Abstract
Human serum albumin (HSA) is the major protein component of blood plasma and serves as a transporter for thyroxine and other hydrophobic compounds such as fatty acids and bilirubin. We report here a structural characterization of HSA–thyroxine interactions. Using crystallographic analyses we have identified four binding sites for thyroxine on HSA distributed in subdomains IIA, IIIA, and IIIB. Mutation of residue R218 within subdomain IIA greatly enhances the affinity for thyroxine and causes the elevated serum thyroxine levels associated with familial dysalbuminemic hyperthyroxinemia (FDH). Structural analysis of two FDH mutants of HSA (R218H and R218P) shows that this effect arises because substitution of R218, which contacts the hormone bound in subdomain IIA, produces localized conformational changes to relax steric restrictions on thyroxine binding at this site. We have also found that, although fatty acid binding competes with thyroxine at all four sites, it induces conformational changes that create a fifth hormone-binding site in the cleft between domains I and III, at least 9 Å from R218. These structural observations are consistent with binding data showing that HSA retains a high-affinity site for thyroxine in the presence of excess fatty acid that is insensitive to FDH mutations.
The hormone thyroxine [3,3′,5,5′-tetraiodo-L-thyronine (T4)] is secreted into the bloodstream by the thyroid gland and converted intracellularly to the active form, triiodothyronine [3,3′,5,-triiodo-L-thyronine (T3); ref. 1]. T4, which acts on almost all cells of the body, is required for normal physical and mental development and is primarily responsible for maintaining the basal metabolic rate. In plasma, T4 is bound by three proteins: thyroxine-binding globulin, transthyretin, and human serum albumin (HSA). Thyroxine-binding globulin, which has the highest affinity for T4 (Kd = 0.1 nM), binds about three-quarters of the hormone carried in the circulation; the remainder is divided more or less equally between transthyretin and HSA. Thyroxine binding globulin appears to be adapted to target the hormone to sites of inflammation because its affinity for T4 is reduced on cleavage by neutrophil elastase (2–4). Albumin binds T4 with a Kd of ≈2 μM and provides an important fast-response reservoir for the hormone during capillary transit (4).
HSA is involved in the transport of several hydrophobic compounds in addition to T4, including nonesterified fatty acids, bilirubin, bile acids, and steroids (5). It is a monomeric protein containing three homologous domains (I–III), each divided into two subdomains (A and B). The number and locations of binding sites for T4 are not well defined (5, 6), although a consensus of most recent studies (6, 7, 8, 9, 10, 11) indicates that there are likely to be high-affinity T4-binding sites in subdomains IIA and IIIA. These subdomains contain the two primary drug-binding sites on the protein (12, 13) and also bind fatty acids (refs. 14 and 15; Fig. 1). Fatty acid binding is known to induce a significant conformational change in the protein that can impact the binding of other HSA ligands (14, 16–18). For a more complete understanding of thyroxine binding to HSA under in vivo conditions where fatty acids are invariably present, the interplay between these two types of ligand needs to be characterized.
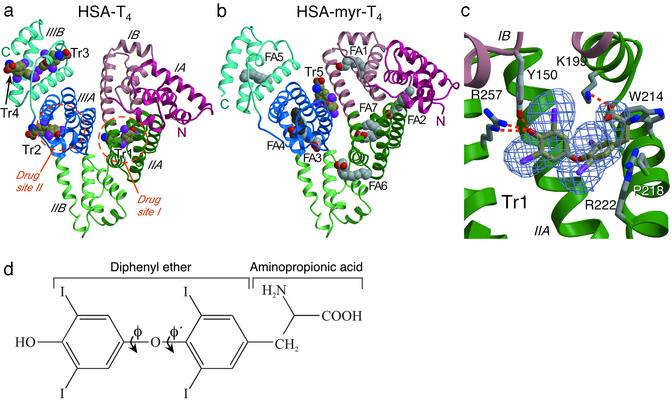
Fig. 1.
Comparison of the structures of HSA–T4 and (a) HSA–myristate–T4 (b). The protein secondary structure is shown schematically with the subdomains color-coded as follows: IA, red; IB, light red; IIA, green; IIB, light-green; IIIA, blue; IIIB, light blue. This color scheme is maintained throughout. Ligands are shown in a space-filling representation, colored by atom type: carbon (fatty acid), gray; carbon (T4), brown; nitrogen, blue; oxygen, red; iodine, magenta. T4-binding sites are labeled Tr1–Tr5; fatty acid-binding sites are labeled FA1–FA7. Except where stated otherwise, molecular graphics were prepared by using bobscript (40) and raster3d (41). (c)An Fobs - Fcalc simulated annealing omit map (29) contoured at 3σ for T4 bound to site Tr1 in subdomain IIA of the R218P mutant. Selected amino acid side chains are colored by atom type. Hydrogen bonds are indicated by dashed orange lines. (d) Schematic structure of T4 hormone, indicating key nomenclature.
The current picture of HSA–thyroxine interactions is further elaborated by the finding that naturally occurring mutants of HSA carrying the substitutions R218H or R218P in subdomain IIA give rise to a 10- to 15-fold enhancement of T4 binding affinity and are the cause of the autosomal-dominant condition familial dysalbuminemic hyperthyroxinemia (FDH) (19–21). Individuals with FDH have normal serum levels of free T4, but their total serum T4 is significantly elevated, a characteristic that can lead to misdiagnosis of hyperthyroidism and inappropriate treatment (22–24). Mutational analysis of R218 and neighboring residues led to a hypothesis that the enhanced binding affinity of the HSA mutants is due to relief of steric forces (6, 10), but this has not yet been tested by direct observation.
In view of the uncertainty surrounding the nature of the interaction of T4 with HSA and the potential for allosteric interactions between ligand-binding sites (14, 16–18), structural information is required to elucidate the locations of T4-binding sites on the protein and the mechanistic basis of FDH.
Materials and Methods
Protein Purification, Complex Formation, and Crystallization. Purified recombinant wild-type HSA (Recombumin) was obtained from Delta Biotechnology (Nottingham, U.K.). HSA mutants were expressed in Pichia pastoris and purified as described (6). Before crystallization in the absence of fatty acid, the protein was defatted and purified by gel filtration using established protocols (14, 25, 26). All crystals were grown by sitting-drop vapor diffusion with protein concentrations of ≈100 mg/ml in 20 mM potassium phosphate, pH 7.0–7.5. HSA–T4 complexes were typically prepared by mixing 100 μl of 100 mg/ml (1.5 mM) HSA with 2.5 ml of 0.3 mM T4 (pH 9.8; a 5-fold molar excess) in water and incubating overnight at room temperature. The resulting complex was concentrated by using a 10-kDa ultracentrifugation device (Millipore) and then diluted (with 0.1 mM T4/50 mM potassium phosphate, pH 8.4) and reconcentrated at least twice to ensure a free T4 concentration of 0.1 mM. The elevated pH values of solutions containing T4 were necessary to keep T4 soluble. The HSA–T4 complexes were crystallized in 10-μl drops (initial volume) over a reservoir containing 24–30% (wt/vol) polyethylene glycol 3350 (Sigma-Aldrich) and 50 mM potassium phosphate (pH 7.0). HSA–myristate complexes were prepared (without prior defatting of the protein) and crystallized as described (14, 15). In all cases the largest crystals were obtained by streak seeding into drops that had been allowed to equilibrate for 5–7 days (27). Crystals were harvested into solutions containing slightly higher PEG 3350 concentrations than were used for crystallization (15). Myristate and T4 were included at 0.1 mM in the harvest buffers where appropriate. Ternary HSA–myristate–T4 complexes were prepared by soaking crystals of HSA–myristate in harvest buffer containing first 0.75 mM, then 1.5 mM T4 over a period of 21 h.
X-Ray Data Collection. X-ray diffraction data were collected at room temperature by using synchrotron radiation sources (Table 1), processed with MOSF LM (Medical Research Council, Laboratory of Molecular Biology, Cambridge, U.K.), and scaled by using the CCP4 suite (28). Previously determined structures of defatted HSA (ref. 25; PDB ID code 1E7A) or HSA–myristate (ref. 15; PDB ID code 1E7G) stripped of its ligands, were used as a starting models for phasing of the x-ray data. Rigid body, positional, and B-factor refinements were performed with CNS (Version 1.1; ref. 29). In structures lacking myristate, a pair of short helices in subdomain IA (residues 77–88) and part of the C-terminal helix in subdomain IIIB (residues 570–585, 577–585, and 576–585 in wild-type HSA, R218H, and R218P, respectively) are disordered and are not included in the refined models; in the crystal, these features lie close to one another on adjacent, symmetry-related molecules. Refinement statistics are summarized in Table 1. Coordinates have been deposited in the Protein Data Bank (ID codes are given in Table 1).
Table 1. Data collection and refinement statistics.
| wt T4 | R218H-T4 | R218P-T4 | wt myr-T4 | R218H-myr-T4 | |
|---|---|---|---|---|---|
| Space group | P21 | P21 | P21 | C2 | C2 |
| a, Å | 59.14 | 58.94 | 59.33 | 189.58 | 190.14 |
| b, Å | 88.57 | 88.39 | 89.28 | 38.91 | 39.04 |
| c, Å | 59.82 | 59.99 | 60.53 | 96.26 | 96.23 |
| β, ° | 102.39 | 103.00 | 100.80 | 105.42 | 105.07 |
| Resolution range, Å | 35.4-2.65 | 34.9-2.8 | 35.4-2.8 | 37.5-2.4 | 46.6-2.8 |
| Independent reflections | 16,851 | 14,703 | 14,600 | 24,201 | 17,211 |
| Multiplicity* | 2.2 (2.2) | 2.7 (2.7) | 3.1 (3.0) | 2.1 (1.7) | 2.4 (2.0) |
| Completeness, % | 95.9 (97.5) | 99.0 (100.0) | 85.1 (97.7) | 90.6 (76.0) | 90.0 (80.0) |
| I/σ1 | 5.7 (1.8) | 5.2 (1.8) | 5.7 (2.0) | 5.6 (2.7) | 5.6 (1.8) |
| Rmerge, %† | 5.4 (36.4) | 7.1 (39.8) | 7.0 (37.0) | 7.9 (22.1) | 8.2 (30.9) |
| Model refinement | |||||
| Nonhydrogen atoms | 4,371 | 4,379 | 4,408 | 4,631 | 4,578 |
| Rmodel, %‡ | 20.0 | 19.0 | 20.3 | 21.6 | 19.9 |
| Rfree, %§ | 25.2 | 24.4 | 24.5 | 26.7 | 25.9 |
| rms deviation from ideal bond lengths, Å | 0.007 | 0.007 | 0.008 | 0.007 | 0.007 |
| rms deviation from ideal angles, ° | 1.25 | 1.25 | 1.27 | 1.18 | 1.26 |
| Average B-factor, Å2 | 62.4 | 65.8 | 73.5 | 52.4 | 54.7 |
| PBD ID code | 1HK1 | 1HK2 | 1HK3 | 1HK4 | 1HK5 |
wt, wild-type; myr, myristate.
Values for the outermost resolution shell are given in parentheses.
Rmerge = 100 × ΣhΣj|Ihj - Ih|/ΣhΣjIhj, where Ih is the weighted mean intensity of the symmetry-related reflections Ihj.
Rmodel = 100 × Σhkl|Fobs - Fcalc|/ΣhklFobs.
Rfree is the Rmodel calculated by using a randomly selected 5% sample of reflection data omitted from the refinement.
Thyroxine-Binding Measurements. Quenching of the fluorescent emission of W214 was used to determine Kd value for T4 binding to recombinant HSA essentially as described (6, 30). In experiments to assay the effect of myristate on T4 binding to HSA, 30 μM myristate was used with 1 μM HSA to ensure that all myristate sites on HSA were occupied by the ligand. Myristate was dissolved in 80% ethanol to a concentration of 10 mM and diluted with PBS (40 mM phosphate/150 mM NaCl, pH 7.4) for use in titration experiments. Kd values and Hill coefficients were determined by nonlinear regression analysis of the binding isotherms with the program PRISM (GraphPad, San Diego) as described (6).
Results and Discussion
Overview of the HSA–T4 Structure. The crystal structures of recombinant wild-type and mutant HSA complexed with T4 and/or myristate were determined by molecular replacement to resolutions between 2.4 and 2.8 Å (Table 1, Fig. 1). The overall structure of the HSA–T4 complex is very similar to that for defatted HSA (Fig. 1a): there are no gross changes in the relative dispositions of the three domains, in contrast to conformational changes induced by fatty acid binding (14, 15, 16, 31). The most significant deviation in main-chain conformation between HSA–T4 and defatted HSA structures (13, 25, 32) occurs in subdomain IIIB. In particular, there are differences in the extended polypeptide segment 502–514 that are probably largely attributable to T4 binding (see below).
Overview and Comparison of the T4-Binding Sites. Four molecules of T4 (Fig. 1d) bind to wild-type HSA, one in subdomain IIA (drug site 1), one in IIIA (drug site 2), and two adjacent to one another in subdomain IIIB, which we will designate sites Tr1–Tr4, respectively (Fig. 1a). In each case, the shape of the difference density and the steric restrictions enforced by the binding environment clearly indicated the position and orientation of the bound T4 (Fig. 1c).
In Tr1, the hormone occupies the same binding location as site 1 drugs (13, 14, 33) and fatty acid site 7 (15). Comparison with the structure of unliganded HSA shows that there are only minor adjustments of the side chains lining the pocket to accommodate the bound hormone; W214, R218, and R222 appear to be most affected by T4 binding (see below). The phenolic hydroxyl of T4 makes specific hydrogen bond interactions with the side chains of Y150 and R257 (Fig. 2a). The substituted phenyl rings are accommodated within a predominantly hydrophobic pocket, although there are also significant hydrophilic contacts, particularly with the iodine atoms, from side chains and main-chain carbonyl oxygens. As observed for both the thyroid receptor and transthyretin, the ether oxygen in the center of the hormone molecule is found in a hydrophobic environment and does not form any hydrogen bonds with the protein (34, 35). The T4 amino group at the mouth of the pocket is solvent-exposed while the carboxylate moiety makes a salt-bridge interaction with K199 (2.9 Å); K195 may also contribute an electrostatic interaction with the T4 carboxylate but is more distant (3.1 Å) and exhibits some disorder at the tip of the side chain.
Fig. 2.
Comparison of the bound configurations of T4 in HSA. (a) Site Tr1 in subdomain IIA. There is only very weak electron density for the side chain of R218 because variation in the position of this residue and atoms beyond Cγ were omitted from the refined model; the position shown in the figure is indicative only but is consistent with the location of the main-chain atoms and steric constraints imposed by neighboring residues and the T4 ligand. (b) Site Tr2 in subdomain IIIA. (c) Sites Tr3 and Tr4 in subdomain IIIB (from the R218H mutant structure, because this model shows the least disorder of the C-terminal helix). (d) Site Tr5 in the interdomain cleft of the HSA–myristate complex. Figures were prepared by using molscript (41) and pymol (42). Selected amino acid side chains are shown colored by atom type. The van der Waals surface of the ligand is represented by a semitransparent magenta surface. Hydrogen bonds are indicated by dashed orange lines.
The T4 hormone is bound to site Tr1 in a cisoid conformation, with the amino-propionic acid and the outer phenolic ring both on the same side of the inner ring of the molecule (ref. 36; Figs. 1 and 2a). The cisoid conformation is dictated by steric constraints due to residues W214, R218, and R222, which project from the helix that forms one side of the mouth of the pocket and prevent rotation of the amino-propionic acid. In contrast, the other three sites all bind T4 in a transoid conformation and are thus similar to the conformations observed for T3 and T4 bound to the thyroid hormone receptor (35) and transthyretin (34, 37), respectively.
In Tr2 (subdomain IIIA) T4 is only partially buried (Fig. 1a). Although subdomain IIIA contains a hydrophobic, T-shaped cavity that can accommodate two fatty acid molecules (14, 15) and also binds drugs (13, 25), T4 is sterically prevented from penetrating this tunnel network and binds at the mouth of one of the fatty acid-binding sites (FA4 in Fig. 1b). The phenolic hydroxyl of T4 and the carboxylate moiety of the fatty acid both hydrogen bond to Y411 and S489 but, whereas the methylene tail of the fatty acid is accommodated within the core of the domain, the T4 molecule points in the opposite direction, out toward the solvent. In what appears to be a novel mode of binding to subdomain IIA, T4 lies in a slot on the surface at the mouth of the fatty acid site that is opened up on binding by movements of the side chains of Q390, N391, and R410. The outer ring of T4 is held in the deepest part of the Tr2-binding site (allowing the phenolic hydroxyl to make hydrogen bonds to the side chains of Y411 and S489). The inner ring makes van der Waals contacts with the side chains of Q390, N391, L394, A406, and R410 (Fig. 2b) but is nevertheless partially exposed to solvent. The amino-propionic acid is fully solvent-exposed and makes no hydrogen bond contacts with the protein; the closest contact to the hormone carboxylate group is from the NE2 atom of N390 (3.9 Å). The incomplete burial of T4 in site Tr2, particularly in contrast to the more enclosed binding configuration observed in Tr1, suggests that it may not bind the hormone with high affinity and may account for the observed 50% occupancy. Although subdomain IIIA is reported to be a high-affinity site for the hormone that is also stereo-selective for L-T4 (8), this does not appear to be fully supported by the crystal structure.
Unexpectedly, we find that T4 binds to two sites in subdomain IIIB. The hormones bound in Tr3 and Tr4 partially overlap the carboxylate and methylene ends, respectively, of fatty acid site 5 (Fig. 1; ref. 14). Occupation of these sites induces significant alterations in the main-chain conformation of the extended polypeptide (residues 502–514) that forms one flank of this subdomain. T4 is largely bound by hydrophobic side-chain contacts in site Tr3 (Fig. 2c). In contrast to the other T4 sites on HSA, Tr3 is open at both ends; the phenolic hydroxyl is exposed to solvent and makes no specific interaction with the protein. In the crystal, the T4 carboxylate group makes a hydrogen bond to a water molecule stabilized by D301 from a symmetry-related HSA molecule. The observation of 100% occupancy at this site must therefore be treated with some caution.
The T4 molecule bound to Tr4 is also apparently 100% occupied, but one face of the binding site is not visible because of disorder of the C-terminal helix (Figs. 1a and 2c), a curious observation because one might expect the ligand to stabilize the helix. However, this is a very flexible part of the structure with high associated temperature factors and one that does not often yield good electron density (14, 25), although the helix is observed to be intact in HSA–myristate structures (Fig. 1b). Although the hormone appears only partially buried in Tr4, with just the outer ring fully contacting the protein (akin to the binding configuration in site Tr2), in solution the helix that is disordered in the crystal may serve to further enclose T4 at this site. However, the possibility that this is a relatively low-affinity site is strengthened by the finding that the phenolic hydroxyl, although buried in the pocket, faces an intrahelical main-chain hydrogen bond (between A528 and L532) and does not have a hydrogen bonding partner.
Because each of the four T4-binding sites at least partially overlaps with a fatty acid-binding site on HSA, high mole ratios of fatty acid to HSA would be predicted to prevent T4 binding. Indeed when crystals of the HSA–myristate complex were soaked in solutions containing up to 1.5 mM T4, the hormone failed to bind to any of the four binding pockets that occur in the absence of fatty acid (Fig. 1b). However, a new site was discovered in the cleft between domains I and III, formed on one side by the upper part of the long helix that connects domains I and II and on the other by a pair of helices from subdomain IIIA (Fig. 2d). This site, designated Tr5, is rather open in comparison to sites Tr1–Tr4; the phenolic hydroxyl and amino-propionic acid groups are both solvent-exposed and the inner ring is only partially shielded by contacts with the protein. As in other sites, the substituted rings are contacted by a combination of hydrophobic and hydrophilic side chains. The formation of this site is clearly dependent on the well characterized fatty acid-induced conformational change (14, 15, 16, 31), which results in significant rotations of domains I and III relative to domain II and thus causes a shift in the relative positions of the two helices that make up the hormone-binding site (Fig. 1).
Although the occupancy of binding site Tr5 appears low (≈30%) in our soaked crystals, affinity measurements in solution in the presence of a 30-fold molar excess of myristate indicate that T4 binds to this site with an affinity similar to that observed for the primary T4 site in defatted HSA (Table 2). In normal physiological conditions only 1–2 mol of fatty acid are bound per mole of HSA (5), a level that is unlikely to induce the conformational change needed to induce site Tr5 (18). Furthermore, the balance of current evidence indicates that whereas subdomain IIA is probably a low-affinity binding site for fatty acid, subdomains IIIA and IIIB may bind fatty acids with high affinity (15, 16). Thus, Tr1 in subdomain IIA may be the most accessible site for T4 binding in vivo.
Table 2. Kd values for T4 binding to HSA in the presence or absence of myristate.
| 0 mM myristate*
|
30 mM myristate
|
|||
|---|---|---|---|---|
| Kd, μM | Hill coefficient | Kd, μM | Hill coefficient | |
| Wild-type HSA | 2.3 ± 0.6 | 0.93 ± 0.11 | 4.9 ± 0.1 | 1.41 ± 0.03 |
| FDH HSA (R218H) | 0.17 ± 0.05 | 0.72 ± 0.08 | 5.72 ± 0.13 | 1.30 ± 0.04 |
| FDH HSA (R218P) | 0.26 ± 0.1 | 1.09 ± 0.17 | — | — |
—, not tested.
Previously published data included for ease of comparison (6).
Effects of Mutations R218H and R218P. How do mutations of R218 enhance the binding of T4 to HSA? R218 is located in the center of the helix that forms the “doorstep” at the entrance to site Tr1 in subdomain IIA. Mutations of R218 probably only affect T4 binding to this site, because the side chain is too far (19–40 Å) from the other three sites to impinge directly on their interactions with the hormone and there is no indication of nonlocal conformational changes as a result of substitutions of this residue. Although the occupancy of T4 in site Tr1 of wild-type HSA refined to ≈50%, for both the R218H and R218P mutants the occupancy of the hormone was 100%, which gives at least a crude indication that the affinity of this site is higher compared with wild-type HSA.
In unliganded wild-type HSA, R218 is flanked by W214 and R222, both of which also project from the doorstep helix in defined configurations. T4 bound to Tr1 makes direct van der Waals contacts with this triad of residues (Figs. 2a and 3) and induces a concerted rearrangement of their side chains, a process that appears to be required to create sufficient room for the ligand. W214 and R222 are both displaced outwards from the pocket by contact with the inner ring of the hormone, whereas R218 is displaced and partially disordered on T4 binding. These movements are all achieved by rotations of side-chain torsion angles.
Fig. 3.
Stereoview of the binding of T4 to site Tr1 in subdomain IIA for wild-type HSA and the FDH mutants R218H and R218P. The secondary structure for wild-type HSA is depicted schematically. Carbon atoms for selected side chains and T4 are color-coded as follows: wild-type, gray; R218H, pink; R218P, cyan.
The mutation R218H places a smaller side chain at position 218 and permits a new configuration of the modified W214–H218–R222 triad that creates additional space for the ligand. In this mutant there is a 10° rotation of torsion angle ϕ within T4 (Fig. 1d) that displaces the inner phenyl ring of the bound T4 toward W214; this side chain undergoes a 20° χ2 rotation to accommodate the new position of the hormone (Fig. 4).
Fig. 4.
Binding curves derived from fluorescence quenching experiments with T4 in the presence of 30 μM myristate. y axis, log of the free T4 concentration (μM); x axis, ratio of bound T4 molecules to HSA molecules. The curves represent the theoretical curves corresponding to the mean Hill coefficients and mean Kd values for three identical titrations.
The mutation R218P introduces an even smaller side chain at 218. As expected for a proline substitution, there is distortion of the helix main chain, but this is tightly localized to the vicinity of the substitution. The effect of the R218P mutation on the conformations of the bound T4 and the side chains of W214 and R222 is distinct from that observed for R218H: the reduced size of the side chain allows a translation of T4 by ≈0.4 Å toward the mutated residue to bring the inner ring into contact with P218 (Fig. 4). This displacement apparently involves slight stretching (0.3 Å) of the hydrogen bonds made by T4 to R257 and K199. Both mutations create additional room for T4 binding to subdomain IIA by introducing smaller side chains at position 218 and allowing adjustments of the conformations of adjacent residues (W214 and R222) on the doorstep helix. The additional volume created at the entrance of the pocket relaxes steric constraints on T4 binding at this site. What is striking is that relatively modest conformational adjustments can increase the binding affinity by at least an order of magnitude (6, 10).
These findings explain a number of additional observations on binding of T4 to HSA. For example, the mutation R218A exhibits the largest observed enhancement of affinity for T4 (≈100-fold; ref. 6); this is likely to be due to the fact that alanine is the smallest side chain to be introduced at this position and therefore provides the greatest volume increase in site Tr1. In addition, mutation of either W214 or R222 to smaller side chains enhances the affinity of HSA for T4 (6), presumably also by increasing the pocket volume. Moreover, the higher affinity of HSA for tetraiodothyroacetic acid (6) is probably due to the decreased susceptibility of this ligand, which is shorter than T4 by one carbon atom, to steric constraints imposed by subdomain IIA. Simple modeling experiments indicate that if tetraiodothyroacetic acid binds with its phenyl rings in the same position as observed for T4, its terminal carboxylate group would lie in a position that is more favorable for electrostatic interactions with R218 and R222, a finding consistent with the observation that mutation of R218 or R222 to glutamic acid reduces the affinity for this ligand (6). Lastly, the distance of closest approach between the side chain of R218 and site Tr5 in the interdomain cleft is 9 Å, which explains why, in the presence of a molar excess of myristate, the affinity of T4 for HSA is unaffected by mutations of this residue (Table 2). In vivo fatty acid binding may therefore attenuate the effects of FDH mutations by redirecting T4 to site Tr5.
The results reported here provide structural insights into the factors governing binding of T4 to HSA, the effects of fatty acid binding, and the impact of FDH mutations and will facilitate new experiments to probe HSA–T4 interactions in greater depth. The results will also assist the development of drugs based on T4 analogues (38, 39). Such compounds are also likely to interact with HSA, and knowledge of the structure of the T4-binding pockets will facilitate efforts to modulate HSA binding to optimize their pharmacodynamic profiles.
Acknowledgments
We thank Delta Biotechnology for purified recombinant HSA (Recombumin) and the staff at Daresbury Synchrotron Radiation Source (Station 9.6) and European Molecular Biology Laboratory/Deutsches Elektronen Synchrotron, Hamburg (X11 and X13), for assistance with data collection. A.A.B. and J.G. are grateful for the award of MRC studentships. This work was supported by a grant-in-aid from the American Heart Association, Hawaii Affiliate (to N.V.B.), and by the Biotechnology and Biological Sciences Research Council (S.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FDH, familial dysalbuminemic hyperthyroxinemia; HSA, human serum albumin; T3, 3,3′,5,-triiodo-L-thyronine; T4, 3,3′,5,5′-tetraiodo-L-thyronine.
Data deposition: The structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 1HK1–1HK5).
References
- 1.Bhagavan, N. V. (2001) Medical Biochemistry (Harcourt Academic, San Diego).
- 2.Pemberton, P. A., Stein, P. E., Pepys, M. B., Potter, J. M. & Carrell, R. W. (1988) Nature 336, 257–258. [DOI] [PubMed] [Google Scholar]
- 3.Jirasakuldech, B., Schussler, G. C., Yap, M. G., Drew, H., Josephson, A. & Michl, J. (2000) J. Clin. Endocrinol. Metab. 85, 3996–3999. [DOI] [PubMed] [Google Scholar]
- 4.Schussler, G. C. (2000) Thyroid 10, 141–149. [DOI] [PubMed] [Google Scholar]
- 5.Peters, T. (1995) All About Albumin: Biochemistry, Genetics, and Medical Applications (Academic, San Diego).
- 6.Petersen, C. E., Ha, C. E., Harohalli, K., Park, D. & Bhagavan, N. V. (1997) Biochemistry 36, 7012–7017. [DOI] [PubMed] [Google Scholar]
- 7.Divino, C. M. & Schussler, G. C. (1990) J. Clin. Endocrinol. Metab. 71, 98–104. [DOI] [PubMed] [Google Scholar]
- 8.Loun, B. & Hage, D. S. (1992) J. Chromatogr. 579, 225–235. [PubMed] [Google Scholar]
- 9.Loun, B. & Hage, D. S. (1995) J. Chromatogr. B Biomed. Appl. 665, 303–314. [DOI] [PubMed] [Google Scholar]
- 10.Petersen, C. E., Ha, C. E., Jameson, D. M. & Bhagavan, N. V. (1996) J. Biol. Chem. 271, 19110–19117. [DOI] [PubMed] [Google Scholar]
- 11.Park, D. S., Petersen, C. E., Ha, C., Harohalli, K., Feix, J. B. & Bhagavan, N. V. (1999) IUBMB Life 48, 169–174. [DOI] [PubMed] [Google Scholar]
- 12.Sudlow, G., Birkett, D. J. & Wade, D. N. (1975) Mol. Pharmacol. 11, 824–832. [PubMed] [Google Scholar]
- 13.He, X. M. & Carter, D. C. (1992) Nature 358, 209–215. [DOI] [PubMed] [Google Scholar]
- 14.Curry, S., Mandelkow, H., Brick, P. & Franks, N. (1998) Nat. Struct. Biol. 5, 827–835. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya, A. A., Grüne, T. & Curry, S. (2000) J. Mol. Biol. 303, 721–732. [DOI] [PubMed] [Google Scholar]
- 16.Curry, S., Brick, P. & Franks, N. P. (1999) Biochim. Biophys. Acta 1441, 131–140. [DOI] [PubMed] [Google Scholar]
- 17.Reed, R. (1977) J. Biol. Chem. 252, 7483–7487. [PubMed] [Google Scholar]
- 18.Vorum, H. & Honoré, B. (1996) J. Pharm. Pharmacol. 48, 870–875. [DOI] [PubMed] [Google Scholar]
- 19.Sunthornthepvarakul, T., Angkeow, P., Weiss, R. E., Hayashi, Y. & Refetoff, S. (1994) Biochem. Biophys. Res. Commun. 202, 781–787. [DOI] [PubMed] [Google Scholar]
- 20.Petersen, C. E., Scottolini, A. G., Cody, L. R., Mandel, M., Reimer, N. & Bhagavan, N. V. (1994) J. Med. Genet. 31, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wada, N., Chiba, H., Shimizu, C., Kijima, H., Kubo, M. & Koike, T. (1997) J. Clin. Endocrinol. Metab. 82, 3246–3250. [DOI] [PubMed] [Google Scholar]
- 22.Croxson, M. S., Palmer, B. N., Holdaway, I. M., Frengley, P. A. & Evans, M. C. (1985) Br. Med. J. (Clin. Res. Ed.) 290, 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming, S. J., Applegate, G. F. & Beardwell, C. G. (1987) Postgrad. Med. J. 63, 273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood, D. F., Zalin, A. M., Ratcliffe, W. A. & Sheppard, M. C. (1987) Q. J. Med. 65, 863–870. [PubMed] [Google Scholar]
- 25.Bhattacharya, A. A., Curry, S. & Franks, N. P. (2000) J. Biol. Chem. 275, 38731–38738. [DOI] [PubMed] [Google Scholar]
- 26.Sogami, M. & Foster, J. F. (1968) Biochemistry 7, 2172–2182. [DOI] [PubMed] [Google Scholar]
- 27.Stura, E. A. & Wilson, I. A. (1990) Methods 1, 38–49. [Google Scholar]
- 28.Collaborative Computing Project No. 4 (1994) Acta Crystallogr. D 50, 760–763.15299374 [Google Scholar]
- 29.Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 30.Petersen, C. E., Ha, C. E., Harohalli, K., Park, D. S., Feix, J. B., Isozaki, O. & Bhagavan, N. V. (1999) Clin. Chem. 45, 1248–1254. [PubMed] [Google Scholar]
- 31.Petitpas, I., Grüne, T., Bhattacharya, A. A. & Curry, S. (2001) J. Mol. Biol. 314, 955–960. [DOI] [PubMed] [Google Scholar]
- 32.Sugio, S., Kashima, A., Mochizuki, S., Noda, M. & Kobayashi, K. (1999) Protein Eng. 12, 439–446. [DOI] [PubMed] [Google Scholar]
- 33.Petitpas, I., Bhattacharya, A. A., Twine, S., East, M. & Curry, S. (2001) J. Biol. Chem. 276, 22804–22809. [DOI] [PubMed] [Google Scholar]
- 34.Wojtczak, A., Cody, V., Luft, J. R. & Pangborn, W. (2001) Acta Crystallogr. D 57, 1061–1070. [DOI] [PubMed] [Google Scholar]
- 35.Darimont, B. D., Wagner, R. L., Apriletti, J. W., Stallcup, M. R., Kushner, P. J., Baxter, J. D., Fletterick, R. J. & Yamamoto, K. R. (1998) Genes Dev. 12, 3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cody, V. (1980) Endocr. Rev. 1, 140–166. [DOI] [PubMed] [Google Scholar]
- 37.Blake, C. C. & Oatley, S. J. (1977) Nature 268, 115–120. [DOI] [PubMed] [Google Scholar]
- 38.Klabunde, T., Petrassi, H. M., Oza, V. B., Raman, P., Kelly, J. W. & Sacchettini, J. C. (2000) Nat. Struct. Biol. 7, 312–321. [DOI] [PubMed] [Google Scholar]
- 39.Baxter, J. D., Goede, P., Apriletti, J. W., West, B. L., Feng, W., Mellstrom, K., Fletterick, R. J., Wagner, R. L., Kushner, P. J., Ribeiro, R. C., et al. (2002) Endocrinology 143, 517–524. [DOI] [PubMed] [Google Scholar]
- 40.Esnouf, R. (1997) J. Mol. Graphics 15, 133–138. [Google Scholar]
- 41.Merrit, E. A. & Bacon, D. J. (1997) Methods Enzymol. 277, 505–524. [DOI] [PubMed] [Google Scholar]
- 42.Delano, W. L. (2002) The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA).