Abstract
In animals, including humans, the source of long-chain saturated fatty acids is de novo synthesis, which is mediated by fatty acid synthase (FAS), ingested food, or both. To understand the importance of de novo fatty acid synthesis, we generated FAS knockout mice. The heterozygous FAS mutants (Fasn+/–) are ostensibly normal. In Fasn+/– mice the levels of FAS mRNA and the FAS activity are ≈50% and 35% lower, respectively, than those of WT mice; hence, FAS levels are affected by gene dosage. When the Fasn+/– mutant mice were interbred, Fasn–/– mice were not produced; thus, FAS is essential during embryonic development. Furthermore, the number of Fasn+/– progeny obtained was 70% less than predicted by Mendelian inheritance, indicating partial haploid insufficiency. Even when one of the parents was WT, the estimated loss of heterozygous progeny was 60%. This loss of Fasn+/– pups appeared to be strain-specific and became more pronounced as the heterozygous females produced more litters. Most of the Fasn–/– mutant embryos died before implantation and the Fasn+/– embryos died at various stages of their development. Feeding the breeders a diet rich in saturated fatty acids did not prevent the loss of homoor heterozygotes. These observations are very important in considering teratogenic consequences of drugs aimed at inhibiting FAS activity, to reduce either obesity or the growth of cancerous tissues.
Fatty acid synthase (FAS; EC 2.3.1.85) of animal tissues is a homodimer and the monomer is a multifunctional protein containing seven catalytic domains and a site for the prosthetic group, 4′-phosphopantetheine. The FAS complex catalyzes the synthesis of the saturated fatty acids myristate, palmitate, and stearate by using the substrates acetyl-CoA, malonyl-CoA, and NADPH (1–4). FAS is highly expressed in tissues like liver, adipose, and lactating mammary glands (5). It is noteworthy that nearly every tissue in the human body has some level of FAS expression (5). FAS plays an important role in energy homeostasis by converting excess food intake into lipids for storage and providing energy when needed via β-oxidation. It is required for the generation of milk lipids during lactation. Besides being the apolar constituent of various membrane lipids required for membrane biogenesis and its functions, the products of FAS myristate (C14) and palmitate (C16) are involved in the myristoylation and palmitoylation of cellular and viral proteins for membrane targeting. The products of FAS, palmitate and stearate (C18), also serve as substrates for chain elongation to produce very-long-chain fatty acids. The latter fatty acids are important constituents of sphingolipids, ceramides, and glycolipids that are needed for cell division progression and brain structures and neurological functions (6). Further, increased FAS levels in cancer tissues indicate a poor prognosis (7–9). However, saturated fatty acids synthesized by FAS are readily available from food sources. Hence, the importance and contribution of the de novo fatty acid synthesis catalyzed by FAS during embryonic development and the general well-being of animals is not known.
To determine the importance of de novo fatty acid synthesis we generated mice with a mutation in the FAS gene (Fasn+/–). When Fasn+/– heterozygotes were interbred, Fasn–/– null mutant mice died at the preimplantation stage. In addition, ≈70% of the heterozygotes died in utero, suggesting haploid insufficiency (i.e., one functional FAS gene is unable to support embryonic development efficiently). Herein we describe these studies.
Materials and Methods
Generation of the Fasn Knockout Construct. A λ-FIX II mouse embryonic stem (ES) cell genomic library (Stratagene) was screened with mouse FAS cDNA probes generated by using the mouse cDNA sequence (10) to isolate a λ-genomic clone that contained the 5′ end sequences of Fasn. The Fasn-targeting vector was constructed as follows. The 3.8-kb BamHI fragment from the 5′ end of this clone was cloned at the BamHI site present at the 3′ end of the hypoxanthine phosphoribosyltransferase (HPRT) mini gene in pPGK-hprtmini-179 vector (Fig. 1A). The 2.2-kb downstream BamHI fragment was blunt-end cloned at the EcoRV site on the 5′ end of the HPRT mini gene. Based on the sequence comparison with rat Fasn (11, 12), the 2-kb BamHI fragment, which is replaced by HPRT minigene in the Fasn knockout construct (Fig. 1 A), contains exons 11–18 and part of intron 18, and codes for the sequences of acetyl and malonyl-transacylases and dehydratase domains (4, 5, 11–13). Whereas HPRT is used for positive selection for both homologous and nonhomologous events, the negative selection for nonhomologous recombination was achieved by cloning the TK gene outside the targeting sequences (14).
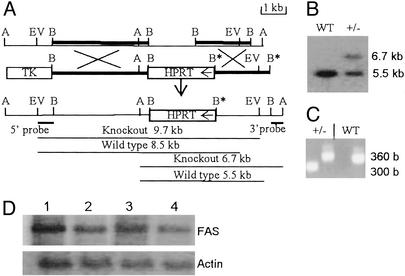
Fig. 1.
Targeted mutation of the Fasn locus and analysis of Fasn knockout mice. (A) Restriction map of the 5′ half of the Fasn gene. The restriction sites shown are: A, Asp718; B, BamHI; EV, EcoRV. The BamHI fragments used in the generation of the disruption construct (line 2) are indicated in line 1 as dark lines. The arrow in the HPRT gene box indicates its direction of transcription. The 5′ and 3′ probes used and the sizes of the restriction fragments they hybridize to identify WT and targeted alleles by Southern blot analysis are indicated. (B) Southern blot analysis of genomic DNAs digested with Asp718 and probed with the 32P-labeled 3′ probe described in A. (C) A typical PCR analysis of the genotypes. Two sets of primers were used to amplify 360-bp WT and 300-bp knockout allele products as described in Materials and Methods. (D) A typical Northern blot analysis of RNA isolated from livers of mice that were either starved for 48 h and refed the fat-free diet for 48 h (lanes 1 and 3) or fed the normal diet (lanes 2 and 4). Total liver RNA from WT (lanes 1 and 2) and heterozygous (lanes 3 and 4) mice were analyzed on agarose gel and the blots were probed with a 32P-labeled mouse FAS cDNA probe. Actin mRNA levels were used to normalize FAS mRNA levels.
Generation of Mice Carrying a Fasntm1sc Allele. AB2.2 ES cells (129/SvEv) were electroporated with the linearized targeting construct (Fig. 1) and were cultured as described (15, 16). For genotyping ES-cell clones, the DNAs were digested with either EcoRV or Asp-718, fractionated on agarose gels, and subjected to Southern blot analysis by using 32P-labeled 5′ and 3′ probes, respectively. The sizes of the expected Fasn fragments are shown in Fig. 1. To obtain chimeric mice, the ES-cell clones that contained a HPRT-disrupted Fasn allele were injected into mouse blastocysts (14–16). Among the pups produced, four high-percentage male chimeras were identified and bred with C57BL/J6 females to generate Fasntm1sc/+ (herein called Fasn+/–) C57/129 hybrid mice or with 129/SvEv females to produce 129-inbred mice. The Fasn+/– hybrid F1 progeny were either interbred or bred with corresponding WT C57 mice or 129 mice. Genotyping of mice was performed by using tail DNAs by Southern blot analysis as described above. For PCR-based genotyping, the primers used were a forward primer for the disrupted allele designed from the complementary sequence of the PGK promoter of the HPRT minigene (GCGCATGCTCCAGACTGC) or a forward WT allele-specific primer (CTCTGCAGGAACCGTGGCGCTG) from the 2-kb BamHI fragment that was deleted in the disrupted allele, and a common reverse primer (GCCCACCTTCAAGGGTGGCCTC) from the 3′ BamHI fragment used in the generation of the disruption construct (Fig. 1). Two separate PCRs were performed, one for WT allele and the other for disrupted allele, using 30 cycles of amplification, with each cycle containing incubations at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, after an initial 2-min, 94°C denaturation. These primers amplify 300- and 360-bp products that are specific to knockout allele and WT allele, respectively.
Miscellaneous Procedures. Routine procedures such as Southern blot analysis (17), measurement of FAS activity (3), SDS/PAGE, and Western blot analysis (13) were performed as described. Total RNA from livers was isolated by using Triazol (Invitrogen) and Northern blotting, and analyses were performed by using the NorthernMax kit (Ambion, Austin, TX) and mouse FAS cDNA probes described above. Whole-mount in situ hybridization with digoxigenin-labeled antisense mouse FAS riboprobes was performed on fixed, WT mouse embryos according to the method of Conlon and Rossant (18). The Anatomical Pathology Department of the Methodist Hospital, Houston, performed the immunohistochemistry of embryo sections.
Results
Generation and Characterization of Fasn Knockout Mice. The Fasn targeting construct that will replace acetyl and malonyl transacylases and dehydratase coding regions of FAS with HPRT (Fig. 1 A) and the generation of the chimeric male mice, Fasn+/– C57/129 hybrid F1 mice, and 129-inbred mice are described in Materials and Methods. The F1 Fasn+/– C57/129 mice were interbred and the progeny genotyped. Because we could not observe any Fasn–/– homozygous mutants, we conclude that de novo fatty acid synthesis by FAS is essential for embryonic development (see below for further analysis of genotypes of F2 progeny). To determine the effect of loss of one of the Fasn genes on FAS expression in heterozygote mice, we analyzed the levels of FAS mRNA, FAS activity, and FAS protein in their livers. Because the levels of FAS expression were very low when the animals were fed a normal diet (Purina 5010) with 9% of the calories coming from fat, WT mice and Fasn+/– heterozygotes were starved for 2 days and refed a fat-free diet (ICN) for 2 days to induce FAS expression in livers. Under these conditions, as determined by densitometric scanning of autoradiographs, the levels of FAS mRNA (Fig. 1D) of the heterozygote mice were found to be 50 ± 10% of those of WT littermates (n = 5). Correspondingly, the specific activity of FAS in the cell-free liver extracts of the heterozygotes was 4.27 ± 0.39 nmol/min/mg, compared with 6.19 ± 0.54 nmol/min/mg for WT FAS, or ≈31% lower FAS activity in the heterozygous mice (P > 0.002). There was no significant difference in the specific activity of FAS between males and females. Both WT mice and heterozygous mice had a very low FAS-specific activity of 1.1 ± 0.3 nmol/min/mg when fed the normal diet. Consistent with these results, the estimated levels of FAS protein as measured by Western blot analysis were also ≈40% lower in Fasn+/– mice than in WT mice (data not shown). Hence, the lower levels of FAS mRNA, FAS activity, and FAS protein in the livers of the heterozygous mice when fed a fat-free diet, as compared with that of WT mice, reflect a gene dosage effect.
Breeding Fasn+/– F1 Hybrid Heterozygote Males and Females and Genotype Analyses of the Progeny. Several breeding cages were set up, each consisting of one Fasn+/– male and two Fasn+/– females, and the resulting F2 progeny were genotyped. Surprisingly, the average litter size of the progeny produced in these matings is only 3.8 pups, compared with an expected normal litter size of 8–10 pups (Table 1). The results of genotype analysis (Table 1) showed that no homozygous FAS null mutant mice were produced, indicating embryonic lethality. Based on the Mendelian inheritance of Fasn+ and Fasn– alleles, the number of Fasn+/– mice obtained from these crosses was ≈70% less than the expected number (Table 1). Even though the litter size is low, the average and the total number of WT progeny produced from these crosses were within the expected range (two to three pups per litter). Hence, the overall small litter size is due to loss of all of the null mutants and the majority of the heterozygotes. Thus, the loss of heterozygote embryos containing only one functional FAS gene can be attributed to Fasn haploid insufficiency.
Table 1. Summary of the genotypes of the progeny produced in the crossing mice with at least one FAS heterozygote parent.
| No. of
|
||||||
|---|---|---|---|---|---|---|
| Cross | Litters | Pups | Average litter size | WT:+/-:-/- | Expected WT pups* | Loss of +/- pups, %† |
| Crosses involving C57BL/6J/129/SvEv hybrid Fasn+/- F1 progeny | ||||||
| +/- ♂ × +/- ♀ | 160 | 600 | 3.8 | 373:227:0 | 320-400 | 70 |
| Ratios observed | 1:0.6:0 | |||||
| +/- ♂ × C57/129 ♀ | 41 | 278 | 6.8 | 196:82 | 164-205 | 60 |
| Ratios observed | 1:0.4 | |||||
| C57/129 ♂ × +/- ♀ | 21 | 138 | 6.6 | 96:42 | 84-105 | 60 |
| Ratios observed | 1:0.4 | |||||
| Crosses involving 129 Fasn+/- F1 progeny | ||||||
| +/- ♂ × 129 WT ♀ | 34 | 138 | 4.1 | 107:31 | 102-136 | 70 |
| Ratios observed | 1:0.3 | |||||
| 129 WT ♂ × +/- ♀ | 10 | 38 | 3.8 | 34:4 | 30-40 | 90 |
| Ratios observed | 1:0.1 | |||||
| 129 +/- ♂ × 129 +/- ♀ | 4‡ | 13 | 3.3 | 7:6 | 6-8 | 60 |
| Ratios observed | 1:0.9 | |||||
The genotype of the progeny was determined as described in Materials and Methods and Fig. 1. Because no FAS null mutants were obtained, the expected ratio of Fasn+/- mutants to WT would be 2:1 when both the parents are Fasn+/- or 1:1 when one of the parents is Fasn+/- and the other WT.
The expected number of WT mice is based on Mendelian inheritance of Fasn alleles, the total number of litters produced, and the expected litter size of 8-10 in the first set of crosses or 6-8 in the second set.
Because the WT progeny were within the expected range, the loss of heterozygotes was calculated according to the Mendelian inheritance ratios.
The number of litters produced in six breeding cages in 18 months.
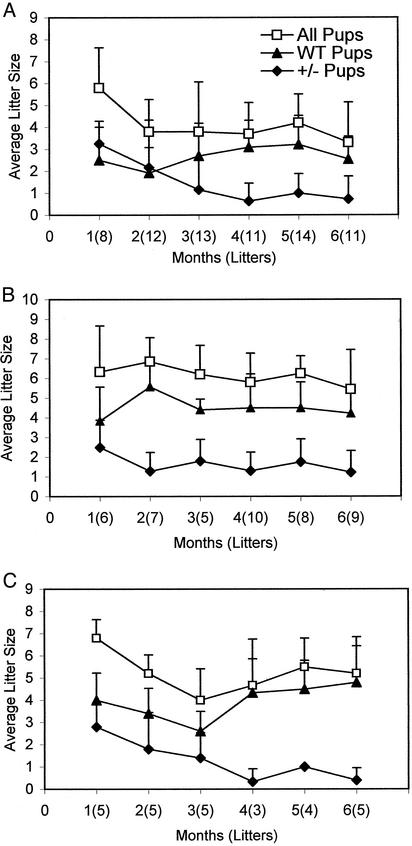
To further understand the relationship between the haploid insufficiency and fecundity, the F2 progeny data of eight cages of heterozygote parents, which were breeding for 6 months, were analyzed. During that period, the number of litters produced varied from 8 to 14, and the first litters tended to be larger litters, averaging about six pups per litter (Fig. 2A). In these first litters, the ratio of the average number of heterozygote (3.3 ± 1) to WT (2.5 ± 1.5) progeny indicates a loss of ≈33% of the heterozygotes, based on the expected ratio of one WT for every two heterozygotes. During the following 5 months, the litter size averaged between three and four pups. Interestingly, these F1 heterozygote parents produced progressively fewer heterozygous pups in their subsequent litters, and in the sixth month the ratio of heterozygous pups (0.7 ± 1) to WT (2.5 ± 1) indicated a loss of ≈80% of the heterozygous pups (Fig. 2 A). However, the average number of two to three WT mice produced per litter during the 6-month period remained within the range expected from the litter size of 8–10 mice (Fig. 2 A). In addition, after a year the breeders tended to produce litters less often and those litters rarely contained heterozygotes (data not shown). These analyses indicate that the heterozygote mice lose their ability to produce heterozygote pups as they produce more litters but do not lose their ability to produce WT pups. This disability could be due to aging, a progressive loss of Fasn– gamete production by either the females or males, or the inability of heterozygote females to support the fatty acid-related nutritional requirements of heterozygote embryos. These issues are addressed below.
Fig. 2.
Analysis of the breeding behavior of F1 Fasn heterozygotes. F1 heterozygotes were either interbred or bred with WT C57/129 hybrid mice, and the number of litters produced and their genotypes were determined. (A) Analysis of litters produced when heterozygous males and females were interbred. (B) Heterozygous F1 males were bred with WT C57/129 females. (C) WT males were bred with F1 hybrid heterozygous females. Only the positive values of the SD of the mean are indicated to reduce crowding of the plot. Genotyping was performed as described in Fig. 1 and Table 1.
The progressive absence of heterozygous mice in litters of F1 parents did not appear to be related to the age of the mice for the following reasons. Because chimeras and several breeders were lost in recent floods in Houston, we were forced to set up several breeding cages that contained 9- to 12-month-old males and females. All of these aged breeders produced litters that included both heterozygotes and WT pups in their first litters, similar to the data shown in Fig. 2 A.
To determine whether the loss of heterozygote F2 progeny in F1 heterozygote matings described above (Table 1 and Fig. 2 A) could be due to defective Fasn– gametes of males or females, or both, because they produce more litters, we set up six cages containing heterozygote males and WT C57/129 females and three cages containing C57/129 WT males and heterozygote females. As reported in Table 1, the litter size increased to about seven compared with about four pups obtained in the crosses with only heterozygote breeders. Also, in these crosses the number of heterozygous mice obtained was 60% less than expected. Analysis of the litters showed that, even in these crosses, as the parents produced more litters there was a progressive decrease in the average litter size and number of heterozygous pups per litter (Fig. 2 B and C). WT litter sizes were relatively stable (Fig. 2 B and C), as observed in crosses of heterozygote parents (Fig. 2 A). However, as the parents produced more litters, the loss of heterozygous pups became more pronounced, especially when the Fasn+/– females were bred with WT males (Fig. 2C). These results suggested that partial haploid insufficiency might be due in part to progressive loss in the Fasn+/– female's ability to produce Fasn– gametes. Because WT progeny were produced at the expected frequency (Table 1, Fig. 2), the prenatal loss of homozygotes, as well as heterozygotes, may also be due to the inability of Fasn mutant embryos to produce desired amounts of fatty acids de novo during their development in the uterus.
It is important to note that the studies described above (Table 1, Fig. 2) were conducted with C57BL/129 F1 hybrids, which were not pure breeders. Because the ES cells that were used to generate Fasn knockout mice were originally from 129/SvEv mice, we generated 129-strain Fasn knockout mice. Unexpectedly, when 129-Fasn+/– F1 heterozygote mice were interbred, no pups were produced for 5 months. However, in two of the eight cages set up, we observed two litters in each: one at 6 months and the other at ≈10 months. The number of heterozygotes in these litters was 10% less than that of WT, representing a loss of 60% of the heterozygotes. In the next 8 months, no litters were observed in these cages (Table 1). When 129-Fasn+/– heterozygote males were bred with WT 129 females, only ≈40% of the expected number of heterozygous pups were produced (Table 1). Surprisingly, when WT 129 males were mated with 129-Fasn+/– females, the loss of the heterozygous pups was ≈90% (Table 1); although, in these matings, the average litter size is about four, the WT litter size is within the range expected of six to eight pups per litter (Table 1). These observations suggested that the Fasn haploid insufficiency becomes very severe in the 129 strain. Even though both heterozygote parents seem to contribute to the haploid insufficiency, female Fasn+/– parents may contribute even more to the partial haploid insufficiency of developing embryos (Table 1).
Analyses of the Genotypes of Embryos and Their Characterization. To determine at what stages of development the homozygote (Fasn–/–) and heterozygote (Fasn+/–) mutant embryos are lost because of FAS insufficiencies during development, timed matings were performed by using C57/129 F2 Fasn+/– males and females. At various embryonic days, the embryos were resected from the uterus and genotyped. The results are summarized in Table 2. We have not observed any Fasn–/– null mutants in any of the implanted embryonic stages. Hence, most of the FAS null mutant embryos seem to die before embryonic day (E) 3.5 (blastocysts). However, based on PCR genotyping, we found two Fasn–/– E3.5 embryos that had different phenotypes. One of them became affixed to the culture dish during the 24-h incubation in ES-cell medium and the other looked normal. Although the ratios of implanted heterozygotes to WT embryos varied, with no corresponding relationship to their developmental stages, it is important to note that the ratio of 1:1.2 for the total number of implanted WT embryos to Fasn+/– embryos (Table 2) is very similar to that of the firstborn litters observed in F1 hybrid matings (Fig. 2 A). In addition, the ratio of the number of WT embryos to the sum of the numbers of implanted heterozygous embryos and resorbed embryos is ≈1:2 (Table 2), which is consistent with Mendelian inheritance of Fasn– and Fasn+ alleles, suggesting that most of the resorbed embryos were Fasn+/– embryos.
Table 2. Analyses of the embryos and the genotypes of the intact embryos at various stages of their development.
| Genotypes
|
|||||
|---|---|---|---|---|---|
| Embryonic stage | Litter size | Resorbed embryos | WT | +/- | -/- |
| E14.5 | 9 | 3 | 2 | 4 | 0 |
| E13.5 | 8 | 3 | 2 | 3 | 0 |
| E12.5 | 9 | 0 | 5 | 4 | 0 |
| E10.5 | 10 | 3 | 3 | 4 | 0 |
| E9.5 | 10 | 2 | 3 | 5 | 0 |
| E8.5 | 9 | 0 | 5 | 4 | 0 |
| 10 | 5 | 3 | 2 | 0 | |
| 3 | 0 | 0 | 3 | 0 | |
| E7.5 | 6 | 0 | 3 | 3 | 0 |
| E6.5* | 10 | 2 without embryos | |||
| 1 resorbed, 1 retarded | |||||
| E5.5* | 8 | 1 abnormal | |||
| 9 | 3 without embryos | ||||
| E3.5† | 23 | 5 | 16 | 2 | |
The C57/129 Fasn+/- males and females were mated, and the day females had vaginal plugs was noted as E0.5. Uteri were removed from the females at various embryonic days after cervical dislocation. The embryos were separated from their yolk sacs and fixed in paraformaldehyde. The yolk sac DNA was used for genotyping by Southern blot analysis. Resorbed embryos could not be genotyped.
The E6.5 and E5.5 embryos were not genotyped, and the litter size for the total embryos refers to decidua. The decidua-containing regions were fixed in paraformaldehyde, and the microscopic observations of the sectioned decidua are described.
Embryos at E3.5 were collected from five mated females and genotyped by PCR followed by Southern blotting analysis with radioactive probes as described in Materials and Methods. The total implanted embryos from E7.5 to E14.5 were: resorbed, 16; +/-, 32; and WT, 26.
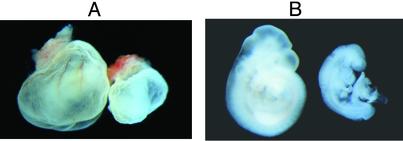
In these analyses we found one E9.5 growth-retarded Fasn+/– embryo that did not complete embryonic turning (Fig. 3). Although embryonic turning is normally finished when the embryo develops 14–16 somites, this abnormal embryo contained 17 somites. The deciduum of the abnormal embryo was smaller and hemorrhagic compared with those of the normal-appearing littermates. Unlike the WT embryos, which had well formed blood vessels on their yolk sacs, the abnormal embryo's yolk sac lacked blood vessels and was pale (Fig. 3A). Additionally, the heart of the abnormal embryo was swollen (Fig. 3B). Although the shape of the allantois was normal, there were several hemorrhagic blocks close to the chorion, suggesting possible defects in the establishment of allantois–chorion circulation. Because we found only one defective nonresorbed Fasn+/– embryo between E7.5 and E14.5, we do not know how this phenotype relates to haploid insufficiency.
Fig. 3.
Photographs of E9.5 embryos. (A) Embryos shown are in their yolk sacs. A WT embryo is on the left and a defective hemorrhagic Fasn+/– embryo is on the right. (B) The embryos dissected from their yolk sacs. Note that the Fasn+/– defective embryo on the right was growth retarded and did not finish turning. (Magnification: × 100.)
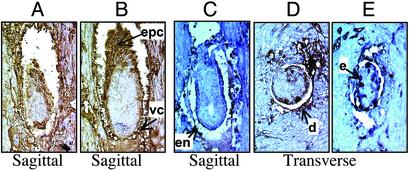
Analyses of FAS Expression Patterns During Embryonic Development. To ascertain the expression pattern of FAS during periimplantation stages, the uteri from pregnant females were collected as above (Table 2). The embryo-containing regions were fixed in Bouin's fixative and sectioned through the decidua. Out of 10 E6.5 embryos examined from a female, two decidua were without embryos, one resorbed, and one embryo was found to be growth-retarded. The sections of the growth-retarded embryo and a normal-looking embryo were immunostained by using anti-FAS antibodies. In both cases, immunostaining for FAS revealed that the endometrium (not shown) and the decidual cells immediately surrounding the embryo, ectoplacental cone, and visceral endoderm have high expression of FAS and may be very active in fatty acid synthesis (Fig. 4 A and B). Although we could not distinguish WT and heterozygote embryos by immunostaining with FAS antibodies, the growth-retarded embryo in Fig. 4A could be a heterozygote. At E5.5 postcoitum, one female contained nine decidua, three of which were devoid of embryos and six of which appeared normal. Another female examined contained one abnormal embryo and seven apparently normal embryos. Compared with the apparently normal embryos (Fig. 4 C and D), this abnormal embryo (Fig. 4E) appeared to be growth-retarded. Because immunostaining with anti-FAS antibodies was positive, this defective embryo is not a null mutant and probably is a heterozygote. Based on the observation of the apparently normal embryos at both stages, the endometrium of the uterus, the decidual cells immediately surrounding the embryo, and the embryonic and extraembryonic endoderm appear to be the sites of intense fatty acid synthesis at these stages of embryonic development.
Fig. 4.
Immunological determination of FAS expression in developing embryos with anti-FAS antibodies. Timed matings were performed and the uteri were resected at E6.5 (A and B) and E5.5 (C–E) postcoitus. The uteri were fixed in Bouin's solution and sectioned through the decidua-containing regions. The sections were treated with anti-FAS goat antibodies, and the immune complexes were detected immunohistochemically with peroxidase-conjugated secondary antibodies (dark brown regions). (A) Small, growth-retarded E6.5 embryo. (B) Normal-looking E6.5 embryo. (C and D) Sagittal and transverse sections of normal-looking E5.5 embryos. (E) Growth-retarded E5.5 embryo. (Magnification: ×400.) Arrows indicate some of the regions expressing high FAS levels. epc, ectoplacental cone; vc, visceral endoderm; en, endoderm; d, decidual cells; e, ectoderm.
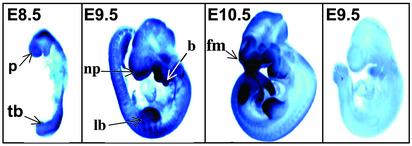
To further understand the expression of FAS during development, we assessed the levels of FAS mRNA expression at later postimplantation stages in WT embryos. Whole-mount in situ hybridization was performed to detect FAS mRNA expression in E7.5–E10.5 embryos, using digoxigenin-labeled antisense FAS mRNA probes. The results indicated that the FAS gene was broadly expressed in the mouse embryo, but the regions of strong FAS mRNA expression varied in the developmental stages examined. Low-level labeling was seen in embryonic and extraembryonic ectoderm as early as E7.5 (data not shown). By E8.5 (Fig. 5), strong expression was seen in the open neural folds at both the anterior and posterior regions of the body. The most anterior neuroectoderm of the prosencephalon and dorsal ridge of the closing neural tube had particularly strong labeling. The epidermis, the most distal region of the branchial arches, and the somites were also well labeled. In contrast, the heart and closed regions of the neural tube in the central portion of the body were relatively unlabeled or poorly labeled. By E9.5, the neural tube was completely closed and the limb buds had appeared (Fig. 5). Labeling was apparent throughout the epidermis. The strongest labeling was seen in the distal epidermis of the limb bud, including the apical ectodermal ridge, an important signaling center for limb outgrowth, and its underlying mesenchyme. The distal portions of the branchial arches and frontonasal mass were similarly strongly labeled. These are all sites of progressive outgrowth and epithelial/mesenchymal interactions during this period of development. Consistent with the earlier stages, the closed neural tube was not strongly labeled, but by E10.5 (Fig. 5), differential labeling was detectable in the forebrain and midbrain neuroepithelium. Robust and sharply defined expression was visualized in the distal edges of the nasal pit, branchial arches, and limb and tail buds.
Fig. 5.
Analysis of FAS mRNA expression in developing embryos. WT embryos of E8.5, E9.5, and E10.5 were probed with antisense FAS mRNA probes. The rightmost E9.5 embryo was hybridized with labeled sense transcripts showing only background labeling. (Magnification: ×100.) Arrows indicate some of the regions expressing high levels of FAS mRNA. p, proencephalon; tb, tail bud; np, nasal pit; b, branchial arches; lb, limb bud; fm, frontonasal mass.
The robust FAS expression pattern in developing tissues, at these later embryonic stages, suggests that the Fasn+/– embryos may not be able to meet the expression levels demanded during their development, because of haploid insufficiency. However, these observations suggest that the sites of the most intense FAS expression coincide with regions known to be undergoing tissue–tissue interactions, tissue outgrowth, and progressive modeling/remodeling. There are also many common features in the molecular pathways used in these areas [e.g., signaling molecules (BMPs, Wnts, Fgfs, etc.) and transcription factors (Dlx and Msx homeobox genes)], which may point to commonalities in fatty acid synthetic requirements.
Role of Dietary Saturated Fatty Acids in Fertility and Fecundity in Mice. In the experiments described above, mice were fed a standard diet (Purina 5010 containing 9% fat). Dietary fat can be a source of long-chain fatty acids (C14, C16, and C18), which are also the products of FAS reactions. To discern the importance of dietary saturated fatty acids in the maternal ability to support developing Fasn+/– embryos, we set up three breeding cages with heterozygotes and provided them with a completely fat-free diet (ICN). In the first month these breeders produced three litters, which contained 5 heterozygote and 10 WT pups. However, these parents produced no litters in the next 5 months. By comparison, in three cages with WT breeders that were fed the same fat-free diet, we observed 11 litters in 3 months with an average litter size of 6 ± 1.2, which is less than the expected litter size of about eight for WT mice fed a normal diet. On this diet, WT mice also did not produce any pups during the following 3 months, and the breeders were losing weight. In addition, three of the litters died before weaning, suggesting that a diet completely free of fat was suboptimal for growth and did not provide the nutritional requirements for the females to support the growing pups. These results suggest that when the heterozygote Fasn+/– mice were deprived of dietary fat, they became incapable of synthesizing enough fatty acids de novo to produce offspring.
In an attempt to compensate for the impaired de novo fatty acid synthesis by the heterozygotes, we set up three breeding cages as described above and provided them with a diet rich in saturated fatty acids (D12451, Research Diets, New Brunswick, NJ). These breeders produced 22 litters containing 102 pups in 8 months, with an average litter size of 4.7 ± 1.5, which is a relative improvement over the 3.8 pups per litter obtained with comparable parents fed a normal diet (Table 1). The parents became fatty during the course of this investigation and stopped breeding after ≈8 months. These litters contained no Fasn–/– null mutants but contained 46 heterozygotes and 56 WT mice, with an estimated loss of ≈59% of heterozygote pups. Although this is a slight improvement compared with the estimated loss of 70% of the heterozygote pups when the heterozygote parents were fed standard diet (Table 1), the loss of heterozygote pups appears to be mainly due to the haploid insufficiency of the growing embryos.
Discussion
In summarizing the results described above, the Fasn–/– mutant embryos die in their early stages of embryonic development. In addition, Fasn+/– embryos die at various stages of their development. Consequently, the number of Fasn+/– mice born is 60–90% lower than that expected based on Mendelian inheritance. However, the number of WT mice produced was always within the range expected based on Mendelian inheritance of the Fasn– and Fasn+ alleles (Table 1). Hence, we concluded that FAS plays an essential role in embryonic development and that one functional Fasn+ allele in heterozygotes is unable to support the demands of de novo fatty acid synthesis during this development, thus creating haploid insufficiency. As shown in Figs. 4 and 5, there is an intense increase in FAS protein and its mRNA levels in embryonic and extraembryonic tissues. In addition, when the adult animals were induced to synthesize fat in the liver from carbohydrates by feeding a fat-free diet, the heterozygous mice contained significantly less FAS mRNA, FAS protein, and its activity, demonstrating the importance of Fasn gene dosage dependence. Hence, the lack of one functional Fasn allele in heterozygous embryos may lead to their inability to meet the demands of the threshold level of fatty acid synthesis required for their development. We do not know how this threshold is met in the ≈30% of heterozygote embryos that develop normally (Table 1). As the heterozygote mice were bred over time, they produced fewer and fewer heterozygous pups (Fig. 2). However, this loss of ability to produce heterozygotes is not age-related, as discussed above. Female heterozygotes produced fewer surviving heterozygous pups when bred to WT males than the reciprocal cross (Fig. 2C). It is possible that Fasn+/– females may lose their ability to produce Fasn– gametes as they produce more litters. It is more probable that the Fasn+/– uterine environment becomes less able to support Fasn+/– embryos. Consistent with this is the high expression of FAS in the endometrium of the uterus and the decidual cells surrounding the implantation site. The penetrance of haploid insufficiency is also strain-specific, as indicated by a failure to produce pups when both parents were 129-derived Fasn+/– mice. The observation that some heterozygote pups survive when the heterozygote parents are of mixed background (C57/129) suggests that other genes modify the penetrance or expressivity of the phenotypes during embryonic development.
Feeding heterozygous mice a diet containing high levels of saturated fatty acids (high-fat diet) did not alleviate the problem of haploid insufficiency, indicating that fatty acids derived from dietary fat do not provide the required fatty acids for embryonic development. Therefore, the generation of fatty acids in situ through FAS is required during development. It has been reported that inhibition of FAS with cerulenin leads to increased levels of malonyl-CoA, one of the substrates of FAS that is produced by acetyl-CoA carboxylase, which causes cytotoxicity (9). This cytotoxicity can be relieved by inhibition of acetyl-CoA carboxylase with 5-(tetradecyloxy)-2-furoic acid (9). However, the transport of fatty acids through placenta is limited and de novo synthesis of fatty acids in embryos through maternal carbohydrate supplements is essential (19, 20). Hence, haploid insufficiency of Fasn+/– embryos is a direct consequence of their inability to produce desired amounts of required fatty acids. Because dietary fat cannot substitute for de novo fatty acid synthesis during embryonic development, it is necessary to generate tissue-specific knockout mice.
Acknowledgments
We thank Rukmini Chirala for immunohistochemical staining of embryo sections, Parichher Kordari for help with genotyping, Zi-Wei Gu for FAS assays, Drs. Simona Varani and Lei Chen for their help with ES cells and blastocyst microinjection, respectively, and Chaomai Liu for in situ hybridizations. This work was supported in part by a Clayton Foundation grant and National Institutes of Health Grant GM-63115.
Abbreviations: En, embryonic day n; ES, embryonic stem; FAS, fatty acid synthase; HPRT, hypoxanthine phosphoribosyltransferase.
References
- 1.Wakil, S. J. (1989) Biochemistry 28, 4523–4530. [DOI] [PubMed] [Google Scholar]
- 2.Smith, S. (1994) FASEB J. 8, 1248–1259. [PubMed] [Google Scholar]
- 3.Stoops, J. K. & Wakil, S. J. (1981) J. Biol. Chem. 256, 5128–5133. [PubMed] [Google Scholar]
- 4.Chirala, S. S., Jayakumar, A., Gu, Z.-W. & Wakil, S. J. (2001) Proc. Natl. Acad. Sci. USA 98, 3104–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayakumar, A., Tai, M.-H., Huang, W.-Y., Al-Feel, W., Hsu, M., Abu-Elheiga, L., Chiral, S. S. & Wakil, S. J. (1995) Proc. Natl. Acad. Sci. USA 92, 8695–8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannun, Y. A. & Obeid, L. M. (2002) J. Biol. Chem. 277, 25847–23850. [DOI] [PubMed] [Google Scholar]
- 7.Kuhajda, F. P., Jenner, K., Wood, F. D., Hennigar, R. A., Jacobs, L. B., Dick, J. D. & Pasternack, G. R. (1994) Proc. Natl. Acad. Sci. USA 91, 6379–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Towsand, C. A., Ronnett, G. V., Lane, M. D. & Kuhajda, F. P. (2000) Science 288, 2379–2381. [DOI] [PubMed] [Google Scholar]
- 9.Pizer, E. S., Thupari, J., Han, W. F., Pinn, M. L., Cherst, F. J., Frehywot, G. L., Townsend, C. A. & Kuhajda, F. P. (2000) Cancer Res. 60, 213–238. [PubMed] [Google Scholar]
- 10.Paulakis, J. D. & Sul, H. S. (1989) Biochem. Biophys. Res. Commun. 158, 690–695. [DOI] [PubMed] [Google Scholar]
- 11.Amy, C. M., Williams-Ahlf, B., Naggert, J. & Smith, S. (1992) Proc. Natl. Acad. Sci. USA. 89, 1105–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck, K. F., Schreglmann, R., Stathopulos, I., Klein, H., Hoch, J. & Schweizer, M. (1992) DNA Seq. 2, 359–386. [DOI] [PubMed] [Google Scholar]
- 13.Chirala, S. S., Huang, W.-Y., Jayakumar, A., Sakai, K. & Wakil, S. J. (1997) Proc. Natl. Acad. Sci. USA 94, 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley, A. (1987) in Production and Analysis of Chimeric Mice, ed. Robinson, E. J. (IRL, Oxford), pp. 113–151.
- 15.Chang, H., Huylebroeck, D., Verschueren, K., Guo, Q., Matzuk, M. M. & Zwijsen, A. (1999) Development (Cambridge, U.K.) 126, 1631–1642. [DOI] [PubMed] [Google Scholar]
- 16.Matzuk, M. M., Finegold, M. J., Su, J. G., Hsueh, A. J. & Bradley, A. (1992) Nature 360, 313–319. [DOI] [PubMed] [Google Scholar]
- 17.Maniatis, T., Fritsch, E. F. & Sambrook, J. (1982) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 18.Conlon, R. A. & Rossant, J. (1992) Development (Cambridge, U.K.) 116, 357–368. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, J. (1965) in Handbook of Physiology, eds. Renold, A. E. & Cahill, G. F., Jr. (Am. Physiol. Soc., Washington, DC), pp. 181–189.
- 20.Hirsch, J. (2002) Proc. Natl. Acad. Sci. USA 99, 9096–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]