford for providing new tuberculins from M. kansasii and M. vaccae, and Glyn Hewinson for providing M. avium and M. bovis PPD.
Financial support was provided by the Wellcome Trust (grant 047340/Z/96/M) with additional support from the British Leprosy Relief Association (LEPRA, grant 7.01.01.01 and grant 7.02.02.58). R.W. was supported by a separate grant from LEPRA.
REFERENCES
- 1.Adams, E., A. Basten, R. Prestidge, and W. J. Britton. 1995. T cell clones from a non-leprosy exposed subject recognize the Mycobacterium leprae 18-kD protein. Clin. Exp. Immunol. 102:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 4.Banasure, K. D., S. H. Basagoudanavar, P. Chaudhury, V. Tiwari, N. S. Parihar, and P. P. Goswami. 2001. Identification and characterization of a gene encoding a 35-kDa protein from Mycobacterium avium subspecies paratuberculosis. FEMS Microbiol. Lett. 196:195-199. [DOI] [PubMed] [Google Scholar]
- 5.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 6.Black, G. F., H. M. Dockrell, A. C. Crampin, S. Floyd, R. E. Weir, L. Bliss, L. Sichali, L. Mwaungulu, H. Kanyongoloka, B. Ngwira, D. K. Warndorff, and P. E. M. Fine. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322-329. [DOI] [PubMed] [Google Scholar]
- 7.Black, G. F., P. E. M. Fine, D. K. Warndorff, S. Floyd, R. E. Weir, J. M. Blackwell, L. Bliss, L. Sichali, L. Mwaungulu, S. Chaguluka, E. Jarman, B. Ngwira, and H. M. Dockrell. 2001. Relationship between IFN-γ and skin test responsiveness to Mycobacterium tuberculosis PPD in healthy, non-BCG-vaccinated young adults in Northern Malawi. Int. J. Tub. Lung Dis. 5:664-672. [PubMed] [Google Scholar]
- 8.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the United Kingdom: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 9.Booth, R. J., D. P. Harris, J. M. Love, and J. D. Watson. 1988. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J. Immunol. 140:597-601. [PubMed] [Google Scholar]
- 10.Booth, R. J., D. L. Williams, K. D. Moudgil, L. C. Noonan, P. M. Grandison, J. J. McKee, R. L. Prestidge, and J. D. Watson. 1993. Homologs of Mycobacterium leprae 18-kilodalton and Mycobacterium tuberculosis 19-kilodalton antigens in other mycobacteria. Infect. Immun. 61:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyn, J., R. Bosmans, M. Turneer, M. Weckx, J. Nyabenda, J. P. Van Vooren, P. Falmagne, H. G. Wiker, and M. Harboe. 1987. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect. Immun. 55:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dockrell, H. M., N. G. Stoker, S. P. Lee, M. Jackson, K. A. Grant, N. F. Jouy, S. B. Lucas, R. Hasan, R. Hussain, and K. P. McAdam. 1989. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57:1979-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine, P. E., J. A. Sterne, J. M. Ponnighaus, and R. J. Rees. 1994. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344:1245-1249. [DOI] [PubMed] [Google Scholar]
- 16.Fine, P. E., S. Floyd, J. L. Stanford, P. Nkhosa, A. Kasunga, S. Chaguluka, D. K. Warndorff, P. A. Jenkins, M. Yates, and J. M. Ponnighaus. 2001. Environmental mycobacteria in northern Malawi: implications for the epidemiology of tuberculosis and leprosy. Epidemiol. Infect. 126:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 18.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhof. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harboe, M., A. S. Malin, H. M. Dockrell, H. G. Wiker, G. Ulvund, A. Holm, M. C. Jorgensen, and P. Andersen. 1998. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 66:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter, S. W., B. Rivoire, V. Mehra, B. R. Bloom, and P. J. Brennan. 1990. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265:14065-14068. [PubMed] [Google Scholar]
- 22.Hussain, R., H. M. Dockrell, A. Kifayet, A. Daud, J. D. Watson, T. J. Chiang, and N. G. Stoker. 1992. Recognition of Mycobacterium leprae recombinant 18-kDa proteins in leprosy. Int. J. Lepr. Other Mycobact. Dis. 60:368-375. [PubMed] [Google Scholar]
- 23.Husson, R. N., and R. A. Young. 1987. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc. Natl. Acad. Sci. USA 84:1679-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 25.Jackett, P. S., G. H. Bothamley, H. V. Batra, A. Mistry, D. B. Young, and J. Ivanyi. 1988. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J. Clin. Microbiol. 26:2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadival, G. V., S. D. Chaparas, and D. Hussong. 1987. Characterization of serologic and cell-mediated reactivity of a 38-kDa antigen isolated from Mycobacterium tuberculosis J. Immunol. 139:2447-2451. [PubMed] [Google Scholar]
- 28.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 30.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 31.Lamb, F. I., A. E. Kingston, I. Estrada, and M. J. Colston. 1988. Heterologous expression of the 65-kilodalton antigen of Mycobacterium leprae and murine T-cell responses to the gene product. Infect. Immun. 56:1237-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb, F. I., N. B. Singh, and M. J. Colston. 1990. The specific 18-kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as a heat-shock protein. J. Immunol. 144:1922-1925. [PubMed] [Google Scholar]
- 33.Lamb, J. R., V. Bal, J. B. Rothbard, A. Mehlert, P. Mendez-Samperio, and D. B. Young. 1989. The mycobacterial GroEL stress protein: a common target of T-cell recognition in infection and autoimmunity. J. Autoimmun. 2(Suppl.):93-100. [DOI] [PubMed] [Google Scholar]
- 34.Lefevre, P., M. Braibant, L. de Wit, M. Kalai, D. Roeper, J. Grotzinger, J. P. Delville, P. Peirs, J. Ooms, K. Huygen, and J. Content. 1997. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J. Bacteriol. 179:2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lein, A. D., C. F. von Reyn, P. Ravn, C. R. Horsburgh, Jr., L. N. Alexander, and P. Andersen. 1999. Cellular immune responses to ESAT-6 discriminate between patients with pulmonary disease due to Mycobacterium avium complex and those with pulmonary disease due to Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 6:606-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozes, E., O. Denis, A. Drowart, F. Jurion, K. Palfliet, A. Vanonckelen, J. De Bruyn, M. De Cock, J. P. Van Vooren, and K. Huygen. 1997. Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria belonging to the MAIS-group. Scand. J. Immunol. 46:16-26. [DOI] [PubMed] [Google Scholar]
- 37.MacFarlane, A., R. Mondragon-Gonzalez, F. Vega-Lopez, B. Wieles, J. de Pena, O. Rodriguez, Y. D. L. T. R. Suarez, R. R. de Vries, T. H. Ottenhoff, and H. M. Dockrell. 2001. Presence of human T-cell responses to the Mycobacterium leprae 45-kilodalton antigen reflects infection with or exposure to M. leprae. Clin. Diagn. Lab. Immunol. 8:604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, E., A. T. Kamath, J. A. Triccas, and W. J. Britton. 2000. Protection against virulent Mycobacterium avium infection following DNA vaccination with the 35-kilodalton antigen is accompanied by induction of gamma interferon-secreting CD4+ T cells. Infect. Immun. 68:3090-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin, E., P. W. Roche, J. A. Triccas, and W. J. Britton. 2001. DNA encoding a single mycobacterial antigen protects against leprosy infection. Vaccine 19:1391-1396. [DOI] [PubMed] [Google Scholar]
- 41.Moudgil, K. D., D. L. Williams, and T. P. Gillis. 1992. DNA hybridization analysis of mycobacterial DNA using the 18-kDa protein gene of Mycobacterium leprae. FEMS Microbiol. Immunol. 4:165-174. [DOI] [PubMed] [Google Scholar]
- 42.Mustafa, A. S., H. K. Gill, A. Nerland, W. J. Britton, V. Mehra, B. R. Bloom, R. A. Young, and T. Godal. 1986. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature 319:63-66. [DOI] [PubMed] [Google Scholar]
- 43.Mustafa, A. S., K. E. Lundin, R. H. Meloen, and F. Oftung. 2000. Cross-reactive epitopes and HLA-restriction elements in human T cell recognition of the Mycobacterium leprae 18-kD heat shock protein. Clin. Exp. Immunol. 120:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oettinger, T., and A. B. Andersen. 1994. Cloning and B-cell-epitope mapping of MPT64 from Mycobacterium tuberculosis H37Rv. Infect. Immun. 62:2058-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 46.Roche, P. W., C. G. Feng, and W. J. Britton. 1996. Human T-cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand. J. Immunol. 43:662-670. [DOI] [PubMed] [Google Scholar]
- 47.Roche, P. W., R. L. Prestidge, J. D. Watson, and W. J. Britton. 1992. Antibody responses to the 18-kDa protein of Mycobacterium leprae in leprosy and tuberculosis patients. Int. J. Lepr. Other Mycobact. Dis. 60:201-207. [PubMed] [Google Scholar]
- 48.Roche, P. W., J. A. Triccas, D. T. Avery, T. Fifia, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 170:1326-1330. [DOI] [PubMed] [Google Scholar]
- 49.Shinnick, T. M., D. Sweetser, J. Thole, J. van Embden, and R. A. Young. 1987. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect. Immun. 55:1932-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh, M., A. B. Andersen, J. E. McCarthy, M. Rohde, H. Schutte, E. Sanders, and K. N. Timmis. 1992. The Mycobacterium tuberculosis 38-kDa antigen: overproduction in Escherichia coli, purification and characterization. Gene 117:53-60. [DOI] [PubMed] [Google Scholar]
- 51.Skjot, R. L., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer, J. S., M. A. Marques, M. C. Lima, A. P. Junqueira-Kipnis, B. C. Gregory, R. W. Truman, and P. J. Brennan. 2002. Antigenic specificity of the Mycobacterium leprae homologue of ESAT-6. Infect. Immun. 70:1010-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sterne, J. A., J. M. Ponnighaus, P. E. Fine, and S. S. Malema. 1995. Geographic determinants of leprosy in Karonga District, Northern Malawi. Int. J. Epidemiol. 24:1211-1222. [DOI] [PubMed] [Google Scholar]
- 54.Thangaraj, H. S., T. J. Bull, K. A. De Smet, M. K. Hill, D. A. Rouse, C. Moreno, and J. Ivanyi. 1996. Duplication of genes encoding the immunodominant 38 kDa antigen in Mycobacterium intracellulare. FEMS Microbiol. Lett. 144:235-240. [DOI] [PubMed] [Google Scholar]
- 55.Thole, J. E., R. Schoningh, A. A. Janson, T. Garbe, Y. E. Cornelisse, J. E. Clark-Curtiss, A. H. Kolk, T. H. Ottenhoff, R. R. De Vries, and C. Abou-Zeid. 1992. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol. Microbiol. 6:153-163. [DOI] [PubMed] [Google Scholar]
- 56.Thole, J. E., B. Wieles, J. E. Clark-Curtiss, T. H. Ottenhoff, and T. F. de Wit. 1995. Immunological and functional characterization of Mycobacterium leprae protein antigens: an overview. Mol. Microbiol. 18:791-800. [DOI] [PubMed] [Google Scholar]
- 57.Triccas, J. A., P. W. Roche, N. Winter, C. G. Feng, C. R. Butlin, and W. J. Britton. 1996. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect. Immun. 64:5171-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Triccas, J. A., N. Winter, P. W. Roche, A. Gilpin, K. E. Kendrick, and W. J. Britton. 1998. Molecular and immunological analyses of the Mycobacterium avium homolog of the immunodominant Mycobacterium leprae 35-kilodalton protein. Infect. Immun. 66:2684-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulrichs, T., P. Anding, S. Porcelli, S. H. Kaufmann, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valle, M. T., A. M. Megiovanni, A. Merlo, G. Li Pira, L. Bottone, G. Angelini, L. Bracci, L. Lozzi, K. Huygen, and F. Manca. 2001. Epitope focus, clonal composition and Th1 phenotype of the human CD4 response to the secretory mycobacterial antigen Ag85. Clin. Exp. Immunol. 123:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vekemans, J., C. Lienhardt, J. S. Sillah, J. G. Wheeler, G. P. Lahai, M. T. Doherty, T. Corrah, P. Andersen, K. P. McAdam, and A. Marchant. 2001. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in The Gambia. Infect. Immun. 69:6554-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vikerfors, T., P. Olcen, H. Wiker, and J. D. Watson. 1993. Serological response in leprosy and tuberculosis patients to the 18-kDa antigen of Mycobacterium leprae and antigen 85B of Mycobacterium bovis BCG. Int. J. Lepr. Other Mycobact. Dis. 61:571-580. [PubMed] [Google Scholar]
- 64.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vosloo, W., P. Tippoo, J. E. Hughes, N. Harriman, M. Emms, D. W. Beatty, H. Zappe, and L. M. Steyn. 1997. Characterisation of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene 188:123-128. [DOI] [PubMed] [Google Scholar]
- 66.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiker, H. G., S. Nagai, R. G. Hewinson, W. P. Russell, and M. Harboe. 1996. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand. J. Immunol. 43:374-380. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson, K. A., K. Katoch, U. Sengupta, M. Singh, K. K. Sarin, J. Ivanyi, and R. J. Wilkinson. 1999. Immune responses to recombinant proteins of Mycobacterium leprae. J. Infect. Dis. 179:1034-1037. [DOI] [PubMed] [Google Scholar]
- 69.Wilkinson, R. J., K. Haslov, R. Rappuoli, F. Giovannoni, P. R. Narayanan, C. R. Desai, H. M. Vordermeier, J. Paulsen, G. Pasvol, J. Ivanyi, and M. Singh. 1997. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J. Clin. Microbiol. 35:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter, N., J. A. Triccas, B. Rivoire, M. C. Pessolani, K. Eiglmeier, E. M. Lim, S. W. Hunter, P. J. Brennan, and W. J. Britton. 1995. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol. Microbiol. 16:865-876. [DOI] [PubMed] [Google Scholar]
- 71.Young, D., L. Kent, A. Rees, J. Lamb, and J. Ivanyi. 1986. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect. Immun. 54:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young, D. B., and T. R. Garbe. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young, D. B., S. H. Kaufmann, P. W. Hermans, and J. E. Thole. 1992. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6:133-145. [DOI] [PubMed] [Google Scholar]
- 74.Young, R. A., V. Mehra, D. Sweetser, T. Buchanan, J. Clark-Curtiss, R. W. Davis, and B. R. Bloom. 1985. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316:450-452. [DOI] [PubMed] [Google Scholar]
- 75.Yuen, K. Y., K. S. Chan, C. M. Chan, B. S. Ho, L. K. Dai, P. Y. Chau, and M. H. Ng. 1993. Use of PCR in routine diagnosis of treated and untreated pulmonary tuberculosis. J. Clin. Pathol. 46:318-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou, A. T., W. L. Ma, P. Y. Zhang, and R. A. Cole. 1996. Detection of pulmonary and extrapulmonary tuberculosis patients with the 38-kilodalton antigen from Mycobacterium tuberculosis in a rapid membrane-based assay. Clin. Diagn. Lab. Immunol. 3:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]