Abstract
Objective: To assess the effect of head position and football equipment (ie, helmet and shoulder pads) on cervical spinal cord space in individuals lying supine on a spine board.
Design and Setting: The independent variables were head position (0-cm, 2-cm, and 4-cm occiput elevation with no helmet and shoulder pads and with helmet and shoulder pads) and cervical spine level (C3, C4, C5, C6, and C7). The 3 dependent variables were sagittal space available for the cord (SAC) (mm), sagittal spinal-cord diameter (mm), and cervical-thoracic angle (°), determined via magnetic resonance imaging.
Subjects: Twelve men (age = 24.3 ± 2.1 years; height = 181.1 ± 5.7 cm; weight = 93.9 ± 3.6 kg).
Measurements: Sagittal space available for the cord was determined by subtracting the sagittal spinal-cord diameter from the corresponding sagittal spinal-canal diameter. The spinal-canal diameter was measured as the shortest distance from the vertebral body to the spinolaminar line at each of the spinal levels. Each measurement was taken 3 times, and the 3 measurements were averaged.
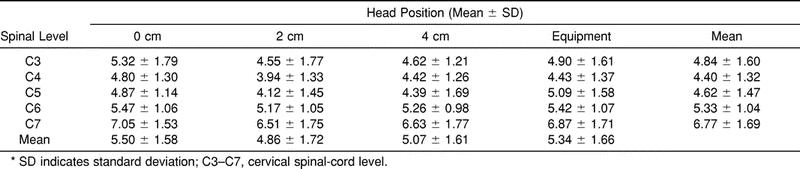
Results: Sagittal space available for the cord was significantly greater (P < .01) for 0-cm (mean = 5.50 mm) than for 2-cm (mean = 4.86 mm) and 4-cm (mean = 5.07 mm) occiput elevation. SAC was also significantly greater (P < .01) for the equipment condition (mean = 5.34 mm) than for the 2-cm and 4-cm elevation levels. No significant difference (P = .093) in SAC existed between 0-cm elevation and the equipment condition.
Conclusions: The helmet and shoulder pads should be left on during spine-board immobilization of the injured football player. Similarly, during spine-board immobilization of an individual without football helmet and shoulder pads, the head should be maintained at 0 cm of occiput elevation. Sagittal spinal-cord space is optimized in both of these conditions.
Keywords: cervical vertebrae injuries, emergency treatment methods, emergency medical services, cervical vertebrae radiography, protective device, equipment
Cervical spine neutral is reported to be the optimal position during spine-board immobilization in football1,2 and least likely to produce further injury during the transportation of the cervical spine-injured athlete.3 De Lorenzo et al4 reported that 2 cm of occiput elevation off the spine board is optimal (neutral) during immobilization because it affords the spinal cord the most space within the spinal canal at the C5 and C6 levels. This is clinically relevant because most cervical spine injuries in football occur at the lower cervical spine levels.5
Several authors6–9 have noted that wearing football equipment (ie, helmet and shoulder pads) during spine-board immobilization elevates the thorax such that the cervical spine is in the neutral position. Swenson et al2 and Palumbo et al1 found no significant differences in cervical spine alignment with and without football equipment during immobilization. Both sets of authors concluded that an “all-or-none” principle be applied to the removal of the helmet and shoulder pads.1, 2 However, their analyses were limited to computed tomography scans and lateral radiographs of the bony alignment of the cervical spine, respectively. These studies were also limited to viewing only the bony anatomy and resultant angle of the cervical spine in varying degrees of flexion and extension. Neither study accounted for the changes occurring to the spinal cord within the cervical spinal canal in various positions of flexion and extension.
If cervical spinal-cord space can be optimized when the injured football player is supine on the spine board, then the chance of neurologic injury during transportation to an emergency facility may be less. Our purpose was to assess the effect of head position and football equipment on the cervical space available for the spinal cord in individuals lying supine on a spine board.
METHODS
Research Design
The research design consisted of a 4 × 5 analysis of variance (ANOVA) with repeated measures on both variables. The independent variables were head position (0-cm, 2-cm, and 4-cm occiput elevations with no helmet and shoulder pads and with helmet and shoulder pads) and cervical spine level (C3, C4, C5, C6, and C7). The 3 dependent variables were sagittal space available for the cord (SAC), sagittal diameter of the spinal cord, and cervical-thoracic angle.
Subjects
Nineteen men volunteered as subjects in this study. However, data from only 12 subjects (age = 24.3 ± 2.1 years, height = 181.1 ± 5.7 cm, weight = 93.9 ± 3.6 kg) were analyzed. (Data on these 12 subjects are also reported in Tierney RT, Maldjian C, Mattacola CG, Straub SJ, Sitler MR. Cervical spine stenosis measures in normal subjects. J Athl Train. 2002;37:190–193.) Five subjects' data were eliminated because of movement in the magnetic resonance imaging (MRI) bore that resulted in unusable images, and 2 subjects did not complete the imaging phase of the study. Subjects had no history of cervical spine injury or disease and were screened for MRI contraindications (eg, claustrophobia, size restrictions in the MRI bore, ferromagnetic implantation). An institutional review board approved the study. All subjects signed a written informed consent before participating in the study.
Instrumentation
The materials used in this study were custom manufactured to meet MRI specifications. The football helmets (model VSR-4) and shoulder pads (model PM86) were manufactured by Riddell Inc (Elyria, OH) and fabricated to contain no metal components. Two plastic blocks (20.32 cm × 20.32 cm × 2 cm, 20.32 cm × 20.32 cm × 4 cm), manufactured by Rohm & Haas (Bristol, PA), were used to elevate the occiput 2 and 4 cm in the sagittal plane. A polycarbonate board (182.88 cm × 39.37 cm × 1.27 cm), manufactured by Rohm & Haas, was used to simulate a wooden spine board and contained no metal supports that would otherwise create “noise” in the MRI scanner. Occipital padding (3-cm thickness, Ferno Inc, Wilmington, OH) was used for the equipment treatment condition to simulate on-field immobilization.
A 1.5-Tesla superconducting MRI scanner (Signa, software 4.7, General Electric Medical Systems, Milwaukee, WI) with body coil was used to collect the data. Magnetic resonance imaging consisted of a volume 3-D, T2-weighted, fast spin-echo pulse sequence (TR = 3000 ms; TE = 105 ms; FOV = 32 cm; 1.3-mm slice thickness; 10 slabs and 6 slices per slab; 256 × 256 matrix; 2 NEX; 62.5-kHz bandwidth; and image time = 9 minutes, 50 seconds). This pulse sequence, because of its superior volume acquisition, provides a greater signal-to-noise ratio than conventional 2-D, fast spin-echo pulse sequences. Thinner slice thickness with the 3-D pulse sequence also adds greater resolution than 2-D, fast spin-echo pulse sequence. The efficacy of 3-D imaging in certain clinical applications has previously been demonstrated.10,11 All imaging was performed and evaluated by the same radiologist.
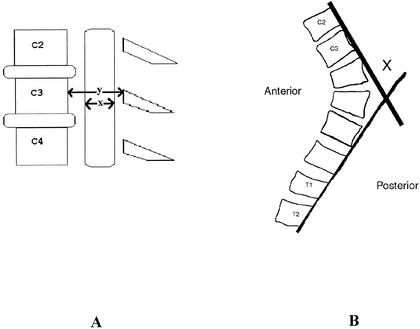
The MRI scans were evaluated midsagittally at each spinal level (C3–C7). The sagittal-diameter spinal-canal and spinal-cord measurements were taken at the midpoint of the vertebral body. Sagittal-diameter spinal-canal and spinal-cord measurements were traced manually, assessed with the General Electric software that accompanied the General Electric Signa Scanner, and recorded in millimeters. The spinal-canal diameter was measured as the shortest distance from the vertebral body to the spinolaminar line (Figure 1A). The spinal-cord diameter was measured at the appropriate spinal level. Each measurement was taken 3 times, and the 3 measurements were averaged. The SAC was determined by subtracting the sagittal-cord diameter from the corresponding sagittal-canal diameter. The cervical-thoracic angle was determined by drawing a line parallel with the dorsal aspect of the C2 and C3 vertebral bodies and a line parallel with the dorsal aspects of the T1 and T2 vertebral bodies. The point of intersection between the 2 lines was the cervical-thoracic angle (Figure 1B).
Figure 1.
A, Sagittal-diameter spinal-canal and spinal-cord measurements: x = sagittal spinal-cord diameter, y = sagittal spinal-canal diameter. B, Cervical-thoracic angle (X).
Data Collection
During a familiarization session, the testing protocol and data-collection procedures were explained to the subjects. Age, weight, and height were also assessed at this time. During this session, the subjects were properly fitted with a football helmet and shoulder pads by the primary investigator, a National Athletic Trainers' Association-certified athletic trainer.
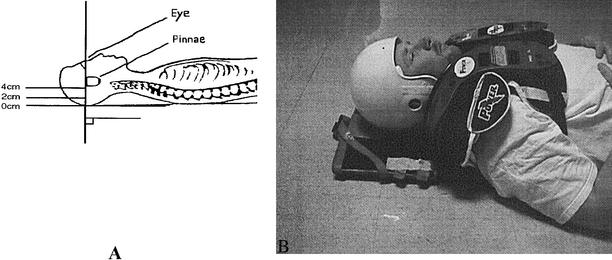
Subjects reported to a university hospital radiology department for MRI scans. Subjects were positioned supine on the spine board for all test conditions. One MRI scan was completed for every subject in each test position (0-cm, 2-cm, and 4-cm of occiput elevation with no helmet and shoulder pads and with helmet and shoulder pads). Testing was completed in sequential order beginning with the 0-cm reference position. For the 3 nonequipment positions, subjects maintained the same upward-facing position such that the lateral canthus of the eye and the superior aspect of the ear defined a line perpendicular to the horizon (Figure 2A).4 For the equipment position, each subject was placed supine on the spine board with the head and neck in an “in-line” neutral position (Figure 2B).
Figure 2.
A, Occiput elevations in upward-facing position. B, Supine subject wearing helmet and shoulder pads. Reprinted with permission of De Lorenzo RA, Olson JE, Boska M, et al. Optimal positioning for cervical immobilization. Ann Emerg Med. 1996;28:301–308.
Statistical Analyses
We analyzed the data using a 4 × 5 analysis of variance with repeated measures. The Statistical Package for the Social Sciences (version 9.0, SPSS, Inc, Chicago, IL) was used for all statistical analyses. Post hoc paired t-test comparisons with Bonferroni correction (.05/4 = .0125) were conducted to determine where significant differences existed. Descriptive statistics were used to analyze sagittal spinal-cord diameters and cervical-thoracic angles. All statistical analyses were conducted in the null form, and the alpha level of P < .05 was determined as statistically significant.
RESULTS
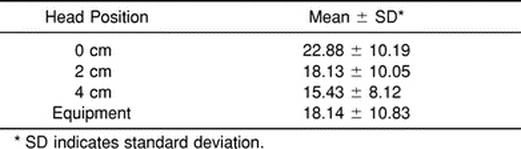
The sagittal-diameter spinal-cord values ranged from 6.22 to 8.89 mm (Table 1). The cervical-thoracic angle averages ranged from 15.43° to 22.88° (Table 2).
Table 1. Sagittal Spinal-Cord Diameter* (mm)
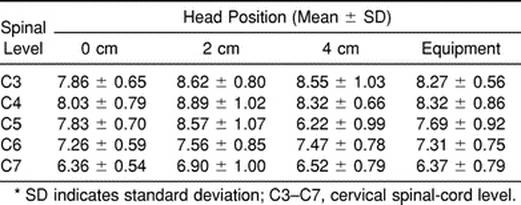
Table 2. Cervical-Thoracic Angle (°)
A significant main effect was noted for head position (F3,33 = 8.34, P < .01) and spinal level (F4,44 = 8.34, P < .01) (Table 3). There was no significant interaction effect (F12,132 = 1.28, P = .237). Post hoc paired t-test comparisons for SAC head position revealed that the SAC was significantly greater (P < .01) for 0 cm (mean = 5.50 mm) than for 2 cm (mean = 4.86 mm) and 4 cm (mean = 5.07 mm). SAC was significantly greater (P < .01) for the equipment condition (mean = 5.34 mm) than for 2 and 4 cm. There was no significant difference in SAC between 0 cm and the equipment condition.
Table 3. Sagittal Space Available for the Spinal Cord (mm)*
DISCUSSION
No occipital padding resulted in significantly more SAC for the subjects lying supine on a spine board compared with 2 and 4 cm of occiput elevation. Similarly, SAC was greater when immobilization was performed with helmet and shoulder pads on than when the occiput was elevated 2 and 4 cm. No significant difference existed in the SAC between 0 cm and the equipment condition.
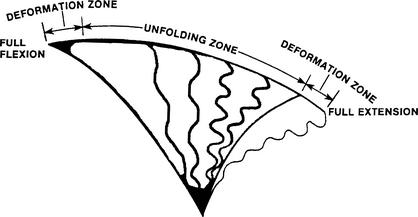
Changes in head position (flexion and extension) result in changes in spinal-cord cross-sectional area. These changes are a function of changes in the spinal cord's sagittal and coronal diameters. During sagittal-plane movement, the coronal diameter of the cord is most responsible for the change in cross-sectional area. As the spine is moved from full extension to full flexion, the spinal-cord shape changes from oval to round, and the total cross-sectional area decreases.12 The converse is also true. This change in cross-sectional area is consistent with the Poisson effect: “any increase in cross-sectional area with a decrease in length, or vice versa, (results in) the total volume remaining the same.”12 Most of the change in cross-sectional area occurs near the end ranges of motion, where elastic deformation occurs.12
In anatomical position, the spinal cord is folded like an accordion.12 During flexion from the anatomical position, the spinal cord first “unfolds” with a small increase in tension, followed by elastic deformation near full flexion. During extension, the cord first “folds” with a small decrease in tension, followed by some elastic compression near full extension (Figure 3).12 Our data show an increase in sagittal-cord diameter with slight flexion (2 cm) and a decrease in sagittal-cord diameter with more flexion (4 cm). More than 4 cm of flexion may result in a greater decrease in sagittal spinal-cord diameter, consistent with the Poisson effect. However, this would not explain the most extended position's (0 cm) resulting in the smallest sagittal-diameter spinal-cord values. An explanation may lie in the fact that our measurements were made in the mid ranges of motion, where the spinal cord is considered folded. Measurements in these mid ranges have previously revealed varying results.4 It has been estimated that this folding mechanism accounts for up to 70% to 75% of the spinal cord's length change and occurs at the mid range of motion. The remaining 25% to 30% of the spinal cord's length change is accounted for by elastic deformation and occurs at the end ranges of motion.12
Figure 3.
Biomechanics of the spinal cord. The spinal cord folds and unfolds in response to compression and tension forces during movement. Reprinted with permission.12
The mean cervical-thoracic angle decreased as the subjects were moved into flexion as a result of the increasing occiput elevations. Our 0- to 4-cm occiput-elevation values were 3° to 5° greater than reported by De Lorenzo et al4 for the same head positions. Differences can be attributed to differences in subjects. Our subjects were all men, whereas in the De Lorenzo et al study, 11 of 19 subjects were women. Men tend to have larger torsos, which result in more extension of the cervical spine while supine.
Cervical spine neutral is reported to be the optimal position for SAC during immobilization4 and has been defined in several ways.1,3,13,14 For example, Curran et al,13 in a pediatric study, defined cervical spine neutral as a Cobb angle of 0°, and Palumbo et al1 defined it as the position of the cervical spine during immobilization on a spine board. These definitions do not take into account the SAC. Data from our study and that of Mazolewski and Manix15 suggest that SAC ranges from approximately 3 to 7 mm in normal subjects. This space may be as little as 0 to 5 mm in subjects with symptoms of cervical cord neurapraxia.16 With trauma to the cervical area, any movement during immobilization increases the risk of neurologic damage.17
We found that 0 cm of occipital padding (the most extended position assessed in this study) resulted in the greatest SAC in the cervical region. The difference between 0 cm and 2 cm and 4 cm of occiput elevations was .64 and .43 mm, respectively. Using MRI to determine spinal canal to spinal-cord cross-sectional area ratios, De Lorenzo et al4 reported that 0 cm of occiput elevation resulted in the least space available for the cord from C3 to C7 for 78% of their subjects. The difference in results may be attributed to differences in dependent variables (type of variable and measurement technique), imaging technique, or measurement variation in the mid range of cervical motion, or a combination of these. Our dependent variable consisted of sagittal measurements, whereas De Lorenzo et al used cross-sectional area measurements. The sagittal spinal-cord diameter may react differently to total cross-sectional area change because most of the change is occurring in the coronal diameter during flexion and extension. This difference may contribute to the contradictory findings.
Data acquisition was also performed differently. In the De Lorenzo et al study,4 the measurement technique consisted of manually tracing (via computer) the spinal canal and cord perimeters to determine cross-sectional area. Reliability for this technique was not reported. We performed our SAC measurement via computer by clicking and dragging the cursor in a straight line from one edge of the structure being measured to the opposite edge (intraclass correlation coefficient = .85, standard error of the measurement = .56). We suggest that the cross-sectional measurement technique is more prone to error because of the increased distance and shape of the trace. Minor mistakes in perimeter tracing can result in large variations in total cross-sectional area calculations. Assuming only one trace per spinal level, the chance for error in the previous study is increased. The data we reported were the average of 3 measurements.
The imaging technique used in De Lorenzo et al4 was also different. They obtained transverse-plane images perpendicular to the anterior-posterior axis of C4. Because the cervical spine has a lordotic curve, cross-sectional area measurements made above and below C4 would be skewed. Our imaging technique consisted of a 3-D gradient echo that allowed images to be reconstructed as needed. This permitted us to make accurate sagittal measurements at each spinal level assessed. The differences between the studies may account for the variance in results, but the mid-range measurements may also account for that variance.
No previous study has examined the effect of football equipment on cervical spinal-cord space. We found no significant difference (P = .093) between the equipment condition and the 0-cm position (neutral). A larger number of subjects might have resulted in a significant difference in SAC between the test conditions. However, previous investigations using small numbers of subjects have similarly reported no significant differences in cervical spine alignment with and without football equipment during immobilization.1,2 In our study, subjects wearing the helmet and shoulder pads elevated the thorax compared with those in the no-equipment condition. Also, SAC was greatest in the 0-cm and equipment conditions at the C5 and C6 spinal levels.
Our data support the literature,6–8,18 as well as the recent recommendations of the Inter-Association Task Force for the Appropriate Care of the Spine-Injured Athlete,19 which advocate not removing football equipment before spine-board immobilization. It is not usually necessary to remove football equipment because injuries can be visualized with the football equipment in place,6,7 and the equipment can be used to stabilize the head and neck.7 If airway access is needed, then the face mask can be removed.6,7,9 Removal of the helmet or shoulder pads (or both) from an athlete with an unstable cervical spine causes movement that could result in neurologic damage.9 Finally, the helmet and shoulder pads place the cervical spine in neutral position.6–9 Our results support the suggestion that immobilization of an athlete at 0-cm occiput elevation or wearing shoulder pads and helmet allows the greatest amount of SAC.
The limitations of this study include (1) a small number of subjects, (2) a measurement of only the sagittal portion of the spinal cord in determining SAC, and (3) head positions varying in the mid ranges only. Future research should be conducted to determine the effect of varying head positions (mid and end ranges of motion) and wearing football equipment on the cross-sectional area of the cord and also its sagittal and coronal components. Future researchers should also analyze the effects of helmets and shoulder pads in other sports (eg, lacrosse, hockey) on SAC.
CLINICAL RELEVANCE
Our results support not removing the helmet and shoulder pads during spine-board immobilization of the cervical spine-injured football player. Also, during the immobilization of an athlete without football helmet and shoulder pad, the head should not be elevated, as SAC is greater at 0 cm than at 2 or 4 cm of occiput elevation.
Acknowledgments
ACKNOWLEDGMENTS
We thank Jack Reed at Rohm & Haas and Ted Monica at Riddell Inc for manufacturing and constructing the custom materials used in this study. We also thank the Department of Radiology at Temple University Hospital and Dr John Kelly for their assistance with this project.
REFERENCES
- Palumbo M A, Hulstyn M J, Fadale P D, O'Brien T, Shall L. The effect of protective football equipment on alignment of the injured cervical spine: radiographic analysis in a cadaveric model. Am J Sports Med. 1996;24:446–453. doi: 10.1177/036354659602400407. [DOI] [PubMed] [Google Scholar]
- Swenson T M, Lauerman W C, Blanc R O, Donaldson W F, 3rd, Fu F H. Cervical spine alignment in the immobilized football player: radiographic analysis before and after helmet removal. Am J Sports Med. 1997;25:226–230. doi: 10.1177/036354659702500216. [DOI] [PubMed] [Google Scholar]
- Nypaver M, Treloar D. Neutral cervical spine positioning in children. Ann Emerg Med. 1994;23:208–211. doi: 10.1016/s0196-0644(94)70032-x. [DOI] [PubMed] [Google Scholar]
- De Lorenzo R A, Olson J E, Boska M, et al. Optimal positioning for cervical immobilization. Ann Emerg Med. 1996;28:301–308. doi: 10.1016/s0196-0644(96)70029-x. [DOI] [PubMed] [Google Scholar]
- Torg J S, Wiesel S W, Rothman R H. Diagnosis and management of cervical spine injuries. In: Torg J S, editor. Athletic Injuries to the Head, Neck, and Face. Lea & Febiger; Philadelphia, PA: 1982. pp. 181–209. [Google Scholar]
- Feld F. Management of the critically injured football player. J Athl Train. 1993;28:206–212. [PMC free article] [PubMed] [Google Scholar]
- Feld F, Blanc R. Immobilizing the spine injured football player. J Emerg Med Serv. 1987;12:38–40. [Google Scholar]
- Patel M N, Rund D A. Emergency removal of football helmets. Physician Sportsmed. 1994;22(9):57–59. doi: 10.1080/00913847.1994.11947694. [DOI] [PubMed] [Google Scholar]
- Segan R D, Cassidy C, Bentkowski J. A discussion of the issue of football helmet removal in suspected cervical spine injuries. J Athl Train. 1993;28:295–305. [PMC free article] [PubMed] [Google Scholar]
- Maldjian C, Adam R J, Akhtar N, et al. Volume fast spin-echo imaging of the cervical spine. Acad Radiol. 1999;6:84–88. doi: 10.1016/S1076-6332(99)80486-3. [DOI] [PubMed] [Google Scholar]
- Maldjian C, Adam R J, Akhtar N, Maldjian J A, Bonakdarpour A, Boyko O. Volume (three-dimensional) fast spin-echo imaging of the lumbar spine. Acad Radiol. 1999;6:339–342. doi: 10.1016/s1076-6332(99)80228-1. [DOI] [PubMed] [Google Scholar]
- White A, 3rd, Panjabi M. Clinical Biomechanics of the Spine. 2nd ed. JB Lippincott; Philadelphia, PA: 1990. p. 67–71,184–186. [Google Scholar]
- Curran C, Dietrich A M, Bowman M J, Ginn-Pease M E, King D R, Kosnik E. Pediatric cervical-spine immobilization: achieving neutral position? J Trauma. 1995;39:729–732. doi: 10.1097/00005373-199510000-00022. [DOI] [PubMed] [Google Scholar]
- Schriger D L, Larmon B, Legassick T, Blinman T. Spinal immobilization on a flat backboard: does it result in neutral position of the cervical spine? Ann Emerg Med. 1991;20:878–881. doi: 10.1016/s0196-0644(05)81430-1. [DOI] [PubMed] [Google Scholar]
- Mazolewski P, Manix T H. The effectiveness of strapping techniques in spinal immobilization. Ann Emerg Med. 1994;23:1290–1295. doi: 10.1016/s0196-0644(94)70354-x. [DOI] [PubMed] [Google Scholar]
- Torg J S, Corcoran T A, Thibault L E, et al. Cervical cord neurapraxia: classification, pathomechanics, morbidity, and management guidelines. J Neurosurg. 1997;87:843–850. doi: 10.3171/jns.1997.87.6.0843. [DOI] [PubMed] [Google Scholar]
- American Academy of Orthopaedic Surgeons . Emergency Care and Transportation of the Sick and Injured. 5th ed American Academy of Orthopaedic Surgeons; Rosemont, IL: 1992. [Google Scholar]
- National Collegiate Athletic Association . NCAA 1995–1996 Sports Medicine Handbook. 8th ed. National Collegiate Athletic Association; Overland Park, KS: 1996. pp. 65–66. [Google Scholar]
- Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete General guidelines. Available at: http://www.nata.org/Departments/communications/recommen.htm. Accessed June 1, 1999.