Abstract
Objective: Anabolic steroid precursors have gained widespread popularity as ergogenic supplements. Advertisements for these supplements claim that they increase endogenous testosterone production and protein synthesis, resulting in increased lean body mass and strength during training. At this time scientific support is limited, but the potential for serious side effects exists and the popularity of these supplements continues to grow. This review provides rationales for the ergogenic claims regarding steroid precursors and compares claims with data from scientifically controlled investigations.
Data Sources: A search of MEDLINE and SPORT Discus from 1960 to 2001 using the key words dehydroepiandrosterone, androstenedione, and androstenediol in combination with testosterone, estrogen, exercise, performance, and side effects.
Data Synthesis: Although fairly new to the athletic community, steroid precursors have been used as ergogenic or anabolic agents for quite some time. Suggested gains in strength and lean body mass are attributed to an increase in the endogenous production of testosterone and enhanced protein synthesis. Most of the scientific data, however, do not support manufacturers' ergogenic claims, and the potential for serious side effects, such as decreased high-density lipoprotein cholesterol and increased estrogen concentrations, has been associated with precursor use. Thus, the safety and efficacy of these supplements must be questioned.
Conclusions/Recommendations: It appears that the risks associated with the use of anabolic steroid precursors outweigh any possible ergogenic benefits. Furthermore, these supplements are banned by most athletic organizations. Thus, it is extremely important that athletic trainers are able to educate athletes on these issues so they can continue to perform at an optimum level in a safe and healthy manner.
Keywords: dehydroepiandrosterone, adrostenedione, androstenediol, ergogenic aid
Anabolic-androgenic steroids (AAS) are synthetic derivatives of testosterone that have been used by athletes for decades to increase lean body mass, strength, and overall athletic performance.1 A number of side effects have been associated with AAS use, including acne, hair loss, increased risk of heart disease, kidney and liver dysfunction, hypertension, and impotence. It is currently illegal to possess AAS without a prescription from a licensed physician.1,2 The legal issues and dangers associated with AAS have resulted in a reluctance to use them on the part of many athletes and a search for a more natural method to improve performance. The result has been an increase in the popularity of nutritional supplements marketed as natural testosterone enhancers or steroid precursors. Although bodybuilders have been using steroid precursors for some time, it was home-run hitter Mark McGuire's well-publicized use that first brought them to the attention of the athletic community and the public. While there are numerous precursor products on the market, the most popular are those containing dehydroepiandrosterone (DHEA) and the various forms of androstenedione (A'dione) and androstenediol (A'diol).
Steroid Precursors
Dehydroepiandrosterone, A'dione, and A'diol are androgenic hormones produced primarily by the adrenal glands and gonads that act as precursors in the endogenous production of testosterone and estrogen.3,4 Because they bind poorly to androgen receptors, these precursors have little inherent androgen action on their own.5 However, they provide an important pool of circulating precursor steroids that can be converted to active androgens and estrogens in the peripheral tissue where they act (Figure).4 Virtually all sex steroids in the human body are made from DHEA, which is the most abundant steroid hormone in circulation. This natural process begins in the adrenal cortex where DHEA is secreted and eventually converted to either A'dione or A'diol, which are both immediate precursors to testosterone.5
Fig. 1.
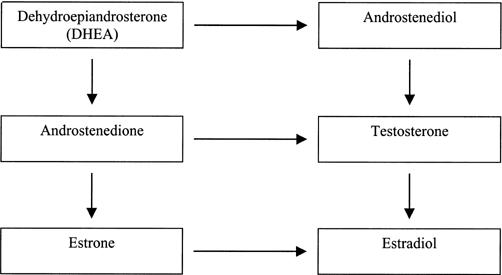
Pathway for endogenous sex-steroid production.
The conversion of A'dione and A'diol to testosterone occurs in the testes and other target tissues via 2-way reactions that are regulated by the enzymes 17γ-hydroxysteroid dehydrogenase and 3γ-hydroxysteroid dehydrogenase, respectively.6,7 This conversion can occur in many cells containing androgen or estrogen receptors, including adipose, bone, muscle, breast, prostate, skin, brain, and liver cells.3,8 In men, approximately 95% of circulating testosterone after puberty is derived from testicular secretion, with the remainder arising from the extragonadal conversion of the steroid precursors.8 In women, circulating levels of testosterone are derived about equally from direct gonadal secretion and indirect peripheral conversion of the precursors.8
Testosterone production is not the only pathway for these precursors, as A'dione and testosterone can also be aromatized to estrone and estradiol (Figure).3,7,9 In normal men, all of the estrone and about 85% of the estradiol can be accounted for by formation from A'dione and testosterone at the extragonadal sites.10 While the gonadal production of testosterone and estrogen is regulated by a negative-feedback system, the peripheral formation of active androgens and estrogens depends on the quantity of precursors and is not subject to any known physiologic regulation.8
Original Research
Like testosterone, the production of DHEA and A'dione peaks in the midtwenties and then declines steadily with age after the third decade of life.11 This decline stimulated great interest in a potential role for a steroid deficiency state in aging. Thus, original research in precursor supplementation focused on its role in replacement therapies to compensate for the age-related decline in their endogenous production.12–15 The suggested benefits from these replacement therapies included stimulation of immunologic and cardiovascular protection, inhibition of carcinogenesis, lowering of body fat and increase in lean body mass, and promotion of physical and psychological well-being.3,16 In fact, DHEA as a supplement first gained popularity for its proposed health and anti-aging properties and was touted as the “fountain of youth.”
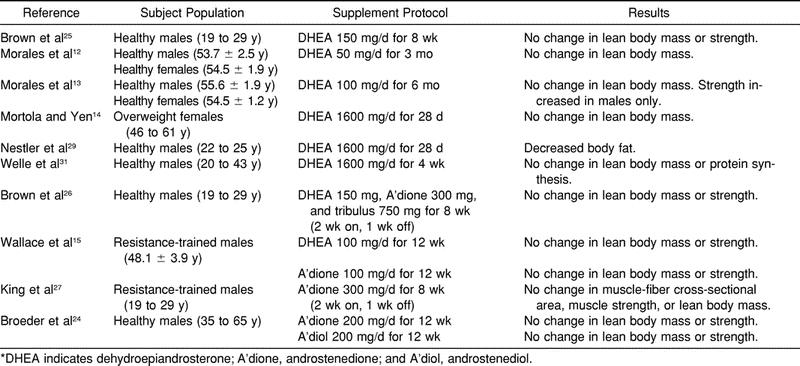
When DHEA was administered (50 mg/d for 3 months) to older men and women (age = 40 to 70 years), DHEA levels were restored to levels found in young adults.13 The women in that study also experienced a 2-fold increase in A'dione and testosterone, while men experienced only a small increase in A'dione. Similar observations were made when larger dosages (100 mg/d) were administered to the same age group over a 6-month period.12 In that study, both sexes experienced increases in DHEA. However, the A'dione and testosterone levels in women reached levels above sex-specific young adult ranges, while no changes were observed in men. Similarly, no changes in testosterone, strength, or lean body mass were observed in a group of men (age = 40 to 60 years) when supplementation with either DHEA or A'dione was combined with resistance training.15 Thus, it appears that only women experience increases in testosterone after precursor supplementation. Finally, Mortola and Yen14 observed 15-, 20-, and 9-fold increases in DHEA, A'dione, and testosterone, respectively, in postmenopausal women (age = 46 to 61 years) after DHEA supplementation (1600 mg/d) for 28 days. Significant increases in estrone and estradiol were also observed. The changes in hormone levels were not associated with any change in lean body mass; however, the subjects did not perform any type of physical training. It is important to note that significant decreases in high-density lipoprotein (HDL) cholesterol were observed in 2 of the previously mentioned studies.13,14
Ergogenic Claims
Increased levels of testosterone have been shown to increase protein synthesis, muscle strength, and lean body mass.17–20 Thus, the role of DHEA and A'dione as testosterone precursors has sparked interest in their potential as ergogenic aids. Results from earlier studies involving an older population have led to the marketing of these products as ergogenic or anabolic supplements capable of increasing testosterone levels and, consequently, lean body mass, strength, and overall athletic performance. As described, the increases in testosterone had only been observed in older women; however, these products are marketed toward young athletic men.
One of the initial studies cited in support of precursor supplementation was published by Mahesh and Greenblatt21 in 1962. The subjects in that study consisted of 4 women (ages were not reported), 2 of whom ingested DHEA while the other 2 ingested A'dione. Although the authors did not perform a statistical analysis, they concluded that ingestion of these supplements increased testosterone levels, with A'dione causing the greater increase. Obviously, with such a small sample, the results are not reliable. It is important to note that women are essentially testosterone deficient when compared with their male counterparts and, as mentioned previously, about 50% of their testosterone is derived from peripheral precursor conversion. A second study commonly cited in support of these supplements involved ovariectomized rats.22 After ovariectomy, significant reductions in plasma A'dione, testosterone, and estradiol were observed. These levels were regained in a dose-responsive manner when the rats were fed A'dione. These are just 2 examples of what has become common practice among supplement manufacturers, as results from deficiency studies and animal studies (and animal deficiency studies) are generalized to a young, healthy, and athletic population.
Research Involving Young Men
When young, healthy men were recruited as subjects, results similar to those previously observed in older women have not been reproduced.23–30 Research published since 1999 has focused on 2 areas: the acute effects of a single dose and the chronic effects of daily ingestion. A summary of the supplementation protocols and results is found in Table 1.
Table 1. Effects of Precursor Supplementation on Hormone Levels*

Acute Hormonal Changes
When investigating the acute hormonal response to DHEA ingestion (50 mg) in young men (age = 19 to 29 years), researchers observed significant elevations in serum A'dione, while no changes in testosterone occurred.25 Similar observations have been made after A'dione ingestion.6,23,27,28,30 These elevations occurred after ingestion of 100,27,28,30 200,6,23 and 300 mg.28 However, only 200-mg and 300-mg doses have been shown to significantly increase serum testosterone levels.6,28 Testosterone levels in a group of young men ingesting 300 mg were significantly greater than those ingesting 100 mg and those in a control group, while no differences were observed between the 100-mg and control groups.28 It was suggested that a significant portion of the ingested A'dione is reduced and conjugated by the liver before it can reach peripheral testosterone-converting tissues. Thus, only higher dosages were able to achieve increases in testosterone. Similarly, a significant increase in testosterone was observed in young men after a 200-mg dose.6 However, significance was only noted when the area under the curve was used as the measure, as no differences were observed when comparing the individual time points (0, 30, 60, and 90 minutes) used to establish the area under the curve.
It must be noted that acute increases in estradiol have also been observed in young men after A'dione ingestion.28,30 Following higher-dose A'dione ingestion (300 mg), a 128% increase in estradiol was observed, while only a 34% increase in testosterone was observed.28 Although lesser doses (100 mg) have failed to raise testosterone levels, they have been observed to increase estradiol levels.28,30 Of 29 subjects ingesting A'dione, 22 experienced estradiol levels above the upper limit of normal for men after ingestion.28 It was suggested by the authors that the local tissue levels of estradiol may have been even greater than those measured in the plasma because aromatase, the enzyme that converts A'dione and testosterone to estrone and estradiol, is found in many human tissues, including muscle and fat.
Chronic Hormonal Changes
When DHEA and A'dione were administered daily to young men using protocols ranging from 2 days to 8 weeks, no changes in resting (before ingestion) testosterone, regardless of dose, have been observed.23,25–30 In a recent study, Rasmussen et al30 showed that 100 mg of A'dione per day for 5 days failed to increase testosterone levels. However, plasma A'dione did increase more than 3-fold. Similarly, 300 mg/d (100-mg doses) for 8 weeks failed to increase testosterone levels, while A'dione levels were elevated by 100%.27 Even a 300-mg daily dose, which did provide an acute increase in testosterone, failed to increase baseline testosterone after 7 days of ingestion.28
Similar to the acute changes after A'dione ingestion, significant increases in estradiol have been observed in young men after chronic ingestion.23,26,27 Although testosterone levels did not change, 300 mg/d produced significant increases in both estrone and estradiol levels.27 Even when precursors were combined with herbal extracts designed to reduce the conversion of androgens to estrogens, significant increases in estrone and estradiol were observed.26
Because of concerns regarding the increases in estrogens observed in men after DHEA and A'dione ingestion, there has been an increase in the number of products containing A'diol. Like A'dione, A'diol is converted from DHEA and is an immediate precursor to testosterone. Unlike A'dione, A'diol cannot be directly converted to estrogen, as it must first be converted to testosterone, which can then be converted to estradiol. However, as in the previous investigations, a single 200-mg dose of A'diol failed to increase serum testosterone levels in young men.6 Also, supplementation with A'diol (200 mg/d) for 12 weeks failed to produce changes in testosterone levels.24 This protocol did produce a significant increase in serum estrone and estradiol concentrations, suggesting that the ingested A'diol is converted to testosterone, which is immediately aromatized to estradiol.
Protein Synthesis
As mentioned previously, ergogenic claims are based on the theory that precursor ingestion will result in increased testosterone levels, which would then stimulate an increase in muscle protein synthesis. However, at this time, there is no scientific support for this theory, as both DHEA31 and A'dione30 ingestion have failed to increase protein synthesis in groups of young men. Furthermore, 8 weeks of A'dione supplementation (300 mg/d) and resistance training failed to increase muscle-fiber cross-sectional area when compared with placebo ingestion and training.27
Androstenedione is a steroid possessing 10% to 20% of the androgenic activity relative to testosterone. Thus, the conversion of A'dione to testosterone may not be necessary to have an anabolic effect. However, this theory also lacks support, as A'dione ingestion failed to affect muscle protein synthesis or muscle-fiber cross-sectional area, even though significant increases in serum A'dione were observed.27,30
Lean Body Mass and Strength
If precursor supplementation elevated testosterone levels, increases in muscle strength and lean body mass, such as those observed after testosterone administration, would be expected. A summary of the performance studies involving precursors and the results is found in Table 2. Nestler et al29 were the first to report anthropometric changes in young men (age = 20 to 25 years) after supplementation, as a significant decrease in body fat was observed after DHEA ingestion (1600 mg/d) for 28 days.29 Because overall body mass remained constant while fat mass decreased, the authors concluded that supplementation resulted in increased lean body mass. However, strength measures were not performed, and diet and physical activity were not controlled or recorded. Thus, the authors' conclusions must be questioned.
Table 2. The Effects of Precursor Supplementation on Performance*
Other than the previously mentioned study, the ergogenic claims regarding precursor supplementation have not been supported. For example, 4 weeks of DHEA supplementation failed to increase lean body mass in a group of young, healthy men.31 Testosterone levels were not measured in that study; thus, whether increases occurred or not is unknown. However, the same supplementation protocol also failed to affect lean body mass in a group of postmenopausal women.14 Although no training or performance testing occurred, a significant increase in testosterone was observed in that group. It is possible that 4 weeks of ingestion were insufficient to observe remarkable body-composition changes, although changes have been observed after just 4 weeks of testosterone administration.19 Yet in a recent study, 8 weeks of A'dione ingestion combined with resistance training also failed to increase muscle strength and lean body mass in a group of young men (age range = 19 to 29 years).27 Although both acute and long-term administration (100 mg/d) failed to increase testosterone levels, A'dione levels did increase. Again, no changes in strength or lean body mass were observed in a similar age group after 8 weeks of DHEA ingestion and resistance training.25
Longer supplementation and training protocols have also failed to support ergogenic claims. Twelve weeks of DHEA and A'dione supplementation combined with resistance training were ineffective in increasing lean body mass and strength in an older group of men (age range = 40 to 60 years).15 Similarly, supplementation with A'diol (200 mg/d) for 12 weeks also failed to produce changes in muscle strength and lean body mass.24 Previous studies have shown that 12 weeks of training provides sufficient time for remarkable training effects to occur.17,20 Thus, the ergogenic claims must be questioned. The failure to increase strength or lean body mass further suggests that exogenous DHEA and A'dione must first be converted to testosterone to achieve an anabolic effect.
Side Effects
Significant reductions in serum HDL cholesterol of 12% and 20% have been observed after A'dione and DHEA supplementation, respectively.14,24,27 Similar changes have been observed after AAS injection and have been associated with the development of cardiovascular disease.32,33 Broeder et al24 administered either A'dione or A'diol (200 mg/d) and observed that both adversely affected HDL-cholesterol levels, low-density lipoprotein (LDL)-to-HDL cholesterol ratios, and coronary heart disease risk. Thus, it is possible that long-term supplementation could have serious side effects similar to those associated with AAS use, such as suppressed testosterone production, liver dysfunction, cardiovascular disease, testicular atrophy, male-pattern baldness, acne, and aggressive behavior.34 If the supplements are taken before puberty, premature closing of the epiphysis and stunted growth could occur. In women, precursor-induced increases in testosterone concentrations could cause lowered voice pitch, hirsutism (changes in hair growth patterns, including facial hair), increased abdominal fat accumulation, and general virilization.34 Furthermore, increases in estrogen concentrations experienced by men could have feminizing effects, including gynecomastia.10
CONCLUSIONS
Although DHEA, A'dione, and A'diol are in fact steroids, they are not classified as anabolic steroids or controlled substances by either the Food and Drug Administration or the Drug Enforcement Agency. The Anabolic Steroid Control Act of 1990 defines an anabolic steroid as any drug or hormonal substance chemically and pharmacologically related to testosterone, other than estrogens, progestins, and corticosteroids, that promotes muscle growth. Under that act, testosterone and anabolic steroids are classified as Schedule III drugs and cannot be sold over the counter or possessed without a prescription. Although DHEA, A'dione, and A'diol are structurally and pharmacologically related to testosterone, they have not been proven to promote muscle growth and are, therefore, not classified as Schedule III drugs. Furthermore, the Dietary Supplement Health and Education Act of 1994 has allowed these products to be sold legally over the counter as natural supplements in the United States.
Unfortunately, the term “natural supplement” implies that it is safe; however, this is not always true. Over-the-counter availability and unrestrained self-medication with steroid precursors create a heightened potential for serious side effects, and the safety of these products must be questioned, as there are no human studies in the medical literature on their long-term safety. Unfortunately, most companies that manufacture and sell nutritional supplements are profit driven and are often misleading with their advertising. One of the primary concerns is that manufacturers are not required to list the ingredients on the labels of natural supplements; thus, the consumer does not always know the true contents of the product. In an analysis of 9 brands of A'dione, 6 contained less than 90% of the amount stated on the label, 1 contained no A'dione, and 1 was actually found to contain 10 mg of testosterone.35 Furthermore, in the same study, 20 of 24 men ingesting A'dione (100 or 300 mg/d) would have tested positive for the banned steroid nandrolone based on levels of 19-norandrosterone (a metabolite of nandrolone) found in the urine. Thus, these men were ingesting a supplement and urinating a drug.
Whenever an athlete is considering using steroid precursors or any ergogenic supplement, 3 questions must be asked: is it safe, does it work, and is it legal? At this time, scientific support for the ergogenic or anabolic use of steroid precursors does not exist. However, numerous studies provide evidence of the possible dangers associated with them, including increased risk of heart disease and increased estrogen concentrations in men. Thus, it appears that the risks far outweigh the benefits. It is also important to note that the International Olympic Committee, the National Collegiate Athletic Association, and the National Football League have banned the use of steroid precursors, and the use of these supplements can be detected in drug tests. The key to performance is a healthy diet and a well-developed training program; there is no “quick fix” or “shortcut to success.” As allied health professionals, it is important that athletic trainers are able to educate athletes regarding the efficacy and safety of nutritional supplements, so they may continue to perform at an optimal level in a safe and healthy manner.
REFERENCES
- Yesalis C E. Anabolic Steroids in Sport and Exercise. 2nd ed Human Kinetics; Champaign, IL: 2000. [Google Scholar]
- Collins R. Anabolic steroids, bodybuilding, and the law: testosterone, androgens, muscle growth, and performance hormones. Available at http://www.steroidlaw.com Accessed January 18, 2002.
- Baulieu E E. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150(Suppl):125. [PubMed] [Google Scholar]
- Miller W L, Chrousos G P. The adrenal cortex. In: Felig P, Frohman L A, editors. Endocrinology and Metabolism. 4th ed. McGraw-Hill; New York, NY: 2001. pp. 387–437. [Google Scholar]
- Earnest C P, Olson M A, Broeder C E, Breuel K F, Beckham S G. In vivo 4-androstene-3,17-dione and 4-androstene-3beta,17beta-diol supplementation in young men. Eur J Appl Physiol. 2000;81:229–232. doi: 10.1007/s004210050035. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin S, et al. The key role of 17β-hydroxysteroid dehydrogenase in sex steroid biochemistry. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- Kretser D M, Risbridger G P, Kerr J B. Functional morphology. In: DeGroot L J, Jameson J L, editors. Endocrinology. 4th ed. WB Saunders; Philadelphia, PA: 1995. pp. 2209–2231. [Google Scholar]
- Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48:2191–2201. doi: 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz A G, Wilson J D. Endocrine disorders of the breast. In: Wilson J D, editor. Williams Textbook of Endocrinology. 9th ed. WB Saunders; Philadelphia, PA: 1985. pp. 402–416. [Google Scholar]
- Orentreich N, Brind J L, Vogelman J H, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Morales A J, Haubrich R H, Hwang J Y, Asakura H, Yen S S. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- Morales A J, Nolan J J, Nelson J C, Yen S S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- Mortola J F, Yen S S. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990;71:696–704. doi: 10.1210/jcem-71-3-696. [DOI] [PubMed] [Google Scholar]
- Wallace M B, Lim J, Cutler A, Bucci L. Effects of dehydroepiandrosterone vs androstenedione supplementation in man. Med Sci Sports Exerc. 1999;31:1788–1792. doi: 10.1097/00005768-199912000-00014. [DOI] [PubMed] [Google Scholar]
- Dehennin L, Ferry M, Lafarge P, Peres G, Lafarge J P. Oral administration of dehydroepiandrosterone to healthy men: alteration of the urinary androgen profile and consequences for the detection of abuse in sport by gas chromatography-mass spectrometry. Steroids. 1998;63:80–87. doi: 10.1016/s0039-128x(97)00138-4. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer T W, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. New Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Ferrando A A, Tipton K D, Doyle D, Phillips S M, Cortiella J, Wolfe R R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275(5 Pt 1):E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- Urban R J, Bodenburg Y H, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269(5 Pt 1):E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Welle S, Jozefowicz R, Forbes G, Griggs R C. Effect of testosterone on metabolic rate and body composition in normal men and men with muscular dystrophy. J Clin Endocrinol Metab. 1992;74:332–335. doi: 10.1210/jcem.74.2.1730811. [DOI] [PubMed] [Google Scholar]
- Mahesh V B, Greenblatt R B. The in vivo conversion of dehydroepiandrosterone and androstenedione to testosterone in the human. Acta Endocrinol. 1962;41:400–406. [Google Scholar]
- Lea C K, Moxham V, Reed M J, Flanagan A M. Androstenedione treatment reduces loss of cancellous bone volume in ovariectomised rats in a dose-responsive manner and the effect is not mediated by oestrogen. J Endocrinol. 1998;156:331–339. doi: 10.1677/joe.0.1560331. [DOI] [PubMed] [Google Scholar]
- Ballantyne C S, Phillips S M, MacDonald J R, Tarnopolsky M A, MacDougall J D. The acute effects of androstenedione supplementation in healthy young males. Can J Appl Physiol. 2000;25:68–78. doi: 10.1139/h00-005. [DOI] [PubMed] [Google Scholar]
- Broeder C E, Quindry J, Brittingham K, et al. The Andro Project: physiological and hormonal influences of androstenedione supplementation in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000;160:3093–3104. doi: 10.1001/archinte.160.20.3093. [DOI] [PubMed] [Google Scholar]
- Brown G A, Vukovich M D, Sharp R L, Reifenrath T A, Parsons K A, King D S. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol. 1999;87:2274–2283. doi: 10.1152/jappl.1999.87.6.2274. [DOI] [PubMed] [Google Scholar]
- Brown G A, Vukovich M D, Reifenrath T A, et al. Effects of anabolic precursors on serum testosterone concentrations and adaptations to resistance training in young men. Int J Sport Nutr Exerc Metab. 2000;10:340–359. doi: 10.1123/ijsnem.10.3.340. [DOI] [PubMed] [Google Scholar]
- King D S, Sharp R L, Vukovich M D, et al. Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men a randomized controlled trial. JAMA. 1999;281:2020–2028. doi: 10.1001/jama.281.21.2020. [DOI] [PubMed] [Google Scholar]
- Leder B Z, Longcope C, Catlin D H, Ahrens B, Schoenfeld D A, Finkelstein J S. Oral androstenedione administration and serum testosterone concentrations in young men. JAMA. 2000;283:779–782. doi: 10.1001/jama.283.6.779. [DOI] [PubMed] [Google Scholar]
- Nestler J E, Barlascini C O, Clore J N, Blackard W G. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988;66:57–61. doi: 10.1210/jcem-66-1-57. [DOI] [PubMed] [Google Scholar]
- Rasmussen B B, Volpi E, Gore D C, Wolfe R R. Androstenedione does not stimulate muscle protein anabolism in young healthy men. J Clin Endocrinol Metab. 2000;85:55–59. doi: 10.1210/jcem.85.1.6322. [DOI] [PubMed] [Google Scholar]
- Welle S, Jozefowicz R, Statt M. Failure of dehydroepiandrosterone to influence energy and protein metabolism in humans. J Clin Endocrinol Metab. 1990;71:1259–1264. doi: 10.1210/jcem-71-5-1259. [DOI] [PubMed] [Google Scholar]
- Kuipers H, Wijnen J AG, Hartgens F, Willems S M. Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders. Int J Sports Med. 1991;12:413–418. doi: 10.1055/s-2007-1024704. [DOI] [PubMed] [Google Scholar]
- Lenders J W, Demacker P N, Vos J A. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, and liver function in amateur body builders. Int J Sports Med. 1988;9:19–23. doi: 10.1055/s-2007-1024972. [DOI] [PubMed] [Google Scholar]
- Medical and nonmedical uses of anabolic-androgenic steroids: AMA Council on Scientific Affairs. JAMA. 1990;264:2923–2927. [PubMed] [Google Scholar]
- Catlin D H, Leder B Z, Ahrens B, et al. Trace contamination of over-the-counter androstenedione and positive urine test results for a nandrolone metabolite. JAMA. 2000;284:2618–2621. doi: 10.1001/jama.284.20.2618. [DOI] [PubMed] [Google Scholar]


