Abstract
YOR068c, termed VAM10 (altered vacuole morphology), lies within the VPS5 gene on the opposite DNA strand. VAM10 deletion causes vacuole fragmentation in vivo. The in vitro fusion of purified yeast vacuoles is stimulated by recombinant Vam10p and blocked by antibody to Vam10p. Vam10p acts early in the priming stage of fusion, independent of Sec18p. After priming, recombinant Vam10p will not stimulate fusion and anti-Vam10p antibodies will not inhibit; Vam10p provides a functional marker for this Sec18p-independent priming step. Pure Vam10p restores normal, Ypt7p-dependent tethering to vacuoles from a vam10Δ strain.
Membrane fusion is regulated and catalyzed by similar factors for each organelle and organism (1). These include Rab/Ypt and Rho family GTPases, their nucleotide exchange and GTPase-activating proteins, other tethering proteins and effectors, soluble N-ethylmaleimide-sensitive fusion proteins (SNAREs), SNARE-associated proteins such as the Munc18/Sec1 family, chaperones, phosphoinositides, sterols, actin, calcium, and calcium-binding proteins. The complexity of regulated trafficking and membrane fusion suggests that additional factors remain to be discovered.
We study membrane fusion mechanisms with yeast vacuoles (2). Homotypic vacuole fusion occurs in three stages: (i) priming, which precedes vacuole association, (ii) tethering and docking, in which vacuoles form clusters, and (iii) fusion, the mixing of the bilayers and luminal contents. Sec18p ATPase drives Sec17p release during priming (3), disassembling the cis–SNARE complex. In addition to Sec18p action, lipid synthesis (4) and acylation of Vac8p (5) occur during priming. Docking occurs in three subreactions: tethering, vertex ring assembly, and trans-SNARE pairing. Tethering is mediated by the Ypt7p GTPase (6). Ypt7p is enriched at a “vertex ring” microdomain that surrounds the apposed membranes of tethered vacuoles (7). Conversion of Ypt7p to its GTP-bound form and remodeling of vacuolar actin allow the SNAREs, the HOPS/VPS class C effector complex, and actin to assemble into the vertex ring (7, 8). The assembled ring allows trans-SNARE pairing. Fusion is initiated by a flux of calcium from the vacuole lumen (9).
A genomic approach has identified genes involved in vacuole fusion (10). We screened a commercially available yeast library of nonessential gene deletions for their vacuole morphology; deletion of a vacuole fusion gene causes fragmented vacuoles. Among the strains with abnormal vacuole morphology (vam phenotype; ref. 11) were 28 ORFs encoding proteins of unknown function.
We now report that one ORF, YOR068c (VAM10), catalyzes an early and novel stage of the priming subreaction, a stage that can proceed independently of Sec18p and ATP-mediated disassembly of the cis–SNARE complex. Vam10p acts during priming to permit vacuole tethering.
Methods
Yeast Strains. Strains BJ3505 and DKY6281 have been described (12). Deletions of yor068c, ydr136w, and vps5 in BY4742 were purchased from Research Genetics (Huntsville, AL). Deletions of pep4 or pho8 were made in vam10 (yor068c)Δ as described (9). Yor068p and Ybr174p were overexpressed in yeast strain Y258, fused to GST-HisX6 at their NH2 termini, and expressed from GAL1 (ref. 13; a gift from M. Snyder, Yale University, New Haven, CT).
Proteins. Proteins tagged with C-terminal maltose binding protein (MBP) were expressed in Escherichia coli BL21(DE3) (Novagen) with pMAL-c2x (New England Biolabs) containing in-frame EcoRI–XbaI (vam10) or EcoRI–BamHI (ydr136c) PCR products. MBP-tagged full-length Yor068p and truncated Ydr136p (61 aa; from “FALPR” to “PPHGE”) were purified by amylose affinity chromatography (New England Biolabs). Whole-cell lysates were prepared as described (14). IgG was prepared by Sephacryl S-200 HR gel filtration (Pharmacia) after isolation on protein A Sepharose CL-4B (Pharmacia). Vam10p antibody was affinity-purified on peptide crosslinked to Affi-Gel 10 (Bio-Rad).
Vacuole Fusion. Standard reactions contained 3 μg of each of frozen vacuoles from BJ3505 and DKY6281 in 30–35 μl of reaction buffer (10 mM Pipes-KOH, pH 6.8/200 mM sorbitol/125 mM NH4Cl/5 mM MgCl2/10 μM CoA/8 μg/ml IB2/1 mM Mg-ATP/40 mM creatine phosphate/0.5 mg/ml creatine kinase/6.6 ng/ml leupeptin/16.6 ng/ml pepstatin/16.6 μM o-phenanthroline/3.3 μM Pefabloc SC). After 90 min at 27°C, alkaline phosphatase was assayed (12).
Yeast Expression of Vam10p and Vps5p. The pRS316 vector, this vector bearing VPS5 and VAM10 (nucleotides 453367–456210) or bearing this fragment with a T-to-A mutation at residue 454213, abolishing VAM10 expression by changing its initiator Met codon to a Lys codon while not changing the amino acyl residues encoded on the opposite strand (for VPS5), was transformed into wild-type, vam10Δ, or vps5Δ yeast. The latter deletion is the 3′ end of the VPS5 gene, shown in the unshaded box in Fig. 3A. To do this, the VPS5 gene with its native promoter and terminator (and with a point mutation in the start codon of VAM10, where indicated) was amplified by PCR, using CCCAAGCTTTCGCGGCAGTCTTC and CCGCTCGAGGTTACACCAACAAAAGA with HindIII and XhoI sites. The point mutation was made by the QuikChange site-directed mutagenesis kit (Stratagene), using CCA A ATACTTCAAAAAGCTTCTCTGGAAGAAGATCCGG and CCGGATCTTCTTCCAGAGAAGCTTTTTGAAGTATTTGG. The plasmid pRS316 (New England Biolabs) without insert or bearing the PCR product encoding both VPS5 and VAM10 or these genes with T454213A was transformed into BY4742, BY4742 bearing deletion of vam10 (and the corresponding 5′ region of the vps5 genes), and BY4742 bearing deletion of nucleotides 454858–455796, the 3′ region of vps5. Transformants were selected on SD-ura (Bio 101) and screened for integration by RT-PCR, using ATGGACTACGAGGATAATCT (5′-VPS5), CTAAAGATTGGTTTGGTAGAA (3′-VPS5), AAGTATTTGGTGAAGTGTTAG (5′-VAN10), and AATATCTACAAATGAAGAAAGAA (3′-VAM10), and by the presence or absence of a HindIII site (AAGCTT).
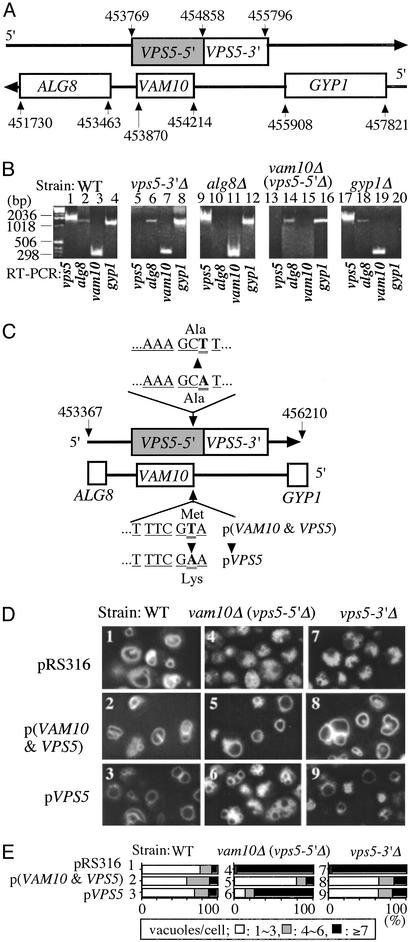
Fig. 3.
Relationship of VPS5 and VAM10. (A) Map of the ALG8, VAM10, VPS5, GYP1 region. The VPS5 gene encompasses 2,028 bp (shaded plus open boxes in the figure), from 453769 to 455796 (19, 20), although only residues 454858–455796 were removed in the vps5Δ strain. The VAM10 gene lies within the VPS5 gene on the opposite strand. (B) Levels of mRNA from ALG8, VAM10, VPS5, and GYP1 genes in BY4742 and BY4742 mutants bearing deletions, which were assayed by RT-PCR by using the oligonucleotides listed in C for VPS5 and VAM10 and GGCTCCATTGTCGCCG (5′-ALG8), TCACTGCCATAGTAATTCGT (3′-ALG8), TACACAGGCCTGTTGTTTG (5′-GYP1), and TTACAGCCAGTGCGACGT (3′-GYP1). RNA preparation used RNase-free DNase I and TRIzol (Invitrogen). Samples also were analyzed without the RT step to confirm the RNA origins of the RT-PCR products (data not shown). (C) Plasmids for gene expression were prepared as described in Methods. (D) Vacuole morphology was examined in WT, vam10Δ (vps5-5′Δ), and vps5-3′Δ strains bearing the control plasmid (pRS316), p(VAM10 and VPS5), or pVPS5 prepared as in B above, which were grown in 0.5 ml of SD-ura medium in 15-ml tubes at 30°C. After 16 h, 1 ml of YPD with 5 μM FM4–64 was added, incubation was continued for 4 h, and vacuole morphology was examined. Pictures of multiple fields were taken for each condition. (E) For each of the strains shown in D, the percentage that have one to three, four to six, or more than seven vacuoles was scored for 120–160 cells.
Results
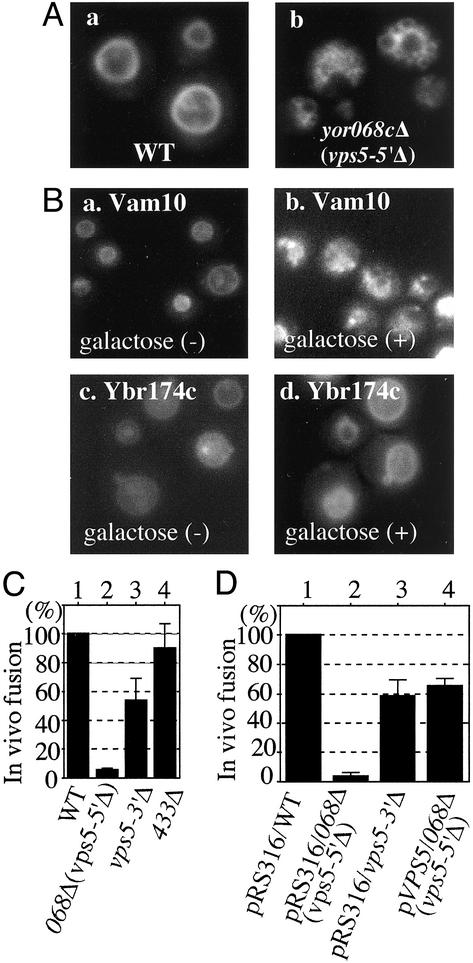
Vacuoles are fragmented when YOR068c is deleted (Fig. 1A; ref. 10) or overexpressed from a plasmid under GAL regulation (Fig. 1B). Vacuoles normally fuse on exposure of cells to low osmolarity (16); these in vivo fusion events were counted during a 30-sec window 3 min after cells were transferred to hypoosmolar medium. Deletion of YOR068c prevented osmotic stress-induced vacuole fusion in vivo (Fig. 1C, lane 2).
Fig. 1.
Vacuole fusion in vivo. (A) Wild-type (WT) strain BY4742 (a) and yor068cΔ (b) were grown in 0.2 ml of yeast extract/peptone/dextrose (YPD) in 15-ml culture tubes at 30°C. After 16 h, 0.4 ml of YPD with 5 μM FM4–64 (15) was added, incubation was continued for 4 h at 30°C, and cells were photographed (7). (B) Vacuole morphology before and after induction of Vam10p (a and b) and Ybr174p (c and d) by galactose. (C) Osmotic stress-induced vacuole fusion. WT strain BY4742 (lane 1) and yor068Δ (lane 2), vps5-3′Δ (lane 3), and ydr433wΔ strains were grown in 0.5 ml of YPD in 15-ml culture tubes at 30°C. After 16 h, 1 ml of YPD with 5 μM FM4–64 was added and incubation was continued at 30°C until OD600 = 5 (4–5 h). Samples (400 μl) were centrifuged (6,000 × g for 30 sec) and cells were resuspended in 350 μl of water. After 3 min at 23°C, in vivo vacuole fusion events were counted for 4 min in eight fields (30 sec per field). The average and SD of five experiments are presented. (D) Vector pRS316-transformed WT strain BY4742 (lane 1) and yor068cΔ (lane 2), vps5Δ (lane 3), and pVPS5-transformed yor068cΔ (lane 4) strains were grown in 0.5 ml of SD-ura medium and analyzed as above.
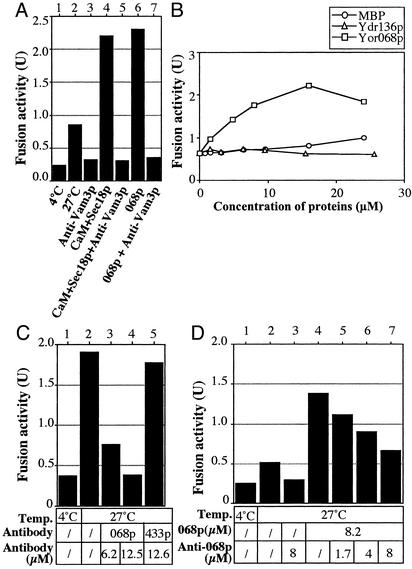
In accord with these in vivo studies, the YOR068c-encoded protein (068p), fused to MBP, stimulates in vitro vacuole fusion. To assay fusion, we isolate vacuoles from two strains, one with normal proteases but lacking the PHO8 phosphatase, the other with a normal PHO8 gene but lacking luminal proteases and, thus, with catalytically inactive proPho8p. On fusion, proPho8p is cleaved to the mature active enzyme (12). Stimulated fusion (Fig. 2A, lane 2 vs. 6) was blocked by antibody to Vam3p (lane 7), showing that it is on the normal fusion pathway. MBP or other protein fusions with MBP did not stimulate (Fig. 2B). Other pure proteins, such as Sec18p and calmodulin, caused similar stimulation of fusion (Fig. 2 A, lane 4; also see ref. 17), but combinations of these proteins with YOR068p did not enhance fusion (data not shown). Antibody to Vam10p (or Fab; data not shown) inhibited the fusion of purified vacuoles (Fig. 2C, lanes 2–4). Inhibition was dose dependent and was reversed by the addition of recombinant Vam10p (Fig. 2D). Further studies also point to a direct role for YOR068c in vacuole fusion, and, therefore, we name this protein Vam10p (11).
Fig. 2.
YOR068p promotes vacuole fusion. (A) Fusion reactions with vacuoles from BJ3505 and DKY6281 were performed with anti-Vam3p antibody (1 μM), calmodulin (CaM; 20 μM), his6-Sec18p (0.1 μM), or MBP-tagged Vam10p (Vam10p; 8 μM) as indicated. (B) Fusion reactions contained the indicated concentrations of MBP, Yor068p, or Ydr136p, each with an MBP tag. (C) Fusion reactions bore control buffer (lanes 1 and 2) or the indicated concentrations of anti-Vam10p (lanes 3 and 4) or anti-Ydr433p antibody. These IgG antibodies were purified as described (18). (D) Standard fusion reactions contained PS buffer (10 mm Pipes-KOH, pH 6.8/200 mm sorbitol; lanes 1 and 2) or the indicated concentrations of anti-Vam10p antibody (IgG fraction; lanes 3 and 5–7) and/or Vam10p (lanes 4–7).
The VAM10 gene is in a cluster of membrane-trafficking genes (Fig. 3A). It lies within VPS5, encoded on the opposite DNA strand. Genetic studies distinguish the functions of VAM10 and VPS5. Deletion of the VAM10 gene causes a large deletion in the 5′ domain of VPS5, which we term vps5-5′Δ, whereas deletion from 454858 to 455796 in the 3′ domain of VPS5 (unshaded VPS5-3′ box; Fig. 3A) leaves the VAM10 gene intact. Yeast with each of these deletions, as well as the isogenic wild type, was purchased. RT-PCR analysis shows that each deletion ablates the corresponding transcript (Fig. 3B). Deletion of VAM10 also abolishes the production of full-length VSP5 transcript (Fig. 3B, lane 13), whereas deletion of the 3′ half of VPS5 allows VAM10 transcription (lane 7). Plasmid p(VAM10 and VPS5) was prepared (Fig. 3C) that expresses both the normal VAM10 and VPS5 genes. A derivative plasmid with a T454213A mutation abolishes the start codon for VAM10 but leaves the normal amino acid sequence of Vps5p (Fig. 3C); this plasmid is termed pVPS5. Control vector, the vector expressing Vam10p and Vps5p, and the vector expressing Vps5p alone were transformed into wild-type BY4742 or into this strain deleted for VAM10 (and, hence, the 5′ domain of VPS5) or for the 3′ domain of VPS5. Vacuole morphology was examined in each of these strains (Fig. 3D). Whereas BY4742 wild-type yeast have one to three vacuoles in most cells (Fig. 3D, 1), vam10Δ (vps5-5′Δ) or vps5-3′Δ causes fragmentation (Fig. 3D, 4 and 7). Coexpression of Vps5p and Vam10p from p(VAM10 and VPS5) cures the vacuole fragmentation in vam10Δ (vps5-5′Δ) or vps5-3′Δ backgrounds (Fig. 3 D5 and D8). However, expression of Vps5p alone cures the vps5-3′Δ phenotype (Fig. 3D9) but does not cure the vam10Δ (vps5-5′Δ) phenotype (Fig. 3D6), establishing a distinct role for Vam10p in vacuole morphology. Quantitation of vacuole number in cells from multiple microscopy fields (Fig. 3E) confirms these visual impressions. Thus, Vam10p and Vps5p each are needed for normal vacuole morphology.
With these strains, we returned to the in vivo assay of osmolarity-induced fusion to distinguish the roles of Vam10p and Vps5p. Vacuole fusion (Fig. 1D, lane 1) was abolished by the deletion of VAM10 and, hence, truncation of VPS5 (lane 2). Intermediate fusion rates were seen when only one of the VPS5 or VAM10 genes was expressed (lanes 3 and 4). Thus, both VAM10 and VPS5 are required for normal vacuole fusion. Further studies may determine whether Vps5p has a direct role in vacuole fusion or only governs vacuole morphology through its role in biosynthetic protein delivery to the vacuole.
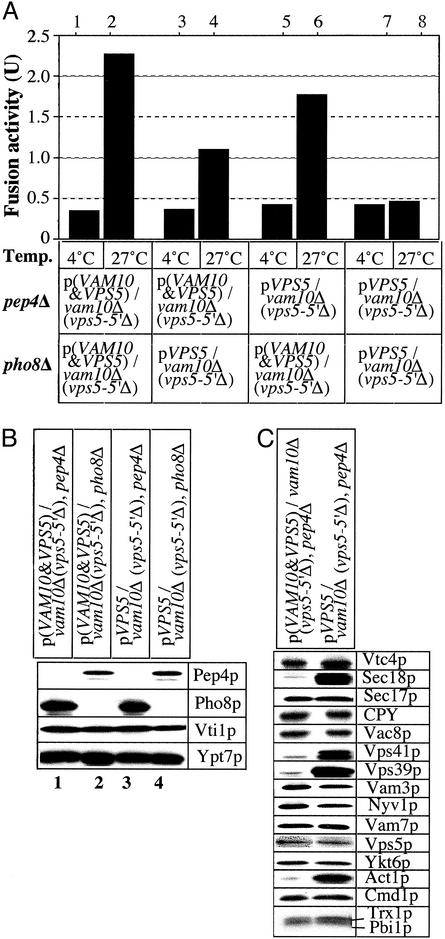
Vam10p is needed on at least one vacuole in a fusion pair (Fig. 4A). Our in vitro fusion assay was performed with vacuoles from pep4Δ and pho8Δ strains that were vam10Δ (vps5-5′Δ), with expression of Vps5p, or both Vps5p and Vam10p, from plasmids. The absence of Vam10p from even one of the two vacuoles inhibited fusion (lanes 4 and 6), but fusion was abolished by the complete absence of Vam10p (lane 8). This was not due to a deficiency in Pep4p or Pho8p (Fig. 4B). Many other proteins known to be required for fusion also were present at normal levels in the absence of Vam10p (Fig. 4C). Carboxypeptidase Y was present at normal levels in vacuoles from cells lacking Vam10p, showing that VAM10 is not a VPS gene. Strikingly, Sec18p, the HOPS subunits Vps39p and Vps41p, and actin were enriched on vacuoles lacking Vam10p (Fig. 4C). Each of these proteins is involved in early stages of the reaction, suggesting that Vam10p may function early in the fusion pathway.
Fig. 4.
Fusion activity and protein composition of vacuoles from vam10Δ cells. (A) Standard fusion assays were performed with vacuoles from VAM10, pep4Δ; VAM10, pho8-Δ; vam10-Δ, pep4-Δ; and vam10-Δ, pho8-Δ strains. (B and C) Vacuoles (6 μg) from each indicated strain were analyzed by immunoblotting.
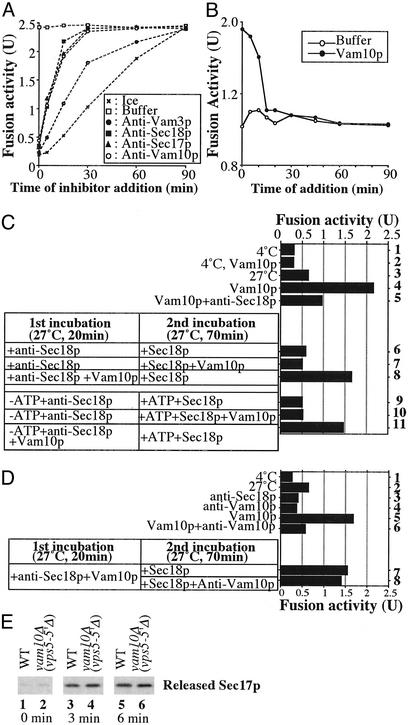
To determine where Vam10p acts, we analyzed the kinetics with which vacuoles acquired resistance to antibodies to Vam10p. Fusion reaction aliquots either were placed on ice, mixed with buffer, or mixed with antibody to Vam10p, to Sec17p or Sec18p (which catalyze priming), or to Vam3p to inhibit docking (Fig. 5A). All incubations were continued to 90 min, when samples were assayed for active, mature Pho8p. Each inhibitor completely blocked fusion when added at the start of the incubation. The reaction rapidly became resistant to anti-body to Sec17p or Sec18p, reflecting their early priming role. Resistance to anti-Vam10p (Fig. 5A) occurred in parallel with resistance to antibody to Sec17p or Sec18p, suggesting that Vam10p functions exclusively during priming. Fusion reactions also were performed with the addition of either recombinant Vam10p or buffer at various times. Fusion was stimulated when Vam10p was added at early reaction times but not after 15 min of incubation (Fig. 5B). Clearly, Vam10p only acts early in the reaction.
Fig. 5.
Vam10p acts at a novel priming stage. (A) Reactions (1,350 μl) had BJ3505 and DKY6281 vacuoles and 4 μM Vam10p. Aliquots (30 μl) were placed on ice or added to inhibitors [anti-Sec17p (final concentration, 1 μM), anti-Sec18p (1 μM), anti-Vam3p (1 μM), and anti-Vam10p (4.9 μM)] at the indicated times. After 90 min, alkaline phosphatase was measured. (B) Aliquots (30 μl) were removed from a standard fusion reaction (300 μl) and added to PS buffer or to Vam10p (final concentration, 8 μM) at the indicated times. After 90 min, phosphatase was assayed. (C) Standard fusion reactions contained 8 μM Vam10p (lanes 2, 4, and 5) and 0.33 μM anti-Sec18p antibody (lane 5) at 4°C (lanes 1 and 2) or 27°C (lanes 3–5). Certain reactions were started with 0.33 μM anti-Sec18p in the presence (lanes 8 and 11) or absence (lanes 6, 7, 9, and 10) of 8 μM Vam10p under plus (lanes 6–8) or minus (lanes 9–11) ATP conditions. After 20 min at 27°C, samples received 8 μM Vam10p (lanes 7 and 10), ATP (lanes 9–11), or PS buffer. After further incubation for 70 min at 27°C, phosphatase was measured. (D) Fusion reactions contained 4 μM Vam10p (lanes 5 and 6), 8 μM anti-Vam10p (lanes 4 and 6), or 0.33 μM anti-Sec18p (lane 3) at 4°C (lane 1) or 27°C (lanes 2–6). Reactions were started with 0.33 μM anti-Sec18p with 4 μM Vam10p (lanes 7 and 8). After 20 min at 27°C, samples received 0.16 μM Sec18p with (lane 7) or without (lane 8) 8 μM anti-Vam10p. After 70 min at 27°C, phosphatase was measured. (E) After incubating fusion reactions with vacuoles from wild-type or yor068Δ strains with 0.018 μM Sec18p for the indicated times, vacuoles were removed by centrifugation and supernatants were analyzed by immunoblotting with anti-Sec17p (3).
Are Vam10p and Sec17/18p activities related? Anti-Sec18p blockade is reversed by the addition of recombinant Sec18p (3). Vacuole fusion (Fig. 5C, lane 3) was stimulated by Vam10p (lane 4) on the normal fusion pathway as it was inhibited by anti-Sec18p (lane 5). A two-step incubation was begun with anti-Sec18p for 20 min followed by recombinant Sec18p. Vam10p stimulation was seen only when Vam10p was present from the start of the reaction (lanes 6–8), even when ATP was omitted from the first incubation (lanes 9–11).
To show that Vam10p can act before Sec18p, a two-step reaction was used, exploiting Sec18p reversal of anti-Sec18p inhibition (3) and the capacity of anti-Vam10p to block the stimulation of fusion by Vam10p (Fig. 5D, lanes 5 and 6). When vacuoles first are incubated with Vam10p in the presence of anti-Sec18p and then mixed with sufficient antibody to Vam10p to block stimulation of fusion and given Sec18p, full fusion activity is seen (lanes 7 and 8). Accordingly, vacuoles from a vam10Δ strain show normal release of Sec17p (Fig. 5E). Vam10p is the first vacuole fusion factor that can act upstream of Sec18p, although our data do not address whether it must act upstream of Sec18p.
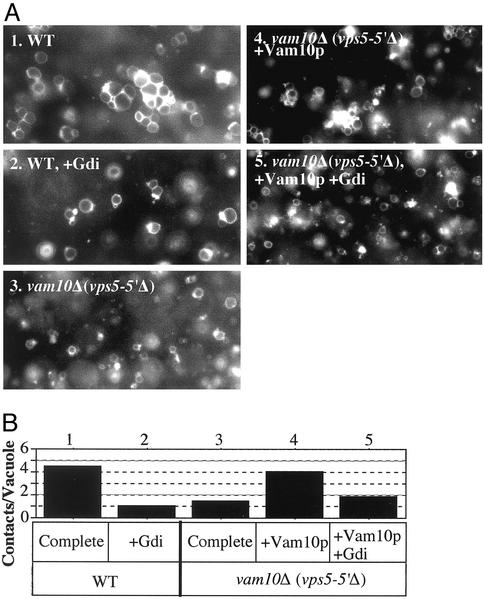
Vacuole priming is a prerequisite for tethering, the first association between vacuoles. Tethering (Fig. 6A, 1) is abolished by Gdi1p (Fig. 6A, 2), which extracts Ypt7p (20). Strikingly, vam10-Δ vacuoles do not tether (Fig. 6A, 3) unless pure Vam10p is added (Fig. 6A, 4). Vam10p-mediated tethering of vam10Δ vacuoles is still fully sensitive to Gdi1p (Fig. 6A, 5). Quantitation of the intervacuole contacts in multiple image fields (Fig. 6B) confirms the visual impressions. Thus, Vam10p is an ATP-independent priming factor, not coupled to Sec18p action or Sec17p release, which supports subsequent vacuole tethering.
Fig. 6.
Vam10p supports tethering. (A) Docking assays (7) had 6 μg of vacuoles from BY4742 (1 and 2)or vam10Δ (3–5), with reaction buffer (10 mM Pipes-KOH, pH 6.8/200 mM sorbitol/40 mM KCl/0.1 mM MgCl2/10 μM CoA/0.3 mM ATP/6 mM creatine phosphate/10 units/ml creatine kinase/7.5 ng/ml leupeptin/37.5 ng/ml pepstatin/3.75 μM o-phenanthroline/7.5 μM Pefabloc SC). After 25 min at 27°C, samples were chilled and stained with 5 μM FM4–64 for 2 min. Half of each sample was mixed with 15 μl of 0.7% low-melt agarose in PS buffer (10 mM Pipes-KOH, pH 6.8/200 mM sorbitol). Aliquots (15 μl) were transferred to prechilled slides and cover slips. After 5 min at 4°C, samples were observed by fluorescence microscopy (five fields recorded per condition). (B) Contacts per vacuole in the focal plane was determined for 80–120 vacuoles for each condition in A.
Discussion
Vam10p is needed for vacuole fusion in vivo and in vitro. The VAM10 gene lies within VPS5, yet it has a distinct function (Fig. 3). VAM10 deletion does not lower vacuolar CPY (Fig. 4C) and thus is not a VPS gene. The in vivo role of Vam10p was confirmed by its being required for osmotic stress-induced fusion and by the vacuole fragmentation induced by Vam10p overexpression. In vitro, we find that Vam10p stimulates vacuole fusion, and antibody to Vam10p blocks fusion during priming. Vam10p has no detectable effect on Sec17p release, suggesting that it affects another early process. This is likely a new function, as the known events of priming all require ATP, whereas Vam10p can act before ATP addition. However, the strongly enhanced vacuolar levels of peripheral membrane proteins such as Sec18p, HOPS, and actin in the absence of Vam10p suggests that Vam10p might function in a release stage of their normal cycling between the vacuole and cytoplasm. Vam10p has no obvious homologs, in yeast or in other sequenced organisms, although it is in a cluster of trafficking genes between ALG8 and GYP1. Because each aspect of vacuole fusion is conserved functionally in other membrane-fusion events (2), Vam10p may have functional, if not sequence, homologs in other fusion reactions.
The stages of priming that are needed for tethering are complex. Tethering needs neither Vam3p nor Vam7p, and the Ypt7p on tethered vacuoles is enriched in vertex rings (8). Ligands to ergosterol (22) and phosphoinositides (4) can inhibit during priming, and these lipids might have a role in establishing the vertex ring of Ypt7p in conjunction with Vam10p. The striking effect of Vam10p on the levels of vacuolar HOPS, Sec18p, and actin is in accord with their roles in early steps of the fusion pathway. (i) Vps41p, a subunit of the HOPS complex, has altered mobility and abundance in the absence of Vam10p (Fig. 4C); because both Ypt7p and Vam10p are needed for tethering, and HOPS is a Ypt7p–effector complex (23), this may reflect the functional role of Vam10p. (ii) Vacuoles from vam10Δ strains bear more Sec18p (Fig. 4C), suggesting a connection between these two priming factors. (iii) Actin disassembly, which is sensitive to jasplakinolide, is needed for an early docking stage of the reaction, because jasplakinolide allows Ypt7p-dependent tethering (14) but fusion becomes jasplakinolide-resistant sometime between Ypt7p action and the completion of docking (14). Our working model is that Vam10p participates in tethering along with Ypt7p, regulating crucial vacuole-bound proteins. Other functions for Vam10p could be to regulate phosphoinositide synthesis or turnover; to control the essential vacuolar gradients of protons, calcium, or other ions that are established in vitro during priming; or to interact with Vtc complex (24), SNARE complex, Ypt7p, actin, or other vacuolar proteins that act during priming and tethering.
Acknowledgments
We thank Drs. Charles Barlowe, Scott Emr, and Stefan Otte for discussions and Dr. Michael Snyder for strains. This work was supported by grants from the National Institute of General Medical Sciences, the Human Frontier Science Program Organization, and the Yokoyama Foundation for Clinical Pharmacology.
Abbreviations: SNARE, soluble N-ethylmaleimide-sensitive fusion protein; YPD, yeast extract/peptone/dextrose.
References
- 1.Rizo, J. & Sudhof, T. (2002) Nat. Rev. Neurosci. 3, 641–653. [DOI] [PubMed] [Google Scholar]
- 2.Wickner, W. (2002) EMBO J. 21, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer, A., Wickner, W. & Haas, A. (1996) Cell 85, 83–94. [DOI] [PubMed] [Google Scholar]
- 4.Mayer, A., Scheglmann, D., Dove, S., Glatz, A., Wickner, W. & Haas, A. (2000) Mol. Biol. Cell 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veit, M., Laage, R., Dietrich, L., Wang, L. & Ungermann, C. (2001) EMBO J. 20, 3145–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer, A. & Wickner, W. (1997) J. Cell Biol. 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, L., Seeley, S., Wickner, W. & Merz, A. (2002) Cell 108, 357–369. [DOI] [PubMed] [Google Scholar]
- 8.Wang, L., Merz, A. J., Collins, K. M. & Wickner, W. (2003) J. Cell Biol. 160, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters, C. & Mayer, A. (1998) Nature 396, 575–580. [DOI] [PubMed] [Google Scholar]
- 10.Seeley, E. S., Kato, M., Margolis, N., Wickner, W. & Eitzen, G. (2002) Mol. Biol. Cell 13, 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada, Y., Ohsumi, Y. & Anraku, Y. (1992) J. Biol. Chem. 267, 18665–18670. [PubMed] [Google Scholar]
- 12.Haas, A., Conradt, B. & Wickner, W. (1994) J. Cell Biol. 126, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, H., Bilgin, M., Bangham, R., Hall, D., Casamayor, A., Bertone, P., Lan, N., Jansen, R., Bidlingmaier, S., Houfek, T., et al. (2001) Science 293, 2101–2105. [DOI] [PubMed] [Google Scholar]
- 14.Eitzen, G., Wang, L., Thorngren, N. & Wickner, W. (2002) J. Cell Biol. 158, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vida, T. A. & Emr, S. D. (1995) J. Cell Biol. 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone, N., Millar, J. B. A., Toda, T. & Armstrong, J. (1998) Curr. Biol. 8, 135–144. [DOI] [PubMed] [Google Scholar]
- 17.Ungermann, C., Wickner, W. & Xu, Z. (1999) Proc. Natl. Acad. Sci. USA 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow, E. & Lane, D. (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 19.Nothwehr, S. F. & Hines, A. E. (1997) J. Cell Sci. 110, 1063–1072. [DOI] [PubMed] [Google Scholar]
- 20.Horazdovsky, B. F., Davies, B. A., Seaman, M. N. J., McLaughlin, A., Yoon, S.-H. & Emr, S. D. (1997) Mol. Biol. Cell 8, 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas, A., Scheglmann, D., Lazar, T., Gallwitz, D. & Wickner, W. (1995) EMBO J. 14, 5258–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato, M. & Wickner, W. (2001) EMBO J. 20, 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seals, D., Eitzen, G., Margolis, N., Wickner, W. & Price, A. (2000) Proc. Natl. Acad. Sci. USA 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller, O., Bayer, M. J., Peters, C., Andersen, J. S., Mann, M. & Mayer, A. (2002) EMBO J. 21, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]