Abstract
Objective: To determine blood serum creatine kinase (CK) levels in football players undergoing 2-a-day football practices and to determine if CK levels are related to fitness. Our hypotheses were that CK levels in each subject would increase over the course of practices and that higher levels of fitness would result in smaller increases in CK.
Design and Setting: Creatine kinase measurements were taken 4 times over 10 days of preseason, 2-a-day practices: before beginning practices (CKM1) and on the mornings of the 4th (CKM2), 7th (CKM3), and 10th (CKM4) days of practice.
Subjects: Twelve male Division I football players from a midwestern university.
Measurements: Fitness tests included percentage of body fat, body mass index, anaerobic capacity, and peak power from a 1-leg step test and 1-repetition maximum bench press and squat lifts. Changes in CK levels were calculated as the difference between the second CK measure (CKM2) and the first CK measure (CKM1).
Results: Differences were significant between the CK measurements (P = .0002). Post hoc analysis revealed that CKM2 and CKM3 levels were statistically higher than CKM1 levels. No other statistically significant differences between CK measures were noted. Pearson product moment correlation coefficients showed that athletes who generated higher peak power during a 15-second step test had smaller increases in CK levels from CMK1 to CMK2 (r = −.64). Although the correlations with anaerobic capacity (r = −.54, P = .071), body mass index (r = −.51, P = .090), and percentage of body fat (r = −.52, P = .082) approached statistical significance, no other correlations were statistically significant. The mean CKM2 level was 5124.7 U·L−1 ± 5518.1, approximately 30 times the norm for men.
Conclusions: Participation in 2-a-day football practices resulted in significant serum CK elevations, which remained elevated for at least 7 days. Participants who had higher peak power had smaller increases in CK.
Keywords: exertional rhabdomyolysis, muscular damage, peak power
Muscular damage resulting from vigorous exercise is a common and normal event. Athletes often feel muscular soreness between 8 and 24 hours postexercise, with peak levels occurring at around 48 hours.1 This soreness is thought to be the result of the muscular damage that can occur with any type of high-intensity workout and has been reported to occur at the level of the sarcolemma, Z discs, or both.2 Although it is normal to sustain muscular damage with exercise, excessive damage can cause a condition known as exertional rhabdomyolysis. Exertional rhabdomyolysis is defined as the degeneration of skeletal muscle caused by excessive unaccustomed exercise and has been known to cause death in healthy athletes.3–5
One of the most valid and reliable methods for assessing muscular damage is to check for increases in blood serum levels of creatine kinase (CK), the primary enzyme regulating anaerobic metabolism, because a high percentage of the body's CK is present in skeletal muscle tissue.2 Assessing CK levels has been commonplace for more than 3 decades in studies investigating muscular damage.6 Creatine kinase is located in the sarcolemma and mitochondrial intermembrane space of healthy muscle cells and is responsible for catalyzing the movement of phosphate from phosphocreatine to adenosine diphosphate, forming adenosine triphosphate (ATP) and creatine.7,8
Creatine kinase is present in the body as 3 isozymes: the skeletal muscle, cardiac muscle, and brain tissue types. In some clinical situations, it may be relevant to check for one of the 3 types of isozymes. If cardiac pathology is suspected, increases in the cardiac muscle type can be expected. In cases of head injury, increases in the brain tissue type can be expected. However, strenuous exercise that damages skeletal muscle cell structure results in an increase in total CK, with a mixture of all 3 isozymes.2,9 Therefore, when analyzing CK, one may mistakenly conclude that physical exercise caused damage to heart or brain tissue. However, most research shows the increase in the cardiac and brain CK isozymes to be a negligible amount of the total exercise-induced increase in CK.9,10 Also, most studies indicate that the CK elevation is from skeletal muscle, not cardiac muscle.2,11
Nosaka and Clarkson12 pointed out that the exact mechanism by which CK enters the general blood circulation is unknown; however, it is thought that when acute damage occurs to the muscle cell structure, CK leaks into the interstitial fluid and is picked up by the lymphatic system. The CK then travels through the lymphatic system and is eventually emptied back into the general blood circulation, which results in an increase in serum levels of CK.9 Thus, increases in blood levels of CK are one indicator of muscular trauma. Normal resting levels of CK for men are 55 to 170 units per liter (U·L−1), and for women, 30 to 135 units per liter (U·L−1).13
When strenuous exercise results in excessive muscular damage, a condition known as exertional rhabdomyolysis can develop. Postexercise CK levels 5 to 10 times the normal limit for men or women have been defined as laboratory evidence that exertional rhabdomyolysis may be present, although it is possible to reach much higher levels.4,14 Line and Rust4 presented the case of a runner who was diagnosed with exertional rhabdomyolysis and had a CK level of 108 000 U·L−1. Epstein2 noted that a high CK level in the blood without other laboratory and physical evidence does not indicate the presence of exertional rhabdomyolysis. Other laboratory evidence of rhabdomyolysis may include increased serum levels of potassium, phosphate, uric acid, creatinine, or lactate dehydrogenase, while blood calcium and pH may be decreased.15 Physical signs include muscular weakness, swelling, pain, cramping, and darkened or tea-colored urine.15
Risk factors for exertional rhabdomyolysis include exercising in very hot and humid environments, not drinking enough water (dehydration), weight lifting, poor physical conditioning, fatigue, and prior history of heat exhaustion.4 Although the prevalence and incidence of exertional rhabdomyolysis are not known due to the inherent fatigue, dehydration, and blunt trauma that occur while playing football, exertional rhabdomyolysis is a primary concern for athletes such as football players.
The purpose of our study was to determine if participation in 9 days of 2-a-day football practices resulted in changes in plasma CK levels. Secondary purposes included examination of the differences in CK levels for the preseason levels, the first 6, the second 6, and the third 6 practices (4th, 7th, and 10th days of practice) and determination if a relationship existed between measures of fitness and CK levels.
Although studies of muscular damage and CK are somewhat common, most have been conducted in the controlled environment of the laboratory. Few studies have been done on athletes participating in their competitive activity. We found no studies that examined CK levels in football players under the stress of high-intensity exercise combined with high levels of heat and humidity (2-a-day football practices) in a literature search of MEDLINE, the Internet, and SPORT Discus. Regarding football players, we found one case report of a player who eventually died due to complications of exertional rhabdomyolysis.5 Of the studies that have been done on athletes on the field, most involved marathon runners.11 As a result, very little is known about the blood CK levels of football players. More data on this subject are needed to aid us in preventing exertional rhabdomyolysis.
METHODS
Subjects
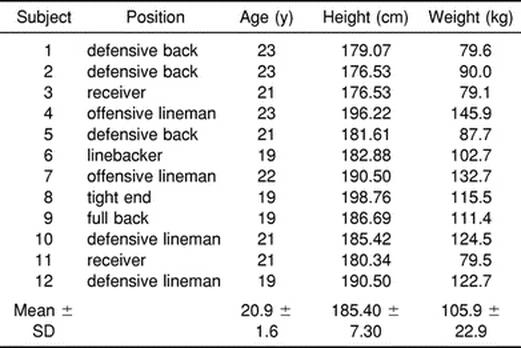
Sixteen sophomore, junior, or senior members of a Division I football team from a large midwestern university were recruited to participate in this study (Table 1). Freshman players did not participate because they were required to perform early morning conditioning sessions in addition to the normally scheduled practices that all players were required to attend. Subjects were volunteers from a pool of athletes that remained on campus for most of the 1997 summer and participated in the team off-season conditioning program designed by the team strength and conditioning coaches. For this reason, we assumed that the participants in the study were acclimated and in proper condition to exercise in the study environment. Four subjects were dropped from the study as a result of musculoskeletal injuries, leaving 12 subjects (75%) to complete the research protocol. Each subject signed an informed consent form in accordance with the institutional review board of the university, which approved the study. The intent of the study and the risks involved were explained before participation.
Table 1. Subjects' Physical Characteristics
Procedures and Protocols
Before beginning 2-a-day practices, each participant completed a questionnaire that was used to help determine whether factors in the lives of the participants other than exercise might be responsible for any increases seen in the CK levels. Each participant also performed the following fitness tests to permit generalizations regarding the relationship of conditioning and CK levels: (1) percentage of body fat assessment using the methods described by Jackson and Pollock with the sum of 7 skinfolds,16,17 (2) body mass index (BMI) assessment (body weight in kg/height in meters)2, (3) 1-repetition maximum (1-RM) strength tests for squat and bench press, (4) 60-second 1-leg step test to estimate anaerobic capacity, and (5) 15-second 1-leg step test to estimate anaerobic peak power.
Skinfolds were measured with Lange skinfold calipers (Beta Technology Inc, Santa Cruz, CA). We took 3 nonconsecutive measures at each of the 7 standard sites on the right side of the body, and the average of the 3 measures was used as the skinfold thickness. Within-day intraclass reliability was calculated to be 0.9998. The 7 sites measured were the chest, subscapular, midaxillary, suprailiac, abdominal, triceps, and thigh areas. Body density was determined from the Jackson and Pollock equation,17 which has been found to be the most valid skinfold body density equation for football players and male athletes aged 18 to 29 years old.16 Percentage of body fat was calculated with the Siri equation.16
The 1-RM strength tests were performed using standard free-weight equipment. The athletes had been on a structured lifting routine for the summer, so each subject, along with the strength and conditioning coaches, already knew his 1-RM weight. After the athlete lifted his former 1-RM weight, weight was added in 2.27-kg (5-lb) increments to determine the true 1-RM weight. Athletes were allowed as much time between lifts as necessary to feel that recovery was obtained.
We obtained peak power and anaerobic capacity using the single-leg step test described by Adams.18 The step test was performed using a steel step 40 cm in height. The subject stood perpendicular to the step, so that one foot was planted on the top of the stepping surface and the other was positioned on the floor at the base of the step. Each step consisted of raising the body to the level of the step (40 cm) and then lowering it back down to the ground in concentric and eccentric fashion. The nonplanted leg was dangled in the air during the upward, concentric stepping action and then lowered back to the ground during the lowering, eccentric stepping action. The subject was not allowed to bounce up from the floor and was cued to keep his back straight at all times. Subjects practiced the stepping procedure for 2 to 3 repetitions. After a short rest period, the athletes were encouraged to perform the stepping procedure with maximal effort for 1 minute. We used the number of steps completed in the first 15 seconds to compute peak power and the number of steps completed in 60 seconds to compute anaerobic capacity, following the procedures of Petersen.19 The anaerobic step test has been found to be a valid and reliable test for the estimation of anaerobic power and capacity.18,19
Creatine kinase levels were assessed by having a blood specimen drawn from an antecubital vein. Approximately 3 to 4 mL of blood were drawn during the preseason physical examination (CKM1), which was the morning before the first day of 2-a-day practices. The same quantity of blood was also drawn the morning before the 4th (CKM2), 7th (CKM3), and 10th (CKM4) days of 2-a-day practices, allowing 6 practices between each blood draw. Blood was drawn before breakfast, between 5:00 AM and 6:00 AM, and practice generally began at 9:00 AM. All blood was collected following Centers for Disease Control and Prevention guidelines. The antecubital fossa was cleaned with an alcohol swab and thoroughly dried with a sterile gauze pad. Either the median cephalic vein or the median basilic vein (whichever was most accessible) was punctured using standard sterile technique, and blood was drawn into the Vacutainer tube (Becton, Dickinson and Co, Franklin Lakes, NJ). A piece of sterile gauze was used to stop bleeding at the blood draw site, and a bandage was placed over the needlestick site. All equipment contaminated with body fluids was handled and disposed of according to the guidelines. Venipuncture was performed by a registered nurse, and CK analysis was performed by the laboratory at the local community hospital. The blood was refrigerated and transported immediately to the laboratory. It was then centrifuged and the serum drawn off for analysis. The laboratory uses the Dade Dimensions CK Analyzer (Dade Behring, Deerfield, IL), which is accurate to ±5%. All necessary blood-drawing equipment was supplied by the hospital. This equipment consisted of Vacutainer tubes and needles, tourniquets, and rubber examination gloves.
We measured temperatures and relative humidity on the field before each practice with a sling psychrometer. Mean temperature was 22.5°C and mean relative humidity was 64% over the 10 days for which measurements were taken.
Statistical Analysis
We performed statistical analyses using the Statistics Package for the Social Sciences (version 6.1, SPSS Inc, Chicago, IL). A one-way, repeated-measures analysis of variance (ANOVA) was calculated to test the differences between the means of each group of CK measures. A Student-Newman-Keuls post hoc comparison test was used to determine where differences existed. We plotted CK group means versus practice day as a line graph to show the rise and fall of CK from measure to measure. Pearson product moment correlation coefficients allowed us to determine whether there was a relationship between the measures of physical fitness and the difference between the first 2 measures of CK. The difference between the first 2 CK measures was used because one of the goals of this research was to determine a factor that was associated with less muscular damage in the football players, as indicated by lower levels of CK in the blood. A within-day reliability coefficient was calculated for the 3 skinfold measures completed at each skinfold site using ANOVA. All alpha levels were set at .05.
RESULTS
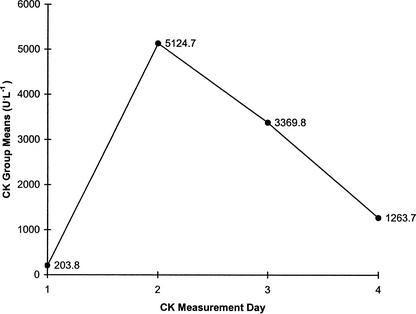
The CK analysis test results for each of the subjects are presented in Table 2. The ANOVA revealed statistically significant main effects for the 4 CK measures (F3,44 = 8.67, P = .0002). Power for the ANOVA was .9831 (alpha = .05 level). The Student-Newman-Keuls post hoc test showed that plasma CK levels at the second (CKM2) and third measures (CKM3) were statistically higher than CK levels at baseline (CKM1) (Figure 1). However, the differences between CKM1 and CKM4, CKM2 and CKM3, and CKM3 and CKM4 were not statistically significant.
Table 2. Creatine Kinase Measures*
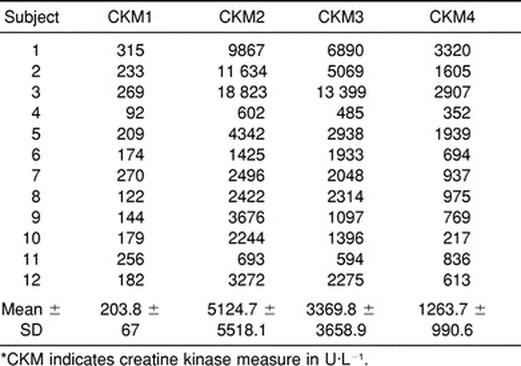
Figure 1.
Mean values for creatine kinase on the four test days. Creatine kinase measure 1 (CKM1) was significantly different than CKM2 and CKM3. There was no significant difference between CKM1 and CKM4, CKM2 and CKM3, and CKM2 and CKM4.
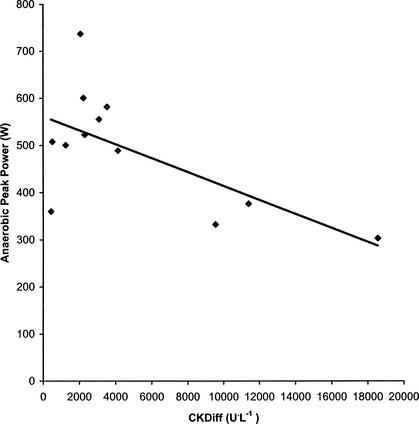
We calculated Pearson product moment correlations (r) to determine whether a relationship existed for the difference between CKM1 and CKM2 (CKDiff = CKM2 − CKM1) and the measures of fitness. Differences between CKM1 and CKM2 were used because the greatest increase was between the first 2 measures, and one of our goals was to determine if there was a relationship between fitness indices and increases in serum CK. Athletes who generated higher peak power during a 15-second step test had lower increases in CK levels from CKM1 to CKM2 (r = −.64, P = .025) (Figure 2). There were no other statistically significant correlations, although the correlations with anaerobic capacity (r = −.54, P = .071), body mass index (r = −.51, P = .090), and percentage of body fat (r = −.52, P = .082) did approach statistical significance.
Figure 2.
Visual representation of the Pearson product moment correlation between anaerobic peak power and CKDiff (r = −.64).
DISCUSSION
A description of normal 2-a-day practices is important in understanding our findings. Generally, the team warmed up with approximately 10 minutes of static stretching and form running. Then, the team broke into groups based on position played and performed position-specific drills that were generally very physical with full contact. The position-specific drills were generally done for approximately 80 minutes, after which the team met for approximately 30 minutes for the heavy contact drills, usually an offense versus defense scenario. Because plasma concentrations of various substances are affected by hydration status, we kept track of temperature and humidity. The average temperature and humidity for the duration of the study were 22.5°C and 64%, respectively. In addition, all blood was taken in the morning before any activity to give the athletes maximal potential for rehydration during the night.
Examination of CKM1 (the prepractice measure for this study) shows that, even though the subjects had not begun 2-a-day practices yet, the mean CK level was 203.8 ± 67.0 U·L−1, which is higher than the published CK norms for healthy men. Norms for CK levels in healthy men are 55 to 170 U·L−1.13,20 The high CK levels found in CKM1 can likely be explained by the summer conditioning program in which the subjects participated. Hortobagyi and Denahan9 showed that it is not uncommon for healthy exercising athletes to have blood CK levels ranging from 100 to 1000 U·L−1. These high resting CK levels were attributed to decreased enzyme removal from the blood, permanently damaged muscle cell membranes as a result of chronic physical stress, higher lean body mass of athletes, higher levels of protein breakdown, or a combination of all these factors. If the muscle damage that occurs is a one-time event, normal clearance of CK from the blood can generally be expected in 2 to 3 days. Peak levels usually occur approximately 18 hours after the injury.13
One of the hypotheses tested in this study was that an increase in serum CK levels would be noted in each consecutive CK measure. However, a statistically significant rise was noted only between CKM1 and CKM2 (Figure 1). A gradual decrease from CKM2 to CKM4 was then demonstrated, even though the intensity, length, and collision content of the practices remained the same for the duration of 2-a-day practices. The difference between CKM2 and CKM3 was not statistically significant, nor was the difference between CKM3 and CKM4. The initial rise in CK levels may have resulted from muscle cell damage due to the unaccustomed activity, coupled with the blunt trauma occurring to the body during tackling and hitting drills. The initial rise followed by a gradual decrease in blood levels of CK seen in this study supports the hypothesis of Byrnes et al21 that there may be a “pool” of injury-prone fibers in the human skeletal muscle system. Byrnes et al21 studied serial CK measures in 3 groups of downhill runners. Each group performed 2 bouts of downhill running with 3, 6, or 9 weeks separating each bout. Both group 1 and group 2 had significantly lower CK increases in the blood after the second bout as compared with the first bout, indicating that a muscular adaptation had taken place. In group 3, however, the postexercise CK increases were similar for the 2 bouts of downhill running. The researchers hypothesized that 9 weeks was too long for the “pool” of injury-prone fibers to maintain the damage-resistant adaptation that had occurred.
Another possible explanation for the high CKM2 reading, combined with the gradually decreasing CKM3 and CKM4 results, is that during the practices between CKM1 and CKM2, the players were required to wear “shells.” “Shells” consist of a helmet, shoulder pads, jersey, and shorts. No thigh or hip padding was worn during this time. After CKM2, full pads were worn for every practice. The consecutively lower blood concentrations in CKM2, CKM3, and CKM4 might be indicative of the protective effects of full pads as opposed to just wearing “shells.” Also, it is possible that a physiologic adaptation occurred to allow greater elimination of CK from the blood more quickly or that the muscle cells adapted to inhibit damage.
All subjects in this study showed laboratory evidence of exertional rhabdomyolysis. Line and Rust4 and Rucker and Tanner14 defined laboratory evidence of exertional rhabdomyolysis as CK levels 5 to 10 times greater than the normal levels for the sex. All athletes had at least a 5-fold increase in serum CK concentrations between CKM1 and CKM2, while 9 of 12 athletes had a more than 10-fold increase between CKM1 and CKM2. Whether the athletes exhibited any physical signs and symptoms of exertional rhabdomyolysis, such as muscular weakness or pain, muscular swelling, cramping, or darkened or tea-colored urine is unknown. Also unknown is whether the athletes in this study exhibited any other laboratory evidence of exertional rhabdomyolysis, such as electrolyte imbalances, pH disturbances, creatinine and lactate dehydrogenase increases, or the presence of myoglobinuria, as these were not tested.15 However, none of the athletes who had large increases in CK had any heat-related illnesses or medical issues that required them to take time away from the practice schedule. The data from this study imply that large increases in CK concentrations can occur in the blood of football players without the athletes' leaving practice secondary to injury of any kind.
Overall, the fitness measures were negatively correlated to CKDiff; however, only 1 of the 6 coefficients was statistically significant, with 3 others showing a trend toward significance. These data show a trend toward lower increases in CK in the athletes who were more fit (including BMI and body fat percentage).
The 2 fitness variables most strongly correlated with CKDiff were peak power (r = −.64, P = .025) and anaerobic capacity (r = −.54, P = .071). Peak power was the only fitness measure that was statistically related to CKDiff. The athletes with the lowest peak power tended to have greater increases in CK concentrations in the blood from CKM1 to CKM2. Although anaerobic capacity and CKDiff were not significantly correlated statistically, the P value obtained was not much higher than the preset alpha of .05. As a result, although not statistically significant, there was a trend for low scores on the anaerobic capacity test to be associated with higher levels of CK in the blood.
If a nonstatistical comparison is made among the 3 athletes who scored the highest on the test of anaerobic power (subjects 7, 9, and 10) with the athletes who scored the poorest on the test (subjects 1, 3, and 11), we find that the athletes who scored high generally had much lower increases in CK (Table 2). Where subjects 7, 9, and 10 had CK values that were more than 14, 21, and 13 times, respectively, the normal limits for men, subjects 1, 3, and 11 had CK values that were more than 58, 110, and 5 times, respectively, the normal limits. We should recall that all the athletes in the study were conditioned in the same manner. When a nonstatistical comparison was made between the high (subjects 5, 9, and 12) and low (subjects 1, 3, and 11) scorers on the test of anaerobic capacity, similar results were noted. Subjects 5, 9, and 12 had 25, 21, and 19 times, respectively, the normal limits for men, while subjects 1, 3, and 11 had, as mentioned earlier, CK values that were more than 58, 110, and 5 times, respectively, the normal limits. These results may suggest that football players who were not as anaerobically fit sustained greater muscular damage, as indicated by higher concentrations of CK in the blood, and thus, were at higher risk for laboratory evidence of exertional rhabdomyolysis. Although the topic does not seem to have been addressed in past literature, our results support the suggestion that training football players to increase anaerobic peak power and anaerobic capacity may offer protection from excessive muscular damage.
Body mass index (r = −.51, P = .090) and percentage of body fat (r = −.52, P = .082), while not statistically significant, were also negatively correlated with CKDiff. There was a trend toward greater muscular damage, as indicated by high CK concentrations in the blood with lower BMI and lower percentage of body fat levels. It may be that the players who had more body fat were afforded extra protection from trauma to the muscles during body contact in football. Thus, the extra body fat may have provided a “cushioning effect,” giving the skeletal muscle more protection. Another plausible explanation for the effect of lower CK levels seen with higher body fat percentages and BMIs is related to position played. It could be that, since the players who had higher levels of body fat were the offensive- and defensive-line players, they were not subjected to the quantity of high-velocity body contact to which some of the players in other positions were subjected.
An interesting contrast to the data included in the above analysis is the data collected on one subject who did not follow the research protocol and was thus not included in the statistical analysis. This subject sustained a neck injury that allowed him to perform conditioning activities (sprinting, jogging, stair stepping, stationary bicycle) with the rest of the participants in the study but kept him from any contact in practice. The CK measures for this subject were 104 U·L−1 (CKM1), 901 U·L−1 (CKM2), 183 U·L−1 (CKM3), and 1651 U·L−1 (CKM4). The initial increase in this participant's CK concentration from CKM1 to CKM2 was caused solely by physical conditioning. Then, even though this athlete continued to condition with the same intensity, his CK concentration dropped drastically (CKM3), presumably due to the same mechanism as the other subjects. Immediately after the blood was drawn for CKM3, the team physician cleared this participant for full physical contact during practice. The dramatic rise in CKM4 for this subject suggests that muscular trauma, resulting from the collisional nature of football, is responsible for the increase in CK in the blood. The data from this subject could be used as a case study of what may have happened to the other subjects under similar circumstances.
As with any study performed in the field, internal validity is sacrificed for external validity, as illustrated by several limitations present in our study. One limitation is the small number of subjects, which limits extrapolation of these results. For example, we do not know if football players at this institution differed from football players at other institutions. Thus, a larger and more representative sample is needed. Other limitations include temperature and humidity variations from practice to practice and day to day, fitness and hydration status of the participants, and nutritional status. Benefits include the fact that these results could be easily applied to a practical situation.
Our findings offer several implications for further study. We measured CK serum levels only. Elevated levels of myoglobin in the blood can have a toxic effect on the glomerular filtration rate, causing potential renal failure, especially in instance of dehydration. Therefore, a more thorough investigation of CK levels in the blood coupled with myoglobin levels, electrolytes, and blood urea nitrogen and creatinine would provide useful information. Also, exploring factors that can affect preseason conditioning status and factors that allow, for example, proactive rehydration strategies for these players in hot and humid environments, would help us to understand if such measures can aid in the prevention of exertional rhabdomyolysis in the football athlete.
Acknowledgments
ACKNOWLEDGMENTS
We thank the Great Lakes Athletic Trainers' Association for their support of this research in the form of a grant.
REFERENCES
- Smith L L, Keating M N, Holbert D, Spratt D J, McCammon M R, Smith S S, Israel R G. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preliminary report. J Orthop Sports Phys Ther. 1994;19:93–99. doi: 10.2519/jospt.1994.19.2.93. [DOI] [PubMed] [Google Scholar]
- Epstein Y. Clinical significance of serum creatine phosphokinase activity levels following exercise. Isr J Med Sci. 1995;31:698–699. [PubMed] [Google Scholar]
- Clarkson P M. Worst case scenarios: exertional rhabdomyolysis andacute renal failure. Available at http://www.gssiweb.com/reflib/15/d0000000200000054.cfm. Accessed January 22, 2002.
- Line R L, Rust G S. Acute exertional rhabdomyolysis. Am Fam Physician. 1995;52:502–506. [PubMed] [Google Scholar]
- Rosenthal M A, Parker D J. Collapse of a young athlete. Ann Emerg Med. 1992;21:1493–1498. doi: 10.1016/s0196-0644(05)80068-x. [DOI] [PubMed] [Google Scholar]
- Dawson D M, Fine I H. Creatine kinase in human tissues. Arch Neurol. 1967;16:175–180. doi: 10.1001/archneur.1967.00470200063005. [DOI] [PubMed] [Google Scholar]
- Clark J F. Creatine and phosphocreatine: a review of their use in exercise and sport. J Athl Train. 1997;32:45–51. [PMC free article] [PubMed] [Google Scholar]
- Moeller J L, McKeag D B. Exercise-induced rhabdomyolysis. Sport Med Arthros Rev. 1995;3:274–279. doi: 10.1097/00132585-199500340-00004. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Denahan T. Variability in creatine kinase: methodological, exercise, and clinically related factors. Int J Sports Med. 1989;10:69–80. doi: 10.1055/s-2007-1024878. [DOI] [PubMed] [Google Scholar]
- Schneider C M, Dennehy C A, Rodearmel S J, Hayward J R. Effects of physical activity on creatine phosphokinase and the isoenzyme creatine kinase-MB. Ann Emerg Med. 1995;25:520–524. doi: 10.1016/s0196-0644(95)70270-9. [DOI] [PubMed] [Google Scholar]
- Young A. Plasma creatine kinase after the marathon—a diagnostic dilemma. Br J Sports Med. 1984;18:269–272. doi: 10.1136/bjsm.18.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka K, Clarkson P M. Relationship between post-exercise plasma CK elevation and muscle mass involved in the exercise. Int J Sports Med. 1992;13:471–475. doi: 10.1055/s-2007-1021300. [DOI] [PubMed] [Google Scholar]
- Pagana K D, Pagana T J. Mosby's Diagnostic and Laboratory Test Reference. 2nd ed Mosby-Year Book; St Louis, MO: 1995. [Google Scholar]
- Rucker K S, Tanner R. Reconditioning after exertional rhabdomyolysis: avoiding setbacks. Physician Sportsmed. 1992;20(10):95–102. doi: 10.1080/00913847.1992.11947505. [DOI] [PubMed] [Google Scholar]
- Harrelson G L, Fincher A L, Robinson J B. Acute exertional rhabdomyolysis and its relationship to sickle cell trait. J Athl Train. 1995;30:309–312. [PMC free article] [PubMed] [Google Scholar]
- Heyward V H, Stolarczyk L M. Applied Body Composition Assessment. Human Kinetics; Champaign, IL: 1996. pp. 143–154. [Google Scholar]
- Jackson A S, Pollock M L. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- Adams G M. Exercise Physiology Laboratory Manual. WCB Publishing; Dubuque, IA: 1990. pp. 99–104. [Google Scholar]
- Petersen M D. Anaerobic Power Norms and the Relationship Between Anaerobic Power Versus Leg Strength and Lean Body Mass [master's thesis] California State University, Fullerton; Fullerton, CA: 1989. [Google Scholar]
- Tilkian S M, Conover M B, Tilkian A G. Clinical and Nursing Implications of Laboratory Tests. 5th ed Mosby-Year Book; St Louis, MO: 1995. [Google Scholar]
- Byrnes W C, Clarkson P M, White J S, Hsieh S S, Frykman P N, Maughan R J. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol. 1985;59:710–715. doi: 10.1152/jappl.1985.59.3.710. [DOI] [PubMed] [Google Scholar]