Abstract
Hematopoietic stem cells rarely contribute to hepatic regeneration, however, the mechanisms governing their homing to the liver, which is a crucial first step, are poorly understood. The chemokine stromal cell–derived factor-1 (SDF-1), which attracts human and murine progenitors, is expressed by liver bile duct epithelium. Neutralization of the SDF-1 receptor CXCR4 abolished homing and engraftment of the murine liver by human CD34+ hematopoietic progenitors, while local injection of human SDF-1 increased their homing. Engrafted human cells were localized in clusters surrounding the bile ducts, in close proximity to SDF-1–expressing epithelial cells, and differentiated into albumin-producing cells. Irradiation or inflammation increased SDF-1 levels and hepatic injury induced MMP-9 activity, leading to both increased CXCR4 expression and SDF-1–mediated recruitment of hematopoietic progenitors to the liver. Unexpectedly, HGF, which is increased following liver injury, promoted protrusion formation, CXCR4 upregulation, and SDF-1–mediated directional migration by human CD34+ progenitors, and synergized with stem cell factor. Thus, stress-induced signals, such as increased expression of SDF-1, MMP-9, and HGF, recruit human CD34+ progenitors with hematopoietic and/or hepatic-like potential to the liver of NOD/SCID mice. Our results suggest the potential of hematopoietic CD34+/CXCR4+cells to respond to stress signals from nonhematopoietic injured organs as an important mechanism for tissue targeting and repair.
Introduction
Recent reports suggest that hematopoietic stem cells (HSCs) of human origin also have hepatic potential. Following clinical liver or bone marrow (BM) transplantation from sex-mismatched donors, BM-derived hepatocytes have been identified (1–3). Murine and rat HSCs can also migrate to and engraft irradiated or injured adult livers, with hepatic differentiation (4–6). Single murine HSC transplantation has resulted in detection of HSC-derived cells in the liver of irradiated recipients with a low percentage of transplanted cells exhibiting immunohistochemical and morphologic properties of hepatic epithelial cells (7), confirmed (as a rare event) in another report using a different protocol (8). This process was not documented in parabiotic mice (8), suggesting that it does not occur under steady-state homeostatic conditions in nonirradiated or nondamaged intact livers. Enriched human BM or cord blood (CB) CD34+ progenitors and sorted primitive human CD34+/CD38– cells, a population highly enriched for HSCs, can also engraft the liver of irradiated immune-deficient NOD/SCID and NOD/SCID/B2m-null mice at low frequencies.
In addition to extensive multilineage hematopoietic differentiation in the murine BM and spleen, transplanted BM or CB CD34+ progenitor cells can also rarely develop into human hepatic-like CD45-negative cells that express hepatocyte-specific antigen, c-met, and cytokeratin 19, produce human albumin, and respond to stimulation induced by liver injury together with HGF in vivo (9, 10). These human albumin–producing cells were detected in the murine liver, but not in other organs such as the murine BM and spleen (9, 10). In addition to its powerful mitogenic effect on hepatocytes, in vitro stimulation with the hepatic cytokine HGF also promotes proliferation, adhesion, and survival (11). HGF stimulation also promotes high-level differentiation into albumin-producing cells with liver-specific lineage markers by hematopoietic CD34+ enriched cells obtained from human BM (12) and CB mononuclear cells (CBMCs) (13). However, evidence for clonal properties of stem cells with both hematopoietic and hepatic potential has been documented only with murine and rat stem cells and not with human repopulating progenitors. In vitro cultured, single rodent stromal progenitors (multipotent adult progenitor cells) also demonstrate both hematopoietic and hepatocytic differentiation potential in vivo in transplanted recipients preconditioned with total body irradiation (14). However, the mechanisms that mediate and regulate the essential first step of migration into and retention of hematopoietic stem and progenitor cells with hepatic potential in the damaged liver are currently unknown.
Although the levels of HSCs that engraft the irradiated liver and develop into hepatocyte-like, albumin-producing cells are very low, this process can be amplified by liver injury or viral inflammation. Under strong selection conditions that exist in fumarylacetoacetate hydrolase–null mice, which have ongoing severe hepatocyte damage due to deficiency of this enzyme, there is enormous amplification of transplanted, purified murine HSCs that demonstrate hepatic morphology and function, with correction of the metabolic disorder (6) by cell fusion (15). Liver repopulation by BM cells from Bcl-2 transgenic mice transplanted into WT recipients, followed by repeated rounds of liver injury and regeneration induced by Fas-mediated apoptosis, represents another example of selective amplification of transplanted BM cells after they have differentiated into hepatocytes (16). High levels of BM-derived hepatocytes were also reported in a liver transplant recipient in whom the transplanted liver became infected with hepatitis C virus (HCV) (2). Liver injury together with HGF stimulation, or partial hepatectomy significantly increased the levels of human albumin–producing hepatocyte-like cells in the murine liver (10, 13). These studies demonstrate that the potential of HSCs to gain hepatic phenotype can be significantly amplified under stress conditions. However, the mechanisms and factors that regulate HSC recruitment to the treated liver and induce their hepatic phenotype are currently unknown.
The chemokine stromal cell–derived factor-1 (SDF-1), also termed CXCL12, the only known powerful chemoattractant of HSCs of both human (17) and murine origin (18), is widely expressed in many tissues during development (19) and adulthood (20–22), including the liver (23–25). We have shown that BM homing and repopulation by sorted human CD34+/CD38–/low stem cells transplanted into the tail vein of irradiated immune-deficient NOD/SCID and NOD/SCID/B2m null mice are dependent on SDF-1/CXCR4 interactions (26, 27). We further demonstrated the involvement of these interactions in G-CSF–induced mobilization of murine and human stem cells (28). We therefore hypothesized that SDF-1/CXCR4 interactions are implicated in stress-induced stem cell trafficking in vivo and in particular sought to identify mechanisms that mediate in vivo migration of hematopoietic progenitor cells into the liver under stress conditions.
Methods
Human cells.
Human CBMCs and adult mobilized PBMCs were obtained after informed consent in accordance with procedures approved by the human ethics committee of the Weizmann Institute of Science. CD34+ cell enrichment was performed using magnetic bead separation as previously described (27). CXCR4 expression was determined by flow cytometry using purified anti–human CXCR4 (clone 12G5; R&D Systems Inc., Minneapolis, Minnesota, USA) and secondary F(ab′)2 fragment of goat anti-mouse IgG-FITC (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA).
Mice.
NOD/SCID mice were bred and housed as previously described (27). All experiments were approved by the animal care committee of the Weizmann Institute of Science. Mice were sublethally irradiated (375 cGy) where indicated, 24 hours before transplantation. CXCR4 neutralization: human CD34+cells were preincubated with anti–human CXCR4 neutralizing mAb 12G5 (10 μg per 0.5 × 106 cells, R&D Systems Inc.) and were injected into the tail vein without washing (0.5 × 106 to 0.6 × 106 CD34+ cells/mouse for homing assays, 2 × 105 CD34+ cells/mouse for engraftment assays). Mice were killed 4 hours, 16 hours, or 5–6 weeks after cell transplantation as indicated. Single-cell suspensions of liver tissues were washed thoroughly with PBS. Homing of human cells was determined as described (27), acquiring 1.5 × 106 cells/sample. Human cell engraftment in the murine liver was determined by Southern blot for human DNA using a human-specific α-satellite probe 5–6 weeks after transplantation (26).
For in vivo SDF-1 injection, nonirradiated NOD/SCID mice were anesthetized as described (27). Human SDF-1α (1 μg/mouse; PeproTech Inc., Rocky Hill, New Jersey, USA) was injected directly into the hepatic parenchyma before human cell transplantation. For liver injury, mice were injected intraperitoneally with 10, 15, or 30 μl/mouse of CCl4. Liver samples were collected within a few hours or 1–2 days later, as indicated. In homing assays, mice were intravenously transplanted with human mobilized PB CD34+ cells (0.6 × 106 cells/mouse) 4 hours before liver collection. Homing was blocked by preincubation of transplanted cells with 10 μg of anti-CXCR4/mouse or by intraperitoneal injection of 100 μg/mouse of MMP-2/MMP-9 Inhibitor III (Calbiochem-Novabiochem Corp., San Diego, California, USA). Human progenitors in the blood circulation of engrafted mice transplanted a month before with human CBMCs (2 × 107 cells/mouse) were quantified by seeding 2 × 105 PBMCs/ml for a CFU assay as described (27). CXCR4 expression was determined by flow cytometry.
ELISA for mouse SDF-1.
Liver extracts (of nonirradiated mice, or 24 and 48 hours after irradiation) were prepared by cell lysis with 25 mM Tris (pH 7.5), 1% Triton X-100, 0.5 mM EDTA, 150 mM NaCl, 10 mM NaF, and protease inhibitor cocktail (1% PMSF; Sigma-Aldrich, St. Louis, Missouri, USA). Total proteins in these extracts were quantified by Bradford assay and equal protein amounts were assayed for SDF-1 as described (28).
Immunohistochemical detection of human proteins.
Frozen 5-μm sections were fixed in acetone and air-dried and the endogenous peroxidase was blocked. The endogenous biotin was further blocked with the avidin-biotin blocking kit (Vector Laboratories Inc., Burlingame, California, USA). Endogenous mouse IgG was blocked using the Mouse-on-Mouse Immunodetection Kit (Vector Laboratories Inc.) according to the manufacturer’s instructions. The primary mAb, anti–human albumin, was diluted 1:50 in MOM working solution and incubated for 30 minutes at room temperature. The secondary Ab, biotinylated anti-mouse IgG1 raised in rabbit (Zymed Laboratories Inc., South San Francisco, California, USA), was diluted 1:100 in MOM working solution and applied for 30 minutes at room temperature. The sections were then incubated with the ABC complex (MOM; Vector Laboratories Inc.) and developed with DAB as substrate.
Five-micrometer sections of formaldehyde-fixed and paraffin-embedded NOD/SCID mouse liver tissue were used for the detection of human SDF-1, CD45, and albumin. The sections were deparaffinized and incubated with these primary Ab’s: mouse anti–human SDF-1 Ab K15C at 1:400; mouse anti–human albumin (1:5,000; Cedarlane Laboratories Ltd., Hornby, Ontario, Canada); and mouse anti–human CD45 (1:40; Dako Corp., Carpinteria, California, USA). Overnight incubation at 4°C was followed by incubation with biotin-labeled rabbit anti-mouse IgG (1:100; Dako Corp.) for 30 minutes at room temperature. The sections were then incubated with ABC complex (Vector Laboratories Inc.) and developed with DAB as substrate. They were counterstained in hematoxylin and covered with coverslips. To rule out false-positive signals contributed by damaged tissue and as a control, liver sections obtained from nontreated mice or from irradiated nontransplanted mice were labeled with human-specific anti-CD45 or anti–SDF-1 Ab. We did not observe human CD45-positive cells in these control tissues, and SDF-1 staining was restricted to the bile ducts (data not shown).
Detection of human albumin mRNA and protein.
Albumin mRNA was detected in total RNA extracted from frozen liver tissue using Trizol reagent (Invitrogen Corp., San Diego, California, USA) according to the manufacturer’s instructions. Reverse transcription was used to prepare cDNA with 2.5 μg DNA-free RNA, 0.5 μg oligo d(T) primer, and SuperScript II RT (Invitrogen Corp.). Reverse-transcribed products were amplified for albumin cDNA sequences using human albumin–specific primers 5′-AACGCCAAGTAAGTGACAGA and 3′-GAAAAAGAAAAACAGATGAA. Amplification was performed at 95°C for 10 minutes, 40 cycles of 94°C for 30 seconds, 50°C for 60 seconds, and 72°C for 60 seconds, with a final extension at 72°C for 7 minutes. PCR products were then separated by 1.0% agarose gel electrophoresis.
Human albumin was identified in protein extracts from the liver of engrafted mice by Western blot analysis using mouse monoclonal anti–human albumin Ab, IgG1 isotype (Cedarlane Laboratories Ltd.), which did not cross-react with mouse albumin. Briefly, aliquots of liver extract containing 50 μg protein were separated by electrophoresis on 10% SDS-polyacrylamide gels, the proteins were transferred to nitrocellulose membranes using the Trans-Blot Semi-Dry Transfer Cell (Bio-Rad Laboratories Inc., Hercules, California, USA), and the membranes were incubated with mouse monoclonal anti–human albumin (Cedarlane Laboratories Ltd.) at a dilution of 1:1,000, followed by incubation with rabbit anti-mouse IgG F(ab′)2 conjugated with alkaline phosphatase (Sigma-Aldrich) at a dilution of 1:3,000. Subsequent color development used the NBT/BCIP system (Bio-Rad Laboratories Inc.).
Zymography.
A whole liver was homogenized with 1 ml PBS, filtered, and centrifuged twice at 4°C at 1,500 g for 10 minutes. Supernatants were kept on ice and protein concentration was measured by Bradford protein assay (Bio-Rad Laboratories Inc.). Five micrograms of liver supernatant was loaded on 10% SDS-PAGE gels containing 1 mg/ml gelatin. Gels were rinsed for 30 minutes in 2.5% Triton X-100, washed with double distilled H2O, and incubated at 37°C for 16 hours with developing buffer consisting of 50 mM Tris (pH 8), 5 mM CaCl2, 200 mM NaCl, and 0.02% Brij (Sigma-Aldrich). Gels were stained with 0.25% Coomassie blue for 3 hours and destained with 5% acetic acid and 10% methanol.
Chemotaxis.
Migration of enriched CD34+cells toward a gradient of SDF-1 was determined by Transwell (Corning, Corning, New York, USA) assay as described (26). In one set of experiments, 10 ng/ml of SDF-1 was added to the lower chamber and conditioned medium of the cell line HT1080, enriched with secreted MMP-2 and MMP-9 (29), was added to the upper Transwells together with CD34+ cells. Cells were incubated with MMP-2/MMP-9 Inhibitor III (100 μM; Calbiochem-Novabiochem Corp.) for 30 minutes at 37°C before migration. When added together, HT1080-conditioned medium and MMP-2/MMP-9 inhibitor were preincubated together (30 minutes at 37°C) before they were added to the cells in the upper Transwell. In another set of experiments, CD34+ cells were incubated for 40 hours in RPMI 1640 supplemented with 10% FCS in the absence of human cytokines or with stem cell factor (SCF) (50 ng/ml; R&D Systems Inc.), HGF (100 ng/ml; PeproTech Inc.), or both cytokines before migration to 125 ng/ml of SDF-1.
Immunocytochemistry.
CB CD34+ enriched cells were incubated for 40 hours in RPMI 1640 supplemented with 10% FCS in the absence of cytokines or with SCF (50 ng/ml; R&D Systems Inc.), HGF (100 ng/ml; PeproTech Inc.), or both cytokines. Cells were plated on glass coverslips coated with fibronectin (10 μg/cm2; Calbiochem-Novabiochem Corp.) and incubated for 2 hours at 37°C in 5% CO2. Cells were then fixed for 25 minutes in 3% paraformaldehyde and permeabilized for 5 minutes in 0.5% Triton X-100, both in PBS. Cells were indirectly immunolabeled with rabbit anti–human CXCR4 polyclonal Ab (Chemicon International, Temecula, California, USA), washed extensively with PBS, and incubated with phalloidin-TRITC (Sigma-Aldrich) and goat anti-rabbit Alexa 488 (Molecular Probes Inc., Eugene, Oregon, USA). Following extensive washing with PBS, samples were mounted in Elvanol (Mowiol 4-88; Aventis, Strasbourg, France); all procedures were carried out in a humidified atmosphere at room temperature. Immunofluorescence was viewed and analyzed using a confocal microscope (Bio-Rad Laboratories Inc.) at a magnification of ×100.
Results
SDF-1/CXCR4 interactions mediate homing and engraftment of the liver by enriched human CD34+ progenitors in transplanted NOD/SCID mice.
To examine the role of SDF-1 in HSC recruitment to the liver, we transplanted irradiated NOD/SCID mice with human CD34+ enriched cells from mobilized peripheral blood or CB, with and without neutralizing CXCR4 Ab’s, and assayed their homing. CXCR4 neutralization significantly inhibited the homing of human CB or MPB CD34+ enriched cells to the BM, spleen, and liver of NOD/SCID recipients 16 hours after transplantation (Figure 1a). The more primitive, undifferentiated CD34+/CD38–/low cells, highly enriched for human HSCs (25) and cells with hepatic-like potential (9, 10), also require SDF-1/CXCR4 interactions for their migration to the murine liver (Figure 1b). Next, human SDF-1 was injected locally into the hepatic parenchyma of nonirradiated NOD/SCID recipients, followed by intravenous infusion of enriched human CD34+ cells. Human SDF-1 increased the homing of CD34+ progenitors, while neutralizing CXCR4 Ab’s almost completely abrogated their homing (Figure 1c). These findings suggest that local tissue expression of SDF-1 plays a chemotactic role in the migration of human stem and progenitor cells to the irradiated murine liver.
Figure 1.
SDF-1/CXCR4 interactions mediate homing and engraftment of irradiated NOD/SCID mouse liver by human CD34+ cells. (a) Homing of human CB or MPB enriched CD34+ cells to the murine BM, spleen (Spl), and liver is inhibited by neutralizing CXCR4. Data present inhibition as percentage of control. P ≤ 0.008, comparing anti-CXCR4–treated samples with their control counterparts. (b) A representative homing experiment shows human CD34+/CD38–/low homing cells (gated) in the liver of mice transplanted with nontreated cells (top), or CXCR4-neutralized cells (middle). A noninjected (Non-inj) mouse served as a negative control (bottom). Numbers indicate human homing cells/1.5 × 106 acquired cells. (c) Four-hour homing of CXCR4-neutralized or nontreated CD34+ cells to the liver of nonirradiated mice. Human SDF-1 was injected into the liver parenchyma as indicated. Cells were collected from the injected lobe to determine the homing of human CD34+ cells. (d) Human cell engraftment of the liver by CB nonstimulated CD34+ cells or CD34+ cells that migrated toward SDF-1, determined by Southern blot specific for human DNA. A representative blot is shown (*mouse transplanted with CD34+ cells migrating toward SDF-1). (e) Data as presented in d, summarizing three independent experiments (n = 37 mice). (f) SDF-1 levels in liver extracts of mice with no irradiation (ctrl), 24 hours and 48 hours after irradiation, determined by ELISA. Data summarize three experiments.
The levels of human DNA in the livers of NOD/SCID mice 5–6 weeks following transplantation with enriched human CB CD34+ progenitors were low but detectable (Figure 1, d and e). Transplantation of enriched CD34+ cells that were selected for their ability to migrate in vitro to a low concentration of SDF-1 prior to transplantation led to significantly higher levels of human DNA in the engrafted liver (Figure 1d, mouse 3, and Figure 1e, right). In contrast, no human DNA could be detected in the livers of mice coinjected with neutralizing CXCR4 Ab’s regardless of their migration potential, demonstrating an essential role for SDF-1/CXCR4 interactions in human HSC engraftment of the murine liver (Figure 1d, mouse 1 and mouse 2, and Figure 1e). Since migration of human progenitors to the murine liver requires SDF-1–mediated signaling, we measured the levels of this chemokine in the livers of irradiated and nontreated mice, and documented a significant increase in SDF-1 expression following total body irradiation (Figure 1f).
SDF-1 expression and human cell localization around the liver bile duct.
Five to six weeks after transplantation of human CD34+ cells, nonhepatic cells were identified in the murine liver adjacent to the bile ducts (Figure 2a). SDF-1 is highly expressed in bile duct epithelial cells of mice 5–6 weeks after irradiation and transplantation of human progenitors (Figure 2b). Of interest, large clusters of cells surrounding the bile ducts of engrafted mice were identified as human CD45+ cells (Figure 2c) in close proximity to the SDF-1–rich microenvironment (Figure 2b). Single human CD45+ cells were also observed in the liver sinusoids scattered throughout the parenchyma (Figure 2d). Since human liver inflammation due to HCV infection has been associated with increased BM-derived hepatic differentiation (2), we also stained normal and HCV-infected human livers for SDF-1 immunoreactivity. The HCV-infected liver displayed very high expression of SDF-1 due to extensive bile duct proliferation, and more importantly, expression of SDF-1 in the inflamed liver bile ductule and other nonparenchymal cells such as oval cells, canal of Hering, or endothelial cells, that were not observed in control healthy human liver (Figure 2, e and f). These results suggest that increased levels of SDF-1 bound to the liver bile duct epithelium and other nonparenchymal cells can attract and retain BM-derived hematopoietic cells within the liver. Of interest, this patient presented significant leukocytic infiltration in the inflamed liver, predominantly by lymphocytes, suggesting involvement of the increased SDF-1 levels in attraction of lymphocytes to the HCV-infected liver (data not shown).
Figure 2.

SDF-1 expression and engrafting cell accumulation within the liver. (a–d) NOD/SCID mice transplanted with CB CD34+ cells. (a) Hematoxylin and eosin staining 5–6 weeks after transplantation shows a bile duct (large arrow) adjacent to a large portal vein (pv) with cells surrounding the duct (dashed arrow) that are not observed in normal mouse liver. (b) Identification of SDF-1 in the bile ducts of NOD/SCID mouse liver. All epithelial cells in the bile ducts are strongly positive for SDF-1 (large arrow); scattered bile ductule cells (arrowheads) are also SDF-1–positive. (c) Human CD45+ hematopoietic cells (small arrows) are present in large numbers surrounding the bile ducts (large arrows) and accumulate to very high density between the ducts and the adjacent portal veins. (d) Human CD45+ cells are also observed as single cells or small clusters in random distribution in the hepatic sinusoids. Original magnification for a–d, ×400. (e and f) SDF-1 expression in normal adult human liver and in the liver of a patient with chronic hepatitis resulting from HCV infection was detected by immunohistochemistry. (e) Normal liver shows a single mature bile duct stained positive for SDF-1 (arrow) and absence of SDF-1 expression in endothelial cells lining a venous channel (arrowheads). (f) Liver from a patient with chronic liver disease from HCV infection shows extensive proliferation of bile ducts positive for SDF-1 expression (arrows) as well as expression of SDF-1 in bile ductule epithelium and/or canal of Hering or oval cells (arrowheads). Magnification for e and f, ×200.
Rare, hepatic-like potential of liver-engrafting human progenitor cells.
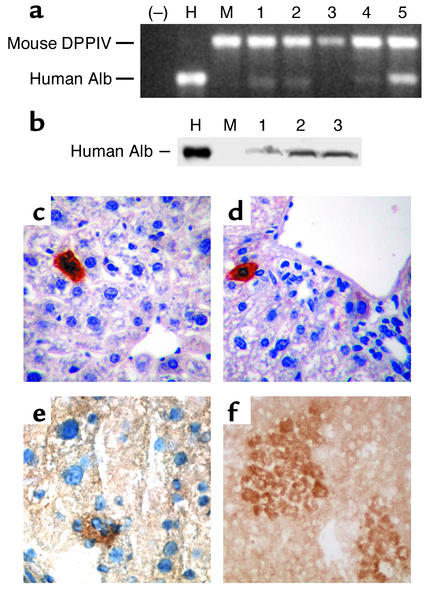
Low levels of human albumin mRNA and/or protein expression were present in more than 50% of engrafted murine livers as detected by RT-PCR and Western blot, respectively (Figure 3, a and b). Using quantitative PCR, the level of human albumin mRNA in the engrafted mouse liver was at or below 0.01% of that observed in normal human liver. Another hepatocyte-specific marker, human α1-antitrypsin mRNA, was also detected in engrafted mouse livers by RT-PCR using human-specific oligonucleotides (data not shown). The hepatic-like potential of CD34+ human-derived cells in the murine liver was also demonstrated by immunohistochemistry showing individual cells and small to medium-sized clusters of cells with hepatocytic morphology and human albumin expression in all sections examined (Figure 3, c–f). Although rare (1–2 cells or cell clusters per section showed human albumin expression in multiple sections), these cells were not present in control mouse livers (data not shown).
Figure 3.
Hepatic differentiation of human albumin–producing cells within the liver of transplanted NOD/SCID mice. (a) Detection of human albumin (Alb) mRNA by RT-PCR using human albumin–specific oligonucleotides and mouse dipeptidyl peptidase IV (DPPIV-specific) oligonucleotides as an internal control. (–), no RNA included; H, human liver RNA; M, RNA from a control, nontransplanted mouse liver; lanes 1, 2, 3, 4, and 5, RNA from the liver of mice transplanted with human CB CD34+ enriched cells. (b) Detection of human albumin by Western blot in the liver of NOD/SCID mice engrafted with human CD34+ CB cells using a mouse mAb specific for human albumin. Shown are a control human liver protein extract (H), liver protein extract from a control nontransplanted mouse (M), and extract from three mice transplanted with human CB CD34+ cells (lanes 1, 2, and 3). (c and d) Sections of formaldehyde-fixed, paraffin-embedded liver tissue analyzed for detection of human albumin using human albumin–specific mAb. (e and f) Sections of frozen liver stained for human albumin, as described in c and d, counterstained with hematoxylin (e) and not counterstained (f). Individual cells (c and d) and small to medium-sized clusters of cells (e and f) with the morphologic appearance of hepatocytes and expressing human albumin were present in the liver of NOD/SCID mice transplanted with human CB CD34+ cells, but not in the liver of control, nontransplanted NOD/SCID mice (data not shown). Original magnification: c, ×200; d, ×100; e and f, ×400.
Stress-induced MMP-2/MMP-9 expression recruits CXCR4+ hematopoietic progenitors to the injured liver.
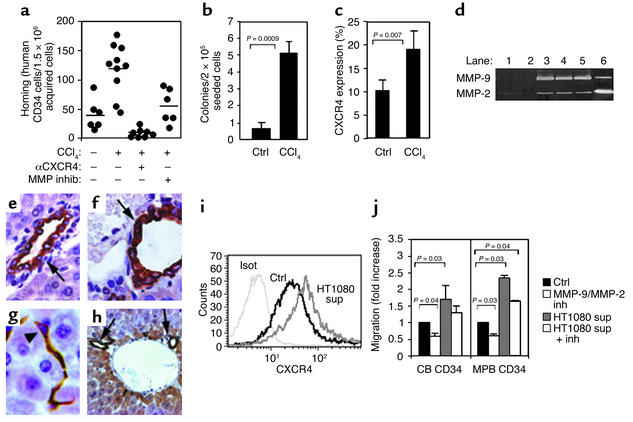
Liver injury has been found to increase the levels of transplanted rodent BM progenitor cells exhibiting a hepatic phenotype in the rat and murine liver (4–6). CCl4-induced liver injury 1 month after transplantation, in combination with HGF stimulation, significantly increased the levels of hepatic-like differentiation and human albumin production in immune-deficient NOD/SCID and NOD/SCID/B2m null mice engrafted with human CD34+ and CD34+/CD38– progenitors, revealing less than 1% human albumin–producing cells in the murine liver 2 months after transplantation (10); this result was supported in another report using a different protocol (13). In our studies, a single injection of CCl4 rapidly induced increased homing of enriched human CD34+ cells to the livers of treated mice in a CXCR4-dependent manner (Figure 4a). CCl4-mediated liver injury also induced the recruitment of human colony–forming progenitors from the BM to the circulation of engrafted NOD/SCID mice (Figure 4b). Unexpectedly, we noticed increased levels of CXCR4 expression on human MNCs in the circulation of CCl4-treated mice (Figure 4c). In addition, CCl4 treatment resulted in increased activity of the proteolytic enzyme MMP-2 and emergence of MMP-9 in the liver of treated NOD/SCID mice (Figure 4d). However, SDF-1 was still strongly expressed on bile duct epithelium (Figure 4e–h, see arrows in Figure 4f and 4h), with additional expression in bile ductule, canal of Hering, and undifferentiated oval cells that are induced to proliferate during liver injury (30) (arrowhead in Figure 4g). This pattern of SDF-1 expression was not detected in livers obtained from control noninjured or nonirradiated mice (Figure 4e). Supernatants from a human cell line (HT1080) that secretes MMP-2 and MMP-9 were also found to increase surface CXCR4 expression on enriched human CD34+ cells (Figure 4i). Moreover, this MMP’s enriched supernatant significantly increased SDF-1–mediated migration of human progenitors in vitro and was inhibited by a specific MMP-2/MMP-9 inhibitor (Figure 4j), demonstrating that these proteolytic enzymes directly affect the motility of enriched human CD34+ progenitors. This inhibitor also reduced the migration of human CD34+ progenitors to the injured liver (Figure 4a), demonstrating a central role for these proteolytic enzymes in SDF-1–mediated recruitment of hematopoietic progenitors to sites of inflammation in the injured liver.
Figure 4.
Stress-induced MMP-2 and MMP-9 increase progenitor cell motility and recruitment to the liver. (a) Four-hour homing of human MPB CD34+ cells to the liver of nonirradiated mice injected 24 hours earlier with 15 μl of CCl4. Anti-CXCR4 pretreatment of transplanted cells or intraperitoneal injection of MMP-2/MMP-9 inhibitor (1.5 hours before transplantation). Bars indicate the mean number of homing cells per 1.5 × 106 acquired cells (n = 3 experiments). (b and c) PBMCs of chimeric mice 1 day after injection of 10 μl CCl4 assayed for the level of human progenitors (b) and human CXCR4 expression (c), (n = 3 experiments). (d) Representative zymography shows MMPs activity in the mouse liver. Control mice (lanes 1 and 2), mice 1 day after injection of 15 μl CCl4 (lane 3), 2 days after 30 μl CCl4 (lane 4), 2 days after 15 μl CCl4 (lane 5), and conditioned media of the human cell line HT1080 (lane 6). (e–h) SDF-1 immunostaining of untreated mouse liver (e) or mouse liver after injection of CCl4 (f–h). Arrows indicate positively stained bile duct epithelium. Arrowhead indicates positively stained bile ductule or canal of Hering oval cells. Original magnifications: ×1,008, ×1,575, ×1,575, and ×500, respectively. (i) CB CD34+ cells were incubated for 5 hours before CXCR4 staining: isotype control (Isot), cells cultured with RPMI 1640 (Ctrl), cells cultured with HT1080 conditioned media (sup). Representative data of three experiments. (j) Transwell migration toward SDF-1 with CB and MPB CD34+ cells, preincubated with RPMI 1640 (Ctrl), MMP-2/MMP-9 inhibitor (inh), HT1080 conditioned media (sup), or HT1080 supernatant together with MMP-2/MMP-9 inhibitor. Data represent fold-increased migration compared with control cells.
Increased HGF-mediated motility and CXCR4-mediated migration by human CD34+ progenitors.
HGF is upregulated in the injured liver following CCl4 administration (31), and the addition of human HGF increases the levels of human albumin–producing cells in CCl4-injured livers of engrafted immune-deficient murine chimeras (10). We therefore hypothesized that HGF may also participate in the regulation of human CD34+ cell migration and recruitment to the injured liver. Enriched CB CD34+ cells were cultured for 40 hours in the absence of cytokines or in the presence of either SCF, which has been shown to induce CXCR4 expression and SDF-1–dependent migration (26), HGF, or a combination of both cytokines. While CD34+ cells cultured without the addition of these cytokines maintain a round shape, cells cultured with SCF are spread and polarized (Figure 5a). Interestingly, HGF by itself induced formation of actin-based protrusions from the cell surface (arrowhead), and the combination of SCF and HGF promoted lamellipodia formation, a phenotype distinct from that observed with SCF or HGF alone (Figure 5a, arrow). Most importantly, these cytoskeletal rearrangements are associated with CXCR4 upregulation (Figure 5b) and a functionally enhanced chemotactic response to SDF-1 (Figure 5c). HGF by itself did not induce chemotaxis of human progenitors (data not shown); however, HGF increased the motility of human progenitors and synergized with SCF to potentiate both CXCR4 expression and SDF-1–induced directional migration. These unexpected findings suggest an important role for HGF in facilitating motility and directional migration of human CD34+ cells in response to injured liver stress signals.
Figure 5.
HGF facilitates CD34+ cell motility, CXCR4 expression, and SDF-1–mediated directional migration. CB CD34+ cells were cultured for 40 hours in RPMI 1640 supplemented with 10% FCS alone (Ctrl) or in the presence of SCF (50 ng/ml), HGF (100 ng/ml), or SCF plus HGF. (a) CD34+ cultured cells indirectly immunolabeled with anti-CXCR4 Ab (green) and stained for polymerized actin (red). Merged images are also presented. Arrowhead indicates cell surface protrusion. Arrow indicates lamellipodia. (b) CXCR4 expression analyzed by flow cytometry. (c) Transwell migration toward a gradient of SDF-1 with CB CD34+ cultured cells. Data represent percentage of migration. (–) indicates spontaneous migration without SDF-1.
Discussion
Recent studies have demonstrated the rare potential of primitive human BM and CB CD34+/CD38– purified subpopulations to engraft the irradiated or injured liver of transplanted NOD/SCID mice and to differentiate into hepatocyte-like cells that express liver-specific proteins (9, 10). In the current research, we provide mechanistic insights concerning stress-induced migration and recruitment of enriched human CB and MPB CD34+ cells to the irradiated or injured liver and reveal for the first time the central roles of the chemokine SDF-1, the proteolytic enzymes MMP-2 and MMP-9, and the cytokine HGF in these processes. Stress can act to facilitate tissue-specific differentiation and provide an advantage to transplanted hematopoietic cells, as others have shown (6), and can also act to induce secretion of signaling mediators that increase migration and guide transplanted cells to the injured organ, as our findings reveal. These sequential events are regulated at least in part by the same mediators, such as HGF, which participates both in human CD34+ progenitor migration and development (10–12).
SDF-1 is locally increased within the BM following total body irradiation or chemotherapy (32), suggesting that it also serves as a stress signal for circulating cells, recruiting them to the damaged tissue. We found increased SDF-1 expression in the liver following total body irradiation. Moreover, in human HCV-infected liver or the injured murine liver, SDF-1 distribution is extended to bile ductule, canal of Hering, and oval cells. Interestingly, SDF-1 on bile duct epithelium of CCl4-treated mice is positively immunostained using the K15C mAb, which binds the signaling N-terminus of the chemokine. Proteolytic cleavage associated with inactivation of SDF-1 was demonstrated by several degrading enzymes (28, 33, 34) including MMP-2 and MMP-9, which cleave the N-terminus (35). In CCl4-treated livers, SDF-1 levels measured by ELISA were only slightly reduced (data not shown). Together, these results imply there is a protective microenvironment or a rapid replacement of proteolytically cleaved chemokine by the liver epithelium and increased expression during liver injury/inflammation. Of interest, using the same Ab during G-CSF–induced mobilization, SDF-1 levels in the BM were significantly reduced (28), documenting differences in stress-induced chemokine expression. Nevertheless, the possibility of SDF-1–positive staining but not function within the CCl4-treated liver has to be considered as well.
A local increment in SDF-1 levels facilitated homing to the liver, whereas impairing SDF-1/CXCR4 interactions abolished both homing and engraftment of this organ by enriched human CD34+ cells. Accumulation of human CD45+ cell clusters around the bile duct/SDF-1–rich microenvironment also suggests involvement of SDF-1/CXCR4 interactions in retention of human progenitors in the treated liver. These interactions mimic those observed between HSCs and the SDF-1–rich BM endosteum region in which hematopoietic progenitors are localized in close proximity to this chemokine (32, 36, 37), which is also a survival factor for both human and murine progenitors (38, 39).
Proteolytic enzymes such as MMPs are widely involved in matrix degradation in the context of motility and in vivo migration of normal and malignant cells. Rat MMP-9 and MMP-2 were activated following CCl4-mediated liver injury (40). In CCl4-treated NOD/SCID mice, we also detected increased MMP-2 secretion and emergence of MMP-9. Could stress-induced secretion of MMPs in the liver transmit signals for hematopoietic cell migration and recruitment to this organ? CCl4 treatment triggered mobilization of human progenitors from the murine BM into the blood circulation, accompanied by increased CXCR4 expression. In addition, we found increased CXCR4-dependent homing of human CD34+ cells to the liver of CCl4-treated mice; this homing was significantly reduced by a specific MMP-2/MMP-9 inhibitor. MMP secretion in response to CCl4 is related to liver sinusoidal stellate cells (41) or other nonparenchymal cells such as hematopoietic monocyte/macrophage/dendritic cells infiltrating the damaged liver (40). MMP-2 and MMP-9 can also increase recruitment of CD34+ cells via other pathways, such as shedding of membrane-bound SCF and autocrine secretion of these enzymes by CD34+ progenitors in response to SDF-1 stimulation (42, 43).
Acute liver injury triggers the expression of other factors, such as the pleiotropic cytokine HGF (31). We demonstrate for the first time that HGF also induces cytoskeleton rearrangement and increases the motility of and potentiates the response of immature CD34+ cells to SDF-1 signaling by inducing CXCR4 upregulation and synergizing with SCF. Interestingly, stellate cells that line the liver sinusoids and secrete MMPs are also the major source of SCF (44) and of HGF production in the liver following CCl4 injury (31). Thus, stellate cells may orchestrate stress signals that recruit CD34+/CXCR4+ progenitor cells from the blood circulation into the SDF-1–rich microenvironment of the liver. While irradiation alone mediated a low frequency of human hepatocyte-like cells (9), significant amplification of human albumin–producing cells in the murine liver was achieved by Wang et al. (10) by combining CCl4 injury with exogenous human HGF administration. We report that in addition to inducing hepatocyte proliferation, HGF also contributes to the recruitment of human CD34+ stem cells to the injured liver, which is an essential first step in primitive human CD34+/CD38– cell differentiation into albumin-producing cells.
Thus, we suggest a general model of regulatory cross-talk between injured tissues and HSCs that is activated by stress signals that increase migration and recruit HSCs to damaged organs and contribute to their repair. SDF-1 was recently shown to be involved in liver allograft rejection (25), ischemic brain (which also displays an expanded distribution of SDF-1 expression to the inflamed endothelium) (45), myocardial infarction (46), and oval cell proliferation (47). We propose SDF-1 as a key molecule in regulation of HSC migration and development. While SDF-1 is widely expressed in many tissues, a local increase in membrane-bound expression of this chemokine, including by ductule epithelial cells, oval cells, and canal of Hering, together with specific local or circulating stress signals, enhances the motility and response sensitivity of CD34+ progenitors to SDF-1 signaling. Together with proteolytic enzymes such as MMP-2 and MMP-9 and cytokines produced by the injured organs, such as HGF and SCF, these factors can amplify the levels of migrating progenitors in the circulation and navigate them to damaged tissue. Our model suggests that an interplay between cytokines, chemokines, and proteolytic enzymes regulates both the migration of HSCs to the injured liver and their differentiation as part of liver repair. This study also demonstrates the potential of human CD34+ stem cells to respond to stress signals from injured/inflamed nonhematopoietic organs such as the liver, and suggests stem cell recruitment to nonhematopoietic organs as an important physiological process.
Acknowledgments
Tsvee Lapidot holds the Pauline Recanati Career Development Chair of Immunology. This work was supported in part by grants from the Ares Serono group, Israel Science Foundation, Minerva Foundation, the Gabriela Rich Center for Transplantation Biology (to T. Lapidot), and NIH (grant R01 DK-17609 to D.A. Shafritz). Special thanks to Cindy Dunbar, Dov Zipori, and Amiela Globerson for critically reviewing this manuscript, and to Norman Iscove and John Dick for fruitful discussions.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: hematopoietic stem cell (HSC); bone marrow (BM); cord blood (CB); hepatitis C virus (HCV); stromal cell–derived factor-1 (SDF-1).
References
- 1.Alison MR, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 3.Korbling M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N. Engl. J. Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 4.Petersen BE, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 5.Theise ND, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 6.Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 8.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 9.Danet GH, et al. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10441–10445. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, et al. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weimar IS, et al. Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+) Exp. Hematol. 1998;26:885–894. [PubMed] [Google Scholar]
- 12.Fiegel HC, et al. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- 13.Kakinuma S, et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 16.Mallet VO, et al. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002;35:799–804. doi: 10.1053/jhep.2002.32530. [DOI] [PubMed] [Google Scholar]
- 17.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev. Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 20.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai K, et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone marrow. Br. J. Haematol. 1999;106:905–911. doi: 10.1046/j.1365-2141.1999.01644.x. [DOI] [PubMed] [Google Scholar]
- 22.Pablos JL, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am. J. Pathol. 1999;155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirozu M, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 25.Goddard S, et al. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72:1957–1967. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- 26.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 27.Kollet O, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 28.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 29.Ginestra A, et al. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J. Biol. Chem. 1997;272:17216–17222. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 30.Oh SH, Hatch HM, Petersen BE. Hepatic oval ‘stem’ cell in liver regeneration. Semin. Cell Dev. Biol. 2002;13:405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- 31.Armbrust T, Batusic D, Xia L, Ramadori G. Early gene expression of hepatocyte growth factor in mononuclear phagocytes of rat liver after administration of carbon tetrachloride. Liver. 2002;22:486–494. doi: 10.1034/j.1600-0676.2002.01731.x. [DOI] [PubMed] [Google Scholar]
- 32.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela-Fernandez A, et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J. Biol. Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 34.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J. Clin. Invest. 2003;111:187–196. doi:10.1172/JCI200315994. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuibban GA, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 36.Weiss L, Geduldig U. Barrier cells: stromal regulation of hematopoiesis and blood cell release in normal and stressed murine bone marrow. Blood. 1991;78:975–990. [PubMed] [Google Scholar]
- 37.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 38.Lataillade JJ, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- 39.Broxmeyer HE, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J. Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 40.Knittel T, et al. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem. Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 41.Nieto N, et al. Rat hepatic stellate cells contribute to the acute-phase response with increased expression of alpha1(I) and alpha1(IV) collagens, tissue inhibitor of metalloproteinase-1, and matrix-metalloproteinase-2 messenger RNAs. Hepatology. 2001;33:597–607. doi: 10.1053/jhep.2001.22520. [DOI] [PubMed] [Google Scholar]
- 42.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp. Hematol. 2000;28:1274–1285. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 44.Gaca MD, Pickering JA, Arthur MJ, Benyon RC. Human and rat hepatic stellate cells produce stem cell factor: a possible mechanism for mast cell recruitment in liver fibrosis. J. Hepatol. 1999;30:850–858. doi: 10.1016/s0168-8278(99)80139-1. [DOI] [PubMed] [Google Scholar]
- 45.Stumm RK, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 47.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1(alpha)/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]