Abstract
The relative value of antibodies and/or T-cell immune responses to Bordetella pertussis antigens in the immunity induced by acellular pertussis (aP) vaccines is still an open issue, probably due to the incomplete knowledge on the mechanisms of protective immunity to pertussis. The relevance of T-cell immune responses in protection from pertussis has been demonstrated in murine and human models of infection; thus, in this study, the ability of different vaccine preparations of three component (pertussis toxin, filamentous hemagglutinin, and pertactin) aP vaccines to induce T-cell responses was investigated in mice. All vaccine preparations examined passed the immunogenicity control test, based on antibody titer assessment, according to European Pharmacopoeia standards, and protected mice from B. pertussis intranasal challenge, but not all preparations were able to prime T cells to pertussis toxin, the specific B. pertussis antigen. In particular, one vaccine preparation was unable to induce proliferation and gamma interferon (IFN-γ) production while the other two gave borderline results. The evaluation of T-cell responses to pertussis toxin antigen may provide information on the protective immunity induced by aP vaccines in animal models. Considering the critical role of the axis interleukin-12-IFN-γ for protection from pertussis, our results suggest that testing the induction of a key protective cytokine such as IFN-γ could be an additional tool for the evaluation of the immune response induced by aP vaccines.
Pertussis is a vaccine-preventable respiratory disease which affects infants and children and is still an important cause of morbidity and mortality in many parts of the world (10). Bordetella pertussis is also a recognized agent of respiratory disease in adolescents and adults (12, 20, 25). The epidemiology of pertussis is not well defined because of the broad spectrum of clinical manifestations. While in infants and children pertussis is characterized by paroxysmal cough, whooping cough, and posttussive vomiting, in adolescents and adults clinical symptoms are atypical and often manifest as protracted cough (42).
Clinical trials have demonstrated that acellular pertussis (aP) vaccines, formulated with different combinations of the putative protective antigens of B. pertussis such as pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae confer protection against whooping cough (15, 19). Thus, these vaccines are increasingly replacing the more reactogenic whole-cell (wP) vaccines, particularly for their use as booster doses (12).
The mechanisms underlying protection conferred by aP vaccines is still a matter of debate (15, 19, 36). In particular the relative value of antibodies (Ab) and/or T-cell mediated immune (CMI) responses to B. pertussis antigens in the mechanisms of the persistent immunity associated with the new aP vaccines is still an open issue, probably due to the incomplete knowledge on the mechanisms of immunity to pertussis (3, 14, 36, 40, 44, 47). The immunogenicity studies performed within the clinical trials did not demonstrate a satisfactory correlation between the presence of Ab to the vaccine antigens and the efficacy of the aP vaccines (1, 23). However, Ab response against PRN, fimbriae, and PT may be associated with protection (16, 43). Moreover, many studies indicate that humoral immunity alone is not sufficient to confer long-term protection against B. pertussis infection and that protection against B. pertussis requires CMI as well as humoral immunity (4, 5, 36, 44).
Using a murine model of infection, it has been demonstrated that adoptive transfer of CD4+ T cells from immune mice confers protection from B. pertussis challenge in the absence of detectable Ab response (34) but also passive Ab transfer protected mice from B. pertussis infection (27). Protection induced with wP or aP vaccines persists after disappearance of specific serum immunoglobulin G (27, 30) and T cells involved in CMI response produce several cytokines that exert a strong regulatory influence on Ab isotype and on activity of macrophages and polymorphonuclear cells (36). Furthermore, some studies have suggested that intracellular survival of B. pertussis is a mechanism for persistence within the respiratory tract (22, 41). These and other data (7, 13, 37) indirectly support the relevance of CMI in protection from pertussis.
The ability of aP vaccines to induce Ab responses to their antigenic constituents in mice is the main assay for the control of the immunogenicity of the vaccine preparations (21, 31, 33); however, it does not assess efficacy, as underlined above, but consistency in the production of different vaccine lots.
Murine respiratory infection models with B. pertussis challenge, either by intranasal instillation or by aerosol delivery, have been proposed by several groups as reliable assays to measure the activity of aP vaccines (11, 24, 35, 46). Some vaccine manufacturing companies have adopted this assay as a premarket assay, in parallel with the immunogenicity assay (17).
In a previous study (32), an in vitro-proliferation assay was performed in aP-vaccinated mice to assess the ability of the vaccines to induce lymphocyte proliferation in response to B. pertussis antigens. Considering the relevance of CMI in pertussis protection, in the present study we investigated whether CMI assessment might provide complementary information to the immunogenicity assay and to protection in the mouse intranasal model, thus allowing a more complete evaluation of the protective immune response induced by aP vaccines.
MATERIALS AND METHODS
Mice.
In evaluating the immunogenicity of aP vaccine preparations, outbred CD1 and inbred BALB/c mice are routinely used in order to analyze the immune response in high or low genetically variable mice (21), and thus in this study two mouse strains were used (female CD1 and BALB/c). The mice were commercially obtained (Charles River S.p.A., Milan, Italy) and maintained under pathogen-free conditions. All mice were 6 to 8 weeks old at the beginning of the experiments.
Immunization and vaccines.
Groups of CD1 and BALB/c mice were immunized intraperitoneally (38) at 0 and 3 weeks with one-half a human dose of three-component (PT, FHA, and PRN) aP vaccine preparations from Glaxo SmithKline Beecham Biologicals (GSK) (Rixensart, Belgium) or Chiron Biocine (CB) (Siena, Italy). In preliminary experiments performed in outbred CD1 mice, the dose of vaccine was found to be optimal, because it minimized the intramouse variability (32). Each vaccine contained inactive PT, FHA, and PRN. In particular, aP from CB contained 2.5 μg of FHA, 2.5 μg of PRN, and 5 μg of genetically inactivated PT. aP from GSK contained 25 μg of FHA, 8 μg of PRN, and 25 μg of formalin-glutaraldehyde-inactivated PT.
All vaccine preparations were kindly provided by the manufacturers as combined diphtheria-tetanus-pertussis (aP) formulations, adsorbed to alum.
Antigen and mitogen.
PT, FHA, and PRN purified proteins (donated by CB) were used as antigens in CMI assessment. To avoid any potential mitogenicity, they were heat inactivated (96°C, 1 h). Heat-killed B. pertussis (strain 18323, ATCC 9797, agglutinogen 1,3) was also used as an antigen. Concanavalin A (ConA) (Sigma, St. Louis, Mo.) was used for mitogenic stimulation.
B. pertussis intranasal infection.
Culture of B. pertussis 18323, grown on Bordet-Gengou agar, was suspended in physiological saline containing 1% casein and was used to infect mice 2 weeks after the second immunization. Fifty microliters (5 × 106 CFU) of B. pertussis was instilled intranasally in mice under light anesthesia. Groups of four animals were killed by cervical dislocation after 2 h or at various time points. The lungs were rapidly removed and homogenized in physiological saline containing 1% casein. Serial 10-fold dilutions of the homogenates were used to determine CFU on Bordet-Gengou agar plates after 3 to 4 days of incubation at 36°C. The limit of detection of the assay was approximately log10 0.5 CFU per lung.
Proliferation and gamma interferon (IFN-γ) assays.
CMI responses were measured by assessing proliferative responses of splenic lymphocytes induced by B. pertussis antigens (2). Mouse spleens from naive and immunized mice were removed and homogenized to obtain a single-cell suspension and depleted of erythrocytes by treatment with Tris-ammonium chloride (9). The cells were washed and cultured at 2 × 106/ml in RPMI 1640 medium with l-glutamine supplemented with 10% nonmitogenic prescreened heat-inactivated fetal calf serum, 1% penicillin, and 1% streptomycin. Proliferation was measured by using 4 × 105 cells/well in 0.2 ml of complete medium, in triplicate, in 96-well flat-bottom trays (Falcon, Becton Dickinson, Lincoln Park, N.J.) in the presence of the predetermined optimal doses of heat-inactivated PT (5 μg/ml), PRN (10 μg/ml), FHA (20 μg/ml) and with heat-killed B. pertussis whole cells (107/ml). Mitogenic response was measured using ConA (2 μg/ml). The cultures were harvested after 5 or 2 days for antigenic or mitogenic stimulation, respectively. [methyl-3H]thymidine (specific activity, 2.5 Ci/mmol; Amersham TRK120) was added to the culture at a final concentration of 0.5 μCi/well, 18 h before cell harvesting with a semiautomatic harvester (Pharmacia, Uppsala, Sweden). DNA synthesis was evaluated by counting [3H]thymidine incorporation with a β-plate counter (Perkin-Elmer/Applied Biosystems, Foster City, Calif.). Data are shown as stimulation index (SI), i.e., the ratio between counts per minute of the antigen-stimulated lymphocyte cultures and those of the unstimulated ones. The counts per minute of unstimulated cultures of spleen cells from the mice under study was 0.2 ± 0.06 (mean ± standard error [SE]). The SE of the counts per minute values of triplicate cultures was below 10%. A CMI proliferative response was considered positive when the SI was greater or equal to 4.
To measure IFN-γ, spleen cells (2 × 106/ml) from naive and immune mice were cultured in the presence of heat-inactivated PT (5 μg/ml), ConA (2 μg/ml), or medium alone. Supernatants were collected after 48 h, and IFN-γ concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (Endogen Inc., Woburn, Mass.). ELISA sensitivity was 2 pg/ml. Results represent picograms of IFN-γ per milliliter obtained in stimulated and unstimulated splenocytes pooled from groups of three mice.
Statistical analysis.
The statistical significance of differences between results from different groups was examined by Student's t test. The results of B. pertussis intranasal infection were analyzed by the parallel-line assay. Statistical analysis was performed using the SPSS statistical software (version 10.0).
RESULTS
Even if Ab levels for each specific antigen were different depending on the vaccine used, all vaccines in this study passed the criteria of the standard immunogenicity assay performed both by manufacturers and official control authorities (data not shown) according to European Pharmacopoeia standards (21).
In the experiments performed in this study, no substantial differences were observed in the performance of either the GSK (two vaccine preparations studied) or CB (four vaccine preparations studied) aP vaccines; thus, the results are reported per single vaccine preparation without specifying the manufacturer.
In order to verify the presence of Ab response to pertussis antigens in our experimental setting, sera of immunized and control mice were collected after the completion of the immunization schedule, i.e., after two immunizations. All vaccine preparations induced high serum immunoglobulin G Ab responses when assessed by the recommended method (21, 31), and the two-dose schedule induced an overimmunization in all mice (data not shown).
Protection in mouse intranasal challenge.
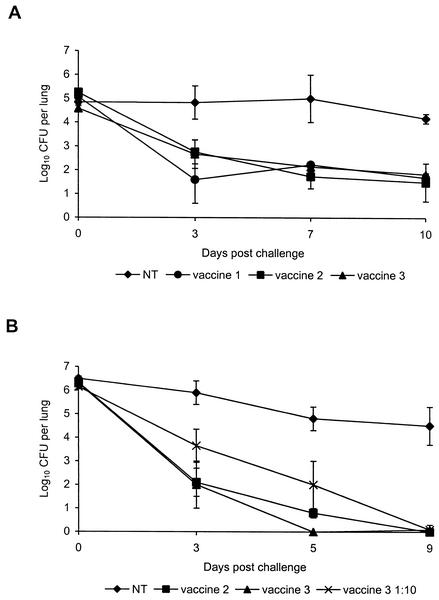
The clearance of the virulent B. pertussis 18323 strain from the lung induced by vaccination in groups of CD1 and BALB/c mice, was determined for three out of six vaccine preparations under study. As shown in Fig. 1A, a mean of 105 CFU were counted in the lung of unvaccinated mice starting from day 0 (2 h after challenge) till day 7 postchallenge. A high level of infection persisted till day 10. On the contrary, CD1 mice immunized with different aP vaccine preparations showed an early decline in bacterial load by day 3 after challenge but failed to eliminate the bacteria up to day 10. As shown in Fig. 1B, CFU counted in the lung of unvaccinated BALB/c mice showed a similar pattern as in CD1 mice; even if, at day 0, CFU counts were higher in BALB/c than in CD1 mice. At day 9 (BALB/c) and day 10 (CD1) CFU counts were comparable in the two strains of mice. Immunization of BALB/c with vaccine 2 or vaccine 3 conferred a complete bacterial clearance by day 9 after challenge, even if kinetics of B. pertussis clearance induced by vaccine 2 was delayed compared to clearance induced by vaccine 3 (for day 9 versus day 5, differences were not statistically significant [Fig. 1B]). A group of mice vaccinated with 1/10 of the vaccine dose of vaccine 3 was included in order to mimic a less-protective vaccine and to measure the sensitivity of the assay (vaccine 3 versus vaccine 3 1:10, P = 0.03) (Fig. 1B).
FIG. 1.
Kinetics of B. pertussis clearance from lungs of intranasally challenged unvaccinated mice (NT) or mice vaccinated with aP vaccine preparations. Groups of CD1 (A) or BALB/c (B) mice were immunized i.p. at 0 and 3 weeks with one-half a human dose. Two weeks after completion of vaccination, mice were challenged with B. pertussis 18323 and CFU counts were performed on individual lung homogenates on the indicated days. Results are means ± SE (error bars) of viable B. pertussis counts from four mice per group at each time point. The results are representative of three experiments performed. The limit of detection of the assay was approximately log10 0.5 CFU per lung.
Differences in kinetics of bacterial clearance between CD1 and BALB/c mice may be related to different genetic background of the two strains of mice as already suggested by Barnard et al. (8).
Proliferative response of spleen cells from immunized mice.
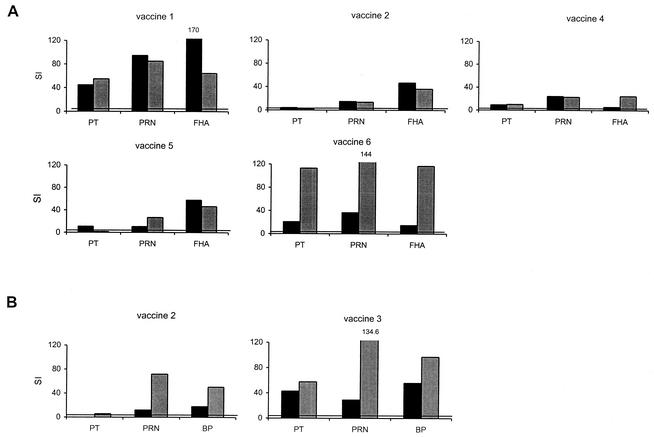
Considering that induction of T-cell response to B. pertussis antigens constitutes a relevant feature of pertussis vaccine immunogenicity, the ability of the six preparations of aP vaccines to induce lymphoproliferative responses was evaluated. Figure 2 shows the results of lymphocyte proliferation to PT, PRN, and FHA antigens in CD1 (Fig. 2A) and to PT, PRN, and B. pertussis antigens in BALB/c mice (Fig. 2B), measured at 15 and 30 days after the completion of vaccine schedule. Three preparations of aP vaccine—vaccine 1 and vaccine 6 (Fig. 2A) and vaccine 3 (Fig. 2B)—gave a high lymphoproliferative response to all antigens tested in CD1 (Fig. 2A) or BALB/C (Fig. 2B) mice. Vaccines 4 and 5 were able to induce a borderline response to PT and a positive proliferation to the other antigens (Fig. 2A), whereas vaccine 2 was not able to elicit a detectable response to PT either in CD1 (Fig. 2A) or BALB/c (Fig. 2B) mice. The SI of spleen cells from unvaccinated mice was below 4 when cultured in the presence of PT, PRN, and FHA antigens (data not shown).
FIG. 2.
Lymphoproliferative response from immune CD1 (A) or BALB/c mice (B) was assessed at 15 (black bars) and 30 (grey bars) days after completion of the immunization schedule. Spleen cells (2 × 106/ml) were stimulated in vitro with the indicated antigens, as detailed in Materials and Methods. DNA synthesis was measured after 5 days by counting [3H]thymidine incorporation (counts per minute, 103). Results are mean values of SI obtained from three mice per group assessed individually and are representative of three experiments performed. The counts per minute (mean ± SE) of unstimulated cultures of spleen cells from the mice under study was 0.2 ± 0.06. The threshold of positive proliferative response is shown (SI = 4).
IFN-γ induction by PT-treated spleen cells from immune mice.
Considering that only PT, the specific antigen of B. pertussis, was able to discriminate the ability of vaccine preparations to induce proliferation of T cells, only the ability of PT to induce IFN-γ, a protective Th1 cytokine in pertussis, was tested in spleen cell cultures of untreated and immunized mice. Table 1 shows the level of IFN-γ, measured in the supernatant of splenocytes obtained 15 days after completion of vaccination and stimulated with PT. The results obtained paralleled those of proliferative assays. PT-stimulated lymphocytes from CD1 or BALB/c mice vaccinated with vaccine 1, vaccine 3, or vaccine 6 secreted elevated amounts of IFN-γ. PT-stimulated lymphocytes from CD1 mice vaccinated with vaccine 4 were able to induce low levels of the cytokine, whereas PT-stimulated lymphocytes from mice vaccinated with vaccine 2 or vaccine 5 were unable to induce IFN-γ release in CD1 (both vaccine preparations were tested) or in BALB/c (vaccine 2 only was tested). Elevated levels of IFN-γ were measured in supernatants of ConA-stimulated lymphocytes from all mice studied.
TABLE 1.
IFN-γ secretion induced by PT in spleen cells from vaccinated micea
| Mouse strain | aP vaccine preparation | IFN-γ induced by spleen cells (pg/ml)
|
||
|---|---|---|---|---|
| Treated with:
|
Untreated | |||
| PT | ConA | |||
| CD1 | Vaccine 1 | 2,470 | 827 | 38 |
| CD1 | Vaccine 4 | 35 | 809 | 5 |
| CD1 | Vaccine 2 | 6 | 1,779 | 11 |
| CD1 | Vaccine 5 | 0 | 869 | 0 |
| CD1 | Vaccine 6 | 200 | 875 | 25 |
| CD1 | Unvaccinated | 12 | 2,071 | 6 |
| BALB/c | Vaccine 2 | 0 | 859 | 0 |
| BALB/c | Vaccine 3 | 480 | 1,890 | 11 |
| BALB/c | Unvaccinated | 5 | 366 | 0 |
Production of IFN-γ by spleen cells from immune mice in response to PT antigen. Spleen cells from unvaccinated or immune CD1 and BALB/c mice (taken 15 days after the completion of vaccination) were cultured with PT (5 μg/ml), ConA (2 μg/ml), or unstimulated (untreated) cells. Supernatants were removed after 48 h and assayed by ELISA. Results were obtained in splenocytes pooled from groups of three mice and are taken from one experiment representative of two performed.
DISCUSSION
Immunogenicity studies performed within clinical trials have shown that there is no clear correlation between the presence of Ab to pertussis antigens and protection from disease (1, 23, 36). Thus, the Ab response, and in particular the assessment of the Ab binding to antigens, may not be sufficient to assure the potency of aP vaccine preparations in animal models. Moreover, measurement of Ab elicited by test vaccines gives information only on the consistency of the vaccine production and not necessarily on the ability of the vaccine to protect from B. pertussis infection.
It is widely accepted that the method applied to wP vaccines to evaluate the potency of vaccine lots is inappropriate for the evaluation of acellular formulations (26). In particular, the conventional intracerebral mouse protection test has not been found to predict clinical performance when applied to aP vaccines (13).
To overcome these problems, a mouse intranasal challenge model has been proposed (11, 24, 35, 46). This method was able to discriminate among aP vaccines that showed a different efficacy in clinical trials (24, 35) and to reveal differences between the mouse lung clearance activities of bi- or tri-component aP pertussis vaccines (24). Furthermore, as also shown in our results, this method was able to discriminate between the lung clearance induced by vaccination with 1/10 of a vaccine dose and with the full dose. An international collaborative study group is now working to formulate recommendations and guidelines for manufacturers to use this method as a possible assay to contribute to the determination of the potency of aP vaccines (17, 18).
The specific goal of this study was to evaluate the CMI in mice to better understand the immunogenicity mechanisms induced by aP vaccines. Growing evidence suggests a relevant role of CMI in protection induced by antipertussis vaccines in children (3, 14, 36, 40, 47). As parameters of CMI responses, two assays were chosen: the T-cell proliferation assay and an assessment of Th1 cytokine IFN-γ by ELISA.
Th1 cytokines are associated with protection in various B. pertussis infection models, and in particular, in humans, protection from pertussis after infection or vaccination is determined by the presence or by the induction of Th1 cytokines such as interleukin-12 (IL-12) and IFN-γ (3-6, 14, 39, 40).
Also, in mice the clearance of B. pertussis or protection induced by wP vaccines is dependent on the production of appropriate Th1 cytokines. Mills and coworkers demonstrated the induction of elevated amounts of IFN-γ after vaccination with wP vaccines in mice, particularly when lymphocytes were activated by killed B. pertussis (35, 38). These authors showed also that protection induced by aP vaccines was associated with the secretion of Th2 cytokines, such as IL-5 (35, 38). But, depending on the mouse strain and on the antigen used to activate lymphocytes, appreciable amounts of IFN-γ were also induced by aP vaccines (35). In addition, the same authors also describe a contribution of IL-12 in vaccine efficacy in the case of aP vaccines (28). Therefore, we thought it would be relevant to measure IFN-γ induction in aP-vaccinated mice, a parameter which could be also used to measure the efficacy of wP cell vaccines.
The results obtained indicate that aP vaccine preparations which had previously passed the routine immunogenicity test and were able to induce the clearance of B. pertussis from the lungs, following intranasal challenge in mice, may behave differently when inducing a CMI response. Indeed, one vaccine preparation was unable and the other two showed a borderline ability to prime mouse T cells to proliferate and to induce IFN-γ secretion when cultured in the presence of PT, the essential constituent of all aP vaccines. These results were in part supported by the results obtained in a recent study on the evaluation of efficacy of Japanese Biken and Takeda aP vaccines. The authors did not find a correlation between the protective effects induced by aP vaccine preparations, measured by respiratory infection protection test, and CMI responses (45).
In conclusion, the results of this study showed that immunization with aP vaccine preparations may not induce an equal CMI response to B. pertussis antigens in mice, especially to PT, which is a critical requirement for long-lasting protection (5, 6, 14, 30). The differences in CMI induction cannot be ascribed to specific vaccine formulation, such as differences in PT inactivation or in protein contents, because the same proportion of vaccine preparations studied, produced either by GSK or CB, was able to induce a positive CMI response. Whether and to what extent this observation is relevant to the assessment of protective capacity of the vaccine preparations in children is not clear at the moment, also in consideration of the low number of vaccine preparations tested.
Considering the critical role of the axis IL-12-IFN-γ for protection from pertussis (28, 29, 36), our results suggest that testing the induction of IFN-γ, as a parameter of CMI responses, could be an additional tool for the evaluation of the immune response induced by aP vaccines. As in the case of the intranasal protection assay, a working group including control laboratories, manufacturers, and research groups could be established to set up and standardize the IFN-γ assay and determine parameters of acceptance for aP vaccine preparations.
Acknowledgments
We thank Antonio Cassone (Istituto Superiore di Sanità, Rome, Italy) for helpful comments and discussion. We gratefully acknowledge Tonino Sofia for editorial assistance.
This work was supported by grants from the Istituto Superiore di Sanità (1011/RI, 2011/RI, and OB/C) and from the Ministry of Health of Italy (99/A and IAF/F3).
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. 1988. Placebo-controlled trial of two acellular pertussis vaccines in Sweden: protective efficacy and adverse events. Lancet i:955-960. [PubMed] [Google Scholar]
- 2.Ausiello, C. M., P. Sestili, C. Locardi, M. Logozzi, P. Rizza, E. Parlanti, L. Yang, A. Modica, A. Modesti, P. Musiani, and F. Belardelli. 1994. Defective response to T cell mitogens in mice injected with human immunodeficiency virus type 1-infected U937 cells. J. Gen. Virol. 75:2789-2794. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., F. Urbani, A. la Sala, R. Lande, and A. Cassone. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausiello, C. M., R. Lande, A. la Sala, F. Urbani, and A. Cassone. 1998. Cell-mediated immune response of healthy adults to Bordetella pertussis vaccine antigens. J. Infect. Dis. 178:466-470. [DOI] [PubMed] [Google Scholar]
- 5.Ausiello, C. M., R. Lande, F. Urbani, A. la Sala, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausiello, C. M., R. Lande, F. Urbani, B. Di Carlo, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 2000. Cell-mediated immunity and antibody responses to Bordetella pertussis antigens in children with a history of pertussis infection and in recipients of an acellular pertussis vaccine. J. Infect. Dis. 181:1989-1995. [DOI] [PubMed] [Google Scholar]
- 7.Barbic, J., M. F. Leef, D. L. Burns, and R. D. Shahin. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 65:4904-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnard, A., B. P. Mahon, J. Watkins, K. Redhead, and K. H. Mills. 1996. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology 87:372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888-895. [PubMed] [Google Scholar]
- 10.Black, S. 1997. Epidemiology of pertussis. Pediatr Infect. Dis. J. 16:S85-S89. [DOI] [PubMed] [Google Scholar]
- 11.Boursaux-Eude, C., S. Thiberge, G. Carletti, and N. Guiso. 1999. Intranasal murine model of Bordetella pertussis infection: II. Sequence variation and protection induced by a tricomponent acellular vaccine. Vaccine 17:2651-2660. [DOI] [PubMed] [Google Scholar]
- 12.Campins-Marti, M., H. K. Cheng, K. Forsyth, N. Guiso, S. Halperin, L. M. Huang, J. Mertsola, G. Oselka, J. Ward, C. H. Wirsing von Konig, and F. Zepp. 2001. Recommendations are needed for adolescent and adult pertussis immunisation: rationale and strategies for consideration. Vaccine 20:641-646. [DOI] [PubMed] [Google Scholar]
- 13.Canthaboo, C., L. Williams, D. K. Xing, and M. J. Corbel. 2000. Investigation of cellular and humoral immune responses to whole cell and acellular pertussis vaccines. Vaccine 19:637-643. [DOI] [PubMed] [Google Scholar]
- 14.Cassone, A., C. M. Ausiello, F. Urbani, R. Lande, M. Giuliano, A. La Sala, A. Piscitelli, S. Salmaso, et al. 1997. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch. Pediatr. Adolesc. Med. 151:283-289. [DOI] [PubMed] [Google Scholar]
- 15.Cherry, J. D. 1997. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr. Infect. Dis. J. 16:S90-S96. [DOI] [PubMed]
- 16.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 17.Corbel, M. J., D. K. Xing, and J. G. Kreeftenberg. 1999. Informal consultation with manufacturers and W.H.O. ad hoc working group on mouse protection models for acellular pertussis vaccines national institute for biological standards, 12 November 1998. Biologicals 27:189-193. [DOI] [PubMed] [Google Scholar]
- 18.Corbel, M. J., P. Mastrantonio, and J. G. Kreeftenberg. 2001. Ad hoc Working Group on acellular pertussis vaccines, World Health Organisation, Geneva, 27-28 July 2000. Vaccine 20:288-291. [DOI] [PubMed] [Google Scholar]
- 19.Edwards, K. M., B. D. Meade, M. D. Decker, G. F. Reed, M. B. Rennels, M. C. Steinhoff, E. L. Anderson, J. A. Englund, M. E. Pichichero, and M. A. Deloria. 1995. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 96:548-557. [PubMed] [Google Scholar]
- 20.Edwards, K. M. 2001. Is pertussis a frequent cause of cough in adolescents and adults? Should routine pertussis immunization be recommended? Clin. Infect. Dis. 32:1698-1699. [DOI] [PubMed] [Google Scholar]
- 21.European Pharmacopoeia. 2002. Pertussis vaccine (acellular, component adsorbed), 4th ed. Monograph 1356. European Pharmacopoeia, Strasbourg, France.
- 22.Friedman, R. L., K. Nordensson, L. Wilson, E. T. Akporiaye, and D. E. Yocum. 1992. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect. Immun. 60:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuliano, M., P. Mastrantonio, A. Giammanco, A. Piscitelli, S. Salmaso, and S. G. Wassilak. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J. Pediatr. 132:983-988. [DOI] [PubMed] [Google Scholar]
- 24.Guiso, N., C. Capiau, G. Carletti, J. Poolman, and P. Hauser. 1999. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine 17:2366-2376. [DOI] [PubMed] [Google Scholar]
- 25.Keitel, W. A., L. R. Muenz, M. D. Decker, J. A. Englund, C. M. Mink, D. A. Blumberg, and K. M. Edwards. 1999. A randomized clinical trial of acellular pertussis vaccines in healthy adults: dose-response comparisons of 5 vaccines and implications for booster immunization. J. Infect. Dis. 180:397-403. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick, P. L., G. Eldering, M. K. Dixon, and J. Misner. 1947. Mouse protection tests in the study of pertussis vaccines: a comparative series using the intracerebral route of challenge. Am. J. Public Health 1947:803-810. [PMC free article] [PubMed] [Google Scholar]
- 27.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahon, B. P., M. S. Ryan, F. Griffin, and K. H. Mills. 1996. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect. Immun. 64:5295-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahon, B. P., B. J. Sheahan, F. Griffin, G. Murphy, and K. H. Mills. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahon, B. P., M. T. Brady, and K. H. Mills. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181:2087-2091. [DOI] [PubMed] [Google Scholar]
- 31.Manclark, C. R., B. D. Meade, and D. G. Burstyn. 1986. Serological response to Bordetella pertussis, p. 388-394. In N. R. Rose, H. Friedman, and J. L. Fahey (ed.), Manual of clinical laboratory immunology, 3rd ed. American Society for Microbiology, Washington, D.C.
- 32.Mastrantonio, P., M. Cerquetti, R. Cardines, R. Lande, C. M. Ausiello, and A. Cassone. 1999. Immunogenicity issues in the quality control of the new acellular pertussis vaccines. Biologicals 27:119-121. [DOI] [PubMed] [Google Scholar]
- 33.Meade, B. D., A. Deforest, K. M. Edwards, T. A. Romani, F. Lynn, C. H. O'Brien, C. B. Swartz, G. F. Reed, and M. A. Deloria. 1995. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics 96:570-575. [PubMed] [Google Scholar]
- 34.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills, K. H. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 37.Petersen, J. W., P. H. Ibsen, K. Haslov, and I. Heron. 1992. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleen of mice aerosol infected with Bordetella pertussis. Infect. Immun. 60:4563-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redhead, K., J. Watkins, A. Barnard, and K. H. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 61:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan, M., G. Murphy, L. Gothefors, L. Nilsson, J. Storsaeter, and K. H. Mills. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246-1250. [DOI] [PubMed] [Google Scholar]
- 40.Ryan, M., G. Murphy, E. Ryan, L. Nilsson, F. Shackley, L. Gothefors, K. Oymar, E. Miller, J. Storsaeter, and K. H. Mills. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saukkonen, K., C. Cabellos, M. Burroughs, S. Prasad, and E. Tuomanen. 1991. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J. Exp. Med. 173:1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senzilet, L. D., S. A. Halperin, J. S. Spika, M. Alagaratnam, A. Morris, and B. Smith. 2001. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin. Infect. Dis. 32:1691-1697. [DOI] [PubMed] [Google Scholar]
- 43.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 44.Tran Minh, N. N., Q. He, A. Ramalho, A. Kaufhold, M. K. Viljanen, H. Arvilommi, and J. Mertsola. 1999. Acellular vaccines containing reduced quantities of pertussis antigens as a booster in adolescents. Pediatrics 104:e70. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe, M., E. Komatsu, T. Sato, and M. Nagai. 2002. Evaluation of efficacy in terms of antibody levels and cell-mediated immunity of acellular pertussis vaccines in a murine model of respiratory infection. FEMS Immunol. Med. Microbiol. 33:219-225. [DOI] [PubMed] [Google Scholar]
- 46.Xing, D. K., R. G. Das, L. Williams, C. Canthaboo, J. Tremmil, and M. J. Corbel. 1999. An aerosol challenge model of Bordetella pertussis infection as a potential bioassay for acellular pertussis vaccines. Vaccine 17:565-576. [DOI] [PubMed] [Google Scholar]
- 47.Zepp, F., M. Knuf, P. Habermehl, J. H. Schmitt, C. Rebsch, P. Schmidtke, R. Clemens, and M. Slaoui. 1996. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect. Immun. 64:4078-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]