Abstract
Objective: To quantify and compare the glenohumeral stiffness response in anterior-directed, posterior-directed, and inferior-directed translations in healthy men and women.
Design and Setting: We used a 2 ⋇ 3 factorial design and employed a device capable of measuring glenohumeral joint displacement as a function of force to gather kinematic data during a single test session.
Subjects: Twenty subjects with healthy nondominant shoulders participated in the study.
Measurements: Force-displacement measures were taken in the anterior, posterior, and inferior translational directions of the glenohumeral joint. These measurements simulated common laxity tests used at the shoulder.
Results: Analysis of variance revealed a nonsignificant sex ⋇ direction interaction effect (P > .05). The main effect for sex and direction was also not significant (P > .05).
Conclusions: Our results suggest that (1) glenohumeral stiffness is widely distributed in healthy shoulders, (2) glenohumeral stiffness is not significantly different between men and women, and (3) glenohumeral stiffness is not significantly different among directions of translations.
Keywords: force displacement, joint laxity, assessment, arthrometer, shoulder
Techniques that employ force-displacement maneuvers are often used in clinical orthopaedic practice. Laxity tests assess the integrity of the joint capsule and ligaments,1–4 and joint mobilizations restore normal range of motion in stiff joints.5,6 With the application of a manual force, the clinician can subjectively gauge the joint's resistance to translation or stiffness.2–4 Glenohumeral (GH) stiffness is a reflection of the articular structures' resistance to humeral head translation and is quantified as the amount of force (N) required to displace a joint by a given amount (mm).7–9 Stiffness measures provide information concerning the structural and mechanical properties of the joint and are considered clinically important when assessing joint stability.7–10 Biomechanical studies have shown that a stiffer joint is able to absorb more force during periods of loading11,12 and, therefore, may decrease the risk of injury such as dislocation or subluxation.
It is important to model the stiffness response of the GH joint as an aid to better analysis and understanding of the joint's arthrokinematic and mechanical behavior. Mechanical springs and dashpots are common models used in clinical biomechanics. Mechanical springs model the stiffness response; dashpots model the strain-rate nature of viscoelastic material. Both mechanical springs and dashpot models have been applied to the shoulder.13–15
Despite a great deal of previous research into the normal and abnormal function of the shoulder, the current information regarding in vivo quantitative force-displacement characteristics of the GH joint is limited. The lack of instrumented arthrometers similar to those used at the knee is cited as the primary limiting factor.9,16–20 Instrumented arthrometers for the knee have enabled researchers to quantify translatory kinematics in various populations.21–26 Furthermore, objective measurement of tibial translation obtained using instrumented knee arthrometers has proven effective for predicting injury status22–24 and the efficacy of various surgical intervention techniques.25–26 Recent publications have underscored the need for more objective means to measure shoulder arthrokinematics.18–20 Therefore, the purpose of our investigation was to quantify and compare GH stiffness response among anterior-directed, posterior-directed, and inferior-directed translations in the shoulders of healthy men and women.
METHODS
Subjects and Design
The data presented in this manuscript are part of a larger study characterizing shoulder kinematics in healthy shoulders.27 Force-displacement data were collected on 20 healthy, nondominant shoulders (11 women, 9 men, mean age = 20.9 ± 3.6 years) during a single test session. Subjects were asymptomatic, with no previous history of shoulder injury involving a physician's visit or formal inpatient or outpatient physical rehabilitation. Subjects also reported no regular, long-term participation in sports involving upper extremity overhead-throwing motion (ie, swimming, baseball, or tennis). Before participating, all subjects read and signed an informed consent explaining the risks, procedures, and benefits of participation. The experimental protocol received institutional review board approval.
Instrumentation
Force-displacement measures were taken in the anterior, posterior, and inferior directions using an instrumented arthrometer.27 A test chair equipped with nylon strapping provided the base of support for testing. Displacement forces were applied to the joint with a custom force applicator. The force applicator consists of a plastic handle mounted to a full-bridge, thin-beam load cell (model LC105–50, Omega Engineering Inc, Stamford, CT) that has a range from 0 to 222 N. A hook attached at the opposite end of the load cell secures the force applicator to an arm cuff. The arm cuff is wrapped around the proximal humerus for the anterior and posterior translations (Figures 1A and B) and around the proximal forearm for inferior translation (Figure 2). The arm cuff is designed to ensure equal force distribution during testing.
Figure 1.
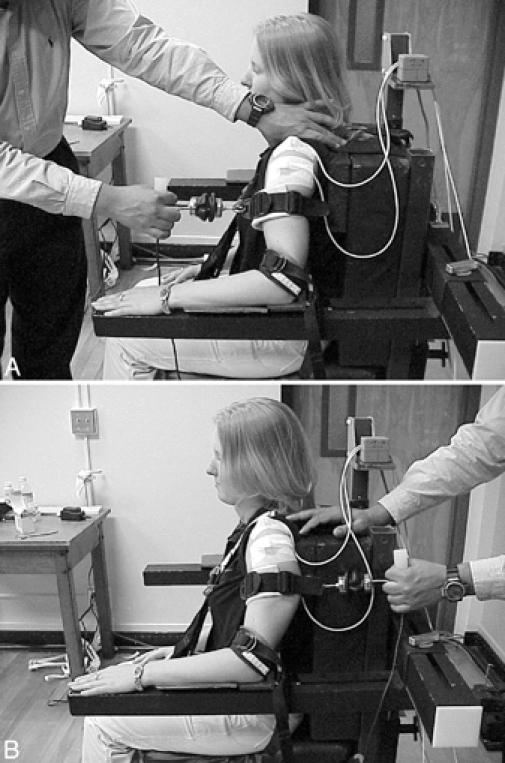
Instrumentation setup and subject positioning for (A) anterior-directed and (B) posterior-directed translations. The transmitter was mounted to the chair frame above and behind the subject, and motion sensors were taped to the acromion process and proximal humerus.
Figure 2.
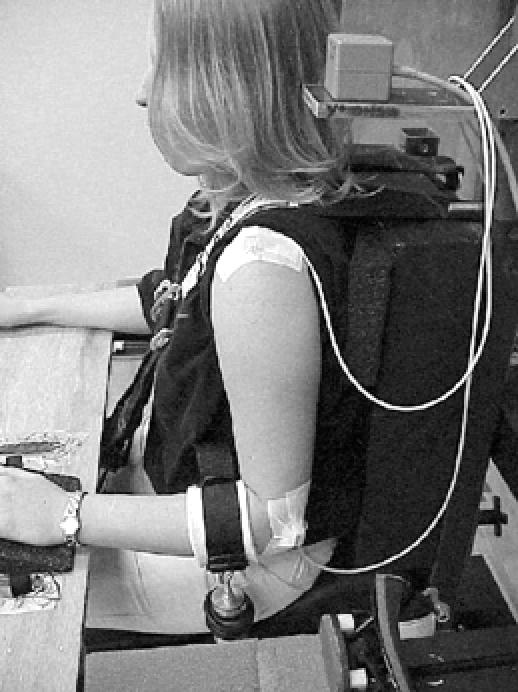
Instrumentation setup and subject positioning for inferior-directed translations. The distal sensor was placed over the lateral epicondyle of the humerus. Figures 1 and 2 reprinted from Borsa EL, Sauers EL, Herling DE, Manzour WF, In vivo quantification of capsular end-point in the healthy glenohumeral joint using an instrumented measuring system, Journal of Orthopaedic & Sports Physical Therapy, 2001, 31, 419–431, with permission of the Orthopaedic and Sports Sections of the American Physical Therapy Association.
Translations were measured using an electromagnetic spatial-tracking device (Polhemus 3Space Fastrak, Colchester, VT) consisting of a transmitter and 2 sensors (receivers). The unit contains the hardware necessary to generate and sense magnetic fields, compute position and orientation, and interface with a host computer. A global x, y, z coordinate system was established by mounting the transmitter on a composite base above and behind the subject, aligned with the cardinal planes of the body. On pre-experiment calibration measures, we found the device to be accurate within 0.1 to 0.2 mm for linear translations.27
Test Procedures
Subjects were seated and secured comfortably in the test chair with nylon strapping. For anterior and posterior translations, the humerus of the nondominant arm was positioned in 20° of abduction with the elbow secured in neutral rotation. The test position is similar to that for the load-and-shift and anterior-posterior drawer tests (Figures 1A and B).1–3 The acromion process was located via palpation, and the acromion sensor was affixed cutaneously to its superior aspect. The humeral head was located via palpation to determine the position for placement of the proximal humeral sensor. The humeral head sensor was then affixed over the lateral aspect of the proximal humerus directly below the acromion sensor. Each sensor was secured to the skin surface with self-adhesive tape (Cover-Roll Stretch, Beiersdorf Inc, Norwalk, CT). Next, the arm cuff was secured firmly around the proximal humerus. The experimenter stabilized the scapula with his thumb (coracoid process) and index finger (scapular spine) during each trial. Anterior and posterior forces were applied to the nondominant arm by pulling linearly in the respective translational direction until a capsular endpoint was achieved.
For inferior translation, the nondominant arm was placed in the resting position with the elbow flexed to 90° and the forearm pronated with the palms resting on a wooden platform (Figure 2). The acromion sensor placement remained the same as for anterior and posterior testing, but the humeral sensor was secured over the lateral epicondyle with self-adhesive tape (Cover-Roll Stretch). The cuff was placed around the proximal forearm just distal to the elbow joint. The examiner applied force to the nondominant arm by pulling downward in line with the longitudinal axis of the humerus until a capsular endpoint was achieved. This test position replicated that for the sulcus or inferior-distraction test.2,3
For each trial, a “start” or zero reference position was determined. Once the start position was secured, a controlled force was applied to the joint using the force applicator until capsular endpoint was reached. The rate of force application to the joint was uniform and did not appear to adversely affect muscle reaction or capsular stiffness. Capsular endpoint was achieved in approximately 3 to 4 seconds. Three trials were completed for each direction, and the average was recorded to the nearest 0.1 mm.
Linear displacements of the acromion process (scapular protraction and retraction) and humeral head (humeral translation) were calculated from the Cartesian coordinates of the 2 sensors. The displacement of the scapula was subtracted from the absolute humeral displacement, giving true humeral head translation.10,16,17 Intraexaminer and interexaminer reproducibility of our technique was established and reported in earlier publications.16,17 Additionally, our technique was validated in a previous study by comparing force-displacement values obtained using our current method of cutaneously applied sensors with a method of directly pinning the sensors to the bony reference points (humeral head and acromion process) in 30 fresh-frozen cadaver shoulders.28 Bone fixation of the displacement sensors enabled accurate measurement of glenohumeral translation and thus served as our “gold standard.” The measures from the cutaneously applied sensors showed good to excellent agreement with the bone-pinned measurements. Correlation coefficients were moderate to good for anterior-directed (P = .79), posterior-directed (P = .68), and inferior-directed (P = .71) translations.28
Data Acquisition and Display
Raw data from the tracking device were fed to the host computer via a serial port, while data from the load cell (force) were fed to the host computer via a data-acquisition card. A custom software program (Visual Basic 6.0, Microsoft Inc, Redmond, WA) simultaneously and sequentially captured force and displacement data for each test trial at intervals of approximately 0.1 second. From these data, a force-displacement curve was displayed for each trial using only the data points from the start of the force application (∼5 N) to a discernable endpoint. Endpoint was identified from our raw data as the point on the force-displacement curve at which increasing the applied force failed to produce associated translation.27 Stiffness values were calculated from the slope of the force-displacement curve (Figure 3). From the force-displacement curve, our custom software program identified the most linear region of the curve and calculated the slope using a simple linear regression analysis. Using Figure 3 as an example, the most linear region of the force-displacement curve was identified, and the slope of the curve was calculated using the associated linear regression equation y = bx−a, where y = predicted value (force), b = slope (stiffness value), x = predictor value (displacement), and a = intercept. From this calculation, the slope was found to be 25.6 N/mm, which was our stiffness value for that trial. This model has been used in previous studies of joint and muscle stiffness.8–13 Three trials were performed for each direction, and the average score from the 3 trials was recorded as the stiffness value.
Figure 3.
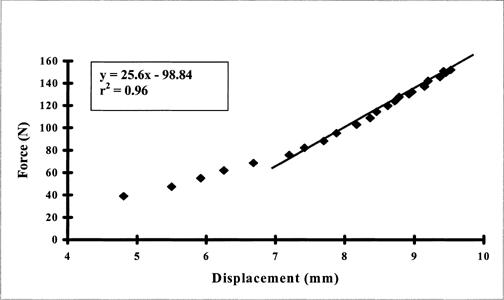
Typical force-displacement curve derived from the available data plots of one trial using the instrumented shoulder arthrometer. Stiffness values were calculated as the slope of the force-displacement curve in the most linear region. For this trial, the stiffness value was 25.6 N/mm.
Statistical Analysis
We used an online power analysis (http://www.math.yorku.ca/SCS/Demos/power/). We entered our design as 2 (sex) ⋇ 3 (direction). Using our previous stiffness data (mean differences/SD) on stiffness, we found our effect size to be 0.8 (medium effect size). For the online calculation, we entered 2 effect sizes (0.5 and 0.75) with alpha set at 0.05. For our design, 20 subjects were necessary to achieve statistical power between .775 and .982.
A 2 (sex) ⋇ 3 (direction) analysis of variance was used to determine significant mean (±SD) differences between sex and directions for stiffness. Tukey Honestly Significant Difference post hoc analyses were used in the presence of a significant interaction or main effect. All data analyses were performed using Statistical Program for Scientific Studies for Windows (version 10.0, SPSS Inc, Chicago, IL). The level of statistical significance was set at .05.
RESULTS
Descriptive data are presented in Table 1. Analysis of variance revealed a nonsignificant sex ⋇ direction interaction effect for joint stiffness (F2,59 = 3.01, P = .06). The main effects for sex (F2,59 = .546, P = .46) and direction (F2,59 = .612, P = .55) were also not significant (Figure 4).
Table 1. Descriptive Data for Glenohumeral Stiffness Collapsed Across Sex*
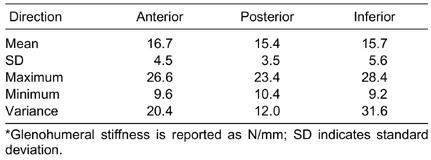
Figure 4.
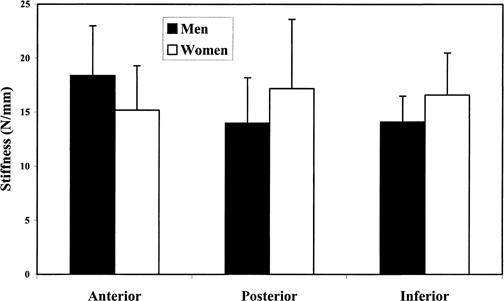
Glenohumeral joint stiffness values for men and women in each translational direction.
DISCUSSION
Stiffness is a physiologic variable that can be used to characterize the mechanical behavior of a joint.8,9,12 Joint-stiffness values can be calculated from the slope of the force-displacement curve using a least squares or linear regression model (Figure 3).7,9,10 The in vivo force-displacement curves reported in this study bear a striking resemblance to those of Markolf et al7,8 in healthy knees and McQuade et al9 in healthy shoulders. On average, our force-displacement curves demonstrated a discernable biphasic pattern of an early, nonlinear response, followed by a linear response after about the 60- to 80-N force level (Figure 3). The early, nonlinear stiffness response is presumed to be the “toe” region, or period when the collagen fibers of the joint capsule are being recruited and are not yet under tension, followed by the mid- to end-range linear stiffness response, indicating that the mechanical restraints are progressively developing tension. The stiffness values reported in this study were calculated from the most linear region of the force-displacement curve, which was most often in the end-range or region of highest force application (Figure 3). This region differed slightly for each individual, depending on the force-displacement response of the individual's joint. During clinical laxity examinations, the clinician gauges joint stiffness at the end-range or endpoint of translation. This is why our aim was to measure end-range stiffness of the joint and not early- and mid-range stiffness.
Interindividual Differences
Mechanical restraint to GH translation is provided by both bony and soft tissue structures.29–34 The angle and depth of the glenoid cavity,29 capsuloligamentous tightness,30–33 and overlying rotator cuff tendons34 contribute to the overall stiffness of the joint. We found GH joint stiffness data to be widely yet normally distributed, with a range of values from 9.2 to 28.4 N/mm, depending on direction of translation in healthy shoulders (Table 1). The interindividual differences in joint stiffness reported in Table 1 are likely to result from variability in any or all of these aforementioned factors.
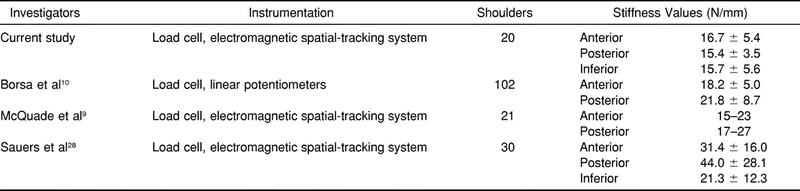
Borsa et al,10 Sauers et al,28 and McQuade et al9 earlier reported on force-displacement measures in healthy shoulders. We10 used a load cell and linear potentiometers to quantify anterior-posterior translation in a large population of healthy shoulders and reported mean force-displacement values ranging from 16 to 22 N/mm. Sauers et al28 quantified humeral translations in the anterior, posterior, and inferior directions using fresh-frozen cadaver shoulders; force-displacement measures were obtained with a load cell and an electromagnetic spatial-tracking system. The sensors were pinned directly to the bony reference points in order to isolate bony movement. Sauers et al28 found mean stiffness values ranging from 21 to 44 N/mm. McQuade et al9 also used a palm-held load cell and an electromagnetic spatial-tracking system to quantify in vivo anterior and posterior humeral translations in multiple degrees of abduction and rotation. McQuade et al9 reported force-displacement curves that closely resembled ours currently and previously10 and those of Sauers et al.28 The mean stiffness values from Sauers et al28 were considerably higher than ours, possibly because they used fresh-frozen cadaveric specimens, which may have had higher resistance properties due to the preservation process. Also, the scapulae of the specimens were rigidly mounted to the test jig, which may have produced additional resistance during force application. Results from these studies are summarized in Table 2.
Table 2. Studies Assessing Glenohumeral Joint Stiffness
Sex and Directional Differences
We did not show any significant difference in GH joint stiffness between men and women. McQuade et al9 also reported no significant sex differences in stiffness. Borsa et al10 found that women with healthy shoulders had significantly less anterior joint stiffness than men with healthy shoulders. These findings at the glenohumeral joint are consistent with those at the knee revealing decreased stiffness in women.8 More research is warranted in order to draw conclusions concerning sex-specific differences in GH joint stiffness.
The capsuloligamentous structures supporting the GH joint have been referred to as a “soft tissue socket.”35 The capsule and ligaments function collectively with the labrum to maintain a centered humeral head and limit excessive translation.36 Translation can occur in any direction as the humeral head moves on the glenoid face during humeral elevation and rotation. Clinically, the most important translation directions to evaluate are anterior, posterior, and inferior1,2 because GH joint instability occurs in these directions.37 In this study of healthy shoulders, mean stiffness values were symmetric with respect to the direction of the applied force. Future studies should compare stiffness between translational directions in shoulders with a documented unidirectional or multidirectional instability.
Clinical Implications
An instrumented technique for measuring force-displacement response similar to the one presented in this study may be clinically useful as an instructional aid9,27 and as an assessment tool in a clinical setting.9,27,38
Stiffness measures are clinically important in the prediction of capsuloligamentous injury and treatment outcomes.7,9,10 Subjectively, a soft end-feel is associated with capsuloligamentous disruption, and a hard or firm end-feel is associated with normal capsuloligamentous tissue.8 During a laxity examination, changes in the end-feel or stiffness are noted along with the patient's history and other physical findings in making a clinical diagnosis.19 Investigators have also evaluated joint stiffness in healthy knees7,8 and anterior cruciate ligament-deficient knees before and after surgical reconstruction.21,22,24,26 From this research, they ultimately concluded that stiffness was of significant diagnostic value at the knee.24,25 Whether stiffness measures are accurate predictors of injury or clinical outcome at the GH joint still needs to be determined.
Study Limitations
We used a simple linear regression model to calculate stiffness only at the most linear region of the force-displacement curve. Because joint stiffness is commonly evaluated at the end-range of translation, we chose to characterize end-range stiffness only. A polynomial regression model would be most appropriate if the goal was to measure the joint's stiffness response over the entire range of translation.
We did not account for the contribution of resting muscle tension on stiffness response of the joint. Subjects appeared to be relaxed and comfortable during test trials, and our examination of force-displacement curves did not reveal any abnormal spiking response, as could occur if the subject “tensed up” during force application. Similarly, we used a slow, controlled load rate in order to prevent any discomfort or abrupt muscle-guarding effects.
Joint stiffness has been reported to depend upon the amount of bulk tissue surrounding the GH joint. We did not account for interindividual differences in bulk tissue size for this study. Forces were applied consistently to each subject regardless of shoulder size. An a priori or post facto method for scaling the force per shoulder size should be considered as a means to account for varying anthropometric characteristics.
We only tested one position of humeral abduction and rotation, compared with McQuade et al,9 who tested several positions of humeral abduction and rotation. Lastly, this study was limited to modeling only static or passive joint stiffness, when, in fact, the role of dynamic factors is suggested to be most critical to overall joint stiffness during functional performance.11,29,39
CONCLUSIONS
Our results suggest the following: (1) glenohumeral stiffness is widely distributed in healthy shoulders, (2) glenohumeral stiffness is not significantly different between men and women, and (3) glenohumeral stiffness is not significantly different among directions of translations.
Acknowledgments
ACKNOWLEDGMENTS
This study was conducted in the Biomechanics Research Laboratory at Oregon State University, Corvallis, OR. The John C. Erkkila Endowment for Health and Human Performance (Corvallis, OR) provided funding for this study.
REFERENCES
- Gerber C, Ganz R. Clinical assessment of instability of the shoulder: with special reference to anterior and posterior drawer tests. J Bone Joint Surg Br. 1984;66:551–556. doi: 10.1302/0301-620X.66B4.6746691. [DOI] [PubMed] [Google Scholar]
- Clarnette R C, Miniaci A. Clinical exam of the shoulder. Med Sci Sports Exerc. 1998;30(4 Suppl):1–6. doi: 10.1097/00005768-199804001-00001. [DOI] [PubMed] [Google Scholar]
- Hawkins R J, Mohtadi G H. Clinical evaluation of shoulder instability. Clin J Sport Med. 1991;1:59–64. [Google Scholar]
- McFarland E G, Torpey B M, Curl L A. Evaluation of shoulder laxity. Sports Med. 1996;22:264–272. doi: 10.2165/00007256-199622040-00005. [DOI] [PubMed] [Google Scholar]
- Conroy D E, Hayes K W. The effect of joint mobilization as a component of comprehensive treatment for primary shoulder impingement syndrome. J Orthop Sports Phys Ther. 1998;28:3–14. doi: 10.2519/jospt.1998.28.1.3. [DOI] [PubMed] [Google Scholar]
- McClure P W, Flowers K R. Treatment of limited shoulder motion: a case study based on biomechanical considerations. Phys Ther. 1992;72:929–936. doi: 10.1093/ptj/72.12.929. [DOI] [PubMed] [Google Scholar]
- Markolf K L, Amstutz H C. The clinical relevance of instrumented testing for ACL insufficiency: experience with the UCLA clinical knee testing apparatus. Clin Orthop. 1987;223:198–207. [PubMed] [Google Scholar]
- Markolf K L, Graff-Radford A, Amstutz H C. In vivo knee stability: a quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60:664–674. [PubMed] [Google Scholar]
- McQuade K J, Shelley I, Cvitkovic J. Patterns of stiffness during clinical examination of the glenohumeral joint. Clin Biomech (Bristol, Avon) 1999;14:620–627. doi: 10.1016/s0268-0033(99)00024-8. [DOI] [PubMed] [Google Scholar]
- Borsa P A, Sauers E L, Herling D E. Patterns of glenohumeral joint laxity and stiffness in healthy men and women. Med Sci Sports Exerc. 2000;32:1685–1690. doi: 10.1097/00005768-200010000-00004. [DOI] [PubMed] [Google Scholar]
- McNair P J, Wood G A, Marshall R N. Stiffness of the hamstring muscles and its relationship to function in anterior cruciate ligament deficient individuals. Clin Biomech. 1992;7:131–137. doi: 10.1016/0268-0033(92)90027-2. [DOI] [PubMed] [Google Scholar]
- Wright V. Stiffness: a review of its measurement and physiological importance. Physiotherapy. 1973;59:107–111. [PubMed] [Google Scholar]
- Kaltsas D S. Comparative study of the properties of the shoulder joint capsule with those of other joint capsules. Clin Orthop. 1983;173:20–26. [PubMed] [Google Scholar]
- Mow V C, Bigliani L U, Flatow E L, Ticker J B, Ratcliffe A, Soslowsky L J. Material properties of the inferior glenohumeral ligament and the glenohumeral articular cartilage. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 29–67. [Google Scholar]
- Lew W D, Lewis J L, Craig E V. Stabilization by capsule, ligaments, and labrum: stability at the extremes of motion. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 69–90. [Google Scholar]
- Borsa P A, Sauers E L, Herling D E. In vivo assessment of AP laxity in healthy shoulders using an instrumented arthrometer. J Sport Rehabil. 1999;8:1–14. [Google Scholar]
- Sauers E L, Borsa P A, Herling D E, Stanley R D. Instrumented measurement of glenohumeral joint laxity: reliability and normative data. Knee Surg Sports Traumatol Arthrosc. 2001;9:34–41. doi: 10.1007/s001670000174. [DOI] [PubMed] [Google Scholar]
- Levy A S, Lintner S, Kenter K, Speer K P. Intra- and interobserver reproducibility of the shoulder laxity examination. Am J Sports Med. 1999;27:460–463. doi: 10.1177/03635465990270040901. [DOI] [PubMed] [Google Scholar]
- Lintner S A, Levy A, Kenter K, Speer K P. Glenohumeral translation in the asymptomatic athlete's shoulder and its relationship to other clinically measurable anthropometric variables. Am J Sports Med. 1996;24:716–720. doi: 10.1177/036354659602400603. [DOI] [PubMed] [Google Scholar]
- Rodkey W G, Noble J S, Hintermeister R A. Laboratory methods of evaluating the shoulder: strength, range of motion, and stability. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 551–582. [Google Scholar]
- Neuschwander D C, Drez D, Jr, Paine R M, Young J C. Comparison of anterior laxity measurements in anterior cruciate deficient knees with two instrumented testing devices. Orthopedics. 1990;13:299–302. doi: 10.3928/0147-7447-19900301-09. [DOI] [PubMed] [Google Scholar]
- Bach B R, Jr, Warren R F, Flynn W M, Kroll M, Wickiewicz T L. Arthrometric evaluation of knees that have a torn anterior cruciate ligament. J Bone Joint Surg Am. 1990;72:1299–1306. [PubMed] [Google Scholar]
- Daniel D, Stone M L, Sachs R, Malcom L. Instrumented measurement of anterior laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13:401–407. doi: 10.1177/036354658501300607. [DOI] [PubMed] [Google Scholar]
- Markolf K L, Kochan A, Amstutz H C. Measurement of knee stiffness and laxity in patients with documented absence of the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66:242–252. [PubMed] [Google Scholar]
- Giannotti B F, Fanelli G C, Barrett T A, Edson C. The predictive value of intraoperative KT-1000 arthrometer measurements in single incision anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:660–666. doi: 10.1016/s0749-8063(96)90167-x. [DOI] [PubMed] [Google Scholar]
- Kochan A, Markolf K L, More R C. Anterior-posterior stiffness and laxity of the knee after major ligament reconstruction. J Bone Joint Surg Am. 1984;66:1460–1465. [PubMed] [Google Scholar]
- Borsa P A, Sauers E L, Herling D E, Manzour W F. In vivo quantification of capsular end-point in the nonimpaired glenohumeral joint using an instrumented measurement system. J Orthop Sports Phys Ther. 2001;31:427–431. doi: 10.2519/jospt.2001.31.8.419. [DOI] [PubMed] [Google Scholar]
- Sauers E L, Borsa P A, Herling D E, Manzour W F, Stanley R D. Validity of an instrumented measurement technique for quantifying glenohumeral joint laxity and stiffness [abstract] J Athl Train. 2001;36:S40. [Google Scholar]
- Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop. 1993;291:20–28. [PubMed] [Google Scholar]
- Harryman D T, Sidles J A, Harris B S, Matsen F A. Laxity of normal glenohumeral joint: a quantitative in vivo assessment. J Shoulder Elbow Surg. 1992;1:66–76. doi: 10.1016/S1058-2746(09)80123-7. [DOI] [PubMed] [Google Scholar]
- O'Brien S J, Schwartz R S, Warren R F, Torzilli P A. Capsular restraints to anterior-posterior motion of the abducted shoulder: a biomechanical study. J Shoulder Elbow Surg. 1995;4:298–308. doi: 10.1016/s1058-2746(05)80024-2. [DOI] [PubMed] [Google Scholar]
- Warner J J, Deng X H, Warren R F, Torzilli P A. Static capsuloligamentous restraints to superior-inferior translation of the glenohumeral joint. Am J Sports Med. 1992;20:675–685. doi: 10.1177/036354659202000608. [DOI] [PubMed] [Google Scholar]
- Turkel S J, Panio M W, Marshall J L, Girgis F G. Stabilizing mechanisms preventing anterior dislocation of the glenohumeral joint. J Bone Joint Surg Am. 1981;63:1208–1217. [PubMed] [Google Scholar]
- Debski R E, Sakane M, Woo S L, Wong K, Fu F H, Warner J J. Contribution of the passive properties of the rotator cuff to glenohumeral stability during anterior-posterior loading. J Shoulder Elbow Surg. 1999;8:324–329. doi: 10.1016/s1058-2746(99)90154-4. [DOI] [PubMed] [Google Scholar]
- Friedman R J. Glenohumeral capsulorrhaphy. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 445–458. [Google Scholar]
- Warner J P. The gross anatomy of the joint surfaces, ligaments, labrum, and capsule. In: Matsen F A, Fu F H, Hawkins R J, editors. The Shoulder: A Balance of Mobility and Stability. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. pp. 7–27. [Google Scholar]
- Silliman J F, Hawkins R J. Classification and physical diagnosis of instability of the shoulder. Clin Orthop. 1993;291:7–19. [PubMed] [Google Scholar]
- Reis M T. Non-invasive measurement of joint translation and range of motion. US Patent 5 961 474. Oct 5, 1999.
- Saha A K. Dynamic stability of the glenohumeral joint. Acta Orthop Scand. 1971;42:491–505. doi: 10.3109/17453677108989066. [DOI] [PubMed] [Google Scholar]


