Abstract
Many surface epithelial cells express adrenomedullin, a multifunctional peptide found in a wide number of body and cell systems. Recently, we and others have proposed that adrenomedullin has an important novel role in host defense. This peptide has many properties in common with other cationic antimicrobial peptides, including the human β-defensins. Upon exposure of human gastric epithelial cells to viable cells of invasive or noninvasive strains of Helicobacter pylori, Escherichia coli, Salmonella enterica, or Streptococcus bovis, a significant increase in adrenomedullin secretion from these cells was demonstrated. Adrenomedullin gene expression was also increased in response to these microorganisms. Similar observations were noted when these cells were incubated with proinflammatory cytokines such as interleukin 1α (IL-1α), IL-6, tumor necrosis factor alpha and lipopolysaccharide. In cultured cells and an animal infection model, increased adrenomedullin peptide and gene expression was demonstrated when exposed to E. coli or Mycobacterium paratuberculosis, respectively. The data suggest there is a strong association between epithelial infection, inflammation, and adrenomedullin expression, which may have clinical relevance. The regulation of adrenomedullin expression may have therapeutic applications, such as improving or enhancing mucosal immunity.
The epithelium constitutes a critical protective interface between external and internal environments and provides the first line of defense against potentially pathogenic microorganisms. In health, the surfaces of higher eukaryotes such as plants, invertebrates, and vertebrates, including humans, possess a normal microflora, which causes no damage to its host. The reason, apart from physical barriers, includes the production of gene-encoded antimicrobial peptides by epithelial cells (17, 39). Studies at the mRNA level suggest that gene expression for these peptides occurs in a rather tissue- or organ-specific manner, which probably relates to their antimicrobial spectra and conditions of expression, and may also define the local microflora (10, 14, 17, 39). Some epithelial antimicrobial peptides are constitutively expressed; others are inducible either directly by the presence of microorganisms or by endogenous proinflammatory cytokines (4, 10, 17, 39). Lipopolysaccharide (LPS)-activated intracellular signaling pathways, which result in NF-κB-mediated induction of human β-defensin-2 (hBD-2), are initiated through CD14 binding (17) and interactions with Toll-like receptors. The role of bacterial components other than LPS in antimicrobial peptide induction has not been well studied, although Krisanaprakornkit et al. (24) observed that LPS was a poor inducer of hBD-2 synthesis by oral epithelial cells and that the up-regulation seen probably occurred via CD14-independent pathways.
There is increasing evidence that antimicrobial peptides are multifunctional molecules of fundamental importance in host defense, serving as signaling molecules communicating between the innate and adaptive immune systems (10, 17). Similarly, inhibition of antimicrobial peptide expression in amphibians increased infection and reduced the control of commensal organisms (40). In humans and mammals, concentrations of neutrophil and epithelial antimicrobial peptides increase to significant levels following infection (17) or injury (11). Overexpression of the human peptide LL-37 resulted not only in the reduced ability of Pseudomonas aeruginosa to colonize the murine lung epithelium and in reduced inflammation and susceptibility to septic shock (3) but also in increased killing of P. aeruginosa in a cystic fibrosis xenograft model (2).
Adrenomedullin (AM) is a multifunctional peptide (19), and studies from our laboratory and others have shown that AM is expressed in key mucosal surfaces and is emerging as an important effector molecule in host defense (1, 17, 22, 34, 42, 43). AM is found in picomolar concentrations in plasma which rise in conditions such as renal failure, hypertension, and septic shock (19, 35). AM is produced by a wide variety of tissues and cells, and it has been demonstrated to accumulate in the apical regions of the normal human bronchial epithelium, in human skin, and in the skin of the Xenopus laevis toad (15, 19, 23, 31, 32). Cameron and Fleming demonstrated the localization of AM mRNA in epithelial cells lining the uterus, bronchioles, and gastrointestinal tract in mice and rats and also indicated novel roles for AM, possibly as an antimicrobial agent (6). AM has many properties in common with other cationic antimicrobial peptides, including hBD-2. Such molecules are defined as peptides of 12 to 50 amino acids with a net positive charge of +2 to +7 (17). Apart from human AM being 52 amino acid residues in length, AM also has a net positive charge owing to an excess of basic amino acids. Both AM and hBD-2 have an amphipathic design, consisting of spatially separated hydrophobic and charged regions, which permit intercalation with bacterial membranes. Chemically, therefore, AM does resemble an antimicrobial peptide; however, its mode of interaction with bacteria is unknown. AM has 30% homology at the genetic level to the cecropin group of antimicrobial peptides and, therefore, might have a similar pore-forming mechanism of action (7).
It is well documented that circulatory levels of AM are raised in many disease states, particularly in sepsis (36). However, to date, there has not been a thorough study of AM expression in models of microbial infection. We have carried out in vitro and in vivo investigations of AM expression, including the use of intestinal epithelium from cows with bovine paratuberculosis, a chronic inflammatory disease caused by infection of the ileum with Mycobacterium paratuberculosis.
MATERIALS AND METHODS
Organisms and culture conditions.
Helicobacter pylori NCTC 11637 (noninvasive, cagA positive), Escherichia coli E35990/O143 (enteroinvasive), E. coli DH5α (noninvasive), Salmonella enterica serovar Dublin JT738 (enteroinvasive), Streptococcus bovis NCTC 8133 (infant feces, noninvasive), and S. bovis ATCC 9809 (infant feces, noninvasive). All cultures were stored for the long term at −70°C on beads in skim milk (Oxoid L31). For experiments, cultures were used at a density of 2 × 107 CFU/ml in phosphate-buffered saline (PBS).
Cell culture.
The human gastric adenocarcinoma cell line AGS (ATCC CRL 1739) was maintained in T 75-cm2 culture flasks containing RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS) and routine antibiotics in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. Twenty-four hours before experiments were carried out, 70% confluent T 75-cm2 flasks of cells were rendered quiescent by placing them in serum-free medium. On the day of experiments, cells were washed in sterile PBS and incubated with 1 ml of microbial suspension in 2 ml of serum-free medium or with various other agents as described in the text. Flasks were then placed in the incubator for various lengths of time as described in Results.
Measurement of AM.
Flasks of cells were incubated with E. coli E35990, E. coli DH5α, H. pylori, S. enterica serovar Dublin, S. bovis NCTC 8133, or S. bovis ATCC 9809 for 0, 1, 4, 8, or 24 h. After these incubation periods, culture supernatants were harvested and stored at −20°C until assayed for AM by enzyme immunoassay (Phoenix Pharmaceuticals, Inc., Belmont, Calif.) (1). Similar experiments were carried out with interleukin 1α (IL-1α) (20 ng/ml), IL-6 (20 ng/ml), tumor necrosis factor alpha (TNF-α) (20 ng/ml), and LPS (E. coli O111:B4. 2 μg/ml).
RNA analysis.
From the experiments described above, optimum incubation periods were determined. Cells were incubated with the various microorganisms for 1 h or with the cytokines or LPS for 4 h. After these incubation periods, total RNA was extracted and 10 μg of total RNA was electrophoresed in a formaldehyde-1% agarose gel and transferred to a Hybond-N nylon membrane (Amersham). After fixation by UV cross-linking, the membrane was hybridized (and sequentially stripped) overnight at 42°C with [γ-32P]dCTP-labeled AM, hBD-2, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes. Washed blots were exposed to Kodak XAR-5 film. Bands underwent scanning densitometry, and the relative ratio of the net intensities of the AM or hBD-2 and GAPDH bands from the same Northern blot reaction was determined to show AM or hBD-2 mRNA expression in response to different treatments (22). All experiments were carried out in triplicate, and bar graphs represent the results obtained by pooling the data together.
Immunocytochemistry.
Gut epithelial cells were removed by trypsinization from a flask when 80% confluent and counted with a hemocytometer to establish the cell number. They were then added to sterile glass coverslips placed inside 24-well microplates at a density of 40,000 cells/well. They were left with 3 ml of medium for 1 day at 37°C to adhere to the coverslips. After this period, medium was aspirated and the cells were washed three times with PBS. One milliliter of E. coli (E35990 or DHα) cell suspension was added to the coverslips and left to incubate for 4 h. After this period, the cells were washed and fixed with 3.7% paraformaldehyde (pH 7.4) for 15 min at 4°C and then permeabilized with 1% Triton X-100 for 5 min at room temperature. Nonspecific sites were blocked with normal rabbit serum, and AM was detected by using a polyclonal anti-rabbit antibody with a secondary biotinylated anti-rabbit immunoglobulin G antibody. The bound antibodies were visualized with vector red (Vector Laboratories, Peterborough, United Kingdom), and coverslips were lightly counterstained with Mayer's hematoxylin. Primary AM antibody, raised in-house (35), was preabsorbed to the antigen it was raised against and served as a negative control. Photographs were taken of the stained cells.
In situ hybridization.
In situ hybridization was performed as described previously (21). The full-length AM cDNA was ligated into the expression vector pcDNA1 and used to generate digoxigenin-labeled sense and antisense riboprobes. Tissue sections were hybridized in a humidified chamber at 46°C for 20 h in a 20-μl volume containing 2.5 ng of probe/μl. Visualization of probes was performed with a digoxigenin detection kit (Roche Diagnostics Ltd., Lewes, United Kingdom).
Western blotting.
Cells were homogenized in a lysis buffer containing 50 mM NaCl, 25 mM Tris-Cl (pH 8.1), 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 10 μg of pepstatin/ml, and 25 μg of aprotinin/ml. The homogenate was centrifuged at 10,000 × g, and the resulting supernatant was used for Western blot analysis. Twenty micrograms of protein was heated to 99°C for 4 min, loaded into sample wells, resolved on a 10 to 20% Tricine sodium dodecyl sulfate-polyacrylamide gel (Novex, San Diego, Calif.), and run at 120 V for 2 h. Transfer blotting was accomplished by using the same apparatus, and proteins were transferred to a polyvinylidene difluoride membrane (Immobilon; Millipore, Watford, United Kingdom) at 30 V for 4 h. Membranes were blocked overnight at 4°C in a solution of 5% dried milk in PBS containing 0.1% Tween 20. Membranes were then washed, incubated for 60 min at room temperature in a 1:100 dilution of rabbit anti-AM polyclonal antibody, washed three times in PBS, and incubated in 1:200 goat anti-rabbit biotinylated immunoglobulin G (Vector Laboratories) for 60 min at room temperature. Membranes were washed three times in PBS, and the signal was amplified and detected by using enhanced chemiluminescence following the manufacturer's instructions (Amersham Pharmacia Biotech).
Collection of paratuberculosis-infected tissue.
Paratuberculosis-infected tissue was collected from two cows testing positive for M. paratuberculosis by cultures of feces and ileum samples. Paratuberculosis-free tissue was obtained from two cows from herds certified to be negative for the disease.
Statistical analysis.
Arithmetic means and standard errors of the mean were calculated. One-way analysis of variance was used to test whether factors had an effect on basal (control) levels of AM expression with the SPSS (version 6.1) statistical software package.
RESULTS
Cytokines, LPS, and microorganisms increase AM production from AGS cells.
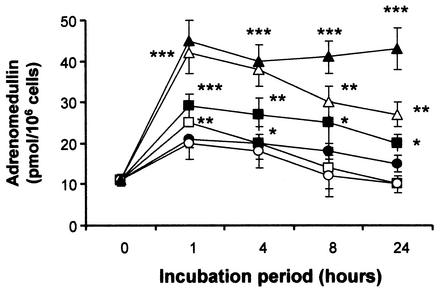
Figures 1 and 2 show that these cells constitutively secrete AM since unstimulated levels of AM were about 12 pmol/106 cells. Figure 1 illustrates the effects of LPS and various cytokines on AM secretion from the gut epithelial cell line AGS. It shows that all agents tested, LPS (2 μg/ml), IL-1α (20 ng/ml), IL-6 (20 ng/ml), and TNF-α (20 ng/ml), caused significant increases in AM production with time. Exposure to these agents for 4 h proved to be the optimum time point in terms of AM response, with LPS and TNF-α being the most potent stimulators of AM production, causing a sixfold increase in AM release. Figure 2 shows the effects of E. coli E35990, E. coli DH5α, H. pylori, S. enterica serovar Dublin, S. bovis NCTC 8133, and S. bovis ATCC 9809 on AM production from these cells. It can be seen that significant levels of AM were secreted from the gut epithelial cells in response to all microorganisms tested. Cells were exposed over a period of 24 h, but as can be seen from Fig. 2, a 1-h exposure was enough to elicit a maximum, significant production of AM. Incubation with H. pylori caused the most significant (4.5-fold) response followed by E. coli E35990 and E. coli DH5α, with S. enterica serovar Dublin and the 2 Streptococcus spp. being the least effective (increases between 1- to 2- to 3-fold).
FIG. 1.
AM secretion from human AGS gut epithelial cells after exposure to LPS (2 μg/ml, open circles), IL-1α (20 ng/ml, open squares), IL-6 (20 ng/ml, filled squares), or TNF-α (20 ng/ml, filled circles) for various lengths of time. Values are means ± standard errors of the means (n = 3). **, P < 0.01; ***, P < 0.001 compared to nonexposure (by analysis of variance).
FIG. 2.
AM secretion from human AGS gut epithelial cells after exposure to a 2 × 107-CFU/ml dose of E. coli E35990 (open triangles), E. coli DH5α (filled squares), H. pylori (filled triangles), S. enterica serovar Dublin (open squares), S. bovis NCTC 8133 (open circles), or S. bovis ATCC 9809 (filled circles) after different lengths of time. Values are means ± standard errors of the means (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to nonexposure (by analysis of variance).
Exposure to cytokines, LPS, and microorganisms caused increased expression of AM and hBD-2 genes.
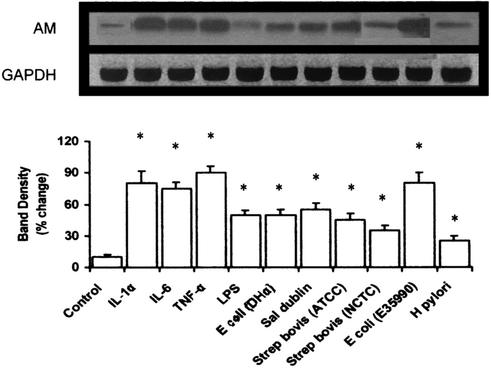
Northern blot analysis was used to study the effect of cytokines, LPS, and the pathogens on AM gene mRNA levels. Figure 3 shows that AM mRNA is constitutively expressed in these cells. Exposure for 4 h to IL-1α, IL-6, TNF-α (all at 20 ng/ml), or LPS (2 μg/ml) elicited a similar level of increased AM gene expression, with LPS causing the least degree of augmentation. Figure 3 also shows that a 1-h exposure to all the microorganisms used in this study resulted in significant increases in AM mRNA expression. The invasive species of E. coli used here was particularly potent in stimulating AM gene levels.
FIG. 3.
AM mRNA expression in human AGS gut epithelial cells after exposure to cytokines, LPS, or pathogens. Representative Northern blot image (upper panel) of AM mRNA levels after treatment with LPS (2 μg/ml), IL-1α (20 ng/ml), IL-6 (20 ng/ml) or TNF-α (20 ng/ml) for 4 h or a 2 × 107-CFU/ml dose of E. coli E35990, E. coli DH5α, H. pylori, S. enterica serovar Dublin, S. bovis NCTC 8133, or S. bovis ATCC 9809 for 1 h. Lower panel, graph showing relative intensity of AM mRNA expression after scanning densitometry and normalization to GAPDH expression. Values are means ± standard errors of the means (n = 3). *, P < 0.05 compared to nonexposure (by analysis of variance).
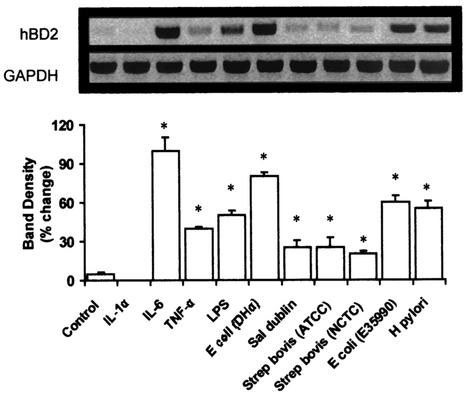
Figure 4 illustrates the expression of hBD-2 mRNA in gut epithelial cells. The gene was expressed constitutively, albeit at a very weak level; however, upon exposure to all microorganisms used in this study, there was significant induction of hBD-2. The only exception to this observation was to the cytokine IL-1α; there appeared to be no increase of the hBD-2 gene in AGS cells upon exposure to IL-1α, if anything there may be a reduction in gene expression.
FIG. 4.
hBD-2 mRNA expression in human AGS gut epithelial cells after exposure to cytokines, LPS, and live pathogens. Representative Northern blot image (upper panel) of hBD-2 mRNA expression after treatment with LPS (2 μg/ml), IL-1α (20 ng/ml), IL-6 (20 ng/ml), or TNF-α (20 ng/ml) for 4 h or a 2 × 107-CFU/ml dose of E. coli E35990, E. coli DH5α, H. pylori, S. enterica serovar Dublin, S. bovis NCTC 8133, or S. bovis ATCC 9809 for 1 h. Lower panel, graph showing the relative intensity of hBD-2 mRNA expression after scanning densitometry and normalization to GAPDH levels. Values are means ± standard errors of the means (n = 3). *, P < 0.05 compared to nonexposure (by analysis of variance).
AGS cells produce AM in response to E. coli.
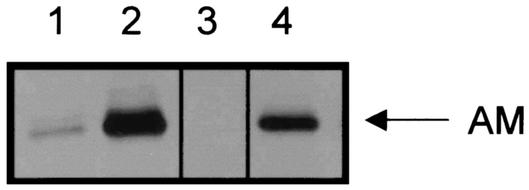
When gut epithelial cells from the AGS cell were serum deprived, AM levels were barely detectable by immunocytochemical techniques as demonstrated in Fig. 5B. When these cells were incubated in the presence of either the invasive (E35990) or noninvasive (DHα) strains of E. coli for 4 h there was positive staining for AM peptide as illustrated in Fig. 5C and D, respectively. Figure 5A represents AGS cells stained in the presence of preabsorbed primary antibody (negative control). This experiment was repeated for Western blot analysis. Figure 6 illustrates more clearly that there appears to be a difference in the levels of AM protein expressed by these cells when exposed to either invasive or noninvasive species of E. coli. Lane 1 shows that there is constitutive expression of AM protein, which is eliminated when the antibody was preabsorbed to a blocking peptide (lane 3). Lanes 2 and 4 are immunoblots for AM peptide from AGS cells exposed to E. coli E35990 or DHα, respectively. There is a large increase in protein expression in response to either microorganism with the invasive species of E. coli (E35990) having the greater effect.
FIG. 5.
Immunostaining for AM in AGS cells. Photographs represent the effect of exposure or nonexposure of the gut epithelial cells to either species of E. coli used in our studies. (A) Preabsorbed primary antibody (negative control) on nontreated cells; (B) nontreated cells; (C) cells exposed to 2 × 107 CFU of E. coli E35990/ml for 1 h; (D) cells exposed to 2 × 107 CFU of E. coli DH5α/ml. Antibody was used at a concentration of 1:100. Cells were fixed and stained as described in Materials and Methods. Magnification, ×56 for panels A, C, and D, and ×100 for panel B.
FIG. 6.
Western blotting for AM peptide in AGS cells. Cells were exposed to either the invasive or noninvasive species of E. coli for 1 h, and cell extracts were immunoblotted for AM. Lane 1, nontreated cells; lane 2, cells exposed to E. coli E35990; lane 3, preabsorbed primary antibody (negative control) on nontreated cells; lane 4, cells exposed to E. coli DH5α.
Increased expression of AM in an animal model of infection.
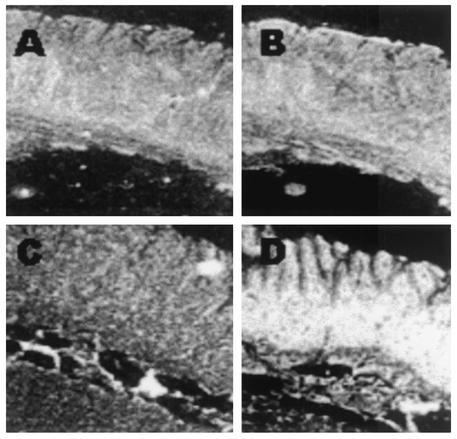
Figure 7 illustrates the expression of AM in the ileum of paratuberculosis-infected cattle. Hybridization of an AM antisense probe revealed a high signal in an area corresponding to the mucosal epithelium (Fig. 7D). Hybridization with ileum derived from noninfected cows showed relatively little signal (Fig. 7B). Examination of AM expression in gastrointestinal epithelium revealed significantly higher mRNA levels in infected versus noninfected cows. Figures 7A and C are control hybridizations with sense AM probes.
FIG. 7.
Expression of AM in ileal tissue of M. paratuberculosis-infected cows. Serial sections of a portion of the ileum of an infection-free cow (A and B) and an infected cow (C and D). A and C, in situ hybridization with sense AM probe; B and D, in situ hybridization with antisense AM probe. Magnification, ×20.
DISCUSSION
The data presented in this study show that gut epithelial cells secrete AM basally and there is a significant increase in production upon exposure to a number of gastric pathogens. In addition, it is clear that the ability to stimulate AM production in these gut epithelial cells is not limited to whether bacteria are gram positive or negative or due to their invasive ability.
If AM is to be considered a peptide antibiotic, then it is important to demonstrate its induction, or increased expression, upon contact with different microorganisms. In the presence of the gastric pathogens H. pylori, enteroinvasive and noninvasive E. coli, S. enterica serovar Dublin, and S. bovis, significant upregulation of AM expression in human gastric epithelial cells was demonstrated. Such interactions between bacteria and AM may be important in selecting the diverse range of microorganisms colonizing the gastric mucosa and other sites and in regulating the pathogenic potential of some organisms.
In addition, and perhaps of greater importance, we have shown that AM is greatly enhanced in disease states. Examination of AM mRNA expression in gut epithelium is increased in infected animals compared to noninfected ones. We investigated this in cows suffering from bovine paratuberculosis, a chronic inflammatory disease caused by infection of the ileum with M. paratuberculosis. Clinical signs of the disease include weight loss, muscle wastage, and chronic diarrhea (8). Tissues obtained from these animals also demonstrated characteristics indicating chronic infection such as the presence of inflammatory cells and mucosal thickening (data not shown). Many giant and epithelial cells often filled with mycobacteria are shed into the intestinal lumen, particularly during the end stages of the disease. Like LPS, components of the mycobacterial cell wall might directly stimulate epithelial cells to produce AM. However, not all microorganisms used in this study contain LPS, and it is clear that increased AM protein and mRNA expression is not LPS dependent but probably involves other mechanisms. On the other hand, mycobacteria could influence gut epithelial AM expression indirectly via inflammatory cytokine production, as demonstrated in other cells. To lend support for the latter hypothesis, we have demonstrated that there is an increase in AM expression in AGS gastric epithelial cells in response to inflammatory mediators. This is an observation seen in skin and oral epithelial cells as well as endothelial and vascular smooth muscle cells (23, 41).
Until recently, the mucosal lining was regarded as a simple physical barrier preventing bacterial invasion and the escape of body fluids. However, it is clear that epithelial cells are highly dynamic cells with the ability to react to changes within their environment. They can interact with other cells and are important during processes such as inflammation and wound healing (26, 27), and they respond by secreting a range of growth factors and cytokines (28, 29). In contrast to the specific immune system, intestinal mucosal cells have developed several nonspecific defense systems for protection against enteropathogens (9, 20, 25, 30). The epithelial cells of intestinal crypts express many antimicrobial agents, such as defensins. However, Marutsuka and coworkers (34) demonstrated that immunoreactive AM was present in surface epithelia of the human colon. Localization of AM to this surface, in combination with its antimicrobial activities, lends strong evidence for its role in mucosal defense (1, 33, 36, 43), and we suggest that AM might be part of a panel of bactericidal peptides in the intestinal mucosa. Although the various antimicrobial peptides and proteins may differ in their molecular specificities and modes of action, they may act cooperatively by attacking multiple essential structures in bacteria or by unmasking structures vulnerable to other components of the mixture. The multiplicity of antimicrobial factors may also broaden the effectiveness of the mixture against the many potential microbial targets and decrease the likelihood of acquired resistance.
Similar studies with hBD-2 have been reported; this was, therefore, used as a control for our studies. Defensins are antimicrobial components of the innate host defense system in higher mammals (10, 14, 17, 39). We and others have shown that hBD-2 expression can be induced at mucosal sites (5, 22, 24, 37). When we studied hBD-2 mRNA levels in AGS cells, we found that these expressed hBD-2 constitutively, albeit at a low level (Fig. 4). This observation supports those found by O'Neill and coworkers (37). This may, of course, be a reflection of using a cell line, since hBD-2 in healthy gastric mucosa is not detectable by reverse transcription-PCR (16). However, a low level of hBD-2 mRNA is present in healthy epithelia, and its expression is greatly upregulated by inflammatory stimuli (18).
The association between epithelial infection, inflammation, and AM expression may have clinical relevance (12, 13, 33, 38). Elsasser and coworkers have suggested that AM has a role in disease stress. In their study they concluded that chronic low-level infection potentiated the severity of metabolic perturbations that arise with additive immune challenge, as can occur with bacterial toxins, and that AM could act as a potential marker for this phenomenon (13). The results described here support a role for AM as an important host effector molecule that is quickly mobilized by the epithelium upon injury. The response appears to be local in nature and may be occurring independently of circulating defending cells. This hypothesis is currently under investigation in our laboratory. These results have important implications in terms of diagnosis and treatment of disease. Production of antimicrobial peptides during host invasion could be used as a local marker of infection sites and subsequent diagnosis of disease. Additionally, regulating the expression of AM, which is not susceptible to existing mechanisms of resistance, may be useful in the prevention and treatment of disease.
Acknowledgments
We thank the Royal Society and The Oral and Dental Research Trust, London, United Kingdom, for grant support.
REFERENCES
- 1.Allaker, R. P., C. Zihni, and S. Kapas. 1999. Antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol. Med. Microbiol. 23:289-293. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R., D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Investig. 103:1113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banyer, J. L., N. H. Hamilton, I. A. Ramshaw, and A. J. Ramsay. 2000. Cytokines in innate and adaptive immunity. Rev. Immunogenet. 2:359-373. [PubMed] [Google Scholar]
- 5.Bonass, W. A., A. S. High, P. Owen, and D. A. Devine. 1999. Expression of β-defensin genes in human salivary glands. Oral Microbiol. Immunol. 14:371-374. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, V. A., and A. M. Fleming. 1998. Novel sites of adrenomedullin gene expression in mouse and rat tissues. Endocrinology 139:2253-2264. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, B., J. Fink, R. B. Merrifield, and D. Mauzerall. 1988. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Natl. Acad. Sci. USA 85:5072-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocito, C., P. Gilot, M. Coene, M. de Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunliffe, R. N., and Y. R. Mahida. 2000. Antimicrobial peptides in innate intestinal host defence. Gut 47:16-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine, D. A., and R. E. W. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:99-110. [DOI] [PubMed] [Google Scholar]
- 11.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 12.Elsasser, T. H., and S. Kahl. 2002. Adrenomedullin has multiple roles in disease states: development and remission of the inflammatory response. Microsc. Res. Tech. 57:120-129. [DOI] [PubMed] [Google Scholar]
- 13.Elsasser, T. H., J. L. Sartin, A. Martínez, S. Kahl, L. Montuenga, L. Pío, R. Fayer, M.-J. Miller, and F. Cuttitta. 1999. Underlying disease stress augments plasma and tissue adrenomedullin (AM) responses to endotoxin: colocalized increases in AM and inducible nitric oxide synthase within pancreatic islets. Endocrinology 40:5402-5411. [DOI] [PubMed] [Google Scholar]
- 14.Ganz, T., and R. I. Lehrer. 1999. Antibiotic peptides from higher eukaryotes: biology and applications. Mol. Med. Today 5:292-297. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez, A., O. Marin, C. Sanchez-Camacho, J. J. Pena, E. Zudaine, A. Martinez, F. Cuttitta, and M. Munoz. 1998. Localization of adrenomedullin-like immunoreactivity in the hypothalamo-hypophysial system of amphibians. Neurosci. Lett. 242:13-16. [DOI] [PubMed] [Google Scholar]
- 16.Hamanaka, Y., M. Nakashima, A. Wada, M. Ito, H. Kurazono, H. Hojo, Y. Nakahara, S. Kohno, T. Hirayama, and I. Sekine. 2001. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 49:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 18.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 19.Hinson, J. P., S. Kapas, and D. M. Smith. 2000. Adrenomedullin, a multifactorial paracrine regulator. Endocrinol. Rev. 21:138-167. [DOI] [PubMed] [Google Scholar]
- 20.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 21.Kapas, S., A. Martínez, F. Cuttitta, and J. P. Hinson. 1998. Local production and action of adrenomedullin in the rat adrenal zona glomerulosa. J. Endocrinol. 156:477-484. [DOI] [PubMed] [Google Scholar]
- 22.Kapas, S., A. Bansal, V. Bhargava, R. Maher, D. Malli, E. Hagi-Pavli, and R. P. Allaker. 2001. Adrenomedullin expression in pathogen-challenged oral epithelial cells. Peptides 22:1485-1489. [DOI] [PubMed] [Google Scholar]
- 23.Kapas, S., M. Tenchini, and P. M. Farthing. 2001. Regulation of adrenomedullin secretion in cultured human skin and oral keratinocytes. J. Investig. Dermatol. 117:353-359. [DOI] [PubMed] [Google Scholar]
- 24.Krisanaprakornkit, S., J. R. Kimball, A. Weinberg, R. P. Darveau, B. W. Bainbridge, and B. A. Dale. 2000. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 26.Li, J., P. M. Farthing, and M. H. Thornhill. 1996. Cytokine regulation of major histocompatibility complex antigen expression by human oral and skin keratinocytes. Arch. Oral Biol. 41:533-538. [DOI] [PubMed] [Google Scholar]
- 27.Li, J., P. M. Farthing, G. W. Ireland, and M. H. Thornhill. 1996. IL-1 alpha and IL-6 production by oral and skin keratinocytes: similarities and differences in response to cytokine treatment in vitro. J. Oral Pathol. Med. 25:157-162. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., G. W. Ireland, P. M. Farthing, and M. H. Thornhill. 1996. Epidermal and oral keratinocytes are induced to produce RANTES and IL-8 by cytokine stimulation. J. Investig. Dermatol. 106:661-666. [DOI] [PubMed] [Google Scholar]
- 29.Li, J., D. L. Mahiouz, P. M. Farthing, D. O. Haskard, and M. H. Thornhill. 1996. Heterogeneity of ICAM-1 expression, and cytokine regulation of ICAM-1 expression, in skin and oral keratinocytes. J. Oral Pathol. Med. 25:112-118. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald, T. T., and S. Pettersson. 2000. Bacterial regulation of intestinal immune responses. Inflamm. Bowel Dis. 6:112-122. [DOI] [PubMed] [Google Scholar]
- 31.Martínez, A., M. J. Miller, E. J. Unsworth, J. M. Siegfried, and F. Cuttitta. 1995. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 136:4099-4105. [DOI] [PubMed] [Google Scholar]
- 32.Martínez, A., T. H. Elsasser, C. Muro-Cacho, T. W. Moody, M. J. Miller, C. J. Macri, and F. Cuttitta. 1997. Expression of adrenomedullin and its receptor in normal and malignant human skin: a potential pluripotent role in the integument. Endocrinology 138:5597-5604. [DOI] [PubMed] [Google Scholar]
- 33.Martínez, A., G. S. Sidhu, R. K. Maheshwari, and F. Cuttitta. 1999. Adrenomedullin involvement in wound repair, p. A1526. In Proceedings of the 81st Annual Meeting of the Endocrine Society. The Endocrine Society, San Diego, Calif.
- 34.Marutsuka, K., Y. Nawa, Y. Asada, S. Hara, K. Kitamura, T. Eto, and A. Sumiyoshi. 2001. Adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp. Physiol. 86:543-545. [DOI] [PubMed] [Google Scholar]
- 35.Müller, F. B., S. Müller-Röver, B. P. Korge, D. B. Holland, W. J. Cunliffe, I. A. Mckay, S. Kapas, J. P. Hinson, and M. P. Philpott. 2003. Adrenomedullin: expression and possible role in human skin and hair growth. Br. J. Dermatol. 148:30-38. [DOI] [PubMed] [Google Scholar]
- 36.Nishio, K., Y. Akai, Y. Murao, N. Doi, S. Ueda, H. Tabuse, S. Miyamoto, K. Dohi, N. Minamino, H. Shoji, K. Kitamura, K. Kangawa, and H. Matsuo. 1997. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit. Care Med. 25:953-957. [DOI] [PubMed] [Google Scholar]
- 37.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 38.Renshaw, D., J. P. Hinson, and S. Kapas. 2002. Adrenomedullin stimulation of cytokines and growth factor release in endothelial cells and skin fibroblasts: a possible role in wound healing. J. Endocrinol. 3:P79. [Google Scholar]
- 39.Schrõder, J. M. 1999. Epithelial peptide antibiotics. Biochem. Pharmacol. 57:121-134. [DOI] [PubMed] [Google Scholar]
- 40.Simmaco, M., M. L. Mangoni, A. Boman, D. Barra, and H. G. Boman. 1998. Experimental infections of Rana esculenta with Aeromonas hydrophila: a molecular mechanism for the control of the normal flora. Scand. J. Immunol. 48:357-363. [DOI] [PubMed] [Google Scholar]
- 41.Sugo, S., N. Minamino, H. Shoji, K. Kangawa, K. Kitamura, T. Eto, and H. Matsuo. 1995. Interleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 207:25-32. [DOI] [PubMed] [Google Scholar]
- 42.Walsh, T. J., A. Martinez, J. Peter, et al. 1998. Antimicrobial activity of adrenomedullin and its gene-related peptides. Clin. Infect. Dis. 23:P96. [Google Scholar]
- 43.Welsch, U., P. Unterberger, E. Hofter, F. Cuttitta, and A. Martinez. 2002. Adrenomedullin in mammalian and human skin glands including the mammary gland. Acta Histochem. 104:65-72. [DOI] [PubMed] [Google Scholar]