Abstract
Objective: To determine the temporal pattern of the repeated bout effect of eccentric exercise on perceived pain and muscular tenderness associated with delayed-onset muscle soreness (DOMS).
Design and Setting: Subjects completed 2 identical eccentric exercise bouts separated by 6, 7, 8, or 9 weeks. The experiment was conducted in a biokinetics research laboratory.
Subjects: Sixteen male and 15 female untrained subjects (age = 24.59 ± 4.42 years, height = 171.71 ± 7.81 cm, weight = 73.00 ± 11.20 kg).
Measurements: Two physiologic characteristics of DOMS were measured immediately before and 0, 24, 48, and 72 hours after each eccentric exercise bout. Perceived pain was measured using a visual analog scale (VAS), and muscular tenderness was measured using a punctate tenderness gauge (PTG).
Results: Two 4 × 2 × 5 (group × bout × time) analyses of variance with repeated measures on the bout and time factors were performed on the VAS and PTG data. Significant (P < .05) main effects were found for group, bout, and time for the VAS and the PTG data. No significant interactions were detected. Post hoc analysis revealed significantly less perceived pain for the 9-week group than the 8-week group. The 7-week group had significantly less and the 8-week group had significantly more muscular tenderness than any other group. Perceived pain and muscular tenderness were significantly less after exercise bout 2 than after exercise bout 1. All subjects had significantly less perceived pain and muscular tenderness pre-exercise than 0 and 24 hours after the eccentric exercise bouts.
Conclusions: An effective prophylaxis for perceived pain and muscular tenderness associated with DOMS is the performance of an eccentric exercise bout 6 to 9 weeks before a similar exercise bout.
Keywords: musculoskeletal injury, eccentric exercise, repeated bout effect, visual analog scale
Delayed-onset muscle soreness (DOMS) affects most active people at some point in their lives. DOMS is a common ailment that in many cases impedes an athlete's performance. As part of a muscle tissue damage continuum, DOMS represents a limited form of muscle tissue damage that, on a larger scale, involves the entire functional muscle unit.1,2 DOMS presents with a dull, aching pain that develops 24 to 48 hours after a novel or unaccustomed exercise bout. The pain typically peaks between 24 and 72 hours postexercise when the muscles are tender and swollen. 3–5 The severity and distribution of pain associated with DOMS are related to the intensity, duration, and type of exercise performed.6,7 DOMS is primarily associated with eccentric exercise or passive lengthening of a contracted muscle.6,7 The deleterious effects of DOMS are well documented,1–11 yet there is no standard treatment or prophylaxis for the condition.
The effects of DOMS are alleviated when a soreness-producing exercise bout is preceded by a similar soreness-producing exercise bout.8,12–15 An adaptive response to one or more bouts of eccentric exercise has been termed the repeated bout effect and appears to be the best known prophylaxis for DOMS.8,12–15 The time frame of the adaptation remains enigmatic. Performance of a single eccentric exercise bout has been shown to reduce muscle soreness after a similar exercise bout up to 6 weeks8,15 but not beyond 9 weeks.15 The extent of the diminished prophylactic response over time is still unknown. Our purpose was to determine the temporal pattern of the repeated bout effect of eccentric exercise on perceived pain and muscular tenderness associated with DOMS when 2 identical exercise bouts were separated by 6, 7, 8, or 9 weeks.
METHODS
Research Design
The study consisted of 3 independent variables and 2 dependent variables. Independent variables were exercise group (6 weeks, 7 weeks, 8 weeks, or 9 weeks), bout (exercise bout 1 or 2), and time (immediately before or 0, 24, 48, or 72 hours after exercise). Dependent variables were perceived pain as measured via a visual analog scale (VAS) and muscular tenderness as measured via a punctate tenderness gauge (PTG). The study was conducted at the Temple University Biokinetics Research Laboratory.
Subjects
Sixteen male and 15 female Temple University students volunteered to participate in the study. Subjects' mean age was 24.59 ± 4.42 years, height was 171.71 ± 7.81 cm, and weight was 73.00 ± 11.20 kg (Table). Subjects were screened and admitted for study participation based on the absence of current upper body weight training, upper extremity injury within one year of the study, and predisposing cardiovascular or cardiorespiratory conditions. Subjects were asked to refrain from performing upper body stretching and exercises, treating the affected arm, and taking anti-inflammatory medications during the data collection periods. One subject did not return to complete the second exercise bout and was dropped from the study. In accordance with the Temple University Institutional Review Board, which approved the study, appropriate subject consent was obtained before data collection.
Table 1.
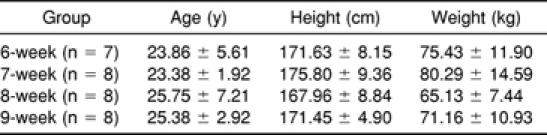
Subjects' Age, Height, and Weight by the 6-, 7-, 8-, and 9-Week Groups (Mean ± SD)
Instrumentation and Equipment
Isokinetic Dynamometer. The Biodex B-2000 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY), was used to perform the exercise bouts, which consisted of eccentric contractions of the wrist extensor muscles of the subject's nonwriting forearm. The dynamometer was calibrated by the primary investigator at the beginning of each data collection session according to the manufacturer's guidelines. Mechanical reliability is 1.0 for eccentric peak torque, average power, and total work, and physiologic reliability ranges from 0.94 to 0.99 for eccentric peak torque, average power, and total work of the quadriceps and hamstrings muscle groups.16
Visual Analog Scale. A variation of the VAS developed by Melzak17 was used to quantify the general level of soreness of the subjects. The VAS consists of a 10-cm line, with no soreness on the left end of the line and extreme soreness on the right end of the line. Subjects were asked to place a slash mark on the line according to the general level of perceived soreness at the time of assessment. A blank scale was used each time to avoid bias from preceding measurements.18 The slash mark was measured from the left end of the line to the nearest 0.1 cm. Scores were recorded as a value between no soreness (0) and extremely sore (10). The VAS is easily and quickly administered19 and has been used as a reliable measurement for determining the intensity of human pain.17,19–21
Punctate Tenderness Gauge. Newham et al6,14,22–24, developed a method to measure tenderness upon palpation using a variation of the PTG. The PTG (Technical Products Co, Caldwell, NJ) used in this study consists of a 2-mm hemispheric metal probe attached to a strain gauge. A plastic grid with holes at 10 evenly spaced sites was placed over the subject's experimental forearm with the elbow flexed to 90° and the forearm fully pronated. The proximal end of the grid was placed snugly in the subject's cubital fossa, with the corners of the grid positioned over the medial and lateral humeral epicondyles and secured with self-adhesive strips. The PTG was inserted into each hole and depressed until the subject reported that the sensation of pressure changed to discomfort or pain. The criterion measure was the amount of pressure required to elicit the response and was recorded in pounds of pressure. Muscular tenderness, as measured using the PTG, and pounds of pressure are inversely related (ie, the more muscular tenderness, the less pressure required to elicit a response). This method of PTG application is comparable with weights traceable to the National Bureau of Standards (r = .99, intraclass correlation coefficient [2,1] = .98 lb; standard error of the mean = .03 lb) (D. C. Meserlian, unpublished data, 1987 to 1991).
Data Collection
Group Assignment and Pre-Exercise Measurements. Each subject was randomly assigned to one of 4 exercise groups: 6 weeks, 7 weeks, 8 weeks, or 9 weeks. The 6-week group performed the second exercise bout 6 weeks after the initial exercise bout, while the 7-, 8-, and 9-week groups performed the second exercise bout 7, 8, or 9 weeks after the initial exercise bout, respectively. The VAS and PTG were administered before the exercise bout.
Exercise Positioning and Stabilization. Subjects were seated upright on the dynamometer accessory chair with the experimental (nonwriting) forearm on the padded armrest, which was positioned immediately proximal to the ulnar styloid process. The forearm was fully pronated and the triquetrum was aligned with the dynamometer axis. The elbow was flexed to 90° with the forearm positioned in the horizontal plane.25 The start position was verified goniometrically by the primary investigator, who performed all goniometric measurements (intratester reliability, r ≥ .81). Straps were positioned across the subject's pelvis, chest, and forearm to ensure stabilization of the arm and elbow during exercise. A folded towel was placed in the axilla to facilitate the adducted position and to allow for greater arm and elbow stabilization. Range-of-motion limits were set by the investigator at 60° of extension and 70° of flexion with 10% range limits in each direction.26
Exercise Bout 1. Standardized instructions were read to each subject before the warm-up and exercise bouts. The warm-up consisted of 5 submaximal and 4 maximal eccentric repetitions, followed by exercise bout 1, which consisted of 5 sets of 50 maximal eccentric repetitions of the wrist extensor muscles with a 60-second rest period between sets. The dynamometer was set in the passive mode to allow the subject to actively resist wrist flexion, producing eccentric tension. After the exercise bout, the subject was instructed to relax and allow the dynamometer to return the wrist to the extended starting position to eliminate concentric work. Total work as a relative measure of muscular effort for the exercise protocol was determined from the computer output and recorded upon completion of the initial exercise bout.
Visual analog scale and PTG tests were administered immediately after exercise bout 1. Subjects were reminded not to massage, stretch, or treat the exercised arm in any way and to refrain from taking anti-inflammatory medications for 3 days postexercise. Subjects returned to the laboratory 24, 48, and 72 hours postexercise for follow-up VAS and PTG administrations. Once the 3 days of follow-up data were obtained, the subjects were scheduled to return to the laboratory 6, 7, 8, or 9 weeks after the initial exercise bout, depending on group assignment.
Exercise Bout 2. Subjects returned to the laboratory for exercise bout 2 after the assigned number of weeks. The VAS and PTG were administered, and then subjects were positioned, stabilized, and exercised as they had been for exercise bout 1. Exercise bout 2 was terminated when the subject had produced the same total work as that of exercise bout 1. Total work output for each subject during exercise bout 2 duplicated the total work recorded for exercise bout 1. The VAS and PTG were administered immediately upon completion of the exercise bout and 24, 48, and 72 hours postexercise.
Data Analysis
Two 4 × 2 × 5 (group × bout × time) analyses of variance with repeated measures on the bout and time factors were performed on the VAS and PTG data. The Biomedical Data Program Statistical Software (University of California Press, Berkeley, CA)27 was used for data analyses. We performed Tukey HSD post hoc tests28 to determine where the significant differences occurred. The alpha level was set at P ≤ .05, and all hypothesis testing was completed in the null form.
RESULTS
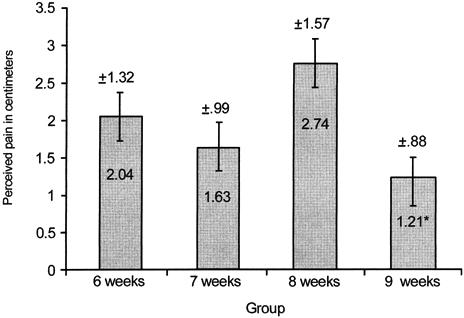
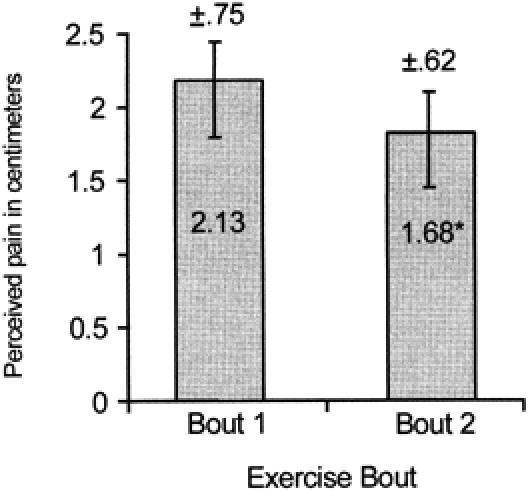
Significant main effects were found for group, bout, and time for the VAS and PTG (Figures 1 and 2). No significant interactions existed. Post hoc analysis revealed that the 9-week group had significantly less perceived pain (F3,27 = 3.12, P < .05) than the 8-week group as measured via the VAS (Figure 1). Significantly less perceived pain (F1,3 = 5.18, P < .05) was found after exercise bout 2 than after exercise bout 1 as measured via the VAS (Figure 2). The time data revealed significantly less perceived pain (F4,12 = 28.39, P < .01) for the pre-exercise test than for the 0-hour and 24-hour tests as measured via the VAS (Figure 3).
Figure 1.
Average perceived pain (cm) for 6-, 7-, 8-, and 9-week groups. *Significantly less perceived pain (P < .05) was found for the 9-week group than for the 8-week group.
Figure 2.
Average perceived pain (cm) at exercise bouts 1 and 2. *Significantly less perceived pain (P < .05) was found for exercise bout 2 than for exercise bout 1.
Figure 3.
Average perceived pain (cm) at pre-exercise and 0, 24, 48, and 72 hours. *Significantly less perceived pain (P < .01) was found at pre-exercise than at the 0- and 24-hour tests.
Post hoc analysis of the PTG data revealed that the 7-week group had significantly less and the 8-week group had significantly more muscular tenderness (F3,27 = 3.35, P < .05) than any other group (Figure 4). Significantly less muscular tenderness (F1,3 = 62.49, P < .01) was found after exercise bout 2 than after exercise bout 1 as measured via the PTG (Figure 5). Significantly less muscular tenderness (F4,12 = 7.47, P < .01) was found for the pre-exercise test than for the 0-hour and 24-hour tests (Figure 6).
Figure 4.
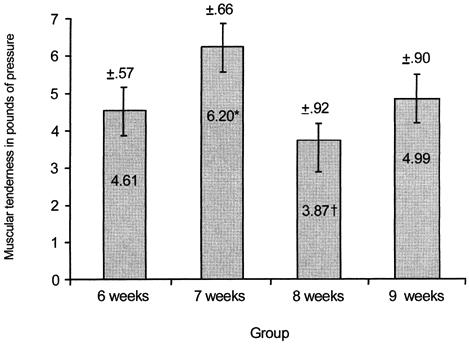
Average muscular tenderness (pounds of pressure) at 6-, 7-, 8-, and 9-week groups. *Significantly less muscular tenderness (P < .05) was found for the 7-week group than for any other group. †Significantly more muscular tenderness (P < .05) was found for the 8-week group than for any other group. Note: values are inversely related to muscular tenderness.
Figure 5.
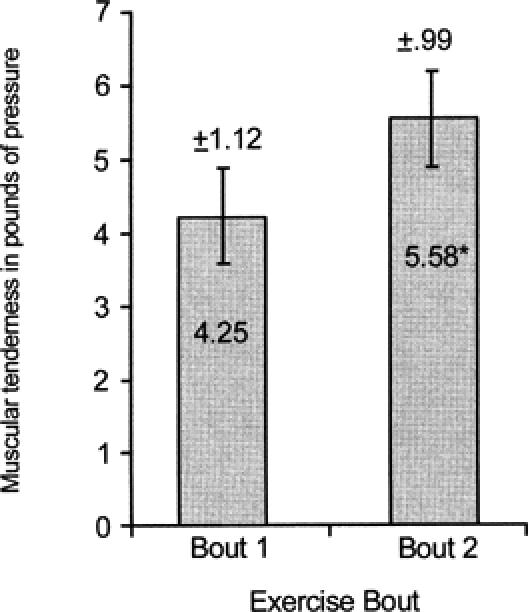
Average muscular tenderness (pounds of pressure) at exercise bouts 1 and 2. *Significantly less muscular tenderness (P < .01) was found for exercise bout 2 than for exercise bout 1. Note: values are inversely related to muscular tenderness.
Figure 6.
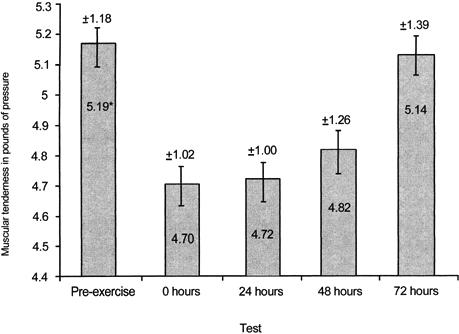
Average muscular tenderness (pounds of pressure) at pre-exercise and 0, 24, 48, and 72 hours. *Significantly less muscular tenderness (P < .01) was found at pre-exercise than at the 0- and 24-hour tests. Note: values are inversely related to muscular tenderness.
DISCUSSION
The expected temporal pattern of DOMS during the 72 hours immediately after the exercise bout is consistent with observations of others.8,11,29–32 Perceived pain and muscle tenderness were both significantly greater than baseline values at 24 and 48 hours after the exercise bout,11,32 with the DOMS resolving by 72 hours (Figures 2 and 5). The acute postexercise discomfort associated with DOMS was most likely due to structural23,24,33,34 and biochemical changes.35, 36–38 Mechanical forces associated with the intense eccentric exercise bout of the present study likely damaged muscle proteins and connective tissue, leading to edema and an inflammatory reaction that caused secondary biochemical damage.37,39 Several mechanisms may be responsible for the adaptive effect observable 6 to 10 weeks after an eccentric exercise perturbation. Healing is usually observable by 72 hours after tissue injury,37 which may be responsible, in part, for the decreases in both perceived pain and muscle tenderness. The formation of protective proteins such as desmin or titin during healing37,39 may have been responsible for the attenuation of DOMS after the second exercise bout8,15 (Figures 1 and 4). Collagen deposition in the myotendinous junction and perimysial areas during maturation and remodeling would increase the strength of connective tissue surrounding the myofiber, providing an additional protective effect.33 Finally, repair of the sarcoplasmic reticulum and sarcolemma, increasing their resistance to damage, would further reduce the calcium-mediated damage and efflux of intramuscular proteins after an eccentric exercise bout.15
We expected progressively higher levels of perceived pain and muscular tenderness when an exercise bout was repeated after 6, 7, 8, and 9 weeks. Group data in the present study revealed differences in the magnitude of the average perceived pain and muscular tenderness incurred by the subjects. Significantly less perceived pain was found for the 9-week group than for the 8-week group (Figure 1). While randomization of subject group assignment would have negated individual variations in the exercise bout response, the relatively small sample size may account for the unexpected results in perceived pain and muscular tenderness of the 7- and 8-week groups.
The exercise bout data in our study were consistent with the expected outcome. Regardless of group, less perceived pain and muscular tenderness occurred after exercise bout 2 than after exercise bout 1. Similarly, Byrnes et al8 found that repeating a bout of downhill running after 3 and 6 weeks resulted in significantly less perceived soreness for the second exercise bout when compared with the first exercise bout. After 9 weeks, perceived soreness ratings for the second exercise bout exceeded those for the first exercise bout. The downhill running was performed at a variable treadmill speed with a constant heart rate (170 beats per minute) and constant duration (45 minutes) for each subject. Nosaka et al15 also found significantly less perceived muscle soreness when an eccentric exercise bout using the elbow flexors was repeated after 6 weeks. No significant decrements in perceived soreness ratings were found when the second exercise bout was performed 10 weeks after the first exercise bout. A similar constant workload was performed during each of the eccentric exercise bouts of the elbow flexors. Additionally, Newham et al14 found progressively more soreness when eccentric exercise bouts of the elbow flexors were repeated every 2 weeks for 6 weeks. Elbow flexors were exercised at a constant workload for all exercise bouts. We used the wrist extensor muscles because these were not eccentrically trained, were used minimally in activities of daily living, and would not incapacitate the subject with pain and reduced function. The relative workload was designed to exactly duplicate the amount of tension produced by the muscles and was measured by the isokinetic dynamometer for each exercise bout for each subject.
CONCLUSIONS
Perceived pain and muscular tenderness associated with an eccentric exercise perturbation can be reduced by performing similar exercise 6, 7, 8, or 9 weeks before beginning an exercise program. Most individuals engaging in physical activity will experience DOMS at some point in their lives. Clinically, exercise and rehabilitation program noncompliance are often attributed to the discomfort associated with DOMS. Performance of an eccentric exercise bout up to 9 weeks before beginning an exercise program can reduce delayed postexercise muscle pain and tenderness. Preseason conditioning may begin up to 2 months before intense physical activity, resulting in reduced delayed muscle pain and tenderness during the first weeks of a competitive sports season. Reduced muscle pain, tenderness, stiffness, and weakness associated with DOMS36 may help improve performance and reduce injury due to unsound biomechanical compensations37 early in a competitive sports season. Sports medicine personnel must emphasize the importance of the prophylactic benefits of the repeated bout effect on DOMS to individuals who are involved in novel or unaccustomed physical activity. Further research is needed to determine other methods of reducing the severity of the signs and symptoms associated with DOMS.
Acknowledgments
ACKNOWLEDGMENTS
We thank Carl Mattacola, PhD, ATC, for reviewing the manuscript.
REFERENCES
- Segan D J, Sladek E C, Gomez J, McCoy H J, Cairns D A. Weight lifting as a cause of bilateral upper extremity compartment syndrome. Physician Sportsmed. 1988;16(10):73–76. doi: 10.1080/00913847.1988.11709623. [DOI] [PubMed] [Google Scholar]
- Stauber W T. Delayed onset muscle soreness and pain. In: Zachazewski J E, Magee D J, Quillen W S, editors. Athletic Injuries and Rehabilitation. WB Saunders Co; Philadelphia, PA: 1996. pp. 92–97. [Google Scholar]
- Faulkner J A, Brooks S V, Opiteck J A. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- Francis K T. Delayed muscle soreness: a review. J Orthop Sports Phys Ther. 1983;5:10–13. doi: 10.2519/jospt.1983.5.1.10. [DOI] [PubMed] [Google Scholar]
- Gulick D T, Kimura I F. Delayed onset muscle soreness: what is it and how do we treat it? Res Rev. 1996;5(3):234–43. [Google Scholar]
- Newham D J. The consequences of eccentric contractions and their relationship to delayed monset muscle pain. Eur J Appl Physiol Occup Physiol. 1988;57:353–359. doi: 10.1007/BF00635995. [DOI] [PubMed] [Google Scholar]
- Byrnes W C, Clarkson P M. Delayed onset muscle soreness and training. Clin Sports Med. 1986;5:605–614. [PubMed] [Google Scholar]
- Byrnes W C, Clarkson P M, White J S, Hsieh S S, Frykman P N, Maughan R J. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol. 1985;59:710–715. doi: 10.1152/jappl.1985.59.3.710. [DOI] [PubMed] [Google Scholar]
- Friden J, Sfakianos P N, Hargens A R. Blood indices of muscle injury associated with eccentric muscle contractions. J Orthop Res. 1989;7:142–145. doi: 10.1002/jor.1100070120. [DOI] [PubMed] [Google Scholar]
- Saxton J M, Clarkson P M, James R, et al. Neuromuscular dysfunction following eccentric exercise. Med Sci Sports Exerc. 1995;27:1185–1193. [PubMed] [Google Scholar]
- Schwane J A, Johnson S R, Vandenakker C B, Armstrong R B. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc. 1983;15:51–56. [PubMed] [Google Scholar]
- Clarkson P M, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- Schwane J A, Armstrong R B. Effect of training on skeletal muscle injury from downhill running in rats. J Appl Physiol. 1983;55:969–975. doi: 10.1152/jappl.1983.55.3.969. [DOI] [PubMed] [Google Scholar]
- Newham D J, Jones D A, Clarkson P M. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Clarkson P M, McGuiggin M E, Byrne J M. The time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol. 1991;63:70–76. doi: 10.1007/BF00760804. [DOI] [PubMed] [Google Scholar]
- Timm K. The mechanical and physiological reliability of the eccentric mode of the Biodex isokinetic dynamometer. Med Sci Sports Exerc. 1990;22:S65. [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Gulick D T, Kimura I F, Sitler M, Paolone A M, Kelly J D., IV Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31:145–152. [PMC free article] [PubMed] [Google Scholar]
- Mattacola C M, Perrin D H, Gansneder B M, Allen J D, Mickey C A. A comparison of visual analog scale and graphic rating scales for pain intensity following DOMS. J Sport Rehabil. 1997;6:38–46. [Google Scholar]
- Lee K A, Kieckhefer G M. Measuring human responses using visual analogue scales. West J Nurs Res. 1989;11:128–132. doi: 10.1177/019394598901100111. [DOI] [PubMed] [Google Scholar]
- Price D D, McGrath P A, Rafii A, Buckingham B. The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Newham D J, Jones D A, Edwards R H. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983;5:380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- Newham D J, McPhail G, Mills K R, Edwards R H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Newham D J, Mill K R, Quigley B M, Edwards R H. Pain and fatigue after concentric and eccentric muscle contraction. Clin Sci. 1983;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Norkin C C, White D J. Measurement of Joint Motion: A Guide to Goniometry. FA Davis; Philadelphia, PA: 1985. [Google Scholar]
- Thorpe M L. Effects of Tennis Elbow Devices on Eccentric Peak Torque Production of Wrist Extensor Muscles [master's thesis] Temple University; Philadelphia, PA: 1990. [Google Scholar]
- Dixon W J. Biomedical Data Program Statistical Software [computer program] University of California Press; Berkeley, CA: 1985. [Google Scholar]
- Bruning J L, Kintz B L. Computational Handbook of Statistics. 3rd ed Scott Foresman; Glenview, IL: 1987. [Google Scholar]
- Diggs Z W. The sickle cell trait in relation to the training assignment of duties in the armed forces, III: hyposthenuria, hematuria, sudden death, rhabdomyolysis and acute tubular necrosis. Aviat Space Environ Med. 1984;55:358–64. [PubMed] [Google Scholar]
- Colliander E B, Tesch P A. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand. 1990;140:31–39. doi: 10.1111/j.1748-1716.1990.tb08973.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G K, Rothstein J M, Mayhew T P, Lamb R L. Exercise-induced muscle soreness after concentric and eccentric isokinetic contractions. Phys Ther. 1991;7:505–513. doi: 10.1093/ptj/71.7.505. [DOI] [PubMed] [Google Scholar]
- Talag T S. Residual punctate soreness as influenced by concentric, eccentric, and static contractions. Res Q. 1973;44:458–469. [PubMed] [Google Scholar]
- Clarkson P M, Byrnes W C, Gillison E, Harper E. Adaptation to exercise-induced muscle damage. Clin Sci. 1987;73:383–386. doi: 10.1042/cs0730383. [DOI] [PubMed] [Google Scholar]
- Stauber W T, Clarkson P M, Fritz V K, Evans W J. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol. 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Friden J. Changes in human skeletal muscle induced by long term eccentric exercise. Cell Tissue Res. 1984;236:365–372. doi: 10.1007/BF00214240. [DOI] [PubMed] [Google Scholar]
- Clarkson P M. Exertional rhabdomyolysis. NCAA News. Oct 26, 1998. pp. 1–3.
- Smith L L. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23:542–551. [PubMed] [Google Scholar]
- Croisier J L, Camus G, Deby-Dupont G, et al. Myocellular enzyme leakage, polymorphonuclear neutrophil activation and delayed onset muscle soreness induced by isokinetic eccentric exercise. Arch Physiol Biochem. 1996;104:322–329. doi: 10.1076/apab.104.3.322.12904. [DOI] [PubMed] [Google Scholar]
- MacIntyre D L, Reid W D, McKenzie D C. Delayed muscle soreness: the inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]


