Abstract
Objective: To determine if 35 days of creatine supplementation (Cr) followed by 28 days of no supplementation altered lower leg anterior compartment pressure (ACP) at rest and after exercise.
Design and Setting: Subjects were divided into 2 treatment groups: (1) high dose (0.3 g Cr·kg body mass−1·d−1 for 7 days followed by 0.03 g Cr·kg body mass−1·d−1 for 28 days), or (2) low dose (0.03 g Cr·kg body mass−1·d−1 for 35 days). After 35 days, supplementation was terminated, and no Cr was ingested for 28 days.
Subjects: Sixteen physically active, healthy, college-aged males (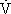 O2max = 47.6 ± 5.1 mL·kg−1·min−1).
O2max = 47.6 ± 5.1 mL·kg−1·min−1).
Measurements: At baseline, 7 days and 35 days of supplementation, and 28 days postsupplementation, ACP was measured preexercise and immediately, 1, 5, 10, and 15 minutes postexercise after a treadmill run at 80% 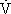 O2max.
O2max.
Results: For ACP, there was no significant group-by-time interaction, but there was a significant time effect for group when the data were combined. ACP was significantly increased at preexercise, immediately postexercise, and 1, 5, and 10 minutes from baseline to 7 days. ACP remained significantly elevated from baseline at 35 days immediately postexercise and 1 minute postexercise. After 28 days of no supplementation, ACP began to return to presupplementation levels, with only the 1-minute postexercise measurement significantly elevated from baseline.
Conclusions: Creatine supplementation increased ACP at rest and after exercise, and ACP began to return to normal after 28 days of no supplementation.
Keywords: ergogenic aids, phosphocreatine, sports medicine, side effects
Pain in the lower leg during or after exercise is a frequent occurrence in athletes and active individuals after an increase in activity.1–5 Typically, this pain is attributed to acute shin splints or periostitis. This discomfort may also be attributed to stress fractures,1,2 accumulation of metabolic waste,6 and soft tissue and arterial injuries.3 Symptoms sometimes diminish after a few weeks of training; however, they may persist and become more severe until exercise becomes exceptionally painful or even impossible. If the symptoms persist and become more severe, a condition known as chronic compartment syndrome can occur.7
Chronic compartment syndrome has been defined as a recurrent, exercise-induced increase in pressure in skeletal muscle accompanied by pain, swelling, and impaired muscle function.8 Chronic compartment syndrome can occur in the anterior, superficial, lateral, or deep posterior (proximal or distal or both) compartment of the lower leg. The anterior compartment is most commonly affected.1,6 Chronic compartment syndrome develops when pressure within skeletal muscle, an inelastic compartment surrounded by fascia, increases. An abnormal increase in compartment pressure greatly limits the space available for the muscles within this compartment.9 As the pressure increases within this inelastic compartment, local tissue circulation and function may become impaired. Functionally, individuals may experience muscle weakness, limited passive motion of distal digits, or even paresthesia with increased activity or sustained exercise.6 These symptoms tend to disappear with cessation of exercise, but in some individuals, they may persist long after exercise has been discontinued. In chronic compartment syndrome, relief typically follows a fasciotomy or fasciectomy of the constricted compartment.
Creatine (Cr) supplementation has been promoted as a useful ergogenic aid for some types of athletic performance10,11 but not all.12–15 Its side effects, especially those related to training and competition, necessitate examination.16 Specific side effects associated with Cr supplementation include gastrointestinal distress and muscle cramping in college-aged athletes.17–19
Recently, we documented an increase in anterior compartment pressure (ACP) in the lower leg after Cr supplementation.20,21 Schroeder et al20 demonstrated a significant increase in resting and postexercise pressure after 6 and 34 days of Cr supplementation compared with a placebo group. We21 also documented exceptionally high pressures at rest and postexercise in the anterior compartment of the lower leg in a young male consuming Cr supplementation.21 A sustained increase in ACP may lead to the development of symptoms resembling those of people with exercise-induced compartment syndrome. The individual's ability to exercise decreases, and he or she may eventually require medical attention. In fact, Robinson19 recently reported on acute quadriceps compartment syndrome and rhabdomyolosis that required several days of hospitalization in a weight lifter using high-dose Cr supplementation.
Creatine supplementation has become widespread, and it is important that the implications and contraindications of this supplement be determined for the health and safety of individual consumers. Based on recent data,19–21 it would seem prudent to determine the effects of varying doses of Cr supplementation and whether discontinuation of supplementation influences ACP in active individuals. Therefore, our purpose was to determine the effects of 35 days of Cr supplementation followed by a 28-day period of no supplementation on lower leg ACP at rest and after a 20-minute run at 80% of 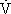 O2max. Additionally, we wanted to examine the influence of Cr supplementation on body mass, body composition, resting blood pressure, and lower leg volume.
O2max. Additionally, we wanted to examine the influence of Cr supplementation on body mass, body composition, resting blood pressure, and lower leg volume.
METHODS
Subjects
Sixteen healthy, physically active male subjects (age = 24.5 ± 3.1 years) participated in this study. In accordance with guidelines set forth by the Advisory Committee for Human Experimentation at the University of Kansas (which approved the study), all subjects read and signed an informed consent form and completed a health history questionnaire. The inclusionary criteria for participation in this study were (1) performed lower body exercise at least 3 days per week for the 6 months prior to participation in the study, and (2) normotensive (resting blood pressure ≤ 139/89 mm Hg). Subjects were excluded from the study if they used Cr supplementation within 3 months of starting the investigation, used anabolic steroids, or were vegetarian dieters. Subjects were also screened for exclusionary criteria consisting of a previous history of lower leg conditions involving the musculoskeletal, neurologic, or vascular structures.
Experimental Design
The subjects reported to the laboratory for testing on 5 separate occasions, having abstained from exercise for 48 hours and from caffeine and alcohol for 24 hours before testing. The initial testing determined the subject's maximal oxygen consumption (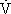 O2max) and familiarized him with the protocol to be used on subsequent testing days. For the 4 experimental testing sessions that followed, the subjects reported to the laboratory for the muscle biopsy and the measurement of body mass, body composition, blood pressure, lower leg volume, and ACP. After baseline testing, subjects were randomly assigned to either a high-dose or a low-dose supplementation group for 35 days. The subjects then stopped consuming the Cr supplementation for 28 days. All dependent measures were taken at baseline, 7 and 35 days of Cr supplementation, and after 28 days of no Cr supplementation.
O2max) and familiarized him with the protocol to be used on subsequent testing days. For the 4 experimental testing sessions that followed, the subjects reported to the laboratory for the muscle biopsy and the measurement of body mass, body composition, blood pressure, lower leg volume, and ACP. After baseline testing, subjects were randomly assigned to either a high-dose or a low-dose supplementation group for 35 days. The subjects then stopped consuming the Cr supplementation for 28 days. All dependent measures were taken at baseline, 7 and 35 days of Cr supplementation, and after 28 days of no Cr supplementation.
Maximal Exercise Testing
During the first visit to the laboratory, the subjects were measured for 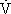 O2max during a graded exercise test on a Quinton treadmill (model 24–72, Quinton Instrument Co, Bothell, WA). The graded exercise test began with a 2-minute warm-up at 180 m·min−1. This was followed by an increase in velocity of 20 m·min−1 every 2 minutes until the subject reached a velocity of 240 m·min−1. Thereafter, the running speed remained constant, and the grade was increased 2% every 2 minutes until the subject reached volitional fatigue. Heart rate, using a Polar Favor monitor (Polar Electro Inc, Woodbury, NY), and rating of perceived exertion, using the 15-point Borg scale,22 were measured and recorded 10 seconds before the end of each 2-minute exercise stage. Expired gases were collected and analyzed by a SensorMedics 2900 metabolic measurement cart (SensorMedics, Yorba Linda, CA). A 3-L syringe and standard gases were used to calibrate the cart prior to each test. For validation of a maximal exercise test, the subject was required to meet 3 of the following 4 criteria: (1) leveling of
O2max during a graded exercise test on a Quinton treadmill (model 24–72, Quinton Instrument Co, Bothell, WA). The graded exercise test began with a 2-minute warm-up at 180 m·min−1. This was followed by an increase in velocity of 20 m·min−1 every 2 minutes until the subject reached a velocity of 240 m·min−1. Thereafter, the running speed remained constant, and the grade was increased 2% every 2 minutes until the subject reached volitional fatigue. Heart rate, using a Polar Favor monitor (Polar Electro Inc, Woodbury, NY), and rating of perceived exertion, using the 15-point Borg scale,22 were measured and recorded 10 seconds before the end of each 2-minute exercise stage. Expired gases were collected and analyzed by a SensorMedics 2900 metabolic measurement cart (SensorMedics, Yorba Linda, CA). A 3-L syringe and standard gases were used to calibrate the cart prior to each test. For validation of a maximal exercise test, the subject was required to meet 3 of the following 4 criteria: (1) leveling of 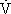 O2 (≤ 2.0 mL·kg−1·min−1) with an increase in exercise intensity; (2) achievement of a heart rate ± 10 beats·min−1 of age-predicted maximal heart rate; (3) respiratory exchange ratio ≥1.10; and (4) rating of perceived exertion ≥18.23
O2 (≤ 2.0 mL·kg−1·min−1) with an increase in exercise intensity; (2) achievement of a heart rate ± 10 beats·min−1 of age-predicted maximal heart rate; (3) respiratory exchange ratio ≥1.10; and (4) rating of perceived exertion ≥18.23
Experimental Measurements
With the subject in shorts and T-shirt, we measured body mass using a calibrated electronic Toledo scale (model 8134, Toledo Scale, Toledo, OH). Body composition was measured using a Lunar dual-energy X-ray absorptiometer (DEXA) (Lunar Corp, Madison, WI). The subject was scanned in a hospital gown after removing all jewelry. He was positioned supine on the DEXA scanning surface with hands palms down, fingers together, and arms tucked against hips. After the scan, the subject remained on the DEXA scanning surface. The same technician then measured resting blood pressure twice using a sphygmomanometer and standard stethoscope. The 2 measurements were averaged for statistical analysis.
Lower leg volume was determined by means of 6 anthropometric measurements and 2 skinfold measurements taken from the right leg while the subject stood erect in the anatomical position. We used a Gulick II measuring tape (Country Technology Inc, Gays Mills, WI) to measure leg circumference at 90° to the longitudinal axis of the lower leg. The 6 sites were the minimal ankle circumference, middle-calf circumference, half the distance between the ankle and calf points, subpatellar circumference, half the distance between the calf and subpatellar circumference, and the knee joint-line circumference. We measured skinfold thickness using Lange calipers (Beta Technology Inc, Santa Cruz, CA) at the medial and lateral sites of the maximal mid-calf circumference. Tibial and femoral intercondylar diameters were measured using anthropometric calipers. Three measurements were taken at each site, and the mean values were used in the calculation of lower leg volume.24
Muscle biopsies were obtained from the superficial portion of the vastus lateralis using the procedures outlined by Bergström,25 with suction applied to ensure adequate sample size.26 After the muscle sample was removed from the needle, it was immediately frozen in liquid nitrogen and stored at −70°C until analysis. Tissue samples were later dissected for removal of connective tissue, freeze dried, powdered, and analyzed for adenosine triphosphate (ATP), Cr, phosphocreatine (PCr), and total creatine (TCr) concentrations.27 Total creatine was calculated as the sum of the PCr and Cr concentrations.
Anterior Compartment Pressure Measurement
After the muscle biopsy was collected, ACP was measured using a Stryker Intracompartmental Pressure Monitor System (Stryker Instruments, Kalamazoo, MI). Subjects were in a supine position for all compartment pressure measurements. A rolled towel supported the subject's knee in 10° of flexion, with the ankle in a neutral position and the first toe of the foot pointed vertically.28 The subject was asked to completely relax the lower leg to avoid producing excessive tissue-fluid pressure. We calibrated the Stryker System before insertion according to guidelines provided by the manufacturer.
The area of skin that was penetrated by the catheter was sterilized with Betadine solution (The Purdue Frederick Co, Norwalk, CT) and anesthetized with 0.5 mL of 1% lidocaine and 0.5 mL of 0.5% bupivicaine hydrochloride. The catheter was inserted into the anterior compartment 15 cm distal to the tibial tuberosity and 2 cm lateral to the tibial crest. The skin and fascia of the lower leg were pierced at approximately a 30° angle to the long axis of the leg. The Stryker System was then directed superiorly at an approximate angle of 45° until it was situated 1 to 4 cm into the anterior compartment. The syringe within the pressure monitor was filled with a saline solution that provided a gradient for the pressure monitor to read. Once the catheter was inserted into the anterior compartment, the pressure was allowed to stabilize, and the value was recorded from the monitor display.
After the pressure was measured, the catheter was removed and the subject began exercising. The subject ran at approximately 50% of 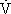 O2max for a 2-minute warm-up. Thereafter, the intensity was increased to 80% of
O2max for a 2-minute warm-up. Thereafter, the intensity was increased to 80% of 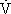 O2max, and the subject ran for 20 minutes. Immediately after the run, the subject positioned himself supine on an examination table and the catheter was reinserted. The time it took for the subject to stop running and have the catheter reinserted was approximately 10 to 15 seconds. Anterior compartment pressure was recorded immediately postexercise and at each minute for 15 minutes postexercise.
O2max, and the subject ran for 20 minutes. Immediately after the run, the subject positioned himself supine on an examination table and the catheter was reinserted. The time it took for the subject to stop running and have the catheter reinserted was approximately 10 to 15 seconds. Anterior compartment pressure was recorded immediately postexercise and at each minute for 15 minutes postexercise.
Creatine Supplementation
Each subject in the high-dose group was given Cr monohydrate (Nutrasense, Shawnee Mission, KS) in tablet form in a dose of 0.3 g Cr·kg body mass−1·d−1 for 7 days followed by a 28-day period of 0.03 g Cr·kg body mass−1·d−1. Each subject in the low-dose group was given 35 days of Cr monohydrate in a dose of 0.03 g Cr·kg body mass−1·d−1. These dosages were based on the work of Hultman et al.29 The subjects consumed the Cr supplement in 2 to 4 equal doses with water throughout the supplementation period. Subjects were instructed to limit caffeine intake during the entire study because this substance has been shown to interfere with the physiologic mechanism causing the ergogenic action of Cr loading.30,31 After the 35-day supplementation period, subjects discontinued Cr supplementation for 28 days. All subjects continued with their normal training regimens throughout the study. We elected to not have a placebo group based on prior research from our laboratory demonstrating no change in ACP during 34 days of placebo supplementation.20
Statistical Analysis
We calculated descriptive statistics, including means and standard deviations, for all data. A 3-factor (group-by-period by-time) analysis of variance with repeated measures on period and time was used to identify significant differences in ACP. A 2-factor (group-by-period) analysis of variance was used to determine significant differences in the remaining dependent variables. Post hoc paired t tests were performed to identify differences among the means when significant F values were obtained. Pearson product moment correlations were calculated to determine if there were any significant relationships among the dependent variables. Significance was set at P ≤ .05 for all statistical tests.
RESULTS
Physical Characteristics and Maximal Aerobic Power
We observed no significant differences between the high-dose and low-dose groups for any physical characteristics or maximal aerobic power (high-dose group age = 24.9 ± 3.2 years, height = 69.5 ± 2.5 cm; low-dose group age = 24.1 ± 3.2 years, height = 69.5 ± 1.6 cm). Maximal aerobic power was 45.5 ± 5.1 mL·kg−1·min−1 for the high-dose and 48.5 ± 3.9 mL·kg−1·min−1 for the low-dose groups.
Body Mass, Body Fat, Leg Volume, and Blood Pressure Measurements
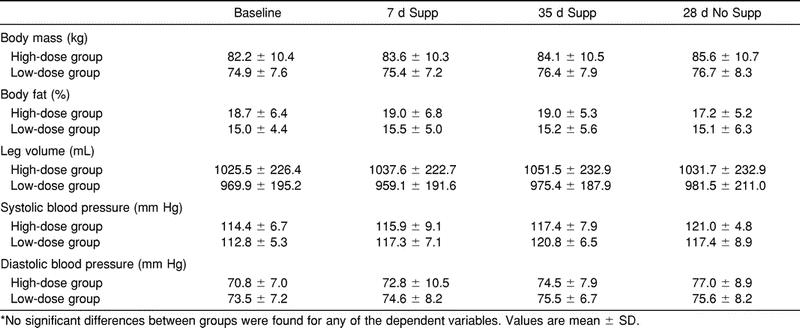
We found no statistically significant differences (F3,11 = 2.48, P >.05) between groups for body mass at baseline, 7 days or 35 days of Cr supplementation, or at 28 days postsupplementation. Body mass increased from baseline to 7 days of supplementation by 1.4 kg in the high-dose group and by 0.4 kg in the low-dose group (Table 1). The increase in body mass was retained throughout the 35-day supplementation period in both groups. The high-dose group demonstrated an overall increase in body mass from baseline to 35 days of supplementation of 1.9 kg, while the low-dose group showed an overall increase of 1.5 kg throughout this period. No statistically significant differences (F3,11 = 2.56, P > .05) between groups for percentage of body fat at any of the measurement times were noted (Table 1). There were also no statistically significant differences (F3,11 = 1.45, P > .05) in leg volume at baseline, 7 days and 35 days of supplementation, and 28 days postsupplementation (Table 1). Leg volume did, however, show an increase from baseline in the high-dose group of 12.1 mL and 26.0 mL after 7 days and 35 days of supplementation, respectively. No statistically significant differences for systolic (F3,11 = 0.55, P > .05) or diastolic (F3,11 = 0.45, P > .05) blood pressures were found between groups at baseline, 7 days and 35 days of supplementation, and 28 days postsupplementation, yet there was a gradual increase in both of these measurements throughout the supplementation period (Table 1).
Table 1. Body Mass, Body Fat, Leg Volume, Systolic Blood Pressure, and Diastolic Blood Pressure at Baseline and After 7 and 35 Days of Creatine Supplementation (Supp), and 28 Days of No Supplementation (No Supp)*
Anterior Compartment Pressure Measurements
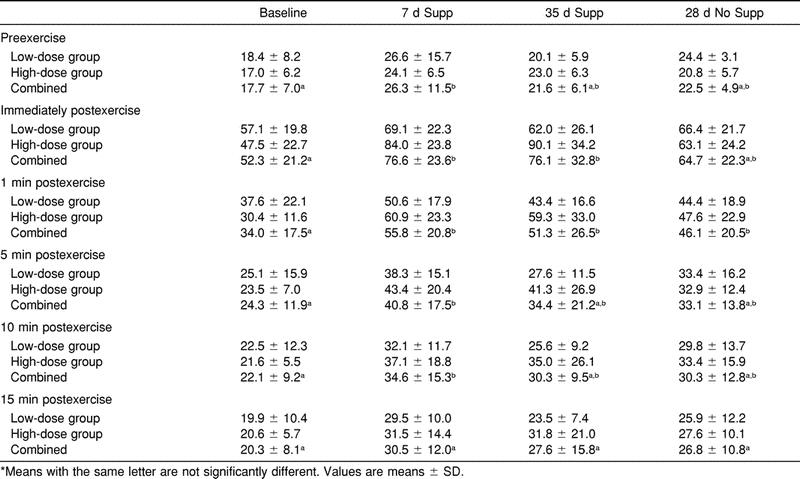
We found no significant differences between groups for compartment pressures (F2,54 = .96, P > .05) at baseline, 7 days and 35 days of supplementation, and 28 days postsupplementation. However, the period-by-time interaction was significant (F2,54 = 318, P < .05). Therefore, the treatment groups were combined and the data are reported as one group (Table 2). Preexercise ACP increased significantly from baseline to 7 days of supplementation. Although still elevated, preexercise ACP was not significantly different from baseline at 35 days of supplementation and 28 days postsupplementation. Immediate postexercise ACP increased significantly from baseline to 7 days of supplementation by 24.3 mm Hg. Immediate postexercise ACP remained significantly elevated from baseline at 35 days of supplementation. Immediate postexercise ACP decreased at 28 days postsupplementation and was not significantly different from the baseline measurement. At 1 minute postexercise, ACP increased significantly above baseline at 7 days and 35 days of supplementation and 28 days postsupplementation by 21.8, 17.3, and 12.1 mm Hg, respectively. The increase in 5-minute postexercise ACP from baseline to 7 days of supplementation of 16.5 mm Hg was significant. No other significant differences were found in 5-minute postexercise ACP after 35 days of supplementation and 28 days postsupplementation when compared with baseline. At 10 minutes postexercise, ACP increased significantly from baseline to 7 days of supplementation by 12.6 mm Hg. We noted no other significant differences in 10-minute postexercise ACP at 35 days of supplementation or at 28 days postsupplementation when compared with baseline. There was no significant difference in 15-minute postexercise ACP at any measurement time.
Table 2. Anterior Compartment Pressures (mm Hg) Measured at Baseline, 7 Days and 35 Days of Supplementation (Supp), and 28 Days of No Supplementation (No Supp) for High-Dose and Low-Dose Groups and Combined*
Muscle Tissue Analysis
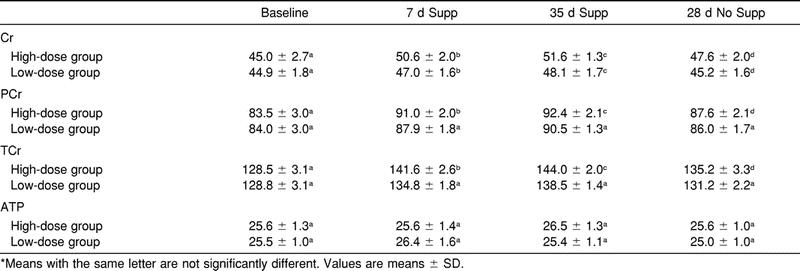
The time effect for muscle Cr (F3,12 = 72.9, P < .05), PCr (F3,12 = 163.4, P < .05), and TCr (F3,12 = 184.9, P < .05) concentrations within both groups was significant (Table 3). For the high-dose group, muscle Cr increased significantly from baseline to 7 days and 35 days of supplementation. We noted no significant difference in Cr between baseline and 28 days postsupplementation. For the low-dose group, Cr increased significantly from baseline to 7 days and 35 days of supplementation. After 28 days postsupplementation, Cr was not significantly different from baseline. There was a significant increase in PCr in the high-dose group from baseline to 7 days and 35 days of supplementation and 28 days postsupplementation. For the low-dose group, PCr increased significantly from baseline to 7 days and 35 days of supplementation, but no significant difference was seen between baseline and 28 days postsupplementation. The TCr increased significantly in the high-dose group from baseline to 7 days and 35 days of supplementation and 28 days postsupplementation. The low-dose group showed a significant increase in TCr from baseline to 7 days and 35 days of supplementation, but no significant difference was noted between baseline and 28 days postsupplementation. The ATP was not significantly different for any of the measurement times between (F3,12 = 2.03, P > .05) or within groups (F3,12 = 1.07, P > .05).
Table 3. Creatine (Cr), Phosphocreatine (PCr), Total Creatine (TCr), and Adenosine Triphosphate (ATP) concentration (mmol·kg−1 dm) of Vastus Lateralis Muscle at Baseline, 7 Days and 35 Days of Supplementation (Supp), and 28 Days of No Supplementation (No Supp)
Relationships Among Dependent Variables
We found no significant correlations among any of the measured dependent variables. These included measures of muscle Cr, PCr, TCr, body mass, blood pressure, lower leg volume, and resting and postexercise ACP.
DISCUSSION
Our principal intentions were to assess the effects of 35 days of Cr supplementation, followed by a 28-day period of no supplementation, on ACP of the lower leg at rest and after exercise. The data from this study indicate that Cr supplementation can significantly increase resting and postexercise ACP above presupplementation levels; ACP begins to return to normal following a 28-day period of no supplementation.
Measuring compartment pressures after the sport activity that may be responsible for increasing those pressures may be the optimal method for identifying exercise-induced chronic compartment syndrome.4 Pedowitz et al32 formulated criteria based on intramuscular ACP recorded with a slit catheter after exercise in 210 muscle compartments without chronic compartment syndrome. For abnormally elevated compartmental pressures, they recommended criteria of (1) a 1-minute postexercise pressure of ≥30 mm Hg, or (2) a 5-minute postexercise pressure of ≥20 mm Hg. According to these criteria, the mean pressures recorded in the high-dose and low-dose groups (and the combined group data) at the baseline measurements were within the normal limits for 1 minute and 5 minutes postexercise. However, after 7 days of Cr supplementation, the preexercise and postexercise measures were abnormally elevated. Furthermore, after 35 days of supplementation, preexercise and postexercise pressures remained abnormally elevated and exceeded the normal criteria. Pedowitz et al32 suggested that compartment pressures measured 15 minutes after exercise should return to normal preexercise levels. Despite declining pressures after exercise at 7 days and 35 days of supplementation, pressures remained elevated above normal. After 28 days of no Cr supplementation, ACP was not significantly different from baseline for any of the measurement times except 1 minute postexercise.
Critical to this investigation was the use of Cr supplementation to establish an increase in muscle Cr, PCr, and TCr and then examining the ACP. Both groups had significant increases in these dependent measures from baseline at 7 days and 35 days of supplementation, and the values were comparable with previously reported data from our laboratory.20 The changes in the intramuscular metabolites agree with previous findings. For example, Harris et al33 showed an approximate 20% increase in PCr in subjects who were supplemented with 5 g of Cr 4 to 6 times per day. Additionally, Hultman et al29 determined that 20 g of Cr for 6 days significantly elevated the TCr and that the ingestion of 2 g Cr·d−1 for 30 days helped maintain the elevated TCr levels.
While the mechanism for creatine's inducing an increase in ACP is not known, we offer the following hypothesis. After creatine is ingested in supplement form, blood creatine concentration increases.34 The transport of creatine from the blood into the muscle fiber requires the cotransport of sodium.35 As long as the blood creatine concentration is elevated, creatine is taken by the muscle fiber until the tissue becomes loaded with creatine and creatine uptake decreases. After creatine supplementation, total body mass increases, which has been attributed to either an increase in total body water10,29 or an increase in fat-free mass.10,31,36 Creatine uptake may contribute to increased water uptake into the muscle fiber during supplementation, which may result in swelling of the muscle fiber. While the mechanism for inducing protein synthesis during Cr supplementation remains unclear, Cr has been demonstrated to increase skeletal and cardiac protein synthesis.37
Due to the rigidity of the anterior compartment of the lower leg, an increase in water content or de novo protein synthesis in the muscle fiber will likely result in higher compartment pressures, both at rest and after exercise. Previous researchers have found a 20% increase in tissue volume of the lower leg compartments during exercise. This increase results from the increased blood flow to that region.38,39 If that particular compartment is unable to allow for this exercise-induced increase in fluid volume, pressure will rise within the osteofascial space.40
Individuals suffering from compartment syndrome may complain of lower extremity aching, cramping, a burning pain, or tightness in the affected compartment.40 Complaints of tightness and burning pain in the region of the anterior compartment during the exercise protocol were noted in subjects from both groups after Cr supplementation and persisted for approximately 5 to 10 minutes after exercise. Consequently, these findings suggest that despite the potential ergogenic benefits of Cr supplementation, the risk of abnormally elevating ACP may contribute to placing those individuals at a higher risk for developing compartment syndrome.
The changes in body mass and percentage of fat observed in the present investigation were similar to those observed previously in our laboratory.20 While resting blood pressure and lower leg volume tended to increase with Cr supplementation, the lack of statistical significance was likely due to the intraindividual variability. Future investigations should focus on the effects of Cr supplementation on these dependent measures, as this is the second investigation that has demonstrated a trend toward increased blood pressure and higher leg volume.
The lack of significant correlations among the major dependent variables was disappointing but not surprising. Several factors, including the physical characteristics of the surrounding tissues, the fluid volume of the interstitial space, and the total amount of fluid in the cells, can contribute to elevations in compartment pressure.41 While our subjects were physically active, the individual variability in those factors that would predispose one to elevated compartment pressures likely contributed to a lack of significant correlations among intramuscular Cr, PCr, or TCr and anterior compartment pressure.
In response to the changes in anterior compartment pressure reported here and previously,20,21 it is extremely important that all sports medicine personnel be aware of this potentially negative side effect associated with Cr supplementation. When evaluating lower leg pain, information about Cr supplementation should be obtained from the patient. This may provide insight into treatment options. For example, cessation of Cr supplementation may alleviate the lower leg pain before more invasive medical procedures are required. In order to elucidate the influence of Cr supplementation on ACP and potential complications, future investigations into such issues as nerve conduction and blood flow should be performed.
Acknowledgments
ACKNOWLEDGMENTS
This research was supported by the National Athletic Trainers' Association Research and Education Foundation Grant #399-A004 (Dallas, TX), Stryker Instruments (Kalamazoo, MI), and The NutraSense Company (Shawnee Mission, KS).
REFERENCES
- Allen M J, Barnes M R. Exercise pain in the lower leg: chronic compartment syndrome and medial tibial syndrome. J Bone Joint Surg Br. 1986;68:818–823. doi: 10.1302/0301-620X.68B5.3782254. [DOI] [PubMed] [Google Scholar]
- Bourne R B, Rorabeck C H. Compartment syndromes of the lower leg. Clin Orthop. 1989;240:97–104. [PubMed] [Google Scholar]
- Dietrich D, Paley K J, Ebraheim N A. Spontaneous tibial compartment syndrome: case report. J Trauma. 1994;37:138–139. doi: 10.1097/00005373-199407000-00026. [DOI] [PubMed] [Google Scholar]
- Leach R E, Hammond G, Stryker W S. Anterior tibial compartment syndrome: acute and chronic. J Bone Joint Surg Am. 1967;49:451–462. [PubMed] [Google Scholar]
- Martens M A, Backaert M, Vermaut G, Mulier J C. Chronic leg pain in athletes due to a recurrent compartment syndrome. Am J Sports Med. 1984;12:148–151. doi: 10.1177/036354658401200211. [DOI] [PubMed] [Google Scholar]
- Ross D G. Chronic compartment syndrome. Orthop Nurs. 1996;15:23–27. [PubMed] [Google Scholar]
- Detmer D E. Chronic shin splints: classification and management of medial tibial stress syndrome. Sports Med. 1986;3:436–446. doi: 10.2165/00007256-198603060-00005. [DOI] [PubMed] [Google Scholar]
- Soderlund K, Balsom P D, Ekblom B. Creatine supplementation and high intensity exercise: influence on performance and muscle metabolism. Clin Sci. 1994;87:120–121. [Google Scholar]
- Detmer D E, Sharpe K, Sufit R L, Girdley F M. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13:162–170. doi: 10.1177/036354658501300304. [DOI] [PubMed] [Google Scholar]
- Balsom P D, Ekblom B, Soderlund K, Sjodin B, Hultman E. Creatine supplementation and dynamic high-intensity intermittent exercise. Scand J Med Sci Sports. 1993;3:143–149. [Google Scholar]
- Volek J S, Kraemer W J, Bush J A, et al. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J Am Diet Assoc. 1997;97:765–770. doi: 10.1016/S0002-8223(97)00189-2. [DOI] [PubMed] [Google Scholar]
- Balsom P D, Harridge S DR, Soderlund K, Sjodin B, Ekblom B. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiol Scand. 1993;149:521–523. doi: 10.1111/j.1748-1716.1993.tb09649.x. [DOI] [PubMed] [Google Scholar]
- Cooke W H, Grandjean P W, Barnes W S. Effect of oral creatine supplementation on power output and fatigue during bicycle ergometry. J Appl Physiol. 1995;78:670–673. doi: 10.1152/jappl.1995.78.2.670. [DOI] [PubMed] [Google Scholar]
- Snow R J, McKenna M J, Selig S E, Kemp J, Stathis C G, Zhao S. Effect of creatine supplementation on sprint exercise performance and muscle metabolism. J Appl Physiol. 1998;84:1667–1673. doi: 10.1152/jappl.1998.84.5.1667. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Gillis N, Van Leemputte M, Van Hecke P, Vanstapel F, Hespel P. Caffeine counteracts the ergogenic action of muscle creatine loading. J Appl Physiol. 1996;80:452–457. doi: 10.1152/jappl.1996.80.2.452. [DOI] [PubMed] [Google Scholar]
- Rawson E S, Wehnert M L, Clarkson P M. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol. 1999;80:139–144. doi: 10.1007/s004210050570. [DOI] [PubMed] [Google Scholar]
- Juhn M S, O'Kane J W, Vinci D M. Oral creatine supplementation in male collegiate athletes: a survey of dosing habits and side effects. J Am Diet Assoc. 1999;99:593–595. doi: 10.1016/s0002-8223(99)00145-5. [DOI] [PubMed] [Google Scholar]
- Greenwood M, Farris J, Kreider R, Greenwood L, Byars A. Creatine supplementation patterns and perceived effects in select Division I collegiate athletes. Clin J Sport Med. 2000;10:191–194. doi: 10.1097/00042752-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Robinson S J. Acute quadriceps compartment syndrome and rhabdomyolysis in a weight lifter using high-dose creatine supplementation. J Am Board Fam Pract. 2000;13:134–137. doi: 10.3122/15572625-13-2-134. [DOI] [PubMed] [Google Scholar]
- Schroeder C A, Potteiger J A, Randall J, et al. The effects of creatine dietary supplementation on anterior compartment pressure in the lower leg during rest and following exercise. Clin J Sport Med. 2001;11:87–95. doi: 10.1097/00042752-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Potteiger J A, Randall J C, Schroeder C, Magee L M, Hulver M W. Elevated anterior compartment pressure in the leg after creatine supplementation: a controlled case report. J Athl Train. 2001;36:85–88. [PMC free article] [PubMed] [Google Scholar]
- Borg G AV. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Howley E T, Bassett D R, Jr, Welch H G. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- Katch V L, Katch F I. A simple anthropometric method for calculating segmental leg limb volume. Res Q. 1974;45:211–214. [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Determination by neutron activation analysis on needle biopsy specimens: a study on normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest. 1962;14(suppl 68):1–110. [Google Scholar]
- Evans W J, Phinney S D, Young V R. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- Harris R C, Hultman E, Nordesjo L O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest: methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Gershuni D H, Yaru N C, Hargens A R, Lieber R L, O'Hara R C, Akeson W H. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66:1415–1420. [PubMed] [Google Scholar]
- Hultman E, Soderlund K, Timmons J A, Cederblad G, Greenhaff P L. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- Styf J F, Korner L M. Diagnosis of chronic anterior compartment syndrome in the lower leg. Acta Orthop Scand. 1987;58:139–144. doi: 10.3109/17453678709146460. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Pedowitz R A, Hargens A R, Mubarak S J, Gershuni D H. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18:35–40. doi: 10.1177/036354659001800106. [DOI] [PubMed] [Google Scholar]
- Harris R C, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Neumann G, Berbalk A, Reuter I. Creatine supplementation in endurance sports. Med Sci Sports Exerc. 1998;30:1123–1129. doi: 10.1097/00005768-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Guimbal C, Kilimann M W. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney: cDNA cloning and functional expression. J Biol Chem. 1993;268:8418–8421. [PubMed] [Google Scholar]
- Pearson D R, Hamby D G, Russel W, Harris T. Long-term effects of creatine monohydrate on strength and power. J Strength Cond Res. 1999;13:187–192. [Google Scholar]
- Ingwall J S. Creatine and the control of muscle-specific protein synthesis in cardiac and skeletal muscle. Circ Res. 1976;38(5 suppl 1):115–123. [PubMed] [Google Scholar]
- Kjellmer I. An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand. 1964;62:31–40. doi: 10.1111/j.1748-1716.1964.tb03948.x. [DOI] [PubMed] [Google Scholar]
- Wallensten R, Eklund B. Intramuscular pressures and muscle metabolism after short-term and long-term exercise. Int J Sports Med. 1983;4:231–235. doi: 10.1055/s-2008-1026040. [DOI] [PubMed] [Google Scholar]
- Varelas F L, Wessel J, Clement D B, Doyle D L, Wiley J P. Muscle function in chronic compartment syndrome of the leg. J Orthop Sports Phys Ther. 1993;18:586–589. doi: 10.2519/jospt.1993.18.5.586. [DOI] [PubMed] [Google Scholar]
- Reneman R S, Jageneau A HJ. The influence of weighted exercise on tissue (intramuscular) pressure in normal subjects and patients with intermittent claudication. Scand J Clin Lab Invest Suppl. 1973;128:37–42. [PubMed] [Google Scholar]


