Abstract
The measurement of antibodies to hepatitis E virus (anti-HEV) has been essential for understanding the epidemiology of hepatitis E. Studies to determine the prevalence of HEV infections require a reliable serologic assay that is sensitive and specific. It is also important to distinguish the acute from the convalescent phase of an infection; this usually requires the detection of the immunoglobulin M (IgM) class of antibody. Few enzyme immunoassays (EIAs) that measure IgM anti-HEV have been described, and most have utilized the sandwich method. The present study describes an EIA that detects IgM anti-HEV by antibody class capture methodology. The assay was validated by using serum and/or plasma panels from experimentally infected nonhuman primates. It was used to demonstrate an anamnestic response and the reappearance of IgM anti-HEV in a chimpanzee experimentally challenged with HEV at two different times 45 months apart. The class capture method was more sensitive than the sandwich EIA when used to test clinical samples from two hepatitis E epidemics in Pakistan; it also had the advantage of distinguishing IgM anti-HEV in the presence of high titers of IgG anti-HEV.
The existence of an enterically transmitted non-A, non-B hepatitis virus was suggested in the 1980s when sensitive serologic assays for hepatitis A excluded hepatitis A virus (HAV) as the etiological agent of waterborne epidemics of hepatitis in India (17, 41). The existence of a new virus was confirmed when a human volunteer immune to hepatitis A developed clinical hepatitis 36 days after ingesting diluted fecal material pooled from nine patients with non-A hepatitis (1). Both the virus and the antibody to it were detected when fecal samples collected from the volunteer were mixed with his convalescent-phase serum and examined by immune electron microscopy. This virus was subsequently designated the hepatitis E virus (HEV). Two major epidemiological differences distinguish HEV infection from HAV infection. First, in countries where both diseases are endemic, seroconversion to HAV usually occurs in young children, whereas seroconversion to HEV occurs mainly in young adults between the ages of 15 and 40. Second, hepatitis E presents a greater risk of fatality in pregnant women, at least in some countries (3, 12, 18-20, 33, 37).
Epidemics of hepatitis E have been reported primarily in developing regions of Africa, the Middle East, and Southeast and Central Asia; one epidemic occurred in North America (Mexico) (3, 4, 6, 14, 17, 39-41). Although sporadic HEV infections have occurred in industrialized nations, there is an unexpectedly high prevalence of antibodies to HEV (anti-HEV) (as high as 21.3%) among blood donors in the United States, where hepatitis E is not endemic (35). In addition, HEV was isolated from swine in the United States and transmitted to nonhuman primates (25, 26). Anti-HEV was found to be ubiquitous in rodents as well as swine (9, 13, 24), suggesting that the virus could possibly be transmitted zoonotically (2, 15, 23). The higher-than-expected prevalence of anti-HEV in industrialized nations and the possibility of zoonotic transmission of the virus suggest that unanswered epidemiological questions still exist. The success of future investigations will greatly depend on the availability of assays that are sensitive and specific for HEV.
The hepatitis E virus genome is a 7.2-kb, positive-sense, single-stranded RNA. It has three open reading frames (ORFs): ORF1 encodes nonstructural proteins, ORF2 encodes the capsid protein, and ORF3 encodes a cytoskeleton-associated phosphoprotein (30, 38, 43). Early immune electron microscopy studies demonstrated serologic cross-reactivity among HEV virions from different regions of the world (4, 21). Later, recombinant peptides expressed from ORF2 and ORF3 of the Mexican strain of HEV were shown to react with sera collected from outbreaks of hepatitis E in Pakistan, Burma, Borneo, the USSR, and Somalia (42). The existence of these so-called type-common epitopes provided the basis for the development of immunoassays that broadly react with antibodies against different HEV strains.
In the early stages of its discovery, hepatitis E was distinguished from hepatitis caused by other viruses by serologic exclusion (non-A, non-B) and absence of parenteral exposure (18, 34). Early serologic assays specific for HEV used antigens based on the sequence of the Burmese strain (29). Unfortunately, the use of different forms of the HEV antigen in different assay systems has resulted in enzyme immunoassays (EIAs) with varied sensitivities and specificities. HEV antigens used in EIAs have differed in composition (recombinant or synthetic), viral strain (Pakistani, Burmese, or Mexican), and viral gene product (ORF2 or ORF3). Furthermore, at least two expression systems (Escherichia coli and baculovirus) have been employed for producing recombinant antigens. A blinded study of 12 different serologic assays showed that recombinant proteins were more sensitive than synthetic antigens for detecting HEV immunoglobulin G (IgG) antibodies (22). An analysis of IgM-specific EIAs indicated very high concordance when similar antigens produced in E. coli and insect cells were compared (concordance, 96.3%; kappa, 0.87) (11). The same study showed that ORF2-based EIAs were significantly more sensitive than ORF3-based EIAs.
Here, we describe a class capture EIA to detect IgM anti-HEV. This assay uses a baculovirus-expressed recombinant protein from the ORF2 region of the Sar-55 strain of HEV.
MATERIALS AND METHODS
Samples.
(i) Negative control blood samples.
Negative control blood samples included 84 random blood donor samples from the United States, 17 chimpanzee samples positive for HAV IgM as measured by a commercial assay (Havab-M EIA; Abbott), 3 chimpanzee samples positive for HBV core IgM as measured by a commercial assay (Corzyme-M [ribosomal DNA] EIA; Abbott), 5 chimpanzee samples positive for antibodies to HCV (HCV EIA 2.0; Abbott), 20 samples from patients previously diagnosed with an autoimmune disease (Millennium Biotech, Inc., Ft. Lauderdale, Fla.), and 20 patient samples containing rheumatoid factor (Millennium Biotech, Inc.).
(ii) Seroconversion panels.
Two HEV-seronegative chimpanzees (CH1374 and CH1375) were intravenously inoculated with 0.5 ml of 10% feces containing 106 monkey infectious doses of a Pakistani strain (Sar-55) of HEV (36). A serum sample and/or a plasma sample was drawn prior to inoculation and weekly thereafter for 16 weeks. Both animals were infected with HEV and developed IgG anti-HEV at the expected time. Panels of serial serum and/or plasma samples from the chimpanzees were used to design and optimize the assay for IgM anti-HEV. Similarly, two rhesus monkeys (RH H461 and RH H572) were intravenously inoculated with 0.5 ml of a dilution of feces containing 104 and 106 monkey infectious doses, respectively, of the Mexican strain of HEV. For these seroconversion panels, one preinoculation sample and nine weekly postinoculation samples were drawn.
(iii) Paired sera from two epidemics.
Fifteen paired sera from a hepatitis E epidemic in Sargodha, Pakistan (6), and eight paired sera from a hepatitis E epidemic in Abbottabad, Pakistan (5), were retested for IgG anti-HEV by using the sandwich method and for IgM anti-HEV by using the class capture method. For both epidemics, the first group of samples was drawn during the epidemic. The second group of Sargodha samples was drawn 19 to 20 months after the first collection, and the second group of the Abbottabad samples was drawn 3 to 4 months after the first collection. These samples had been tested previously for anti-HEV with a sandwich enzyme-linked immunosorbent assay (ELISA) also based on baculovirus-expressed ORF2 (5, 6). Selection of the samples was based on the following criteria: (i) a sufficient volume was available, (ii) the sample drawn at the time of the epidemic was positive for IgM anti-HEV (sandwich EIA) in the original study, and (iii) the second sample of the pair was negative for IgM anti-HEV (sandwich EIA).
(iv) Sequential inoculation of CH1313.
Chimpanzee CH1313 was intravenously inoculated twice with HEV, first with the Pakistani strain and then with the Mexican strain 45 months later (36). The sample series used in this study consisted of a sample drawn before the second inoculation (45 months after the first inoculation) and six subsequent weekly samples.
Optimization.
Optimal concentrations were determined for each reagent of the test (starting from the solid phase to the detection phase of the assay), based on results from checkerboard titrations.
Preparation of goat anti-human IgM (μ chain specific) capture plate.
A 96-well streptavidin-coated polystyrene plate (Pierce 15125) was inoculated with 100 μl (0.1 μg) of biotin-conjugated goat μ chain-specific anti-human IgM (Pierce 31778) in carbonate-bicarbonate buffer. The plate was incubated overnight (18 to 22 h) at room temperature (18 to 22°C), washed twice with KPL wash solution (0.02% Tween 20 [Fisher BP337-500] in 0.002 M imidazole-buffered saline [KPL 50-63-00]), and then blocked by adding 120 μl of gelatin solution (0.5% gelatin, 1% goat normal serum [KPL 71-00-27], and 0.05% Tween 20 in phosphate-buffered saline [PBS]) before incubation at 37°C for 1 h.
Class capture IgM anti-HEV assay.
The blocked plate was washed twice with wash solution (KPL). Samples of test and control sera or plasma were diluted to 1:100 with gelatin solution, and 100 μl of sample or control was inoculated into assigned wells. The plate was incubated at 37°C for 1 h and washed five times with the KPL wash solution. One hundred microliters of HEV recombinant 56-kDa antigen, expressed from ORF2 of the Sar-55 strain of HEV in insect cells and purified as described previously (31), was added to each well at a concentration of 2 to 4 μg/ml depending on the titration of each lot. The plate was incubated overnight at room temperature and washed five times with KPL wash solution. One hundred microliters of a 1:1,000 dilution of mouse monoclonal anti-ORF2 (generously provided by GlaxoSmithKline) was added to each well. The plate was incubated at 37°C for 30 min and washed five times with KPL wash solution. One hundred microliters of peroxidase-labeled goat anti-mouse IgG [γ F(ab′)2] conjugate (0.25 μg/ml) per well was added. The plate was incubated at 37°C for 30 min and washed five times prior to the color development step. One hundred microliters of ABTS substrate [0.3 g of 2,2′-azino-di(2-ethyl-benzthiazoline-6-sulfonate) per liter with 0.01% H2O2 in glycine-citric acid buffer (KPL 50-66-000)] was added to each well. The absorbance was read at 405 nm for 40 min at 10-min intervals.
Characterization of the assay.
(i) Linearity.
The analytical response curve was determined by results obtained from twofold serial dilutions of a sample with high levels of IgM anti-HEV.
(ii) Intra-assay precision.
Three separate samples that represented three levels of IgM anti-HEV (low-positive, mid-positive, and high-positive levels) were tested in a microtiter plate (for each level, n = 28).
(iii) Interassay precision.
Three samples were diluted 1:100, frozen at −70°C in 120-μl aliquots, and tested in separate microtiter plates (for each level, n = 12). Means, standard deviations (SDs), and coefficients of variation (CVs) were calculated.
Quality control.
Three levels of quality control were used to evaluate each test plate. A negative specimen was used as the negative control. Low-positive and intermediate-positive precision study samples were used as quality controls, and the interassay precision data were used to determine the acceptable ranges. Control parameters were set at 2.5 SDs from the mean. The acceptability of each plate (reading) was decided according to the quality control results.
Development of a cutoff equation.
First, samples presumed to be negative for IgM anti-HEV were tested with the class capture assay. Using the raw optical densities (ODs) of these samples, a target cutoff value above the mean was arbitrarily set. Second, a negative sample and a positive sample were chosen based on the seroconversion panel of CH1375. Third, a cutoff equation was derived to approximate the target cutoff OD based on the negative and positive samples. These samples were included with each plate.
Quantification of the assay.
The World Health Organization (WHO) HEV antibody standard (constructed from plasmapheresis units obtained at intervals during the early to late convalescence of a patient with hepatitis E [see below]) (10) was tested in a twofold dilution series for IgM anti-HEV according to the protocol for the class capture IgM anti-HEV assay.
Sandwich IgM anti-HEV.
Data from the IgM anti-HEV sandwich EIA were published previously (36).
Sandwich IgG anti-HEV.
The sandwich EIA for IgG anti-HEV was modified from that described by Tsarev et al. (36). One hundred microliters of HEV recombinant 56-kDa antigen (Sar-55 ORF2) (0.25 to 0.50 μg/ml) was incubated in each well of a polystyrene plate (Falcon 353228) overnight at room temperature. After being washed twice, the wells were blocked with 120 μl of gelatin solution. The plate was then incubated at 37°C for 1 h. Next, the plate was washed and the sample, diluted 1:100 in gelatin solution, was added. The plate was incubated at 37°C for 30 min, washed, and then incubated with peroxidase-labeled goat anti-human IgG (heavy plus light chains) at 1 μg/ml (KPL 074-1006) for 30 min at 37°C. After washing, 100 μl of ABTS substrate was added per well, and the absorbance was read at 405 nm after 10 min. The cutoff was determined by comparison with the OD of 0.010 U of WHO standard (95/584) per ml, which was provided by Morag Ferguson, National Institute of Biological Standards and Control, Hertfordshire, England (10). Results were reported as the quotient of the OD signal and the cutoff.
Inactivation of IgM by treatment with reducing agent.
Samples were mixed with an equal volume of 0.01 M dithiothreitol (DTT) and incubated at 37°C for 2 h to destroy IgM reactivity (27, 28). As a control, PBS-treated samples were tested in parallel. Samples were tested for IgM anti-HEV with the class capture assay and for IgG anti-HEV with the sandwich assay.
RESULTS
Characterization of the assay.
Multiple determinations were performed with the same sample on the same and on different plates in order to determine the reproducibility of the system. An unacceptably high intra-assay precision (mean CV, 13%) with other polystyrene plates prompted the use of a protein avidin-biotin capture system. With this system, the intra-assay precision (CV) improved to less than 5%, with an interassay precision of less than 7% (Table 1). At the 405-nm wavelength, the absorbance of the assay was linear up to a value of 1.6 (data not shown).
TABLE 1.
Precision of the class capture IgM anti-HEV EIA
| Assay | IgM concn | No. of each sample testeda | OD
|
||
|---|---|---|---|---|---|
| Mean | SD | % CV | |||
| Intraplate precision | Low | 28 | 0.461 | 0.022 | 4.800 |
| Intermediate | 28 | 1.418 | 0.062 | 4.371 | |
| High | 28 | 2.154 | 0.036 | 1.674 | |
| Interplate precision | Low | 12 | 0.469 | 0.029 | 6.263 |
| Intermediate | 12 | 1.585 | 0.065 | 4.124 | |
| High | 12 | 2.146 | 0.079 | 3.671 | |
All samples were from the same pool. For the interplate precision assay, samples were aliquoted, frozen, and tested in separate plates.
Development of a cutoff formula.
In order to define a cutoff value, it was necessary to test a large number of sera that were negative for anti-HEV. Since hepatitis E is not endemic in the United States, it was assumed that random blood donors who tested negative for IgG anti-HEV were highly unlikely to have been infected with HEV in the period just prior to their donating blood and therefore would not have IgM anti-HEV, so their blood could be considered a negative sample. Negative blood samples were also collected from chimpanzees that had been born and raised in captivity and had not been exposed to HEV. Samples from chimpanzees that had been experimentally infected with HAV, HBV, or HCV were chosen to rule out cross-reactivity, and samples from patients with autoimmune disease or rheumatoid factor were included, since such sera can give false-positive results in some serologic tests.
The ODs of the random blood donor samples ranged from 0.081 to 0.235 (Table 2). All other samples had ODs within this range, except for one sample containing rheumatoid factor that gave an OD of 0.358 (Table 2). This sample tested negative for IgG anti-HEV (data not shown). The SD for all samples was 0.032. The target cutoff value (0.429) was arbitrarily set at 10 SDs above the mean OD of the negative samples. A cutoff equation was formulated by using a negative serum and a positive serum, based on the seroconversion panel of CH1375 (Fig. 1). This equation, (negative control OD/2) + (positive control OD/4.4), yielded cutoff values of 0.404, 0.467, and 0.404, respectively, for the three plates used to test the negative samples. The highest OD of the negative samples (0.358) was below the calculated cutoff value of the plate (0.404) and was considered negative for IgM anti-HEV.
TABLE 2.
OD values of samples used to establish the cutoff
| Samples | No. | OD
|
|||
|---|---|---|---|---|---|
| Range | Mean | SD | |||
| Random blood donorsa | 84 | 0.081-0.235 | 0.106 | 0.026 | |
| Autoimmune diseasea | 20 | 0.094-0.176 | 0.111 | 0.024 | |
| Rheumatoid factora | 20 | 0.089-0.358 | 0.121 | 0.058 | |
| Hepatitis Ab | 17 | 0.089-0.178 | 0.112 | 0.025 | |
| Hepatitis Bb | 3 | 0.088-0.121 | 0.103 | 0.025 | |
| Hepatitis Cb | 5 | 0.083-0.109 | 0.093 | 0.007 | |
| Total | 149 | 0.081-0.358 | 0.109 | 0.032 | |
Human sera.
Chimpanzee sera.
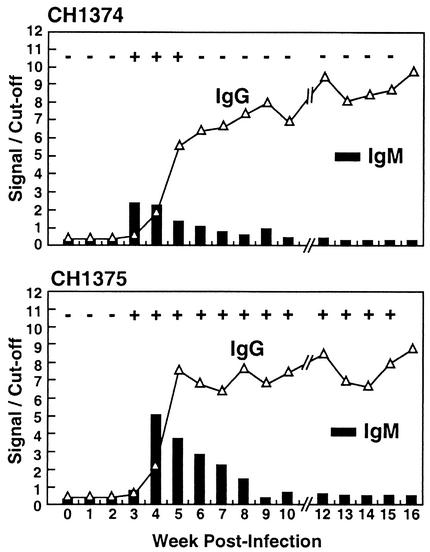
FIG. 1.
Both chimpanzees used were inoculated with 0.5 ml of 10% feces containing Sar-55, a Pakistani strain of HEV (genotype 1). The week zero sample was drawn prior to injection, and subsequent samples were drawn weekly thereafter, except for week 11. Signal/cutoff values of above 1.000 indicated positive results. Plus and minus signs at the top are results from the IgM anti-HEV sandwich EIA, as previously published (36).
Seroconversion panels for two chimpanzees infected with Sar-55.
Two naive chimpanzees were infected with the Sar-55 strain of HEV, and weekly serum and/or plasma samples were assayed to determine if the expected IgM response could be detected. The results for the two chimpanzees were very similar. IgM anti-HEV was first detected 1 week before (CH1374) or coincident with (CH1375) seroconversion to IgG anti-HEV (Fig. 1). In both cases, the highest IgM test value was obtained with the initial positive serum sample and then IgM values gradually decreased over the following 3 or 4 weeks. All samples were IgM negative thereafter. In contrast to the transient IgM response, the IgG anti-HEV response was robust and remained strongly positive throughout the duration of the experiment.
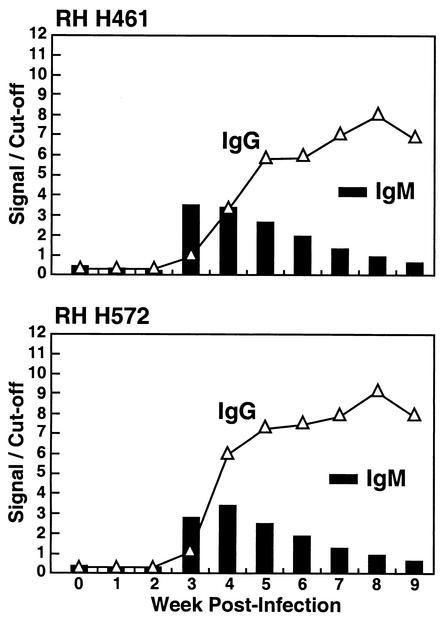
Seroconversion panels for two rhesus monkeys infected with Mex-14.
Additional seroconversion panels from two rhesus monkeys experimentally infected with the genetically divergent Mexican strain of HEV (Mex-14) were used to determine the specificity and utility of the class capture IgM assay. In spite of the fact that sera from the Mex-14-infected animals were tested with the heterologous Sar-55 antigen, the results from the rhesus seroconversion panels were basically identical to those obtained following infection of the chimpanzee with Sar-55 (Fig. 2). Seroconversion to IgM anti-HEV was detected at week 3 postinoculation, and seroconversion to IgG anti-HEV occurred at the same time or 1 week later. The IgG response in the rhesus monkeys was typical and was indistinguishable from that in the chimpanzees.
FIG. 2.
. Both rhesus monkeys used were infected with the Mexican strain of HEV (genotype 2). The week zero sample was drawn before the inoculation, and subsequent samples were drawn on a weekly basis. Signal/cutoff values of greater than 1.000 indicated positive results.
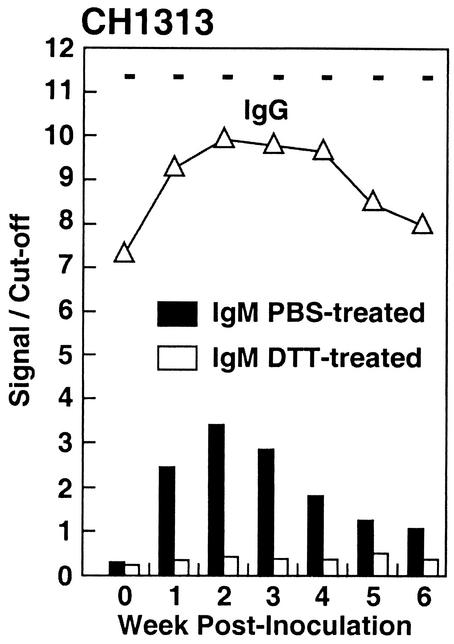
Detection of IgM anti-HEV in the presence of a high titer of IgG anti-HEV.
CH1313 had been previously infected with the Sar-55 strain of HEV and still had a high level (titer of 1:10,000) of IgG anti-HEV when inoculated with the Mex-14 strain 45 months later (Fig. 3). The OD for the IgG anti-HEV test increased fivefold the week following inoculation (Table 3), indicating an anamnestic response. A transient IgM anti-HEV response was also observed. It appeared to be typical of those seen previously except that seroconversion occurred at week 1 postinoculation, again consistent with an anamnestic response.
FIG. 3.
CH1313 was inoculated twice with HEV, first with the Pakistani strain and then, 45 months later, with the Mexican strain (36). Week zero represents a convalescent-phase sample before the second inoculation, and subsequent samples were drawn weekly. Samples were tested for IgM anti-HEV by the class capture method and for IgG anti-HEV by the sandwich assay. Samples were also treated with 0.01 M DTT before being tested for IgM anti-HEV by the class capture method. Samples treated with DTT and samples treated with PBS were analyzed on the same plate. Plus and minus signs at the top are results from the IgM anti-HEV sandwich EIA, as previously published (36).
TABLE 3.
Specific inactivation of IgM anti-HEV by DTT
| Sample | OD of sample drawn at wk:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| IgMa + PBS | 0.103 | 0.895 | 1.255 | 1.045 | 0.659 | 0.452 | 0.386 |
| IgMa + DTT | 0.082 | 0.121 | 0.157 | 0.137 | 0.131 | 0.177 | 0.130 |
| IgGb + PBS | 0.195 | 1.008 | 1.921 | 1.772 | 1.787 | 1.617 | 1.565 |
| IgGb + DTT | 0.175 | 0.754 | 1.747 | 1.796 | 1.504 | 1.474 | 1.721 |
Same results presented as signal/cutoff in Fig. 3.
Serum diluted 1:10,000 prior to testing.
In order to rule out the possibility that the high levels of IgG anti-HEV present in CH1313 were providing low false-positive IgM anti-HEV values, a variation of the test was performed. Since IgM, but not IgG, is destroyed by treatment with reducing agents, the sera were incubated with DTT and then retested for IgM (Fig. 3). The IgM anti-HEV activity was almost totally destroyed by the DTT treatment, and the OD values decreased as much as 87%. As a control to confirm that DTT treatment was specific for IgM and did not adversely affect IgG detection, the sera were diluted with PBS to lower the ODs to within the linear range of the IgG anti-HEV assay (OD of <2.0), and then an untreated sample and a duplicate DTT-treated sample were tested in parallel for IgG anti-HEV. The raw ODs of the paired samples were very similar (Table 3). The greatest decrease (0.283 OD unit, week 4) was only 16%.
Paired sera from two epidemics.
In order to compare the class capture assay and a sandwich IgM assay, previously tested paired sera from two epidemics of hepatitis E in Pakistan were retested. For each pair, the sample drawn during the epidemic had been IgM anti-HEV positive by the sandwich test (5, 6) and the corresponding convalescent-phase serum had been negative for IgM anti-HEV and strongly positive for IgG anti-HEV. The earliest sample of each pair tested in the class capture assay was positive for IgM anti-HEV, as it had been in the sandwich assay (Tables 4 and 5). The lowest ODs in the class capture test were 0.882 for the Sargodha samples and 0.632 for the Abbottabad samples (2.1 and 1.5 times higher, respectively, than the cutoff value for that plate).
TABLE 4.
IgM anti-HEV levels of paired sera from the Sargodha epidemic of 1987a
| Sample | Group 1 (March-April 1987)
|
Group 2 (December 1988)
|
||||
|---|---|---|---|---|---|---|
| Sandwich EIA (previously published)b | Class capture EIA (OD)c
|
Sandwich EIA (previously published)b | Class capture EIA (OD)c
|
|||
| PBS treated | DTT treated | PBS treated | DTT treated | |||
| 137d | + | 0.914e | 0.081e | − | 0.518 | 0.074 |
| 151d | + | 1.299f | 0.086f | − | 0.463 | 0.146 |
| 79d | + | 1.553f | 0.138f | − | 0.461 | 0.069 |
| 143 | + | 1.309e | 0.178e | − | 0.400 | 0.106 |
| 135 | + | 1.620f | 0.089f | − | 0.340 | 0.086 |
| 84 | + | 0.882f | 0.076f | − | 0.335 | 0.212 |
| 141 | + | 1.444e | 0.107e | − | 0.323 | 0.084 |
| 187 | + | 1.285e | 0.137e | − | 0.323 | 0.164 |
| 138 | + | 1.203f | 0.092f | − | 0.317 | 0.150 |
| 147 | + | 1.436e | 0.151e | − | 0.310 | 0.139 |
| 136 | + | 1.247e | 0.083e | − | 0.305 | 0.153 |
| 69 | + | 1.348 | 0.170 | − | 0.282 | 0.150 |
| 152 | + | 1.679e | 0.175e | − | 0.275 | 0.088 |
| 159 | + | 1.508f | 0.088f | − | 0.239 | 0.146 |
| 153 | + | 1.640f | 0.083f | − | 0.170 | 0.126 |
Numbers in boldface indicate that both samples of the pair were positive for IgM anti-HEV.
From reference 6.
PBS-treated and DTT-treated sera were tested on the same plate.
IgG anti-HEV OD values for group 2 sera 137, 151, and 79 were 2.192, 2.480, and 2.577, respectively.
The sample was diluted 1:100 before testing (final dilution of 1:10,000).
The sample was diluted 1:10 before testing (final dilution of 1:1,000).
TABLE 5.
IgM anti-HEV levels of paired sera from the Abbottabad epidemic of 1988a
| Sample | Group 1 (August-September 1988)
|
Group 2 (December 1988)
|
||||
|---|---|---|---|---|---|---|
| Sandwich EIA (previously published)b | Class capture EIA (OD)c
|
Sandwich EIA (previously published)b | Class capture EIA (OD)c
|
|||
| PBS treated | DTT treated | PBS treated | DTT treated | |||
| 62d | + | 1.120e | 0.091e | − | 0.512 | 0.104 |
| 83d | + | 0.814f | 0.073f | − | 0.477 | 0.094 |
| 37d | + | 0.805 | 0.081 | − | 0.462 | 0.103 |
| 92 | + | 1.520e | 0.163e | − | 0.393 | 0.077 |
| 84 | + | 1.406e | 0.195e | − | 0.377 | 0.071 |
| 31 | + | 1.572f | 0.149f | − | 0.371 | 0.109 |
| 90 | + | 0.632e | 0.089e | − | 0.365 | 0.106 |
| 57 | + | 1.516f | 0.114f | − | 0.356 | 0.138 |
Numbers in boldface indicate that both samples of the pair were positive for IgM anti-HEV.
From reference 5.
PBS-treated and DTT-treated sera were tested on the same plate.
The IgG anti-HEV values for sera 62, 83, and 37 were 1.319, 0.899, and 0.746, respectively.
The sample was diluted 1:100 before testing (final dilution of 1:10,000).
The sample was diluted 1:10 before testing (final dilution of 1:1,000).
In 17 of 23 cases, the convalescent-phase serum was negative by the class capture assay, in agreement with the previously published results (Tables 4 and 5). However, 6 of the 23 sera were positive by the class capture assay, whereas they had been negative by the sandwich assay. In all six cases, the OD readings were low and ranged from 0.005 to 0.106 OD unit above the cutoff of 0.457 or 0.412 OD unit. These six paired sera were treated with DTT and tested again for IgM anti-HEV. The OD of each DTT-treated serum was reduced by >50% compared to that of the PBS-treated control. Eleven convalescent-phase sera that had OD levels below the cutoff value were also reduced by >50% after DTT treatment (Tables 4 and 5). Because of their low OD values, these samples were scored as negative despite the 50% reduction in OD after the DTT treatment.
Quantification of IgM anti-HEV.
Although the class capture IgM anti-HEV assay was designed principally for qualitative diagnosis, we examined the test for its ability to quantify IgM anti-HEV in the WHO standard for anti-HEV. Although the standard was developed for quantification of total (principally IgG) anti-HEV, we found, as have others, that the standard contains small amounts of IgM anti-HEV (32). Using the cutoff derived here, the WHO standard had an IgM anti-HEV OD value of 0.773 and a titer of 1:800. To determine the high-end sensitivity of this assay for detecting IgM anti-HEV in acute-phase serum samples from patients with hepatitis E, we determined the end point reciprocal titers of three acute-phase sera listed in Tables 4 and 5, based on twofold dilutions (data not shown). The reciprocal titers of the three sera by class capture EIA ranged from 5 × 104 to 2 × 105.
DISCUSSION
The presence of specific antibody of the IgM class is diagnostic for recent or ongoing infection. Current EIAs that measure IgM anti-HEV use the sandwich method of detection, which consists of the adsorption of HEV antigen onto a solid phase, followed by the binding of all classes of antigen-specific antibodies in the sample. Enzyme-labeled conjugate specific for IgM antibodies is then added, and color develops upon addition of substrate. Although this method is simple, sensitivity is compromised when the corresponding IgG titers are disproportionately higher than those of the IgM antibodies (16), since the IgG competes for binding sites on the antigen. In the IgM class capture system, competing IgG antibodies in the sample are eliminated at the beginning of the assay, thus enhancing the reaction between the IgM anti-HEV and the HEV antigen.
The assay described here uses biotin-conjugated goat anti-human IgM (μ chain specific) adsorbed to a streptavidin-coated polystyrene plate to capture IgM antibodies selectively in the sample. After the removal of unbound antibodies, HEV ORF2 antigen is added to the microtiter wells. This antigen will bind only to those captured IgM antibodies with specificity for the HEV capsid antigen. Mouse monoclonal IgG anti-HEV, which also binds to the captured antigen, is added next. Finally, peroxidase-labeled anti-mouse conjugate and ABTS substrate are added to produce a colored product. Compared to the sandwich method, the IgM anti-HEV class capture assay was more sensitive, especially in detecting IgM in the presence of high concentrations of IgG antibodies.
Since there are numerous ways to determine a cutoff value, different published IgM anti-HEV EIAs have different cutoff formulas. For example, for some EIAs (e.g., HEV IgM ELISA kit; Genelabs Diagnostic) the cutoff is calculated by adding a constant factor to the mean of the negative control values, and others calculate the cutoff by adding a specified number of SDs to the OD of the negative samples (7, 8). In addition, the absence of a “gold standard” and of a reference method to measure IgM anti-HEV, together with the unexpectedly high seroprevalence of IgG anti-HEV in communities where the diseases is not endemic, posed difficulty in defining true positive and negative samples for IgM anti-HEV. In order to achieve optimum specificity, the target cutoff value of the IgM anti-HEV class capture assay was arbitrarily set at 10 SDs above the mean OD of samples from random blood donors and samples with markers for acute non-E hepatitis, rheumatoid factor, and autoimmune disease, which were presumed to be negative for IgM anti-HEV (Table 2). Establishing a cutoff value in this manner set the specificity of the assay at 100% for these samples. After a target cutoff value was established, a cutoff formula was developed by using a negative sample and a positive sample from the seroconversion panel of CH1375. Values from a positive sample were included in the cutoff equation in order to represent the upper end of the linearity curve. In addition, inclusion of a positive sample produced a plate cutoff value that was sensitive to intertest variability. Both the negative and the positive samples were tested in each assay to produce a tailored cutoff for each plate.
The sandwich method and the class capture method could be compared because the same seroconversion panels for CH1374, CH1375, and CH1313 tested in this study were previously tested for IgM anti-HEV by the sandwich method (36). The results from the seroconversion panels of CH1374 and CH1313 suggested that the class capture procedure was as or slightly more sensitive than the sandwich method for detecting IgM anti-HEV. Both methods first detected IgM anti-HEV at week 3 in CH1374, but the class capture method remained positive 1 week longer than the sandwich method. The sandwich assay did not detect IgM anti-HEV in CH1313 at all, whereas the class capture assay was positive for 6 weeks. Moreover, the sandwich method and the class capture method produced different IgM anti-HEV response patterns for CH1375. The previous results by the sandwich method were positive from week 3 to week 15, with two IgM anti-HEV peaks (a major one at week 4 and a minor one at week 9). In contrast, the class capture assay detected an IgM response which began with a peak value at week 4 and then gradually decreased until it became negative at week 9 and thereafter. The results from the IgM anti-HEV sandwich method were unusual because of the double-peak IgM response pattern. The double-peak IgM pattern was seen when the samples were tested at the 1:20 dilution but not when they were tested at a higher dilution (1:400). This behavior is characteristic of an interfering substance. A test such as the DTT treatment was not performed to confirm that the sandwich assay was detecting true IgM in this animal.
The specificity of the class capture EIA was assessed further by testing sera from animals that had been infected with a strain of HEV from a genotype other than that used as the test antigen. In this study, seroconversion panels of two rhesus monkeys infected with the Mexican strain were tested for IgM anti-HEV by using the Sar-55 antigen. The Mex-14 strain is the human strain most divergent from Sar-55, with 93% identity at the amino acid level in ORF2 (26). The class capture method detected IgM anti-HEV in both monkeys at 3 weeks after the inoculation of HEV, and a typical response pattern was observed. In addition, the high levels of IgG anti-HEV detected from week 5 onwards did not mask the natural decrease of the IgM anti-HEV levels.
Paired human sera from HEV epidemics in Sargodha and Abbottabad were tested with the newly developed assay (5, 6). Samples were chosen for this study based on availability and previous data from sandwich EIAs. The samples chosen had been IgM anti-HEV positive by the sandwich method at the time of the epidemic (group 1) and negative in the follow-up sample (group 2). All group 1 samples also tested positive with the class capture assay. Thus, the class capture and sandwich method were highly and equally sensitive in detecting IgM anti-HEV in acute phase sera. In most cases, the follow-up sample was also negative in the class capture assay. However, 6 of 23 samples in the second group, which were previously negative when tested with the sandwich assay, had a significant reduction in the OD between the first and second samples but were still positive when tested with the class capture assay. Since the second group of samples in the Abbottabad epidemic were drawn only 3 to 4 months after the outbreak, the anti-HEV in the latter samples was most likely due to incomplete decay of IgM. In contrast, the convalescent samples from the Sargodha epidemic were drawn 19 to 20 months after the epidemic had occurred, at a time when IgM normally was expected to have disappeared. However, since the ODs were reduced by greater than 65% following DTT treatment (Table 4), it appeared that IgM was being measured in these samples.
Seriwatana et al. (32) recently compared the ratio of HEV-specific IgM and IgG with sandwich EIAs specific for IgM and total anti-HEV, respectively, in serial serum samples from Nepalese patients who had HEV viremia at the time that the initial sample was collected. In the majority of cases, the ratio of IgM to total anti-HEV was high, as expected for a primary infection. However, in eight patients the level of IgM anti-HEV was low and that of total anti-HEV was very high; the resulting low ratio in a recently infected patient was interpreted as signifying an anamnestic response following reinfection. However, since the time of the alleged first infection was unknown and since a serum sample collected prior to the second infection was not available, it was not possible to prove that a reinfection had actually occurred.
In contrast, in the case of CH1313, a detailed history of the animal and serum samples taken just prior to the second exposure to HEV were available. Therefore, it was possible to demonstrate an anamnestic response as well as the reappearance of IgM anti-HEV. CH1313 was initially inoculated with a Pakistani strain of HEV and then challenged with the divergent Mexican strain 45 months later. Following the inoculation with the Mexican strain, the IgG levels rose fivefold in the first week, indicating an anamnestic response (Table 3). Despite the high IgG anti-HEV levels present at the time of the second inoculation, the class capture assay detected an IgM response that was confirmed by retesting the samples following DTT treatment (Fig. 3). The IgM anti-HEV test was negative just prior to inoculation but was clearly positive the following week, thus confirming the anamnestic response of the IgM anti-HEV also. When the same panel had been tested previously for IgM anti-HEV with the sandwich method (36), these samples were negative, presumably because of masking of the IgM anti-HEV due to high titers of IgG anti-HEV. Thus, the class capture assay should prove useful for detecting IgM anti-HEV during reinfection, especially when the IgG titer is high and/or the IgM antibody level is low.
When we tested the WHO anti-HEV standard for IgM anti-HEV content, we found that it was weakly positive, as did Seriwatana et al. (32). We found that the titer of IgM anti-HEV in this sample was approximately eightfold greater than its endpoint titer, based on the cutoff for our ELISA, and therefore slightly greater than was observed by Seriwatana. Thus, these two EIAs for IgM anti-HEV appeared to have similar sensitivities when acute-phase samples were tested. Because these tests are so sensitive, they apparently can detect IgM anti-HEV long after the primary infection, as well as in probable reinfections. To make our assay useful for the diagnosis of recent infections, we recommend a modified OD cutoff value of 0.600. As seen in Tables 4 and 5, this value discriminates between recent infections and more temporally distant exposures to HEV. Under such circumstances, the WHO standard, at a dilution of approximately 1:200, could be used as an internal low-titer positive control for the test.
In summary, the class capture assay developed in this study provides a reliable method for detecting IgM anti-HEV. The class capture and sandwich methods had comparable specificities when acute-phase sera with high IgM anti-HEV levels were tested. The class capture method, however, demonstrated higher sensitivity for samples with low IgM anti-HEV concentrations or with high concentrations of IgG anti-HEV. The DTT procedure was used to confirm the presence of IgM in samples with high IgG titers.
REFERENCES
- 1.Balayan, M. S., A. G. Andjaparidze, S. S. Savinskaya, E. S. Ketiladze, D. M. Braginsky, A. P. Savinov, and V. F. Poleschuk. 1983. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 20:23-31. [DOI] [PubMed] [Google Scholar]
- 2.Balayan, M. S., R. K. Usmanov, N. A. Zamyatina, D. I. Djumalieva, and F. R. Karas. 1990. Brief report: experimental hepatitis E infection in domestic pigs. J. Med. Virol. 32:58-59. [DOI] [PubMed] [Google Scholar]
- 3.Belabbes, E., A. Bouguermouh, and J. Pillot. 1988. Waterborne non-A, non-B hepatitis in Algeria: epidemiological study and development of a test, p. 152-153. In A. J. Zuckerman (ed.), Viral hepatitis and liver disease. Alan R. Liss, New York, N.Y.
- 4.Bradley, D., A. Andjaparidze, E. H. Cook, Jr., K. McCaustland, M. Balayan, H. Stetler, O. Velazquez, B. Robertson, C. Humphrey, M. Kane, et al. 1988. Aetiological agent of enterically transmitted non-A, non-B hepatitis. J. Gen. Virol. 69:731-738. [DOI] [PubMed] [Google Scholar]
- 5.Bryan, J. P., M. Iqbal, S. Tsarev, I. A. Malik, J. F. Duncan, A. Ahmed, A. Khan, A. Khan, A. R. Rafiqui, R. H. Purcell, and L. J. Legters. 2002. Epidemic of hepatitis E in military unit in Abbottabad, Pakistan. Am. J. Trop. Med. Hyg. 67:662-668. [DOI] [PubMed] [Google Scholar]
- 6.Bryan, J. P., S. A. Tsarev, M. Iqbal, J. Ticehurst, S. Emerson, A. Ahmed, J. Duncan, A. R. Rafiqui, I. A. Malik, R. H. Purcell, et al. 1994. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J. Infect. Dis. 170:517-521. [DOI] [PubMed] [Google Scholar]
- 7.Dawson, G. J., K. H. Chau, C. M. Cabal, P. O. Yarbough, G. R. Reyes, and I. K. Mushahwar. 1992. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J. Virol. Methods 38:175-186. [DOI] [PubMed] [Google Scholar]
- 8.Favorov, M. O., Y. E. Khudyakov, E. E. Mast, T. L. Yashina, C. N. Shapiro, N. S. Khudyakova, D. L. Jue, G. G. Onischenko, H. S. Margolis, and H. A. Fields. 1996. IgM and IgG antibodies to hepatitis E virus (HEV) detected by an enzyme immunoassay based on an HEV-specific artificial recombinant mosaic protein. J. Med. Virol. 50:50-58. [DOI] [PubMed] [Google Scholar]
- 9.Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 181:449-455. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, M., D. Walker, E. Mast, and H. Fields. 2002. Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hepatitis E virus. Biologicals 30:43-48. [DOI] [PubMed] [Google Scholar]
- 11.Ghabrah, T. M., S. Tsarev, P. O. Yarbough, S. U. Emerson, G. T. Strickland, and R. H. Purcell. 1998. Comparison of tests for antibody to hepatitis E virus. J. Med. Virol. 55:134-137. [PubMed] [Google Scholar]
- 12.Hussaini, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O'Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepat. 4:51-54. [DOI] [PubMed] [Google Scholar]
- 13.Kabrane-Lazizi, Y., J. B. Fine, J. Elm, G. E. Glass, H. Higa, A. Diwan, C. J. Gibbs, Jr., X. J. Meng, S. U. Emerson, and R. H. Purcell. 1999. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61:331-335. [DOI] [PubMed] [Google Scholar]
- 14.Kane, M. A., D. W. Bradley, S. M. Shrestha, J. E. Maynard, E. H. Cook, R. P. Mishra, and D. D. Joshi. 1984. Epidemic non-A, non-B hepatitis in Nepal. Recovery of a possible etiologic agent and transmission studies in marmosets. JAMA 252:3140-3145. [DOI] [PubMed] [Google Scholar]
- 15.Karetnyi Iu, V., D. I. Dzhumalieva, R. K. Usmanov, I. P. Titova, I. Litvak Ia, and M. S. Balaian. 1993. The possible involvement of rodents in the spread of viral hepatitis E. Z. Mikrobiol. Epidemiol. Immunobiol. 4:52-56. [PubMed]
- 16.Kemeny, D. M. 1992. Titration of antibodies. J. Immunol. Methods 150:57-76. [DOI] [PubMed] [Google Scholar]
- 17.Khuroo, M. S. 1980. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am. J. Med. 68:818-824. [DOI] [PubMed] [Google Scholar]
- 18.Khuroo, M. S., W. Duermeyer, S. A. Zargar, M. A. Ahanger, and M. A. Shah. 1983. Acute sporadic non-A, non-B hepatitis in India. Am. J. Epidemiol 118:360-364. [DOI] [PubMed] [Google Scholar]
- 19.Khuroo, M. S., S. Kamili, and S. Jameel. 1995. Vertical transmission of hepatitis E virus. Lancet 345:1025-1026. [DOI] [PubMed] [Google Scholar]
- 20.Khuroo, M. S., M. R. Teli, S. Skidmore, M. A. Sofi, and M. I. Khuroo. 1981. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 70:252-255. [DOI] [PubMed] [Google Scholar]
- 21.Krawczynski, K., and D. W. Bradley. 1989. Enterically transmitted non-A, non-B hepatitis: identification of virus-associated antigen in experimentally infected cynomolgus macaques. J. Infect. Dis. 159:1042-1049. [DOI] [PubMed] [Google Scholar]
- 22.Mast, E. E., M. J. Alter, P. V. Holland, R. H. Purcell, et al. 1998. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatology 27:857-861. [DOI] [PubMed] [Google Scholar]
- 23.Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33:842-845. [DOI] [PubMed] [Google Scholar]
- 24.Meng, X. J., S. Dea, R. E. Engle, R. Friendship, Y. S. Lyoo, T. Sirinarumitr, K. Urairong, D. Wang, D. Wong, D. Yoo, Y. Zhang, R. H. Purcell, and S. U. Emerson. 1999. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J. Med. Virol. 59:297-302. [PubMed] [Google Scholar]
- 25.Meng, X. J., P. G. Halbur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 72:9714-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuno, T., and N. Kondelis. 1978. Evaluation of dithiothreitol (DTT) for inactivation of IgM antibodies. J. Clin. Pathol. 31:1152-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirofsky, B., and E. R. Rosner. 1974. DTT test: a new method to differentiate IgM and IgG erythrocyte antibodies. Vox Sang 27:480-488. [DOI] [PubMed] [Google Scholar]
- 29.Reyes, G. R., M. A. Purdy, J. P. Kim, K. C. Luk, L. M. Young, K. E. Fry, and D. W. Bradley. 1990. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 247:1335-1339. [DOI] [PubMed] [Google Scholar]
- 30.Reyes, G. R., P. O. Yarbough, A. W. Tam, M. A. Purdy, C. C. Huang, J. S. Kim, D. W. Bradley, and K. E. Fry. 1991. Hepatitis E virus (HEV): the novel agent responsible for enterically transmitted non-A, non-B hepatitis. Gastroenterol. Jpn. 26(Suppl. 3):142-147. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, R. A., W. H. Burgess, S. U. Emerson, R. S. Leibowitz, S. A. Sosnovtseva, S. Tsarev, and R. H. Purcell. 1998. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 12:75-84. [DOI] [PubMed] [Google Scholar]
- 32.Seriwatana, J., M. P. Shrestha, R. M. Scott, S. A. Tsarev, D. W. Vaughn, K. S. Myint, and B. L. Innis. 2002. Clinical and epidemiological relevance of quantitating hepatitis E virus-specific immunoglobulin M. Clin. Diagn. Lab. Immunol. 9:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, S., A. Mohanty, Y. K. Joshi, et al. 2001. Outcome of hepatitis E virus infection in Indian pregnant women admitted to a tertiary care hospital. Indian J. Med. Res. 113:35-39. [PubMed] [Google Scholar]
- 34.Tandon, B. N., B. M. Gandhi, and Y. K. Joshi. 1984. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B virus infection in north India. Bull. W. H. O. 62:67-73. [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35:1244-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsarev, S. A., T. S. Tsareva, S. U. Emerson, A. Z. Kapikian, J. Ticehurst, W. London, and R. H. Purcell. 1993. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J. Infect. Dis. 168:369-378. [DOI] [PubMed] [Google Scholar]
- 37.Tsega, E., B. G. Hansson, K. Krawczynski, and E. Nordenfelt. 1992. Acute sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin. Infect. Dis. 14:961-965. [DOI] [PubMed] [Google Scholar]
- 38.Tyagi, S., S. Jameel, and S. K. Lal. 2001. Self-association and mapping of the interaction domain of hepatitis E virus ORF3 protein. J. Virol. 75:2493-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida, T., T. T. Aye, X. Ma, F. Iida, T. Shikata, M. Ichikawa, T. Rikihisa, and K. M. Win. 1993. An epidemic outbreak of hepatitis E in Yangon of Myanmar: antibody assay and animal transmission of the virus. Acta Pathol. Jpn. 43:94-98. [DOI] [PubMed] [Google Scholar]
- 40.Velazquez, O., H. C. Stetler, C. Avila, G. Ornelas, C. Alvarez, S. C. Hadler, D. W. Bradley, and J. Sepulveda. 1990. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986-1987. JAMA 263:3281-3285. [PubMed] [Google Scholar]
- 41.Wong, D. C., R. H. Purcell, M. A. Sreenivasan, S. R. Prasad, and K. M. Pavri. 1980. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet ii:876-879. [DOI] [PubMed] [Google Scholar]
- 42.Yarbough, P. O., A. W. Tam, K. E. Fry, K. Krawczynski, K. A. McCaustland, D. W. Bradley, and G. R. Reyes. 1991. Hepatitis E virus: identification of type-common epitopes. J. Virol. 65:5790-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 71:9045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]