Abstract
Objective: To provide an overview of currently available sensorimotor assessment techniques.
Data Sources: We drew information from an extensive review of the scientific literature conducted in the areas of proprioception, neuromuscular control, and motor control measurement. Literature searches were conducted using MEDLINE for the years 1965 to 1999 with the key words proprioception, somatosensory evoked potentials, nerve conduction testing, electromyography, muscle dynamometry, isometric, isokinetic, kinetic, kinematic, posture, equilibrium, balance, stiffness, neuromuscular, sensorimotor, and measurement. Additional sources were collected using the reference lists of identified articles.
Data Synthesis: Sensorimotor measurement techniques are discussed with reference to the underlying physiologic mechanisms, influential factors and locations of the variable within the system, clinical research questions, limitations of the measurement technique, and directions for future research.
Conclusions/Recommendations: The complex interactions and relationships among the individual components of the sensorimotor system make measuring and analyzing specific characteristics and functions difficult. Additionally, the specific assessment techniques used to measure a variable can influence attained results. Optimizing the application of sensorimotor research to clinical settings can, therefore, be best accomplished through the use of common nomenclature to describe underlying physiologic mechanisms and specific measurement techniques.
Keywords: proprioception, neuromuscular, assessment
The rapid growth of the athletic training profession has been accompanied by an equally rapid increase in focus on basic and clinical research. Many elements of the profession, such as the boost in research submissions to the Journal of Athletic Training and increase in the size of the Free Communications Program of our National Athletic Trainers' Association Annual Meeting and Clinical Symposia, provide the supporting evidence for this statement. It is essential, however, that as more research is conducted within the profession, such research be completed in a manner that allows for common understanding between researchers and clinicians. Therefore, the purpose of our article is to provide an overview of the currently available sensorimotor measurement techniques in an attempt to initiate a basis for the needed understanding. For each measurement technique discussed, the major underlying physiologic mechanisms, influential factors, and location of the variable within the sensorimotor system will be identified. Additionally, in the context of the current article, we will give a few representative examples of investigations using each technique that have led to advancements in our understanding of the system in either normal or pathologic states.
Maintaining functional joint stability through complementary relationships between static and dynamic restraints is the role of the sensorimotor system.1–3 The sensorimotor system encompasses all of the sensory, motor, and central integration and processing components involved in maintaining functional joint stability.1 In our previous articles,2,3 we reviewed the anatomy and physiology of the entire sensorimotor system. As can be surmised through those reviews, the complex interactions and relationships among the individual components of the sensorimotor system make measuring and analyzing specific characteristics and functions extremely difficult. Adding further complexity are the numerous compensatory mechanisms interspersed throughout the system. For example, the normal ability to close one's eyes during stance without loss of postural equilibrium resides with the ability of the somatosensory and vestibular senses to provide sufficient afferent information despite the absence of visual input. Similarly, vestibular sense–deficient persons are able to maintain equilibrium as long as visual or somatosensory (or both) inputs are available.4 If we were to assess postural stability in these patients, we might not detect a vestibular sense deficiency unless visual or somatosensory (or both) contributions were eliminated or reduced. Many similar compensatory mechanisms exist throughout the various areas of the sensorimotor system. In research involving surgical manipulation of animal models (eg, decerebrate animals), isolation of specific sensorimotor components and mechanisms can be performed. In contrast, investigations involving human subjects usually require the use of groups with known or specific deficiencies or the induction of temporary alterations (eg, nerve blocks), or both. Although many different measurement techniques and instruments are currently available for in vivo human research, only a few can purely evaluate the target variable of interest in isolation.
Most assessment techniques currently available evaluate the integrity and function of sensorimotor components by measuring variables along the afferent or efferent pathways or the final outcome of skeletal muscle activation or a combination of these. Currently, no direct assessment methods are available to isolate the higher central integrating and processing centers. From a physiologic perspective, we stress the importance of being as specific as possible in referring to both the variable and suspected mechanisms. It is essential that both factors be considered during any interpretation of results. We use assessment of reflex latency in response to an imposed joint perturbation with electromyography (EMG) as an example. The variable being measured is onset of muscle activity, a variable located on the efferent pathway. In this example, it is necessary to recognize the presence of both the underlying mechanism and influencing factors. The major underlying mechanism leading to elicitation of the variable involves the afferent acquisition and transmission to central integration or processing centers (or both), where the propagation of an efferent neural signal to the muscle can be initiated. The pathway through the central nervous system can range from a simple monosynaptic relay to the efferent neurons' more complex polysynaptic reflex pathways that include transmission through the brain stem to voluntary activation initiated by the motor cortex. Many factors influence this mechanism, such as the integrity of the mechanoreceptors and the level and type (facilitatory or inhibitory) of descending supraspinal control over the neural pathways. All of these factors must be considered in the final interpretation of the variable.
Additional factors that confound valid and reliable variable measurement are the specific techniques used in data collection, processing, and analysis. Each of these can have profound effects on the final outcome of a measurement and thereby influence the reported results. Reverting back to our joint perturbation example, such factors include details of subject instruction, anticipation and expectations, number of trials, data sampling frequencies, and filtering and smoothing techniques. Although controversy will always surround many of the measurement techniques, there is no substitute for clearly describing the exact procedures used. Attention to each of these will facilitate the common understanding of both clinicians and researchers.
PERIPHERAL AFFERENT ACQUISITION AND TRANSMISSION MEASUREMENTS
Proprioception
Several different testing techniques have been developed to measure the conscious submodalities of proprioception. Because there are 3 submodalities (joint position sense [JPS], kinesthesia, and sense of tension), clarification is required to distinguish the target variable of the assessment. The JPS test measures the accuracy of position replication and can be conducted actively or passively in both open and closed kinetic chain positions. Both direct measurements of replicated joint angles5–8 (goniometers, potentiometers, video) and indirect measures9 (visual analog scales) have been used. Kinesthesia testing is conducted by measuring the threshold to detection of passive motion (TTDPM), or more specific testing can be conducted by using the criterion of threshold to detection of passive motion direction (TTDMD).10–12 The TTDMD assesses one's ability to not only detect motion but also detect in which direction the motion is occurring. Slow speeds, ranging from 0.5 to 2°/s, are used to target the slow-adapting mechanoreceptors, such as Ruffini endings or Golgi-type organs.13 The sense of tension is measured by comparing the ability of subjects to replicate torque magnitudes produced by a group of muscles under varying conditions.
Common to all currently available proprioception testing methods are dependencies on conscious appreciation (perception) of mechanoreceptor signals. As detailed in our previous article,3 proprioceptive information travels to the higher brain centers through the dorsal lateral tracts (conscious appreciation) and the spinocerebellar pathways (stimulation and regulation of motor activities). The precise quantities being conveyed to both ascending tracts from each type of mechanoreceptor, as well as the temporal relationship between arrival at the cerebellum and the somatosensory cortex, remain unknown. Additionally, whether the quantity necessary for conscious perception is identical to the requirements for motor control is unknown.
The sources of conscious proprioceptive information potentially include joint, muscle, and cutaneous mechanoreceptors.14–23 Existing evidence supports the receptors in each tissue as the primary source, so this topic remains very controversial.3 In addition, visual and auditory signals can provide additional cues to JPS, TTDPM, and TTDMD. For example, seeing the position or movement of the limb (vision) or hearing the instrumentation begin to move the joint (auditory) prevents conclusions from being accurately drawn regarding conscious proprioceptive acuity. In addition, if an assessment is attempting to focus on the integrity of capsular mechanoreceptors, appropriate precautions are needed to reduce supplemental proprioceptive information arising from cutaneous mechanoreceptors. In other words, the effects of a deafferentated joint on proprioceptive acuity might go undetected without specific attention to reducing or eliminating supplemental sources of information. A good example would be stimulation of cutaneous mechanoreceptors caused by stabilization straps.
Unfortunately, discrimination between muscle and joint afferents cannot be accomplished without more sophisticated experimental manipulation. Methods used to reduce inputs from cutaneous, muscle, and joint mechanoreceptors include anesthesia and ischemia.24,25 Vibration is a technique that has been specifically used to induce stimulation of the muscle spindle afferents, thereby changing muscle tone and, ultimately, the information provided by the muscle spindles.26,27 With respect to conscious proprioception perception, vibration could be incorporated into assessments to potentially determine muscle spindle contributions.
A wide variety of equipment and instrumentation, including commercial isokinetic dynamometers, electromagnetic tracking devices, and custom-made jigs, has been developed to measure conscious appreciation of proprioception. In our laboratory, we use a motor-driven proprioception testing device that can passively move the limb for both kinesthetic and passive JPS assessment (Figure 1). Subjects are fitted with a blindfold, headphones, and pneumatic cuff to eliminate confounding cues to motion detection and JPS, including vision, audible sensing of the motor-driven apparatus, and vibration induced by motor on the limb. A unique feature of the device is its ability to conduct assessments at very slow speeds (<0.5°/s), unlike most isokinetic devices with minimum speeds of 2°/s.
Figure 1.
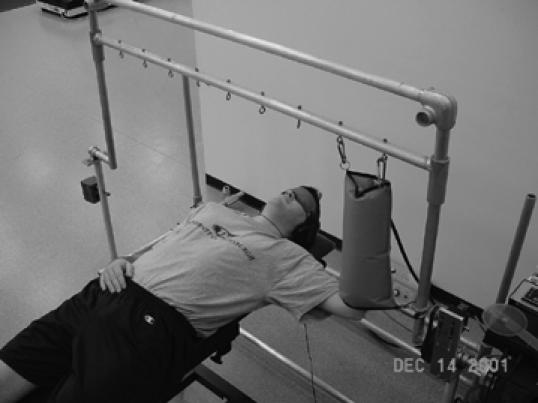
The proprioception testing device is a motor-driven jig used to test both position sense and kinesthesia. Subjects are fitted with a blindfold, headset containing white noise, and pneumatic sleeve to negate visual, audio, and tactile cues.
In addition to the proprioception testing device, we have also used an electromagnetic tracking system to measure the ability to actively reproduce given joint positions or paths of motion (Figure 2). The big advantage of such a device is that subjects have free, unrestricted movement, unlike in the proprioception testing device, where they are limited to 1 degree of freedom (eg, knee flexion-extension or humeral rotation). This is especially important at the shoulder joint, where natural movement patterns involve multiplanar movements.
Figure 2.
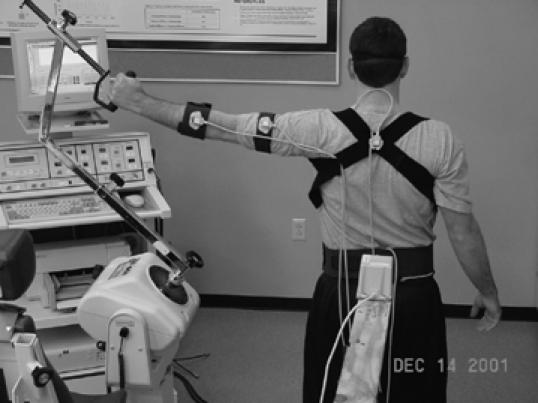
The electromagnetic motion tracking device is used to assess position sense and replicate movement patterns and for 3-dimensional kinematic analysis of movement.
Numerous studies using the previously mentioned approaches have been conducted to compare conscious proprioceptive acuity (JPS and kinesthesia) between normal and pathologic groups at the ankle,28–32 knee,7,9,10,12,33–36 and shoulder8,37 joints. Although some of these investigators have found deficits,8,10,12,28,31,32,34–37 others have not.7,30,33 Possible explanations for the different results include the failure to control any of the previously mentioned confounding factors, inherent instrumentation differences (eg, position of the patient with respect to gravity), varying methodologic approaches (eg, angular positions, speed of passive movements), and different subject characteristics (eg, pathologic group compared with control group versus bilateral comparison).
In addition to comparisons between pathologic and healthy joints, research considering conscious appreciation of proprioception has been conducted in several related areas. The ability of surgical intervention to restore conscious proprioceptive acuity along with mechanical stability has been examined.7,8,38 Additionally, the suggestion that a decrease in proprioception may predispose one to joint injury prompted investigators to prospectively consider proprioceptive acuity before an athletic season39 and after varying intensities and modes of exercise.6,40,41 Lastly, investigators have examined the relationships between conscious proprioceptive acuity and functional activity tests,34,36 functional rating scores,9,42 and hamstrings:quadriceps peak torque ratios43 to determine the degree to which conscious proprioceptive acuity relates to more functional measures. Future research directions include validating conscious proprioceptive acuity through simultaneous measurement of afferent pathway action potentials (ie, microneurography) and correlating decreases in conscious proprioception with deficits in sensorimotor control over dynamic joint stability.
Somatosensory Evoked Potentials
Evoked potentials are methods of testing the integrity of afferent pathways to the cerebral cortex. These techniques, which are traditionally and predominantly used in neurology to confirm and localize sensory abnormalities, involve measuring neurophysiologic and electroencephalographic responses to stimulation of sensory sources (somatosensory, visual, and vestibular).44 The cortical evoked responses are complex waveforms that represent the sensory impulse traveling to the sensory cortex.45 Specific to the somatosensory afferent pathways, the technique is referred to as somatosensory evoked potentials (SEPs). The SEPs can be elicited either through transcutaneous electrical stimulation of peripheral nerves and sensory organs or more physiologic stimuli such as joint movement.46 Once a stimulus is given peripherally, measurements can be made along the afferent pathways. For example, after an electric stimulus is delivered to the wrist (median nerve), the nerve action potentials can be detected as they propagate centrally at the level of the brachial plexus (Erb point), midcervical spinal cord (fifth cervical vertebrae), upper midbrain-thalamus, and somatosensory cortex.44
The techniques are performed by introducing an electric potential with known characteristics (eg, peak characteristics, amplitude, and wavelength) to the afferent pathway. How these characteristics change along the pathway is then assessed. Common variables assessed include the amplitude changes, wavelength changes, and latencies between introduction of the potential and measurement of the potential along the pathway. The luxury of this technique is that it allows for the establishment of objective evidence of abnormality or deficiency by identifying if and where lesions occur along the afferent pathway.46 Unfortunately, neurophysiologists have a difficult time correlating sensory deficits with results from SEP testing because it is difficult to evaluate the submodality of the sensory system by simple stimulation of peripheral afferent nerves.46
Several recent investigations using SEPs have made some important contributions to our understanding of the sensorimotor system. By selectively inducing ischemia at the base of the finger and shoulder, Mima et al46 confirmed the importance of muscle afferents for the dynamic aspect of proprioception. Pitman et al45 demonstrated a direct link between the anterior cruciate ligament (ACL) and the sensory cortex, with the greatest potentials being recorded on stimulation of the ligament's midsubstance. Additionally, significant correlations between kinesthetic deficits (ie, deficits in detecting joint motion) and SEP abnormalities from the afferent pathways from the ACL have been demonstrated in ACL-deficient individuals.47 The patterns of alterations in SEPs led the authors to speculate that the central nervous system had undergone modification and reorganization processes after the peripheral inputs were lost. Lastly, Barrack et al48 recently used SEPs to suggest the occurrence of reinnervation in central-third patellar tendon grafts. At the shoulder, Tibone et al49 demonstrated that no differences exist in evoked potentials between people who are unstable at the shoulder and a healthy population. As such, the decreased proprioception that was demonstrated at the shoulder probably results from the increased tissue laxity decreasing mechanoreceptor stimulation rather than tissue deafferentation. Further research is needed using SEPs to advance our understanding of peripheral afferent receptors projecting on the cortex and the alterations and modifications demonstrated by higher central nervous system areas in their absence.
EFFERENT TRANSMISSION MEASUREMENTS
Nerve Conduction Testing
Nerve conduction testing (NCT) is an objective method of assessing the functional status of the peripheral alpha motor neuron system.50 The basis for NCT resides with the proximal and distal reaction propagation that occurs along an entire nerve after electric stimulation. Motor neurons that are readily measured include the median, ulnar, common peroneal, and posterior tibial.51
In addition, NCT is performed using both an electric current generator and EMG recording. An electric current with known characteristics (eg, amplitude and wavelength) is applied to the efferent, the neural pathway, usually on the innervating nerve. Then EMG recordings are taken distal to the applied current, usually on the desired muscle. For example, ulnar nerve motor nerve conduction testing is performed by stimulating the ulnar nerve at the wrist while recording changes in the induced current at the fifth finger and hypothenar eminence.52 Two limitations exist with NCT. First, the technique is often performed with needle-type electrodes. This can be very uncomfortable for the patient. Second, the timing of the test is critical.53 It may take as long as 3 weeks after injury for deficits to manifest in an NCT, even in the presence of positive clinical findings.53 This can be extremely problematic in the sports medicine setting, where there is often pressure for quick return-to-play decisions. As with many conditions, a lack of objective signs and subjective symptoms does not always mean the patient is injury free.
Also, NCT can assess several variables. Commonly, nerve conduction velocity is the assessment that is erroneously mentioned by physicians when, in fact, they are assessing other variables.52 The change in amplitude is far more important for diagnosis of neuronal lesions than are the velocity changes.52 Conduction velocity is measured by calculating the velocity between the stimulation of one point and the recording of the introduced current.53 The limitation of conduction velocity assessment is that alterations in velocity may only manifest in lesions that cause focal slowing.52 Unlike conduction velocity, amplitude changes indicate not only myelination changes but also loss of intact axons, no matter what type of lesion exists.52 Amplitude assessment indicates the number of intact axons that exist along the nerve innervating a muscle (ie, a decrease in intact axons results in a decrease in amplitude between the introduced and recorded current characteristics). Often, these results are compared with the uninvolved, contralateral limb for a measure of control.
With respect to an orthopaedic application, numerous reports have been published demonstrating impaired motor nerve conduction velocity after injury.54–57 Kleinrensink et al57 reported alterations in the superficial and deep peroneal nerves after inversion ankle injury, suggesting a possible contributing factor to functional ankle instability. Di Benedetto and Markey58 used nerve conduction velocity testing to assess motor deficits in football players with diagnosed brachial plexopathies. Nerve conduction was determined for the muscles supplied by the long thoracic, suprascapular, musculocutaneous, axillary, lateral pectoral, and thoracodorsal nerves. Conduction slowing was present in 16 of the 18 injured football players tested. With NCT, the authors were able to conclude that the abnormalities most likely resulted from compression of the most superficially located fibers of the brachial plexus at the Erb point. The results suggested that the most significant causative factor was ill-fitting shoulder pads against the neck during tackling.58 Further researchers should consider alterations in NCT as an objective assessment tool and possible factor in functional joint instability.
Muscle Activation Patterns
Electromyography is a tool that provides for the detection of electric activity accompanying skeletal muscle activation.59–61 The information gathered through EMG can be used to determine the initiation, cessation, and magnitude of muscle activity. Generally, 3 fundamental types of variables arise from EMG: onset, amplitude, and frequency. Although initially EMG may appear to be a straightforward process, closer inspection quickly reveals a complicated and tedious assessment technique. Confounding factors that arise from physiologic, anatomical, and technical elements surround both signal acquisition and processing. These elements can directly influence the apparent results attained. Effective EMG use and interpretation requires one to understand as much as possible the sources of each of these elements and their influences on EMG signals.60 Several comprehensive discussions and monographs detailing the current understandings and developments have been written.59–61 Further, because the acquisition and processing methods used will influence EMG signals, clinicians and researchers should make extensive efforts to meet the recommended publication standards advocated by the Journal of Electromyography and Kinesiology.
Electromyography measures the myoelectric event associated with muscle contraction.61,62 On receiving an action potential from the motor neuron, a muscle action potential propagates bidirectionally along the muscle fibers.61,62 Electromyography uses electrodes to detect and record the depolarization wave front and subsequent repolarization that occurs as part of the muscle action potential.61
The 2 electrode types commonly used in neuromuscular and biomechanical research are surface and fine wire. Generally, surface electrodes are used for superficial muscles and tend to record a greater muscle area than fine-wire electrodes63; however, because of their large collection area, the risk of collecting muscle activity from unwanted muscles (eg, cross-talk) is high. To decrease this risk, standardized electrode positions are helpful in isolating the desired muscles. Unfortunately, only one relatively recent article has addressed electrode placement.64 However, Basmajian and Blumenstein65 provided a general description of surface-electrode placement for clinical biofeedback assessment. Generally, a site halfway between the innervation zone and the distal myotendinous junction is recommended.60 In conjunction with a bipolar configuration, electrodes should be placed parallel to the direction of the muscle fibers, with a 1-cm interelectrode distance.60 The parallel orientation is critical to ensuring phasic delays between the 2 electrodes.67 Silver-silver chloride electrodes are considered the optimal materials for surface electrode construction because of their electrochemical stability.60 An additional consideration associated with surface EMG is adequate skin preparation. Komi and Buskirk63 established the reliability of surface-electrode EMG as an intraclass correlation coefficient of 0.88 to 0.91 within sessions and 0.64 to 0.73 between testing sessions for amplitude characteristics of the signal.
To assess muscles that cannot be recorded with surface electrodes because of their location, fine-wire electrodes are advocated.63 Fine-wire electrodes consist of some type of conducting wire that is inserted intramuscularly through a cannula (Figure 3A). For example, because of the deep anatomical orientation of the rotator cuff muscles, fine-wire EMG is necessary to measure their muscle firing characteristics (Figure 3B). The fine-wire electrodes can be constructed with either a single-wire or dual-wire design.62,68 The dual fine-wire configuration described by Basmajian et al62,69 is currently the gold standard in biomechanical-neuromuscular research. The reliability of fine-wire electrode use is somewhat less than that for surface-electrode EMG. Komi and Buskirk63 reported that reliability coefficients for amplitude characteristics of the signal within sessions were approximately 0.62, whereas the between-day coefficients were approximately 0.55. Compromised reliability may result from fine-wire electrode movement or fracture within the muscle. Jonsson and Bagge70 reported that fine-wire electrodes may migrate as much as 14.6 mm and fracture with movement. Both Basmajian and DeLuca62 and Jonsson and Bagge70 recommended performing several contractions of the desired muscle before data collection to fix the electrode within the muscle tissue. In terms of fracture prevention, Jonsson and Bagge70 suggested that 0.05-mm fine wire is less likely to fracture than 0.025-mm wire. Fortunately, the risk of pain and infection associated with wire fractures is minimal, and they can often be left untreated.70 Like surface EMG, fine-wire EMG requires correct placement to avoid cross-talk. Fortunately, a plethora of literature describes fine-wire electrode placement.71–75 Giroux and Lamantagne76 reported that EMG data collected with both fine-wire and surface electrodes were statistically similar.
Figure 3.
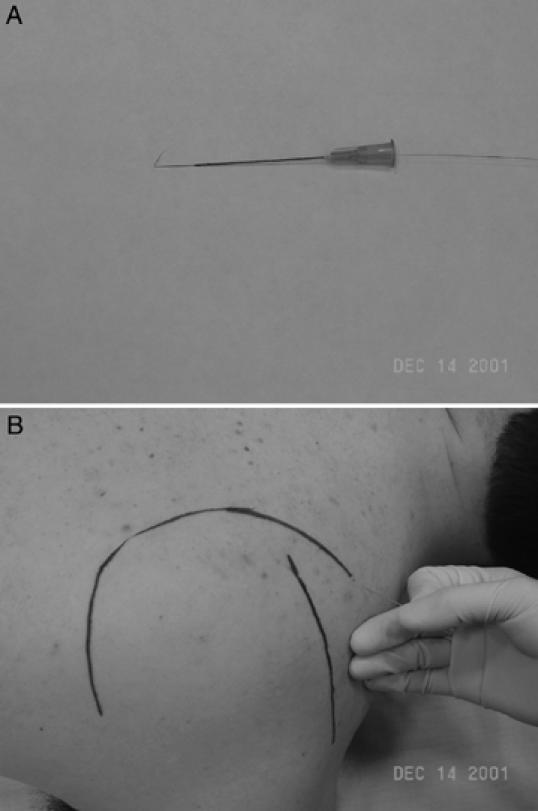
A, Dual fine-wire electrode used for electromyographic assessment of muscle activity. B, Electrode insertion for assessment of deep muscles; the supraspinatus muscle is shown.
Other important EMG hardware considerations include the use of on-site preamplifiers (active electrodes) to reduce artifact and noise,77 high-quality differential amplifiers with high common-mode rejection ratios,61 appropriate antialiasing filters, and adequate sampling frequency during analog-to-digital conversion. Based on the frequency spectrum of EMG presented by Winter,61 surface EMG data need to be sampled at 1000 Hz, whereas fine-wire data should be sampled at higher rates (>2000 Hz).
In addition to varied hardware components and characteristics, a wide variety of data processing approaches have been used in the literature, each aimed at extracting pertinent information. Because the resulting muscle forces and joint torques are of much lower frequencies than raw EMG signals, the most common processing approach involves amplitude demodulation (linear envelope detection).67 In this process, the raw biphasic EMG signal is first full-wave rectified and then subsequently undergoes some form of smoothing function (Figure 4). Frequently used to smooth the signal are low-pass filters such as Butterworth, Chebycheev, or Paytner. The lower the cutoff frequency of the filter, the smoother the signal. The tradeoff to smoother signals is increased phase distortions. Thus, many researchers use zero-phase lag filters during the creation of the linear envelope. Once the linear envelope is created, the signals can be time and amplitude normalized (Figure 4) and variables of interest can be calculated.
Figure 4.
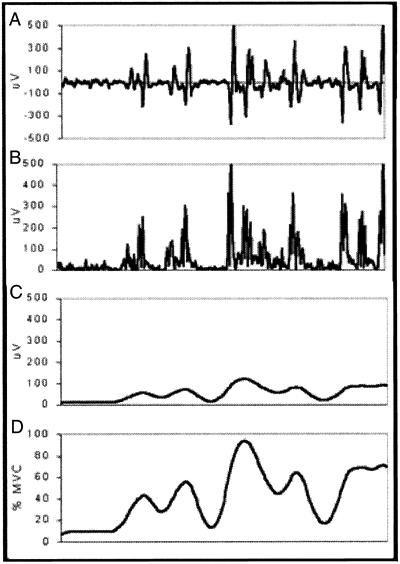
Raw electromyographic signal (A) that has been full-wave rectified (B), smoothed using a 10-Hz low-pass filter (C), and amplitude normalized to a maximum voluntary contraction (D).
Because EMG is specific to the sensorimotor system, it provides a means to examine several aspects of the dynamic restraint mechanism. First, EMG has been used to measure the reflexive responses to ankle (Figure 5A) and knee joint perturbations.78–80 Three characteristics of reflexive responses are often considered: onset latency, sequence of activation, and peak activation (Figure 5B). Onset latency refers to the time between stimulus and the initiation of muscle electrical activity as detected through EMG. Sequence of activation refers to the order in which each muscle is activated. Peak activation refers to the maximum amplitude the EMG signal reaches during the reflexive response. For example, in Figure 5, the sequence of activation is peroneus brevis, peroneus longus, and anterior tibialis. Although reflexes in response to joint perturbation have been traditionally considered to arise from direct connections between ligamentous and capsular mechanoreceptors and alpha motor neurons,78,80,81 more recent research supports the premise of muscle spindles as the initiating sensory organs.82 As our previous articles2,3 detailed, ligamentous and capsular mechanoreceptors are essential for modulating muscle-spindle sensitivity via the gamma efferent system. In other words, stimulation of gamma motor neurons heightens muscle-spindle sensitivity, which in turn increases the level of muscle activation existing in the muscle at a given instant. Whether surface EMG is sensitive enough to detect differences in levels of muscle activation both before and after a perturbation stimulus as a result of gamma-system modulation over muscle-spindle response sensitivity remains unknown. An additional influential consideration in reflex testing that is not under experimental control in vivo is the descending brain stem and cortical commands modulating alpha and gamma motor neuron pool excitability. For example, anticipation and expectations based on prior experience, both of which arise at the cortical level, have been demonstrated to alter postural reflex latencies and sequences of activation.83
Figure 5.
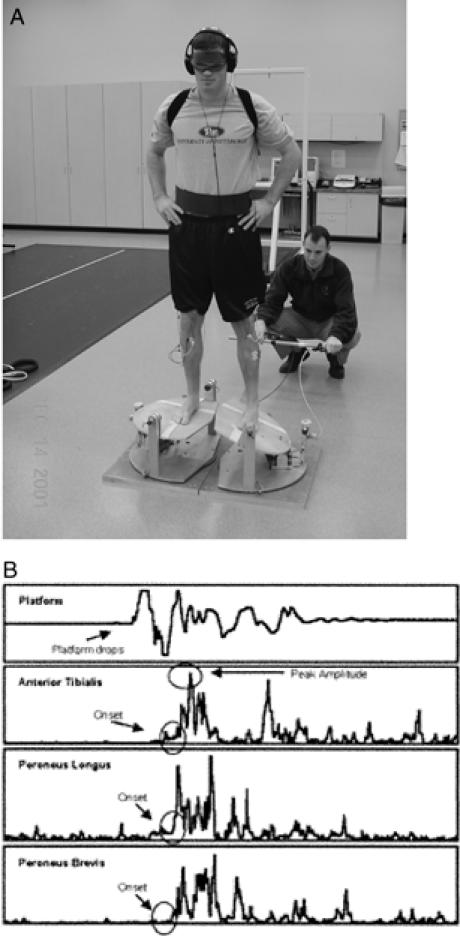
A, Ankle perturbation device used to assess muscle reflex characteristics of the ankle joint. B, Typical muscle reflex characteristics during an inversion perturbation trial.
During more functional tasks involving both the lower and upper extremities, such as walking (Figure 6A), landing, and throwing, EMG enables quantification of muscle activation sequences, amplitude, and duration.84–87 Often a task is subdivided into phases according to joint loading to determine preparatory and reactive muscle activity (Figure 6B). Preparatory activity is often operationally defined as the activity occurring before foot contact, whereas reactive activity encompasses the area of muscle activity occurring after foot contact. Several investigators have considered differences in muscle activation sequences and amplitudes84,85,88–90 and sex differences91–93 between normal subjects and groups with various conditions. In addition to comparing normal and pathologic groups, several investigators have considered the effects of braces and orthoses on EMG activity during functional tasks.94
Figure 6.
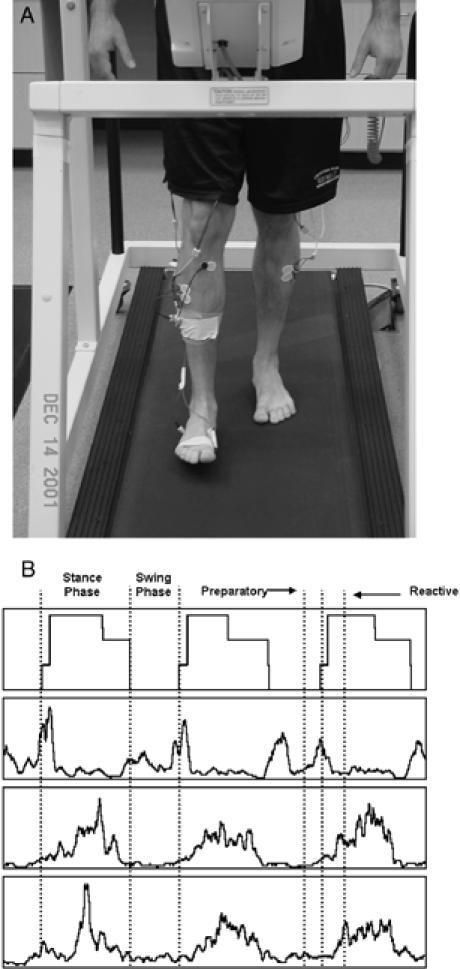
A, Electromyographic analysis of the lower leg muscles during a gait activity on a treadmill. B, Phase delineation of typical muscle activity during gait.
Muscle activation patterns have also been examined during voluntary commands of specific motor patterns. With respect to rehabilitation exercise, identifying the specific muscle activation patterns characteristic of a particular exercise helps to provide a scientific rationale for its use.95–98 For example, most recently, Henry98 qualified the degrees of coactivation accompanying 6 selected shoulder rehabilitation exercises. Similarly, by combining EMG with isokinetic assessments, information can be attained about coactivation patterns accompanying voluntary muscle activation99 and the ratio between EMG activity and force production.61,100
Muscle Performance Characteristics
Measuring muscle performance characteristics has been an integral component of sensorimotor system assessment for many years. Several different assessment approaches involving different types of muscle contractions are available, with isokinetics being the most popular.101 Isokinetics involves keeping the angular speed of a moving limb constant throughout the range of motion,102,103 independent of magnitude103 and velocity of muscle contractions.104 Although isokinetic contractions have been criticized as a nonfunctional mode of muscle contraction, they continue to be used extensively because of the ease of quantifying torque, work, and power in a clinical setting. A thorough review of isokinetic testing, assessment interpretation, and application has been published.101
It is important to recognize isokinetic measures as representative of the resultant body segment torque produced by voluntary skeletal muscle activation. Isokinetic torque does not immediately or directly reflect muscle force production but rather the final outcome of a descending neural command on the muscles across a limb segment. In other words, torque is a function of many factors, such as level of muscle activation, muscle dynamics (length and velocity), joint geometry (moment arm length and joint congruency), limb weight (inertia), and movement velocity.102 As a joint is moved though a range of motion by muscle activation, several of these factors change, giving rise to varying torque production capabilities despite similar activation levels. Additionally, different combinations of compressive and rotary forces result from similar activation levels as a joint moves through the full range of motion. This has been hypothesized to affford muscles the ability to provide dynamic stability at end ranges while remaining a prime mover through the midranges of motion.105
Sufficient voluntary activation of muscle (timing and magnitude) does not guarantee that the same muscle will perform as an adequate dynamic stabilizer for a mechanically unstable joint. Several studies have demonstrated the absence of a relationship between isokinetic peak torque and functional abilities in ACL-deficient subjects106 and healthy individuals.107 Further research is needed to consider the relationships between voluntary muscle activation and force production capabilities and the function of the dynamic restraint mechanisms during functional activity.
Kinetic and Kinematic Measurements
Function and maintenance of the body's structures requires balancing forces that originate from both the environment and within the body. Sources of environmental forces include gravity, friction, and contact with other objects, whereas internal forces most often originate from muscle activation and restraint provided by ligaments. It is the science of biomechanics that studies the effects of forces acting on or being produced by the body during human movement through measurement techniques such as kinetic and kinematic analyses.61
Kinetics is the study of forces that cause movement and resulting energetics.61 Although forces can be measured directly through surgically implanted transducers, they are more commonly measured indirectly using force platforms. Force platforms can assess force in 3 orthogonal vectors (2 horizontal, 1 vertical) and the moments around each vector. From these force data, variables such as peak force, time to peak force, and impulse can be calculated to describe the forces associated with acceleration of the body's center of mass (Figure 7).
Figure 7.
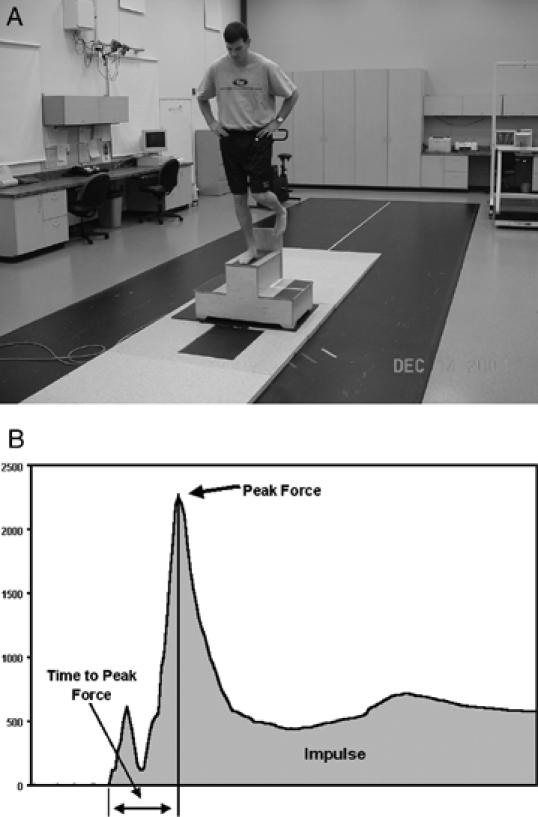
A, Assessment of ground reaction forces during a landing task. B, Typical vertical ground reaction forces during landing.
In comparison, kinematics is the study of motion independent of the causative forces and includes measurement of linear and angular displacements, velocities, and accelerations.61 Kinematic measurements are accomplished by tracking the displacement of specific body segments during motion. This can be accomplished with devices such as high-speed cameras,108,109 electromagnetic tracking systems,110,111 electrogoniometers,112,113 and accelerometers.114,115
Using reflective markers that reflect either natural lighting or infrared light, depending on the system, high-speed video cameras can capture movement of these markers both digitally and on a videocassette tape during functional activities such as hitting a golf ball (Figure 8A). From the tracking of these markers, segment models can be created for the assessment of the desired segment (Figure 8B). One limitation of video-based systems is the enormous amount of time associated with digitizing the videotaped footage for segment analysis after collection. Electromagnetic tracking systems use an electromagnetic transmitter that emits a spherical electromagnetic field with a radius of 91.44 to 365.7 cm and receivers fixed to the desired limb segments. From the 3-dimensional position vectors of these sensors, as well as orientation (yaw, pitch, and roll) within the electromagnetic field, segmental kinematic data can be calculated. The major limitation of this assessment technique is that all movements must be performed within the emitted electromagnetic field for accurate measurement. Electrogoniometers are instrumented strain gauges that provide relative joint angle data. The limitation of such devices is their lack of reliability among observers and the fact that angular changes of less then 10° may provide invalid results.116 Finally, accelerometers, as the name would suggest, measure acceleration. From these acceleration data, both velocity and position of the desired limb can be calculated through derivative calculations. Combining synchronized kinetic and kinematic data with anthropometric data will allow calculations and predictions concerning joint-reaction forces and muscle moments to be made through the process of link-segment modeling.61
Figure 8.
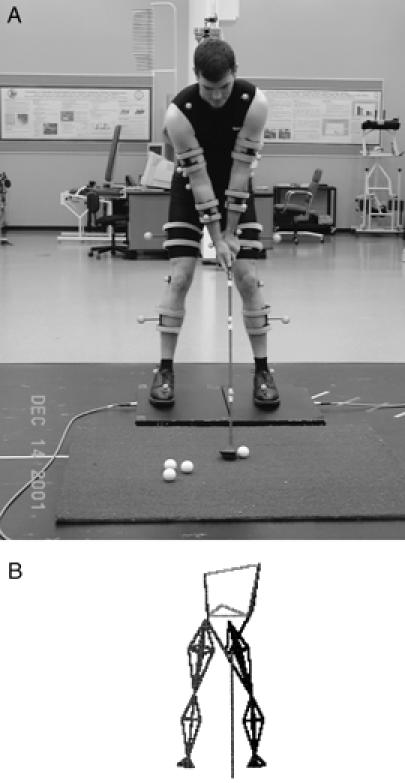
A, Kinematic analysis of the golf swing using a high-speed video camera system. B, Three-dimensional representation of the golf swing for kinematic analysis.
Kinetic and kinematic measurements have been widely used to identify functional adaptations in patients with mechanically unstable joints. For example, video motion analysis of patients with an ACL rupture revealed increased knee flexion during hopping and walking, suggesting that these individuals exhibit a “quadriceps-avoidance gait.”117,118 By providing measures for the outcomes of muscle activation during functional tasks and movement sequences, these assessment tools will continue to increase our understanding of successful and unsuccessful motor adaptations secondary to joint instability.
Postural Control Measures
Postural control has been one of the most misconstrued concepts within the sensorimotor system. Deficits in postural control after orthopaedic injury have been largely attributed to disruptions in the integrity of the afferent information that arises from ligamentous and capsular mechanoreceptors, despite the importance of articular information for postural control being largely unknown. Although the exact significance of proprioceptive information for postural control remains unknown, the somatosensory system as a whole has been demonstrated to play a major role.4,119–121 Postural control combines sensory input from 3 sources (somatosensory, visual, and vestibular) within the central nervous system to develop postural control strategies executed by the joints throughout the kinetic chain. Thus, postural control can become disrupted after articular injury not only from diminished afferent articular information but also by virtue of central strategy selection changes (eg, central inhibitions) or deficiencies in the motor systems (eg, strength, mechanical stability) or both.
During postural control assessments, because each of the sensory sources (somatosensory, visual, and vestibular) can compensate for reductions in the contributions from the remaining sources,4 specific techniques must be used for inferences to be drawn concerning the integrity of each source. Using unstable, compliant, or moving support surfaces is believed to alter somatosensory input that arises from foot contact with the support surface. Other methods of diminishing or altering mechanoreceptor inputs include local anesthetic injection,122,123 inducing ischemia122,123 or hypothermia,124,125 and vibration.26,27 Altering visual inputs is usually done by eliminating visual information (eg, eye closure) or providing inaccurate visual information through sway referencing126,127 or conflict domes.128 Vestibular inputs have been altered through head tilting129 and galvanic stimulation.130
Because postural control is specific to the task,61,102,131 another important consideration in conducting postural control assessments is the type of task used. Generally, the task involved with an assessment can be considered to either remain in equilibrium or to maintain equilibrium while another activity is performed. Assessing the ability to remain in equilibrium is frequently done during periods of quiet stance122,132 or after support surface perturbations4,133,134 or bodily delivered perturbations.112,135 The size and shape of the base of support are commonly manipulated. Single-leg assessments provide a means for bilateral comparisons, an often important application in orthopaedic settings. In addition, single-leg stance requires the body's center of gravity to be reorganized over a narrow and short base of support, thereby increasing the importance of segmental control in the frontal plane. In contrast to the assessment task of maintaining equilibrium, conscious attention is not normally required or centered on maintaining postural control during activities of daily living. Typically, a conscious motor command is initiated (eg, running) with the specific details of the movement (eg, sequence of muscle activation) programmed by various areas of the central nervous system, whereas the conscious can shift focus to another thought. Thus, it naturally follows that postural control assessments should include circumstances that attempt to duplicate similar scenarios. An example of this type of task is the single-leg hop stabilization test.136
In addition to a variety of assessment tasks, many different measurement techniques have been used to quantify postural stability and the types of strategies selected for maintaining equilibrium (Figure 9). Generally, postural control measurement techniques can be considered as either clinical or instrumented. Clinical measures are obtained without sophisticated equipment. Examples include error scoring systems136,137 and measurement of the length a person can reach138 or the time one can maintain equilibrium in a given stance (or both).131,139 Instrumented measures are frequently obtained from support surface sensors, with force platforms being the most dominant tool used. Force plates provide the opportunity to monitor center of pressure and variability in horizontal and vertical reaction forces associated with corrective muscular actions. In addition to measuring changes in postural control through the support surface, kinematic methods can be used to determine the types of movements that occur at each limb segment. Lastly, by incorporating EMG measures, levels of coactivation and characteristics of muscle responses to postural perturbations can be determined. Many of these measurement techniques can also be used during rehabilitation after injury.140
Figure 9.
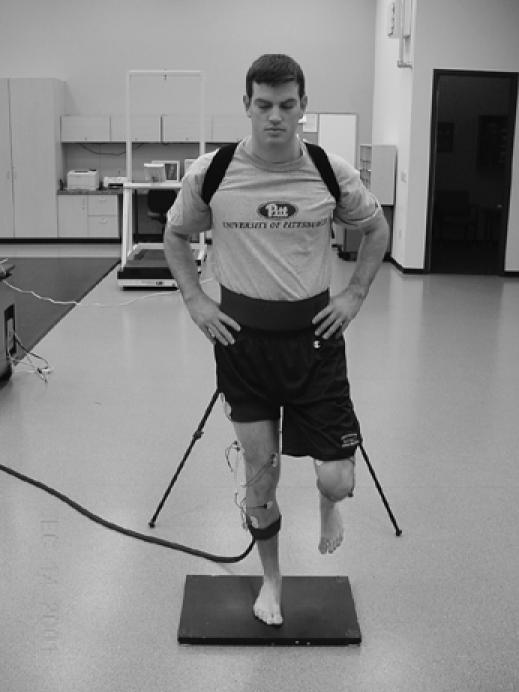
Multivariate assessment of postural control using force plate, electromyographic, and motion analysis.
Studies of disruptions in postural control after orthopaedic injury as measured through force plates during static stance have yielded controversial results. Although some investigators have found decreases in postural stability after joint injury,132,141–144 others have failed to elicit significant differences.145–147 Since force plates depend on center-of-pressure changes and forces exerted against the platform, they may fail to reveal alterations that occur at proximal limb segments. Several researchers have reported alterations in postural control strategies during quiet stance132 and after perturbation.133,134 These results may support the idea that a pathologic joint condition disrupts postural control not only from a sensory perspective but also via the central integration processes or deficiencies in the motor system (or both). An additional use of postural control measures in athletic training research is the area of mild head injury. Several reports have documented changes in postural stability in athletes who sustain mild head injury using both clinical and instrumented measures.126–128,148 Further research is needed to consider postural control through force plate, kinematic, and EMG measures during more dynamic and functional activities.
Muscle and Joint Stiffness
Muscle stiffness, defined as the ratio of change in force per change in length,149,150 is beginning to receive attention from several perspectives within orthopaedic research. Interestingly enough, the closely related characteristic of joint stiffness has been a subject of interest for many years in the rheumatology field.151,152 In contrast to muscle stiffness, which refers specifically to the stiffness properties exhibited by tenomuscular tissues, joint stiffness involves the contributions of all of the structures located within and over the joint (muscles, tendons, skin, subcutaneous tissue, fascia, ligaments, joint capsule, and cartilage).151,152 In our previous articles,2,3 we reviewed the theoretic importance of stiffness to functional joint stability and the role of joint mechanoreceptors in stiffness regulation.
Several testing models have been used to measure stiffness during various levels of muscle activation. The first method measures the resistance to passive movement of the joint and, therefore, reflects the stiffness characteristics of all structures that span the joint (joint stiffness) (Figure 10A).151,152 Data regarding angular position and resistance are related using a polynomial equation, with the slope of the line representing stiffness due to elasticity (Figure 10B).152 Recently, this model was applied in an orthopaedic investigation determining the effects of sex and joint angle on the contribution of the gastrocnemius muscle to ankle joint stiffness.153
Figure 10.
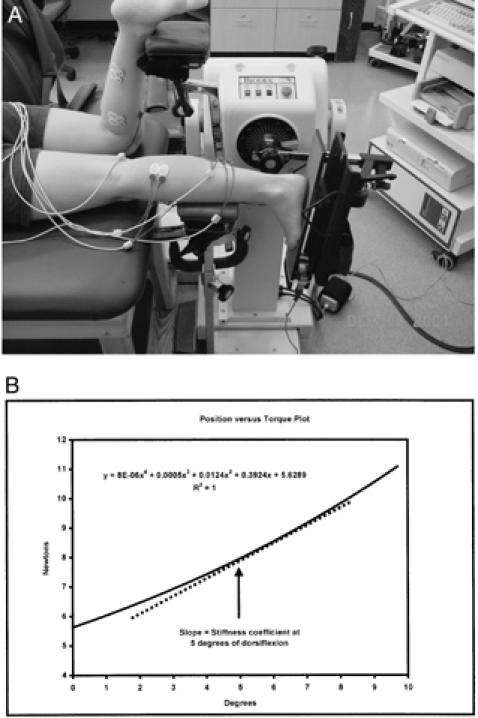
A, Ankle joint stiffness assessed during passive movement using position data from an isokinetic dynamometer and torque data from an externally fixed load cell. B, Typical joint stiffness derived from the slope of the position versus torque data.
Sinkjaer et al154–156 have used a complex version of this testing design with a high-speed, servo-controlled motor to produce angular perturbations. The motor-driven device applies a high-velocity, low-amplitude dorsiflexion movement to the ankle. The perturbation device uses potentiometers to measure the resistance of the ankle for the dorsiflexion movement and ankle joint position and EMG to measure reflexive muscle activity. Through their series of studies, Sinkjaer et al154–156 have been able to quantify not only the contributions of intrinsic stiffness (stiffness before sensorimotor activation of the stretch reflex) but also the role the stretch reflex plays in providing joint stiffness (extrinsic stiffness). Extrinsic stiffness data may suggest that although joint stiffness is increased when compared with intrinsic stiffness alone, the reflex may not react quickly enough to support the joint, indicating that intrinsic stiffness may be a more vital component of stability. Intrinsic stiffness provides the first line of defense for joint stability when force is applied to the joint.149,156–160 Similarly, Kirsch, Kearney, and Hunter161–163 have used similar methods to determine the influence of activation levels and angular position on joint mechanics and stiffness.
Another stiffness testing approach focuses more on the stiffness of the musculotendinous complex crossing a particular joint by using a single-degree-of-mass spring system with a damping component.149,164,165 With this method, 2 different approaches have been used. Oatis164 assessed stiffness by measuring the damping of joint motion during muscle relaxation. For example, the subject was seated with the lower leg hanging off the table. Each trial consisted of the tester holding the relaxed leg of the subject, then dropping the limb, allowing for free pendulum motion. From knee-flexion data obtained with an electrogoniometer, as well as anthropometric assessment of limb characteristics, knee stiffness was calculated. Unlike Oatis,164 who calculated stiffness in the absence of muscle contraction, McNair et al149 and Wilson et al165 measured the damping to induced oscillations under varying degrees of muscle contraction. McNair et al149 positioned subjects prone with the knee and hip flexed at 30° of flexion. By having subjects contract at 30%, 45%, and 60% of a hamstring maximum voluntary contraction, gentle downward force was applied to the posterior aspect of the limb. McNair et al149 calculated stiffness by measuring the oscillation characteristics of the limb using an accelerometer. As one would expect, stiffness increased as a function of muscle contraction because of increased cross-bridge activation.149 McNair et al149 found a moderate correlation between hamstring muscle stiffness and functional ability in ACL-deficient individuals. These results suggest that the hamstrings may resist anterior translation of the tibia in the absence of the ACL.
Lastly, stiffness has been measured during functional tasks such as running,166,167 hopping,168,169 and landing.170 Stiffness during these activities has been calculated by determining either the relationship between the vertical ground reaction force and center-of-mass displacement167 or the natural frequency of the equivalent mass-spring system.170 The advantage to these methods is being able to assess stiffness during functional movements. Future researchers should consider using these methods to advance the findings of McNair et al with respect to ACL-deficient participants and to possibly explain the increased incidence of noncontact ACL injuries in females.
CONCLUSIONS
Collectively, the techniques we have discussed in this article provide a means to evaluate the integrity and function of sensorimotor components by measuring variables along the afferent or efferent (or both) pathways and the final outcome of skeletal muscle activation. In most of the studies, these techniques have been used in isolation to compare normal and abnormal groups. However, conducting comprehensive comparisons of variables located on both the afferent and efferent pathways in patients with different combinations of mechanical and functional stability status may better solidify our understanding of the sensorimotor system. These types of investigations have the potential advantages of identifying the coexistence of sensorimotor deficits after injury and the successful compensatory patterns developed in patients maintaining functional joint stability in the absence of mechanical stability.
Once deficits and effective compensatory patterns are identified, investigators can begin to examine the efficacy of management strategies, both conservative and surgical, in restoring functional joint stability. The measurement techniques discussed in this article also can be applied to prospective and preventive considerations of joint injury. Current major research trends include identifying sex differences and the influence of fatigue as predisposing factors to joint injury. Optimizing the application of sensorimotor research to clinical settings requires the use of common nomenclature and techniques understood by both clinicians and researchers. Our purpose was to initiate a bridge of understanding by providing an overview of the currently available sensorimotor measurement techniques and procedures.
REFERENCES
- Lephart S M, Riemann B L, Fu F H, Lephart S M, Fu F H, editors. Proprioception and Neuromuscular Control in Joint Stability. Human Kinetics; Champaign, IL: 2000. Introduction to the sensorimotor system; pp. xvii–xxiv. [Google Scholar]
- Riemann B L, Lephart S M. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37:80–84. [PMC free article] [PubMed] [Google Scholar]
- Riemann B L, Lephart S M. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37:71–79. [PMC free article] [PubMed] [Google Scholar]
- Horak F B, Nashner L M, Diener H C. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Refshauge K M, Fitzpatrick R C. Perception of movement at the human ankle: effects of leg position. J Physiol. 1995;488:243–248. doi: 10.1113/jphysiol.1995.sp020962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B C, McMeeken J M, Macdonell R AI. Aftereffects of resisted muscle contractions on the accuracy of joint position sense in elite male athletes. Arch Phys Med Rehabil. 1998;79:1250–1254. doi: 10.1016/s0003-9993(98)90270-2. [DOI] [PubMed] [Google Scholar]
- Dvir Z, Koren E, Halperin N. Knee joint position sense following reconstruction of the anterior cruciate ligament. J Orthop Sport Phys Ther. 1988;10:117–120. doi: 10.2519/jospt.1988.10.4.117. [DOI] [PubMed] [Google Scholar]
- Lephart S M, Warner J P, Borsa P A, Fu F H. Proprioception of the shoulder joint in healthy, unstable and surgically repaired shoulders. J Shoulder Elbow Surg. 1994;3:371–380. doi: 10.1016/S1058-2746(09)80022-0. [DOI] [PubMed] [Google Scholar]
- Barrett D S. Proprioception and function after anterior cruciate reconstruction. J Bone Joint Surg Br. 1991;73:833–837. doi: 10.1302/0301-620X.73B5.1894677. [DOI] [PubMed] [Google Scholar]
- Lephart S M, Kocher M S, Fu F H, Borsa P A, Harner C D. Proprioception following anterior cruciate ligament reconstruction. J Sport Rehabil. 1992;1:188–196. [Google Scholar]
- Barrack R L, Skinner H B, Brunet M E, Cook S D. Joint kinesthesia in the highly trained knee. J Sports Med Phys Fitness. 1984;24:18–20. [PubMed] [Google Scholar]
- Barrack R L, Skinner H B, Buckley S L. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17:1–6. doi: 10.1177/036354658901700101. [DOI] [PubMed] [Google Scholar]
- Lephart S M, Pincivero D M, Giraldo J L, Fu F H. The role of proprioception in the management and rehabilitation of athletic injuries. Am J Sports Med. 1997;25:130–137. doi: 10.1177/036354659702500126. [DOI] [PubMed] [Google Scholar]
- Edin B B, Abbs J H. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin B B. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Matthews P BC. Where does Sherrington's “muscle sense” originate? Muscles, joints, corollary discharges? Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- Clark F J, Burgess P R. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol. 1975;38:1448–1463. doi: 10.1152/jn.1975.38.6.1448. [DOI] [PubMed] [Google Scholar]
- Grigg P. Articular neurophysiology. In: Zachazewski J, Magee D, Quillen W, editors. Athletic Injuries and Rehabilitation. WB Saunders Co; Philadelphia, PA: 1996. pp. 152–169. [Google Scholar]
- Proske U, Schaible H G, Schmidt R F. Joint receptors and kinaesthesia. Exp Brain Res. 1988;72:219–224. doi: 10.1007/BF00250245. [DOI] [PubMed] [Google Scholar]
- Burgess P R, Wei J Y, Clark F J, Simon J. Signaling of kinesthetic information by peripheral sensory receptors. Annu Rev Neurosci. 1982;5:171–187. doi: 10.1146/annurev.ne.05.030182.001131. [DOI] [PubMed] [Google Scholar]
- Burgess P R, Clark F J. Characteristics of knee joint receptors in the cat. J Physiol. 1969;203:317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G M, McCloskey M, Matthews P BC. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia S C, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer W Z, D'Almeida A. Joint position sense: the effects of muscle contraction. Brain. 1980;103:1–22. doi: 10.1093/brain/103.1.1. [DOI] [PubMed] [Google Scholar]
- Roland P E, Ladergaard-Pedersen H. A quantitative analysis of sensations of tension and of kinesthesia in man: evidence for a peripherally originating muscular sense and for a sense of effort. Brain. 1977;100:671–692. doi: 10.1093/brain/100.4.671. [DOI] [PubMed] [Google Scholar]
- Enbom H, Magnusson M, Pyykko I, Schalen L. Presentation of a posturographic test with loading of the proprioceptive system. Acta Otolaryngol Suppl. 1988;455:58–61. doi: 10.3109/00016488809125058. [DOI] [PubMed] [Google Scholar]
- Pyykko I, Enbom H, Magnusson M, Schalen L. Effect of proprioceptor stimulation on postural stability in patients with peripheral or central vestibular lesion. Acta Otolaryngol. 1991;111:27–35. doi: 10.3109/00016489109137351. [DOI] [PubMed] [Google Scholar]
- Garn S N, Newton R A. Kinesthetic awareness in subjects with multiple ankle sprains. Phys Ther. 1988;68:1667–1671. doi: 10.1093/ptj/68.11.1667. [DOI] [PubMed] [Google Scholar]
- Glencross D, Thornton E. Position sense following injury. J Sports Med Phys Fitness. 1981;21:23–27. [PubMed] [Google Scholar]
- Gross M T. Effects of recurrent lateral ankle sprains on active and passive judgements of joint position. Phys Ther. 1987;67:1505–1509. doi: 10.1093/ptj/67.10.1505. [DOI] [PubMed] [Google Scholar]
- Forkin D M, Koczur C, Battle R, Newton R A. Evaluation of kinesthetic deficits indicative of balance control in gymnasts with unilateral chronic ankle sprains. J Orthop Sports Phys Ther. 1996;23:245–250. doi: 10.2519/jospt.1996.23.4.245. [DOI] [PubMed] [Google Scholar]
- Lentell G, Baas B, Lopez D, McGuire L, Sarrels M, Snyder P. The contributions of proprioceptive deficits, muscle function, and anatomic laxity to functional instability of the ankle. J Orthop Sports Phys Ther. 1995;21:206–215. doi: 10.2519/jospt.1995.21.4.206. [DOI] [PubMed] [Google Scholar]
- Harter R A, Osternig L R, Singer K M. Knee joint proprioception following anterior cruciate ligament reconstruction. J Sport Rehabil. 1992;1:103–110. [Google Scholar]
- Borsa P A, Lephart S M, Irrgang J J, Safran M R, Fu F H. The effects of joint position and direction of joint motion on proprioceptive sensibility in anterior cruciate ligament–deficient athletes. Am J Sports Med. 1997;25:336–340. doi: 10.1177/036354659702500311. [DOI] [PubMed] [Google Scholar]
- Skinner H B, Barrack R L. Joint position sense in the normal and pathologic knee joint. J Electromyogr Kinesiol. 1991;1:180–190. doi: 10.1016/1050-6411(91)90033-2. [DOI] [PubMed] [Google Scholar]
- Carter N D, Jenkinson T R, Wilson D, Jones D W, Torode A S. Joint position sense and rehabilitation in the anterior cruciate ligament deficient knee. Br J Sports Med. 1997;31:209–212. doi: 10.1136/bjsm.31.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R L, Brunolli J. Shoulder kinesthesia after anterior glenohumeral joint dislocation. Phys Ther. 1989;69:106–112. doi: 10.1093/ptj/69.2.106. [DOI] [PubMed] [Google Scholar]
- Myers J B, Lephart S M, Riemann B L, Bradley J P, Fu F H. Evaluation of shoulder proprioception following thermal capsulorrhaphy. Med Sci Sports Exerc. 2000;32:S123. [Google Scholar]
- Payne K A, Berg K, Latin R W. Ankle injuries and ankle strength, flexibility, and proprioception in college basketball players. J Athl Train. 1997;32:221–225. [PMC free article] [PubMed] [Google Scholar]
- Lattanzio P J, Petrella R J, Sproule J R, Fowler P J. Effects of fatigue on knee proprioception. Clin J Sport Med. 1997;7:22–27. doi: 10.1097/00042752-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Myers J B, Guskiewicz K M, Schneider R A, Prentice W E. Proprioception and neuromuscular control following muscle fatigue. J Athl Train. 1999;34:362–367. [PMC free article] [PubMed] [Google Scholar]
- Borsa P A, Lephart S M, Irrgang J J. Comparison of performance-based and patient-reported measures of function in anterior-cruciate-ligament-deficient individuals. J Orthop Sports Phys Ther. 1998;28:392–399. doi: 10.2519/jospt.1998.28.6.392. [DOI] [PubMed] [Google Scholar]
- Corrigan J P, Cashman W F, Brady M P. Proprioception in the cruciate deficient knee. J Bone Joint Surg Br. 1992;74:247–250. doi: 10.1302/0301-620X.74B2.1544962. [DOI] [PubMed] [Google Scholar]
- Nuwer M R. Fundamentals of evoked potentials and common clinical applications today. Electroencephalogr Clin Neurophysiol. 1998;106:142–148. doi: 10.1016/s0013-4694(97)00117-x. [DOI] [PubMed] [Google Scholar]
- Pitman M I, Nainzadeh N, Menche D, Gasalberti R, Song E K. The intraoperative evaluation of the neurosensory function of the anterior cruciate ligament in humans using somatosensory evoked potentials. Arthroscopy. 1992;8:442–447. doi: 10.1016/0749-8063(92)90005-v. [DOI] [PubMed] [Google Scholar]
- Mima T, Terada K, Maekawa M, Nagamine T, Ikeda A, Shibasaki S. Somatosensory evoked potentials following proprioceptive stimulation of finger in man. Exp Brain Res. 1996;111:233–245. doi: 10.1007/BF00227300. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Lazzaro V D, Franceschi F, Fabbriciani C, Tonali P. Central nervous system modifications in patients with lesion of the anterior cruciate ligament of the knee. Brain. 1996;119:1751–1762. doi: 10.1093/brain/119.5.1751. [DOI] [PubMed] [Google Scholar]
- Barrack R L, Lund P J, Munn B G, Wink C, Happel L. Evidence of reinnervation of free patellar tendon autograft used for anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:196–202. doi: 10.1177/036354659702500210. [DOI] [PubMed] [Google Scholar]
- Tibone J E, Fechter J, Kao J T. Evaluation of a proprioception pathway in patients with stable and unstable shoulders with somatosensory cortical evoked potentials. J Shoulder Elbow Surg. 1997;6:440–443. doi: 10.1016/s1058-2746(97)70050-8. [DOI] [PubMed] [Google Scholar]
- DeLisa J A, Mackenzie K, Baran E M. Manual of Nerve Conduction Velocity and Clinical Neurophysiology. Raven Press; New York, NY: 1994. [Google Scholar]
- Lenman J AR, Ritchie A E. Clinical Electromyography. Churchill Livingstone; New York, NY: 1987. [Google Scholar]
- Wilbourn A J. Electrodiagnostic testing of neurologic injuries in athletes. Clin Sports Med. 1990;9:229–245. [PubMed] [Google Scholar]
- Feinberg J H, Nadler S F, Krivickas L S. Peripheral nerve injuries in the athlete. Sports Med. 1997;24:385–408. doi: 10.2165/00007256-199724060-00004. [DOI] [PubMed] [Google Scholar]
- Streib E W. Traction injury of peroneal nerve caused by minor athletic trauma: electromyographic studies. Arch Neurol. 1983;40:62–63. doi: 10.1001/archneur.1983.04050010082029. [DOI] [PubMed] [Google Scholar]
- Nobel W. Peroneal palsy due to hematoma in the common peroneal nerve sheath after distal torsional fractures and inversion ankle sprains. J Bone Joint Surg Am. 1966;48:1484–1495. [PubMed] [Google Scholar]
- Meals R A. Peroneal-nerve palsy complicating ankle sprain: report of two cases and review of the literature. J Bone Joint Surg Am. 1977;59:966–968. [PubMed] [Google Scholar]
- Kleinrensink G J, Stoeckart R, Meulstee J, et al. Lowered motor conduction velocity of the peroneal nerve after inversion trauma. Med Sci Sports Exerc. 1994;26:877–883. [PubMed] [Google Scholar]
- Di Benedetto M, Markey K. Electrodiagnostic localization of traumatic upper trunk brachial plexopathy. Arch Phys Med Rehabil. 1984;65:15–17. [PubMed] [Google Scholar]
- Kamen G, Caldwell G E. Physiology and interpretation of the electromyogram. J Clin Neurophysiol. 1996;13:366–384. doi: 10.1097/00004691-199609000-00002. [DOI] [PubMed] [Google Scholar]
- De Luca C J. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- Winter D A. Biomechanics and Motor Control of Human Movement. 2nd ed John Wiley & Sons Inc; New York, NY: 1990. [Google Scholar]
- Basmajian J V, DeLuca C J. Muscles Alive: Their Functions Revealed by Electromyography. 5th ed Williams & Wilkins; Baltimore, MD: 1985. [Google Scholar]
- Komi P V, Buskirk E R. Reproducibility of electromyographic measurements with inserted wire electrodes and surface electrodes. Electromyography. 1970;10:357–367. [PubMed] [Google Scholar]
- Zipp P. Recommendation for the standardization of lead positions in surface electromyography. Eur J Appl Physiol Occup Physiol. 1982;50:41–54. [Google Scholar]
- Basmajian J V, Blumenstein R. Electrode placement in electromyographic biofeedback. In: Basmajian J V, editor. Biofeedback: Principles and Practice for Clinicians. 3rd ed. Williams & Wilkins; Baltimore, MD: 1989. pp. 369–382. [Google Scholar]
- Yang J F, Winter D A. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65:517–521. [PubMed] [Google Scholar]
- Hillstrom H J, Triolo R J. EMG theory. In: Craik R, Oatis C, editors. Gait Analysis: Theory and Application. Mosby-Year Book Inc; St Louis, MO: 1995. pp. 271–292. [Google Scholar]
- Kelly B T, Cooper L W, Kirkendall D T, Speer K P. Technical considerations for electromyography research on the shoulder. Clin Orthop. 1997;335:140–151. [PubMed] [Google Scholar]
- Basmajian J V, Stecko G. A new bipolar electrode for electromyography. J Appl Physiol. 1962;17:849. [Google Scholar]
- Jonsson B, Bagge U E. Displacement, deformation and fracture of wire electrodes for electromyography. Electromyography. 1968;8:329–347. [PubMed] [Google Scholar]
- Geiringer S R. Anatomic Localization for Needle Electromyography. 2nd ed Hanley & Belfus Inc; Philadelphia, PA: 1998. [Google Scholar]
- Goodgold J. Anatomical Correlates of Clinical Electromyography. 2nd ed Williams & Wilkins; Baltimore, MD: 1984. [Google Scholar]
- Kadaba M P, Cole A, Wootten M E, et al. Intramuscular wire electromyography of the subscapularis. J Orthop Res. 1992;10:394–397. doi: 10.1002/jor.1100100312. [DOI] [PubMed] [Google Scholar]
- Perotto A O, Delagi E F, Iazzetti J, Morrison D. Anatomical Guide for the Electromyographer. 3rd ed Charles C Thomas; Springfield, MO: 1994. [Google Scholar]
- Nemeth G, Kronberg M, Brostrom L A. Electromyogram (EMG) recordings from the subscapularis muscle: description of a technique. J Orthop Res. 1990;8:151–153. doi: 10.1002/jor.1100080120. [DOI] [PubMed] [Google Scholar]
- Giroux B, Lamantagne M. Comparison between surface electrodes and intramuscular wire electrodes in isometric and dynamic conditions. Electromyogr Clin Neurophysiol. 1990;30:397–405. [PubMed] [Google Scholar]
- Gerleman D G, Cook T M. Instrumentation. In: Soderberg G, editor. Selected Topics in Surface Electromyography for Use in the Occupational Setting: Expert Perspectives. US Department of Health and Human Services; Washington, DC: 1992. pp. 44–68. [Google Scholar]
- Wojtys E M, Huston L J. Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. Am J Sports Med. 1994;22:89–104. doi: 10.1177/036354659402200116. [DOI] [PubMed] [Google Scholar]
- Konradsen L, Olesen S, Hansen H M. Ankle sensorimotor control and eversion strength after acute ankle inversion injuries. Am J Sports Med. 1998;26:72–77. doi: 10.1177/03635465980260013001. [DOI] [PubMed] [Google Scholar]
- Beard D J, Kyberd P J, Fergusson C M, Dodd C AF. Proprioception after rupture of the anterior cruciate ligament: an objective indication of the need for surgery? J Bone Joint Surg Br. 1993;75:311–315. doi: 10.1302/0301-620X.75B2.8444956. [DOI] [PubMed] [Google Scholar]
- Solmonow M, Baratta R, Zhou B H, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15:207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- Hogervorst T, Brand R A. Mechanoreceptors in joint function. J Bone Joint Surg Am. 1998;80:1365–1378. doi: 10.2106/00004623-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Horak F B, Diener H C, Nashner L M. Influence of central set on human postural responses. J Neurophysiol. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- Ciccotti M G, Kerlan R K, Perry J, Pink M. An electromyographic analysis of the knee during functional activities, II: the anterior cruciate ligament–deficient and –reconstructed profiles. Am J Sports Med. 1994;22:651–658. doi: 10.1177/036354659402200513. [DOI] [PubMed] [Google Scholar]
- Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry T. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70:220–226. [PubMed] [Google Scholar]
- Jobe F W, Tibone J E, Perry J, Moynes D. An EMG analysis of the shoulder in throwing and pitching: a preliminary report. Am J Sports Med. 1983;11:3–5. doi: 10.1177/036354658301100102. [DOI] [PubMed] [Google Scholar]
- Kronberg M, Nemeth G, Brostrom L A. Muscle activity and coordination in the normal shoulder: an electromyographic study. Clin Orthop. 1990;257:76–85. [PubMed] [Google Scholar]
- Sinkjaer T, Arendt-Nielsen L. Knee stability and muscle coordination in patients with anterior cruciate ligament injuries: an electromyographic approach. J Electromyogr Kinesiol. 1991;1:209–217. doi: 10.1016/1050-6411(91)90036-5. [DOI] [PubMed] [Google Scholar]
- van Lent M ET, Drost M R, vd Wildenberg F A. EMG profiles of ACL-deficient patients during walking: the influence of mild fatigue. Int J Sports Med. 1994;15:508–514. doi: 10.1055/s-2007-1021096. [DOI] [PubMed] [Google Scholar]
- Souza D R, Gross M T. Comparison of vastus medialis obliquus:vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Phys Ther. 1991;71:310–316. doi: 10.1093/ptj/71.4.310. [DOI] [PubMed] [Google Scholar]
- Huston L J, Wojtys E M. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- Swanik C B, Lephart S M, Giraldo J L, Fu F H. Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. J Athl Train. 1999;34:121–129. [PMC free article] [PubMed] [Google Scholar]
- DeMont R G, Lephart S M, Giraldo J L, Swanik C B, Fu F H. Muscle preactivity of anterior cruciate ligament-deficient and -reconstructed females during functional activities. J Athl Train. 1999;34:115–120. [PMC free article] [PubMed] [Google Scholar]
- Branch T P, Hunter R, Donath M. Dynamic EMG analysis of anterior cruciate deficient legs with and without bracing during cutting. Am J Sports Med. 1989;17:35–41. doi: 10.1177/036354658901700106. [DOI] [PubMed] [Google Scholar]
- Moseley J B, Jr, Jobe F W, Pink M, Perry J, Tibone J. EMG analysis of the scapular muscles during a shoulder rehabilitation program. Am J Sports Med. 1992;20:128–134. doi: 10.1177/036354659202000206. [DOI] [PubMed] [Google Scholar]
- Townsend H, Jobe F W, Pink W, Perry J. Electromyographic analysis of the glenohumeral muscles during a baseball rehabilitation program. Am J Sports Med. 1991;19:264–272. doi: 10.1177/036354659101900309. [DOI] [PubMed] [Google Scholar]
- Wilk K E, Escamilla R F, Fleisig G S, Barrentine S W, Andrews J R, Boyd M L. A comparison of tibiofemoral joint forces and electromyographic activity during open and closed chain exercises. Am J Sports Med. 1996;24:518–527. doi: 10.1177/036354659602400418. [DOI] [PubMed] [Google Scholar]
- Henry T. An electromyographic analysis of dynamic stabilizing exercises for the shoulder. J Athl Train. 1998;33:S74. [Google Scholar]
- Grabiner M D, Koh T J, Miller G F. Further evidence against a direct automatic neuromotor link between the ACL and hamstrings. Med Sci Sports Exerc. 1992;24:1075–1079. [PubMed] [Google Scholar]
- Perry J, Bekey J A. EMG-force relationships in skeletal muscle. Crit Rev Biomed Eng. 1981;7:1–22. [PubMed] [Google Scholar]
- Perrin D H. Isokinetic Exercise and Assessment. Human Kinetics; Champaign, IL: 1993. [Google Scholar]
- Enoka R M. Neuromechanical Basis of Kinesiology. 2nd ed Human Kinetics; Champaign, IL: 1994. [Google Scholar]
- Hislop H J, Perrine J J. The isokinetic concept of exercise. Phys Ther. 1967;47:114–117. [PubMed] [Google Scholar]
- Hinson M N, Smith W C, Funk S. Isokinetics: a clarification. Res Q. 1979;50:30–35. [PubMed] [Google Scholar]
- Lieber R L, Friden J. Neuromuscular stabilization of the shoulder girdle. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 91–105. [Google Scholar]
- Lephart S M, Perrin D H, Fu F H, Gieck J H, McCue F B, Irrgang J J. Relationship between selected physical characteristics and functional capacity in the anterior cruciate ligament-insufficient athlete. J Orthop Sports Phys Ther. 1992;16:174–181. doi: 10.2519/jospt.1992.16.4.174. [DOI] [PubMed] [Google Scholar]
- Cordova M L, Ingersoll C D, Kovaleski J E, Knight K L. A comparison of isokinetic and isotonic predictions of a functional task. J Athl Train. 1995;30:319–322. [PMC free article] [PubMed] [Google Scholar]
- Dixon S J, Collop A C, Batt M E. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919–1926. doi: 10.1097/00005768-200011000-00016. [DOI] [PubMed] [Google Scholar]
- James C R, Dufek J S, Bates B T. Effects of injury proneness and task difficulty on joint kinetic variability. Med Sci Sports Exerc. 2000;32:1833–1844. doi: 10.1097/00005768-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Cornwall M W, McPoil T G. Three-dimensional movement of the foot during the stance phase of walking. J Am Podiatr Med Assoc. 1999;89:56–66. doi: 10.7547/87507315-89-2-56. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Gransberg L, Knutsson E, Nolen P. A new system for three-dimensional gait recording using electromagnetic tracking. Gait Posture. 1997;6:63–75. [Google Scholar]
- Berger W, Trippel M, Discher M, Dietz V. Influence of subjects' height on the stabilization of posture. Acta Otolaryngol. 1992;112:22–30. doi: 10.3109/00016489209100778. [DOI] [PubMed] [Google Scholar]
- Kleiber M, Horstmann G A, Dietz V. Body sway stabilization of human posture. Acta Otolaryngol. 1990;10:168–174. doi: 10.3109/00016489009122533. [DOI] [PubMed] [Google Scholar]
- Sommer H J., III . Dublin, Ireland: Dual axis cranial accelerometry to assess postural stability. Paper presented at: 12th Conference of the European Society of Biomechanics. August 27-30 2000. [Google Scholar]
- Ladin Z, Flowers W C, Messner W. A quantitative comparison of a position measurement system and accelerometry. J Biomech. 1989;22:295–308. doi: 10.1016/0021-9290(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Rome K, Cowieson F. A reliability study of the universal goniometer, fluid goniometer, and electrogoniometer for the measurement of ankle dorsiflexion. Foot Ankle Int. 1996;17:28–32. doi: 10.1177/107110079601700106. [DOI] [PubMed] [Google Scholar]
- Andriacchi T P. Dynamics of pathological motion: applied to the anterior cruciate deficient knee. J Biomech. 1990;23(suppl 1):99–105. doi: 10.1016/0021-9290(90)90044-4. [DOI] [PubMed] [Google Scholar]
- Gauffin H, Tropp H. Altered movement and muscular-activation patterns during the one-legged jump in patients with an old anterior cruciate ligament rupture. Am J Sports Med. 1992;20:182–192. doi: 10.1177/036354659202000215. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann G A, Berger W. Significance of proprioceptive mechanisms in the regulation of stance. Prog Brain Res. 1989;80:419–423. doi: 10.1016/s0079-6123(08)62238-4. [DOI] [PubMed] [Google Scholar]
- Inglis J T, Horak F B, Shupert C L, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res. 1994;101:159–164. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- Riemann B L, Guskiewicz K M. Contribution of peripheral somatosensory system to balance and postural equilibrium. In: Lephart S M, Fu F H, editors. Prioprioception and Neuromuscular Control in Joint Stability. Human Kinetics; Champaign, IL: 2000. pp. 37–51. [Google Scholar]
- Hertel J, Guskiewicz K M, Kahler D M, Perrin D H. Effect of lateral ankle joint anesthesia on center of balance, postural sway and joint position sense. J Sport Rehabil. 1996;5:111–119. [Google Scholar]
- DeCarlo M S, Talbot R W. Evaluation of ankle proprioception following injection of the anterior talofibular ligament. J Orthop Sports Phys Ther. 1986;8:70–76. doi: 10.2519/jospt.1986.8.2.70. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Wiklund J. Significance of pressor input from the human feet in lateral postural control: the effect of hypothermia on galvanically-induced body-sway. Acta Otolaryngol. 1990;110:321–327. doi: 10.3109/00016489009107450. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Pyykko I. Significance of pressor input from the human feet in anterior-posterior postural control: the effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol. 1990;110:182–188. doi: 10.3109/00016489009122535. [DOI] [PubMed] [Google Scholar]
- Riemann B L, Guskiewicz K M. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35:19–25. [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K M, Riemann B L, Perrin D H, Nashner L M. Alternative approaches to the assessment of mild head injury in athletes. Med Sci Sports Exerc. 1997;29:S213–S221. doi: 10.1097/00005768-199707001-00003. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K M, Perrin D H, Gansneder B. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31:300–306. [PMC free article] [PubMed] [Google Scholar]
- Diener H-C, Dichgans J. On the role of vestibular, visual and somatosensory information for dynamic postural control in humans. Prog Brain Res. 1988;76:253–262. doi: 10.1016/s0079-6123(08)64512-4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia S C. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl C, Jarnlo G B, Andersson S I. Standing balance in healthy subjects: evaluation of a quantitative test battery on a force platform. Scand J Rehabil Med. 1989;21:187–195. [PubMed] [Google Scholar]
- Tropp H, Odenrick P. Postural control in single-limb stance. J Orthop Res. 1988;6:833–839. doi: 10.1002/jor.1100060607. [DOI] [PubMed] [Google Scholar]
- Brunt D, Andersen J C, Huntsman B, Reinhert L B, Thorell A C, Sterling J C. Postural responses to lateral perturbations in healthy subjects and ankle sprain patients. Med Sci Sports Exerc. 1992;24:171–176. [PubMed] [Google Scholar]
- Pintsaar A, Brynhildsen J, Tropp H. Postural corrections after standardized perturbations of single limb stance: effect of training and orthotic devices in patients with ankle instability. Br J Sports Med. 1996;30:151–155. doi: 10.1136/bjsm.30.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson L I, Whipple R, Amerman P, Kleinburg A. Stressing the postural response: a quantitative method for testing balance. J Am Geriatr Soc. 1986;34:845–850. doi: 10.1111/j.1532-5415.1986.tb07256.x. [DOI] [PubMed] [Google Scholar]
- Riemann B L, Caggiano N A, Lephart S M. Examination of a clinical method of assessing postural control during functional performance task. J Sport Rehabil. 1999;8:171–183. [Google Scholar]
- Riemann B L, Guskiewicz K M, Shields E W. Relationship between clinical and forceplate measures of postural stability. J Sport Rehabil. 1999;8:71–82. [Google Scholar]
- Hertel J, Miller S J, Denegar C R. Intratester and intertester reliability during the Star Excursion Balance Tests. J Sport Rehabil. 2000;9:104–116. [Google Scholar]
- Crotts D, Thompson B, Nahom M, Ryan S, Newton R A. Balance abilities of professional dancers on select balance tests. J Orthop Sports Phys Ther. 1996;23:12–17. doi: 10.2519/jospt.1996.23.1.12. [DOI] [PubMed] [Google Scholar]
- Guskiewcz K M, Perrin D H. Research and clinical applications of assessing balance. J Sport Rehabil. 1996;5:45–63. [Google Scholar]
- Guskiewicz K M, Perrin D H. Effect of orthotics on postural sway following inversion ankle sprain. J Orthop Sports Phys Ther. 1996;23:326–331. doi: 10.2519/jospt.1996.23.5.326. [DOI] [PubMed] [Google Scholar]
- Freeman M A. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47:669–677. [PubMed] [Google Scholar]
- Tropp H, Odenrick P, Gillquist J. Stabilometry recordings in functional and mechanical instability of the ankle joint. Int J Sports Med. 1985;6:180–182. doi: 10.1055/s-2008-1025836. [DOI] [PubMed] [Google Scholar]
- Zatterstrom R, Friden T, Lindstrand A, Moritz U. The effect of physiotherapy on standing balance in chronic anterior cruciate ligament insufficiency. Am J Sports Med. 1994;22:531–536. doi: 10.1177/036354659402200416. [DOI] [PubMed] [Google Scholar]
- Bernier J, Perrin D H, Rijke A. Effect of unilateral functional instability of the ankle on postural sway and inversion and eversion strength. J Athl Train. 1997;32:226–232. [PMC free article] [PubMed] [Google Scholar]
- Isakov E, Mizrahi J. Is balance impaired by recurrent sprained ankle? Br J Sports Med. 1997;31:65–67. doi: 10.1136/bjsm.31.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E L, Duenkel N, Dunlop R, Russell G. Evaluation of single-leg standing following anterior cruciate ligament surgery and rehabilitation. Phys Ther. 1994;74:245–252. doi: 10.1093/ptj/74.3.245. [DOI] [PubMed] [Google Scholar]
- Riemann B L, Guskiewicz K M. Assessment of mild head injury utilizing measures of balance and cognition: a case study. J Sport Rehabil. 1997;6:283–289. [Google Scholar]
- McNair P J, Wood G A, Marshall R N. Stiffness of the hamstring muscles and its relationship to function in anterior cruciate deficient individuals. Clin Biomech. 1992;7:131–137. doi: 10.1016/0268-0033(92)90027-2. [DOI] [PubMed] [Google Scholar]
- Johansson H, Sjolander P. The neurophysiology of joints. In: Wright V, Radin E, editors. Mechanics of Joints: Physiology, Pathophysiology and Treatment. Marcel Dekker Inc; New York, NY: 1993. pp. 243–290. [Google Scholar]
- Helliwell P S. Joint stiffness. In: Wright V, Radin E, editors. Mechanics of Human Joints: Physiology, Pathophysiology and Treatment. Marcel Dekker Inc; New York, NY: 1993. pp. 203–218. [Google Scholar]
- Wright V. Stiffness: a review of its measurement and physiological importance. Physiotherapy. 1973;59:107–111. [PubMed] [Google Scholar]
- Riemann B L, DeMont R G, Ryu K H, Lephart S M. The effects of sex, joint angle, and the gastrocnemius muscle on passive ankle joint complex stiffness. J Athl Train. 2001;36:369–377. [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Nielsen J, Toft E. Mechanical and electromyographic analysis of reciprocal inhibition at the human ankle joint. J Neurophysiol. 1995;74:849–855. doi: 10.1152/jn.1995.74.2.849. [DOI] [PubMed] [Google Scholar]
- Toft E, Sinkjaer T, Kalun S, Esperson G T. Biomechanical properties of the human ankle in relation to passive stretch. J Biomech. 1989;22:1129–1132. doi: 10.1016/0021-9290(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, Hornemann B C. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol. 1988;60:1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Akazawa K, Aldridge J W, Steeves J D, Stein R B. Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol. 1982;329:553–567. doi: 10.1113/jphysiol.1982.sp014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa K, Milner T E, Stein R B. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol. 1983;49:16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Blanpied P, Smidt G L. Human plantarflexor stiffness to multiple single stretch trials. J Biomech. 1992;25:29–39. doi: 10.1016/0021-9290(92)90243-t. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Hayashi R. Regulation of wrist stiffness by the stretch reflex. J Biomech. 1989;22:1133–1140. doi: 10.1016/0021-9290(89)90215-7. [DOI] [PubMed] [Google Scholar]
- Kirsch R F, Kearney R E. Identification of time-varying stiffness dynamics of the human ankle joint during an imposed movement. Exp Brain Res. 1997;114:71–85. doi: 10.1007/pl00005625. [DOI] [PubMed] [Google Scholar]
- Hunter I W, Kearney R E. Invariance of ankle dynamic stiffness during fatiguing muscle contractions. J Biomech. 1983;16:985–991. doi: 10.1016/0021-9290(83)90099-4. [DOI] [PubMed] [Google Scholar]
- Kirsch R F, Kearney R E, MacNeil J B. Identification of time-varying dynamics of the human triceps surae stretch reflex. Exp Brain Res. 1993;97:115–127. doi: 10.1007/BF00228822. [DOI] [PubMed] [Google Scholar]
- Oatis C A. The use of a mechanical model to describe the stiffness and damping characteristics of the knee joint in healthy adults. Phys Ther. 1993;73:740–749. doi: 10.1093/ptj/73.11.740. [DOI] [PubMed] [Google Scholar]
- Wilson G J, Wood G A, Elliott B C. The relationship between stiffness of the musculature and static flexibility: an alternative explanation for the occurrence of muscular injury. Int J Sports Med. 1991;12:403–407. doi: 10.1055/s-2007-1024702. [DOI] [PubMed] [Google Scholar]
- McMahon T A, Valiant G, Frederick E C. Groucho running. J Appl Physiol. 1987;62:2326–2337. doi: 10.1152/jappl.1987.62.6.2326. [DOI] [PubMed] [Google Scholar]
- Farley C T, Gonzalez O. Leg stiffness and stride frequency in human running. J Biomech. 1996;29:181–186. doi: 10.1016/0021-9290(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Farley C T, Blickhan R, Saito J, Taylor C R. Hopping frequency in humans: a test of how springs set stride frequency in bouncing gaits. J Appl Physiol. 1991;71:2127–2132. doi: 10.1152/jappl.1991.71.6.2127. [DOI] [PubMed] [Google Scholar]
- Ferris D P, Farley C T. Interaction of leg stiffness and surfaces stiffness during human hopping. J Appl Physiol. 1997;82:15–22. doi: 10.1152/jappl.1997.82.1.15. [DOI] [PubMed] [Google Scholar]
- Cavagna G A. Elastic bounce of the body. J Appl Physiol. 1970;29:279–282. doi: 10.1152/jappl.1970.29.3.279. [DOI] [PubMed] [Google Scholar]


