Abstract
Objective: Arthrogenic muscle inhibition (AMI) is a presynaptic, ongoing reflex inhibition of joint musculature after distension or damage to the joint. The extent to which therapeutic interventions affect AMI is unknown. The purpose of this study was to verify that the vastus medialis (VM) is inhibited using the knee joint effusion model and to investigate the effects of cryotherapy and transcutaneous electric nerve stimulation (TENS) on AMI using this model.
Design and Setting: A 3 × 6 analysis of variance was used to compare Hoffmann-reflex data for treatment groups (cryotherapy, TENS, and control) across time (preinjection, postinjection, and 15, 30, 45, and 60 minutes after injection).
Subjects: Thirty neurologically sound volunteers (age = 21.8 ± 2.4 years; height = 175.6 ± 9.6 cm; mass = 71.5 ± 13.3 kg) participated in this study.
Measurements: Hoffmann-reflex measurements were collected using a percutaneous stimulus to the femoral nerve and surface electromyography of the VM.
Results: Hoffmann-reflex measurements from the cryotherapy and TENS groups were greater than measurements from the control group at 15 and 30 minutes after injection. Measurements from the cryotherapy group were greater than for the TENS group, and measurements for the TENS group were greater than those for the control group at 45 minutes. At 60 minutes, the cryotherapy group measurements were greater than the TENS and control group measures. Measurements at 15, 30, 45, and 60 minutes after injection were reduced compared with the preinjection and postinjection measurements in the control group. Measurements in the cryotherapy group at 30, 45, and 60 minutes were greater than the preinjection, postinjection, and 15-minute data. No differences between time intervals existed in the TENS group.
Conclusions: Artificial knee joint effusion results in VM inhibition. Cryotherapy and TENS both disinhibit the quadriceps after knee joint effusion, and cryotherapy further facilitates the quadriceps motoneuron pool. Cryotherapy treatment resulted in facilitation of the VM motoneuron pool during the post-treatment phase. The TENS treatment failed to disinhibit the VM motoneuron pool by 30 minutes postinjection.
Keywords: Hoffmann reflex, joint effusion, neuromuscular
Arthrogenic muscle inhibition (AMI) is a presynaptic, ongoing reflex inhibition of musculature surrounding a joint after distension or damage to that joint.1–4 Arthrogenic muscle inhibition is a natural response designed to protect the joint from further damage. The presence of AMI retards rehabilitation despite complete muscle integrity. However, if the affected joint can be protected from further damage, active exercise can be employed to expedite the rehabilitation process. Early active exercise in the rehabilitative process is essential for decreased healing time,5 increased structural strength and stiffness of ligaments,6 increased collagen synthesis in tendons,7 increased proteoglycan content in articular cartilage,8 and periosteal expansion of bone tissue.9
Arthrogenic muscle inhibition not only slows strength gains during rehabilitation, it also slows gains in proprioception10,11 and increases susceptibility to further injury.3,4,10–12 Many therapeutic techniques have been developed to safely increase strength and neuromuscular control during joint rehabilitation, but these techniques are of little benefit if we cannot overcome AMI.
Several researchers13–18 have shown a decrease in both muscle force output and motoneuron recruitment of the quadriceps using an experimental knee joint effusion model. This model, involving the injection of saline into the knee joint capsule to mimic effusion, allows for a simple, controlled measure of AMI. A pretest or baseline Hoffmann-reflex (H-reflex) measurement is obtained before injection and compared with the postinjection measurement. The H reflex is a measure of motoneuron pool recruitment. A percutaneous stimulus is applied to a mixed nerve, resulting in depolarization of large afferents and, ultimately, depolarization of motoneurons in the anterior horn of the spinal cord. This results in a twitch contraction of the effector muscle, which can be measured by electromyography (EMG). As the stimulus is increased, more afferent fibers reach threshold, and more motoneurons are recruited within the motoneuron pool.1,19 This is represented by an increased amplitude in the twitch contraction.
The difference between the maximum baseline H-reflex measurement and postinjury measurement represents inhibition caused by excitation of mechanoreceptors within the knee joint capsule4,11,20 and stimulation of the Ib inhibitory interneurons.19 Inhibition, or a decrease in the availability of motoneurons within a pool, is reflected by a decreased H reflex, while facilitation is represented by an increased H reflex. The effusion model allows for mechanical inhibition in the absence of perceived pain.2 Pain is a confounding variable in injury that is difficult to control.2
Despite evidence that AMI exists, few clinicians or researchers3,4,21 have even attempted to suggest ways to overcome or neutralize AMI in a clinical setting. Cryotherapy or transcutaneous electric nerve stimulation (TENS) may be effective therapeutic interventions in slowing or modifying AMI. According to Knight,22 ice not only decreases general nerve conduction velocity, muscle spasm, and pain, but it has a definite slowing and blocking effect on sensory nerve fibers at certain nerve tissue temperatures (10°C). The relationship appears to be linear; the cooler the nerve becomes, the more slowly the impulse is carried. It seems that any cooling of a mixed nerve would have the same effect on both motor and sensory nerves, but the results of studies exploring the effects of cryotherapy on strength and torque output are varied.22–27 Furthermore, cryotherapy seems to have no effect on more functional measures such as agility.28 Clinically, cooling an acutely sprained ankle improves the patient's ability to perform active exercise.22 These factors suggest that cooling has a greater effect on the sensory function of the peripheral nervous system than on the motor component.
Transcutaneous electric nerve stimulation is an intervention that could reduce AMI by postsynaptically inhibiting the Ib inhibitory interneurons. This process would decrease activity of the interneurons responsible for mediating inhibition of the motoneuron pool and decrease AMI. Iles29 reported that stimulation of cutaneous nerve branches reduced presynaptic inhibition of the soleus muscle. Transcutaneous electric nerve stimulation has been shown to produce a small increase in quadriceps maximum voluntary contraction after anterior cruciate ligament reconstruction and after open meniscectomy.30,31 Arvidson et al30 attributed this effect to a small decrease in pain, but Stokes et al31 showed some dissociation between pain and AMI.
The purpose of this study was to verify that the quadriceps are inhibited as measured by H reflex using the knee joint effusion model and to investigate the effects of cryotherapy and TENS on AMI using this model.
METHODS
A 3 × 6 factorial design was used to compare treatment groups across time intervals. The independent variables included treatment groups (cryotherapy, TENS, and control) and measurement intervals (pretreatment, post-treatment, and 15, 30, 45, and 60 minutes post-treatment). The dependent variable was the H reflex. Control variables included surface temperature measurements of the knee and EMG electrode sites.
Subjects
Volunteers (age = 21.8 ± 2.4 years; height = 175.6 ± 9.6 cm; mass = 71.5 ± 13.3 kg) were 30 (19 male, 11 female) neurologically sound, physically active college students with no lower extremity conditions resulting in surgery in the last 2 years, no lasting lower extremity pathology in the previous 6 months, and a measurable vastus medialis (VM) H-reflex measurement. Each subject was determined to be free from neurologic disorders and symptoms through a preparticipation questionnaire. A preparticipation questionnaire and an H reflex screening were used to assess other inclusion criteria. Thirty-two subjects were excluded because a measurable VM H reflex could not be obtained due to instrumentation limitations. Subjects gave informed consent to participate in this study. Human subject approval was obtained from the School of Health and Human Performance Human Subjects Committee at Indiana State University.
Instrumentation
H-reflex measurements were collected using surface EMG (MP100, BIOPAC Systems Inc, Santa Barbara, CA). Signals were amplified (DA100B, BIOPAC Systems Inc) from disposable, pregelled Ag-AgCl electrodes. The EMG measurements were collected at 2000 Hz. The BIOPAC stimulator module (STM100A, BIOPAC Systems Inc) was used with a 200-volt (maximum) stimulus isolation adapter (STMISOB, BIOPAC Systems Inc) and a shielded bar electrode (EL503, BIOPAC Systems Inc).
Surface temperature measurements were collected using a portable Datalogger (MSS-3000, Commtest Instruments Ltd, Christchurch, New Zealand) with 30-gauge, exposed-junction thermocouples with Kapton insulated leads (TX-31, Columbus Instruments, Columbus, OH).
Orientation
A 30-minute orientation and screening was held for all volunteers approximately 1 week before testing. A general explanation of the study and its significance was given, along with an explanation of the measurement protocol. Vastus medialis H-reflex measurements were recorded to ensure that the volunteer had a measurable H reflex for data collection. If a maximum measurable H reflex was obtained, all risks involved in the study were explained, and the subject was randomly assigned to a group (control, cryotherapy, or TENS). If no measurable H reflex could be obtained, the subject was dismissed.
Subject Preparation
Two locations were shaved, debrided (abraded with fine sandpaper), and cleaned with isopropyl alcohol for application of the EMG electrodes (10-mm Ag-AgCl, BIOPAC Systems Inc) for each volunteer. Surface electrodes were centered on the greatest bulk of the VM, superomedial to the patella, as found during an isometric contraction. The electrodes were placed in line with the muscle fibers and spaced 2 cm apart from center to center. The ground location was on the ipsilateral medial malleolus.
A stimulating electrode was placed over the femoral nerve in the femoral triangle of the test leg. First, the femoral pulse was located. The electrode was then placed over the femoral nerve, located just lateral to the femoral artery. Adhesive collars were applied to each pole of the stimulating electrode to maintain the position over the nerve for the duration of the data collection. An elastic wrap was applied over the electrode and around the waist to apply pressure and to hold the electrodes in place during measurements.
Thermocouples were placed on the center of the patella and on the skin lateral to the junction of the 2-surface EMG electrodes. The thermocouples were held in place with a 4-cm strip of athletic tape.
H-Reflex Procedure
The volunteer was positioned supine with the knee and hip slightly flexed and the heel of the involved leg resting in a secure pad, designed to keep the heel stable and the lower extremity in a fixed position while at rest. Factors such as head position, eye position, and hand movements may affect H-reflex amplitude.32 For this reason, every attempt was made to control positions and movements of the entire body. Subjects placed their open hands at their sides. They focused on a small picture on the ceiling with their heads forward, and they listened to wave sounds through headphones. In previous work on soleus H-reflex measurements, this protocol resulted in excellent reliability (intraclass correlation coefficient [3,1] = 0.938).33
A series of short duration (0.3-millisecond), high-intensity (100 to 200 V) stimuli with 20-second rest intervals was delivered to the volunteer with varying amplitudes in order to find the maximum H-reflex. These stimuli were delivered using a trial-and-error method for finding the peak H-reflex. Stimuli were increased in 2-volt increments. The number of trials necessary to find a maximum H reflex ranged from 5 to 12. A maximum H reflex was present (Figure 1) in the near absence of a direct motor response (M response), and as the M response increased, the H reflex decreased. The peak of the H reflex was between 19 and 23 milliseconds, while the M response was between 8 and 15 milliseconds (Figure 1). With the stimulating amplitude set at the maximum H-reflex level, 5 measurements were taken, with a 20-second rest period between measurements.
Figure 1.
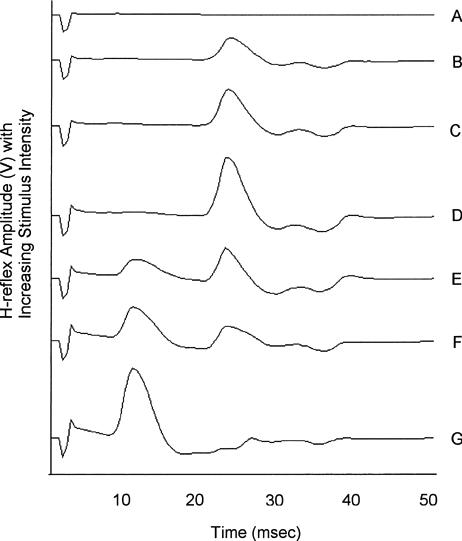
Succession of H-reflex measurements with increasing stimulus intensity. H waves (B-F) are located at approximately 24 milliseconds preceded by M waves (E-G) at approximately 10 milliseconds. D represents a maximum H-reflex measurement.
Joint Effusion Procedure
An area superolateral to the patella was cleaned with alcohol and Betadine (Purdue Frederick Co, Norwalk, CT). Using a sterile, disposable syringe, a physician injected 2 cc of lidocaine subcutaneously for anaesthetic purposes. With a second disposable syringe, 60 mL of sterile saline was injected into the superolateral knee joint capsule. The physician performed an effusion wave and ballotable patella test to ensure that the effusion was within the knee joint capsule. The physician wore a new pair of sterile disposable gloves for each volunteer. All materials were disposed of in the proper containers according to Occupational Safety & Health Administration guidelines.34
Testing Procedure
Three volunteers, 1 from each treatment group (control, cryotherapy, and TENS), reported for each injection session. Electrode-placement sites were prepared as previously described. A baseline H-reflex measurement (preinjection) was recorded for each volunteer. Each volunteer was injected with saline. Once the effusion was confirmed, H-reflex measurements (postinjection) were recorded, followed by measurements every 15 minutes for 1 hour. Measurement time allotments for each subject were 5 minutes, maintaining a 15-minute measurement session for each time interval. Subjects in the treatment groups had a treatment applied immediately after the postinjection measurement and removed before the 30-minute measurement.
Cryotherapy Group
After the postinjection H-reflex measurement, 2 plastic bags containing 1.5 L of partially crushed ice were placed directly on the anterior and posterior surfaces of the knee, at least 3 cm distal to the VM electrodes. The ice remained on the knee for 30 minutes. H-reflex measurements were recorded at 15-minute intervals during the cryotherapy treatment. Subsequent measurements were recorded at 30 minutes (immediately after ice removal). Measurements taken at 45 and 60 minutes represented a post-treatment phase.
Surface temperature measurements were recorded from the knee and EMG electrode sites on the VM at each interval. These measurements were recorded to ensure that cooling was not taking place at the EMG electrode sites, which could potentially affect the measurement.
TENS Group
Four 3 × 3 cm reusable adhesive electrodes (Axelgaard Manufacturing Co Ltd, Fallbrook, CA) were applied to the superior anterolateral and anteromedial and the inferior anterolateral and anteromedial areas of the knee with approximately 5 to 7 cm distance between them, forming a square around the patella.35 A typical TENS protocol was used, including a continuous, asymmetric, biphasic square-pulse wave with a pulse width of 100 and a pulse rate of 120.35 The stimulus intensity was increased until a visible contraction of the VM was apparent. The intensity was then decreased until no contraction was seen or felt by the volunteer. The treatment session lasted 30 minutes, with measurement intervals mimicking those of the cryotherapy treatment. The TENS treatment was briefly terminated during the 15-minute measurement so it would not interfere with the H-reflex amplitude.
Control Group
Measurements were recorded at each of the intervals (preinjection, postinjection, and 15, 30, 45, and 60 minutes postinjection). Subjects in all groups remained in a supine position on the treatment table throughout the entire data-collection session. No treatment or intervention was applied to the control group.
Statistical Analyses
Means were computed from the 5 trials of each measurement for statistical analysis. A 2-way analysis of variance with repeated measures on time was performed to test for overall differences between treatment groups over time, and the Tukey honestly significant difference test was used for post hoc comparisons (P < .05 for all tests). Descriptive statistics were computed for temperature data.
RESULTS
H-reflex (V) means for each time interval and treatment group (Table) are expressed as percentage change from the preinjection measurement (Figure 2). An overall difference was detected in H-reflex measures between treatment groups over time (F 10, 135 = 13.68, P ≤ .0001). At 15 and 30 minutes, the control group had lower H-reflex amplitudes than the cryotherapy and TENS groups (Tukey, P < .05). At 45 minutes, H-reflex amplitudes of all groups were different; cryotherapy was greater than TENS, which was greater than control (Tukey, P < .05). At 60 minutes, the H-reflex amplitudes of the cryotherapy group were greater than those of the TENS and control groups (Tukey, P < .05). The 15-, 30-, 45-, and 60-minute H-reflex measurements decreased compared with the preinjection and postinjection measurements in the control group (Tukey, P < .05). H-reflex measurements at 30, 45, and 60 minutes increased compared with the preinjection, postinjection, and 15-minute measurements in the cryotherapy group (Tukey, P < .05). No differences were found between time intervals in the TENS group.
Table 1.
H-reflex (V) Amplitudes (Mean ± SD) for Each Group Over Time
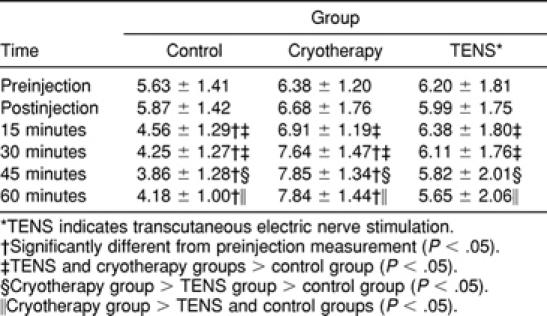
Figure 2.
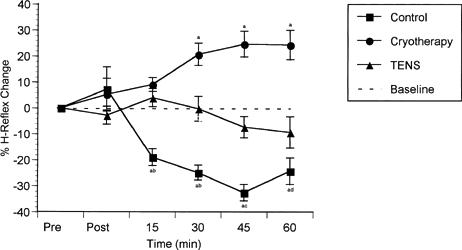
H-reflex amplitudes over time expressed as percentage change from the preinjection measurement (±SE). Values greater than 0 indicate facilitation; values less than 0, inhibition.
Descriptive statistics of surface temperature measurements from the anterior surface of the knee and from the EMG electrode site (Figure 3) suggest that the temperature remained constant at the EMG electrode sites, ensuring that the EMG measurement was not affected by possible cooling of the tissue beneath the electrodes.
Figure 3.
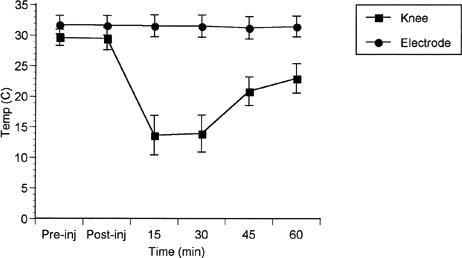
Surface temperatures collected from the anterior knee and the EMG electrode sites.
DISCUSSION
Knee effusion has been shown to cause inhibition of the quadriceps muscle.13,15–17,36–39 Our control group data support this finding (Figure 2). This inhibition is likely the result of increased activity of slowly adapting Ruffini endings in the knee joint capsule.2,17,40 Activity from these receptors stimulates Ib inhibitory interneurons,41 resulting in inhibition of the quadriceps motoneuron pool29,42,43 and facilitation of the hamstrings and triceps surae.2,9,19,44 The Ib interneurons seem to be the integration point for sensory information received from joint mechanoreceptors, resulting in AMI.2
H-reflex measurements were used in this study to compare the availability of motoneurons within the quadriceps motoneuron pool before effusion and over a period of time after joint effusion. A change in the excitability of the motoneuron pool is represented by a change in H-reflex amplitude as more or fewer motoneurons are stimulated from a given stimulus intensity. The H-reflex measurement, which was taken at rest, was very reliable within (ICC[3,1] = .94) and between (ICC[3,1] = .93) sessions using our protocol.33 This measurement does not directly equate to changes in functional strength, but it does equate to state changes to the motoneuron pool, which affect strength, muscle wasting, and mobilization.4
The effusion model was chosen to mimic injury without the effects of perceived pain. Pain is often blamed for inhibition.45 While pain undoubtedly plays some role in AMI, the joint effusion model demonstrates that quadriceps inhibition occurs in the absence of pain.17,38 In previous work, mean pain scores after knee joint effusion were 1.18 ± 0.82 out of 78 possible points using the McGill pain questionnaire.2 “Tight” was one of the McGill pain questionnaire terms that could be selected to describe pain and was the term chosen most often.2 These data were collected after the injection of saline at each H-reflex measurement. They were not representative of the injection itself, only the effusion created by the injection. The knee effusion model allows for “mechanical” inhibition of the quadriceps in the absence of pain. Pain can be a difficult variable to quantify and interpret in studying joint injury. Therefore, this model reduces any variability that may be associated with pain.
The postinjection measurement from the control and cryotherapy groups demonstrated an excitatory trend in the cryotherapy and control groups (Figure 2). The TENS group did not display this same effect. While these data seemed to be inconsistent, occurring in fewer than half of the volunteers, they are worthy of explanation. The volunteers who showed this increased measurement after injection of saline into the knee joint seemed the most anxious and nervous about the injection. This nervousness or anxiety could result in an increased H-reflex measurement. A sympathetic response, triggered by hypothalamic stimulation, would include such physiologic effects as increased heart rate, increased heart contractility, increased blood flow to somatic muscles, sweat, pupil dilation, and increased salivary secretion.46,47 These physiologic reactions could easily increase the variability in the measurement. The only explanation as to why the TENS group postinjection measurement did not increase compared with the other groups is that those randomly assigned subjects may not have been as anxious or nervous about the injection of saline.
Data from the TENS group showed no change from the preinjection measurement over time (Figure 2). TENS decreased the amount of inhibition resulting from knee effusion (disinhibition) during and shortly after the treatment (15-, 30-, and 45-minute intervals). Clinical evidence supports a small increase in voluntary activation of the quadriceps from TENS after ACL reconstruction and meniscectomy.30 While TENS disinhibited the quadriceps motoneuron pool during the treatment phase (Figure 2), it seemed to become less effective after treatment. This is evident by the downward trend at 45 and 60 minutes. The mechanism by which TENS reduces inhibition caused by joint effusion is not known. Because it caused measurements to return only to baseline levels, and it resulted in disinhibition during the treatment followed by an inhibitory trend, it seems likely that TENS primarily affects inhibition by having a direct effect on the interneuron that mediates the process. Afferent activity from TENS may result in inhibition of the Ib inhibitory interneuron. TENS could also cause excitation of the Ia excitatory interneuron, resulting in an excitatory potential at the motoneuron pool. Lastly, TENS could stimulate supraspinal centers to negate the effects of AMI through descending inhibitory fibers synapsing on the Ib interneuron. Each of these explanations is a possible mechanism for TENS-induced disinhibition of the VM after knee joint effusion. More data are needed to determine the exact mechanism.
Placing ice on an effused knee resulted not only in disinhibition but facilitation of the motoneuron pool beyond baseline measures (Figure 2). This facilitation continued for up to 30 minutes even after the ice was removed, a finding supported by data previously collected in our laboratory on healthy subjects.48 Cooling has a slowing effect on nerve conduction velocity of sensory afferent fibers.22 Cooling also slows the discharge rate of mechanoreceptors in muscle.49 Joint mechanoreceptors should react to cooling in the same way. Several authors50–52 have shown a decrease in intra-articular temperature during application of ice. Oosterveld et al52 reported a decrease of 9.4°C in intra-articular temperature after a 30-minute application of chipped ice. They noted that even though the ice was removed at 30 minutes, intra-articular temperatures continued to decrease for up to 45 minutes. A decrease in nerve conduction velocity and a slowing in discharge rate of joint mechanoreceptors would result in less information being delivered to the spinal cord in a given period of time, and therefore, a decrease in inhibition.
Since cryotherapy stimulates cutaneous receptors, including mechanoreceptors (pressure) and thermoreceptors, these receptors may play a role in facilitating the quadriceps motoneuron pool. Quickly adapting mechanoreceptors excite the Ia interneurons, resulting in excitation of the quadriceps motoneuron pool.19 This mechanism would counteract inhibition mediated through the Ib interneurons. Perhaps large amounts of information reaching the spinal cord from several different sensory receptors, stimulated by the effusion and the ice, create an environment in which supraspinal centers intercede. Additionally, since the quadriceps motoneuron pool was facilitated beyond the baseline measurement, supraspinal activity is likely involved. A decrease in inhibition would return H-reflex values to a baseline (preinjection) level. Measurements above the baseline level must be modulated by factors outside the reflexive loop.
Supraspinal pathways generally modulate spinal reflexes.47 In other words, reflexive activity produced from afferent traffic is reduced by supraspinal pathways to allow for controlled movement.47 Perhaps this tonic activity is reduced during cryotherapy treatment, allowing for less supraspinal control over reflexive activity. Cervero et al53 discussed a descending tonic spinal inhibition that occurs as a mechanism limiting the amount of motoneuron inhibition caused by trivial environmental stimulation of cutaneous and subcutaneous mechanoreceptors. This idea is contrary to that discussed previously. They reported that, during joint injury, descending tonic spinal inhibition is reduced, allowing for increased AMI.53 In this case, since descending tonic spinal inhibition is thought to be initiated by environmental factors, ice could increase this process, thereby decreasing AMI. Further study is needed to better understand the supraspinal influences and the process by which ice facilitates motoneuron recruitment within the VM motoneuron pool.
Clinical observation provides support for cryotherapeutic facilitation of the motoneuron pool. During cryokinetics, injured athletes seem able to perform exercises that were not possible before a cryotherapy treatment. Knight22 attributed this finding to a decrease in residual pain from the injury. However, these data show that changes to the motoneuron pool take place after knee effusion and cryotherapy treatment, and previous work suggested that this model facilitates the process in the absence of pain.2 Ice has a direct effect on diminishing AMI, not just pain.
The results of studies investigating the effects of cryotherapy on strength vary, with studies showing increased,54,55 decreased,25,27 and unchanged56,57 force production. These decreases in strength after cryotherapy were a product of cooling muscle, not the joint. Ruiz et al27 reported that isokinetic concentric and eccentric strength decreased immediately after cryotherapy treatment. However, there were no decreases in concentric strength at 20 and 40 minutes post-treatment. The differences reported in strength measurements after cooling were a product of variability in the type of measurement and the times and temperatures at which the measurements were taken. Additionally, each of these studies was conducted using healthy subjects. A population with abnormal joints may respond differently.
Functional measures of motor activity may decrease25 or be unaffected28 by cryotherapy. Cross et al25 reported that vertical jump decreased and shuttle run times increased immediately after ice immersion (foot and ankle). However, vertical jump was decreased by 1.1 cm and shuttle run times increased by 0.2 seconds. These small changes could have been due to a number of factors, including the temporary stiffness often experienced immediately after ice immersion.22 Our data suggest that recruitment within the motoneuron pool increases during and immediately after joint cooling and continues to increase during the post-treatment phase. Knee extensor torque decreases after knee effusion,14,39 which corresponds to our H-reflex measures in the control group. However, this is not a direct indication that a more functional measure of motoneuron pool recruitment would increase with cryotherapy, TENS treatments, or both. Further study is needed to determine if H-reflex measurement changes correspond to functional changes in force output over the same period of time in injured subjects.
Since cooling the skin may affect the electrical conductance properties of the tissue, it was necessary to measure surface temperatures at the site of the recording EMG electrodes to maintain the integrity of our measurement (Figure 3). Temperatures at the EMG electrode sites remained constant throughout the treatment (15 minutes, 31.52 ± 1.79°C, and 30 minutes, 31.49 ± 1.80°C) and post-treatment periods (45 minutes, 31.23 ± 1.84°C, and 60 minutes, 31.44 ± 1.69°C). Knee surface temperature measurements were also collected, showing a normal22 decline in temperature during the cooling phase and a rise in temperature during the post-treatment phase. With these data, we are confident that cooling the knee did not affect our measurements from the VM.
More than 53% of the screened subjects were excluded because we could not measure a maximum H reflex. A stimulator that produces a high voltage stimulus (100 to 200 V) with a pulse duration of at least 1.0 milliseconds would be ideal for the H-reflex measurement. Because our stimulator could only produce a pulse duration of 0.3 milliseconds at that intensity, we were unable to measure a maximum H reflex before the stimulator reached its maximum intensity. Larger amounts of subcutaneous fat in some subjects also made it difficult to obtain a stimulus intensity great enough to elicit a maximum H-reflex measurement.
In conclusion, the knee joint effusion model allows for investigation of neuromuscular changes associated with joint injury. The quadriceps motoneuron pool is inhibited in the absence of muscular injury or pain. Cryotherapy and TENS effectively disinhibit the quadriceps motoneuron pool after knee joint effusion, and cryotherapy further facilitates the motoneuron pool. Reduction of AMI by these therapeutic modalities may permit earlier activation of musculature during joint rehabilitation, allowing for active exercise and its positive effects on healing and the rehabilitation process. Further work will allow us to determine if a reduction in AMI allows the injured athlete to return to competition faster and with less susceptibility to further injury.
REFERENCES
- Hopkins J T, Ingersoll C D. Arthrogenic muscle inhibition: a limiting factor in knee joint rehabilitation. J Sport Rehabil. 2000;9:135–159. [Google Scholar]
- Hopkins J T, Ingersoll C D, Edwards J E, Cordova M L. Changes in soleus motoneuron pool excitability after artificial knee joint effusion. Arch Phys Med Rehabil. 2000;81:1199–1203. doi: 10.1053/apmr.2000.6298. [DOI] [PubMed] [Google Scholar]
- Stokes M, Young A. Investigations of quadriceps inhibition: implications for clinical practice. Physiotherapy. 1984;70:425–432. [Google Scholar]
- Young A. Current issues in arthrogenous inhibition. Ann Rheum Dis. 1993;52:829–834. doi: 10.1136/ard.52.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Frankel V. Basic Biomechanics of the Muskuloskeletal System. 2nd ed Vol. 323. Lea & Febiger; Philadelphia, PA: 1989. [Google Scholar]
- Woo S L, Gomez M A, Woo Y K, Akeson W H. Mechanical properties of tendons and ligaments, II: the relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- Zamora A J, Marini J F. Tendon and myo-tendonous junction in an overloaded skeletal muscle of the rat. Anat Embryol (Berl) 1988;179:89–96. doi: 10.1007/BF00305103. [DOI] [PubMed] [Google Scholar]
- Saamanen A, Tammi M, Kiviranta I, Helminen H, Jurvelin J. Moderate running increases but strenuous running prevents elevation of proteoglycan content in canine articular cartilage. Scand J Rheumatol. 1986;60:45. [Google Scholar]
- Forwood M R, Burr D B. Physical activity and bone mass: exercises in futility? Bone Miner. 1993;21:89–112. doi: 10.1016/s0169-6009(08)80012-8. [DOI] [PubMed] [Google Scholar]
- Barrack R L, Lund P J, Skinner H B. Knee joint proprioception revisited. J Sport Rehabil. 1994;3:18–42. [Google Scholar]
- Johansson H, Lorentzon R, Sjolander P, Sojka P. The anterior cruciate ligament: a sensor acting on the gamma-muscle spindle systems of muscles around the knee joint. Neuro-Orthopedics. 1990;9:1–23. [Google Scholar]
- Stokes M, Young A. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin Sci (Lond) 1984;67:7–14. doi: 10.1042/cs0670007. [DOI] [PubMed] [Google Scholar]
- Baxendale R H, Ferrell W R, Wood L. Knee joint distension and quadriceps maximal voluntary contraction in man. J Physiol. 1985;367:100P. doi: 10.1113/expphysiol.1988.sp003147. [DOI] [PubMed] [Google Scholar]
- DeAndrade J R, Grant C, Dixon A J. Joint distension and reflex muscle inhibition in the knee. J Bone Joint Surg Am. 1965;47:313–322. [PubMed] [Google Scholar]
- Jensen K, Graf B K. The effects of knee effusion on quadriceps strength and knee intraarticular pressure. Arthroscopy. 1993;9:52–56. doi: 10.1016/s0749-8063(05)80343-3. [DOI] [PubMed] [Google Scholar]
- McNair P J, Marshall R N, Maguire K. Swelling of the knee joint: effects of exercise on quadriceps muscle strength. Arch Phys Med Rehabil. 1996;77:896–899. doi: 10.1016/s0003-9993(96)90277-4. [DOI] [PubMed] [Google Scholar]
- Spencer J D, Hayes K C, Alexander I J. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171–177. [PubMed] [Google Scholar]
- Wood S A, Gregory J E, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497:279–290. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash M. Neurophysiological Basis of Movement. 1st ed Vol. 267. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- Johansson H, Sjolander P, Sojka P. Activity in receptor afferents from the anterior cruciate ligament evokes reflex effects on fusimotor neurons. Neurosci Res. 1990;8:54–59. doi: 10.1016/0168-0102(90)90057-l. [DOI] [PubMed] [Google Scholar]
- Morrissey M C. Reflex inhibition of thigh muscles in knee injury causes and treatment. Sports Med. 1989;7:263–276. doi: 10.2165/00007256-198907040-00004. [DOI] [PubMed] [Google Scholar]
- Knight K L. Cryotherapy in Sport Injury Management. Vol. 301 Human Kinetics; Champaign, IL: 1995. [Google Scholar]
- Barnes W M, Larsen M R. Effects of localized hyper and hypothermia on maximal isometric grip strength. Am J Phys Med. 1985;64:305–347. [PubMed] [Google Scholar]
- Brask B, Lueke R H, Soderberg G L. Electromyographic analysis of selected muscles during the lateral step-up exercise. Phys Ther. 1984;64:324–329. doi: 10.1093/ptj/64.3.324. [DOI] [PubMed] [Google Scholar]
- Cross K M, Wilson R W, Perrin D H. Closed chain performance following lower extremity ice immersion. J Athl Train. 1995;30:S8. [PMC free article] [PubMed] [Google Scholar]
- Davies C T, Young K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J Appl Physiol. 1983;55:191–194. doi: 10.1152/jappl.1983.55.1.191. [DOI] [PubMed] [Google Scholar]
- Ruiz D H, Myrer J W, Durrant E, Fellingham G W. Cryotherapy and sequential exercise bouts following cryotherapy on concentric and eccentric strength in the quadriceps. J Athl Train. 1993;28:320–323. [PMC free article] [PubMed] [Google Scholar]
- Evans T A, Ingersoll C D, Knight K L, Worrell T. Agility following the application of cold therapy. J Athl Training. 1995;30:231–234. [PMC free article] [PubMed] [Google Scholar]
- Iles J F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson I, Eriksson E. Postoperative TENS pain relief after knee surgery: objective evaluation. Orthopedics. 1986;9:1346–1351. doi: 10.3928/0147-7447-19861001-06. [DOI] [PubMed] [Google Scholar]
- Stokes M, Shakespeare D, Sherman K, Young A. Transcutaneous nerve stimulation and post-meniscectomy quadriceps inhibition. Int J Rehabil Res. 1985;8:248. [Google Scholar]
- Kameyama O, Hayes K C, Wolfe D. Methodological considerations contributing to variability of the quadriceps H-reflex. Am J Phys Med Rehabil. 1989;68:277–282. doi: 10.1097/00002060-198912000-00004. [DOI] [PubMed] [Google Scholar]
- Hopkins J T, Ingersoll C D, Cordova M L, Edwards J E. Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr Clin Neurophysiol. 2000;40:89–94. [PubMed] [Google Scholar]
- National Athletic Trainers' Association Blood-borne pathogens guidelines for athletic trainers. J Athl Train. 1995;30:203–204. [PMC free article] [PubMed] [Google Scholar]
- Prentice W E. Therapeutic Modalities in Sports Medicine. 3rd ed Vol. 409. Mosby; St Louis, MO: 1994. [Google Scholar]
- Fahrer H, Rentsch H U, Gerber N J, Beyeler C, Hess C W, Grunig B. Knee effusion and reflex inhibition of the quadriceps: a bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–638. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- Iles J F, Roberts R C. Inhibition of monosynaptic reflexes in the human lower limb. J Physiol. 1987;385:69–87. doi: 10.1113/jphysiol.1987.sp016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J C, Alexander I J, Hayes K C. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- Wood L, Ferrell W R, Baxendale R H. Pressures in normal and acutely distended human knee joints and effects on quadriceps maximal voluntary contractions. Q J Exp Physiol. 1988;73:305–314. doi: 10.1113/expphysiol.1988.sp003147. [DOI] [PubMed] [Google Scholar]
- Iles J F, Stokes M, Young A. Reflex actions of knee joint afferents during contraction of the human quadriceps. Clin Physiol. 1990;10:489–500. doi: 10.1111/j.1475-097x.1990.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A. Interneurons in the spinal cord. Trends Neurosci. 1981;3:230–233. [Google Scholar]
- Iles J F, Pisini J V. Cortical modulation of transmission in spinal reflex pathways of man. J Physiol. 1992;455:425–446. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Raunest J, Sager M, Burgener E. Proprioceptive mechanisms in the cruciate ligaments: an electromyographic study on reflex activity in the thigh muscles. J Trauma. 1996;41:488–493. doi: 10.1097/00005373-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Eriksson E. Rehabilitation of muscle function after sport injury: major problem in sports medicine. Int J Sports Med. 1981;2:1–6. doi: 10.1055/s-2008-1034575. [DOI] [PubMed] [Google Scholar]
- Rhoades R A, Tanner G A. Medical Physiology. Vol. 839 Little Brown & Co; Boston, MA: 1995. [Google Scholar]
- Burt A M. Textbook of Neuroanatomy. Vol. 541 WB Saunders Co; Philadelphia, PA: 1993. [Google Scholar]
- Krause B A, Hopkins J T, Ingersoll C D. The relationship of ankle temperature during cooling and re-warming to the human soleus H reflex. J Sport Rehabil. 2000;9:253–262. [Google Scholar]
- Eldred E, Lindsley D F, Buchwald J S. The effect of cooling on mammalian muscle spindles. Exp Neurol. 1960;2:144–157. doi: 10.1016/0014-4886(60)90004-2. [DOI] [PubMed] [Google Scholar]
- Bocobo C, Fast A, Kingery W, Kaplan M. The effect of ice on intra-articular temperature in the knee of the dog. Am J Phys Med Rehabil. 1991;70:181–185. doi: 10.1097/00002060-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Ohkoshi Y, Ohkoshi M, Nagasaki S, Ono A, Hashimoto T, Yamane S. The effect of cryotherapy on intraarticular temperature and postoperative care after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:357–362. doi: 10.1177/03635465990270031601. [DOI] [PubMed] [Google Scholar]
- Oosterveld F G, Rasker J J, Jacobs J W, Overmars H J. The effect of local heat and cold therapy on the intraarticular and skin surface temperature of the knee. Arthritis Rheum. 1992;35:146–151. doi: 10.1002/art.1780350204. [DOI] [PubMed] [Google Scholar]
- Cervero F, Schaible H G, Schmidt R F. Tonic descending inhibition of spinal cord neurones driven by joint afferents in normal cats and in cats with an inflamed knee joint. Exp Brain Res. 1991;83:675–678. doi: 10.1007/BF00229846. [DOI] [PubMed] [Google Scholar]
- McGown H L. Effects of cold application on maximal isometric contraction. Phys Ther. 1967;43:185–192. doi: 10.1093/ptj/47.3.185. [DOI] [PubMed] [Google Scholar]
- Oliver R A, Johnson D J. The effect of cold water baths on posttreatment leg strength. Physician Sportsmed. 1976;4(11):67–69. [Google Scholar]
- Cornwall M W. Effect of temperature on muscle force and rate of muscle force production in men and women. J Orthop Sports Phys Ther. 1994;20:74–80. doi: 10.2519/jospt.1994.20.2.74. [DOI] [PubMed] [Google Scholar]
- Kimura I F, Gulick D T, Thompson G T. The effect of cryotherapy on eccentric plantar flexion peak torque and endurance. J Athl Train. 1997;32:124–126. [PMC free article] [PubMed] [Google Scholar]


