Abstract
Caveolin-1 and -2 are the two major coat proteins found in plasma membrane caveolae of most of cell types. Here, by using adenoviral transduction of either caveolin-1 or caveolin-2 or both isoforms into cells lacking both caveolins, we demonstrate that caveolin-2 positively regulates caveolin-1-dependent caveolae formation. More importantly, we show that caveolin-2 is phosphorylated in vivo at two serine residues and that the phosphorylation of caveolin-2 is necessary for its actions as a positive regulator of caveolin-1 during organelle biogenesis in prostate cancer cells. Mutation of the primary phosphorylation sites on caveolin-2, serine 23 and 36, reduces the number of plasmalemma-attached caveolae and increases the accumulation of noncoated vesicles, but does not affect trafficking of caveolin-2, interaction with caveolin-1 or its biophysical properties. Thus, the phosphorylation of caveolin-2 is a novel mechanism to regulate the dynamics of caveolae assembly.
Caveolae or “small caves” were identified as 50- to 100-nm flask-shaped, non-clathrin-coated invaginations of the plasma membrane (1–4). These organelles are present in many, but not all, mammalian cells and their abundance varies among cell types (5–7). The functions of caveolae are potentially diverse, including cholesterol transport (8, 9), endocytosis (10), potocytosis (11), and signal transduction (12–15). A significant advance in understanding the roles of caveolae was revealed by identification of the caveolin gene family, VIP21/caveolin-1, caveolin-2, and caveolin-3 (16–20). Caveolin-1, found in a variety of cells, was both shown as a major component of the vesicular transport system in the trans-Golgi network (17) and as a structural component of the caveolar coat (21, 22). Later, caveolin-2 was identified as an isoform expressed in the same cells as caveolin-1 (18, 23), and caveolin-3 was identified as a muscle-specific isoform (19). Caveolins are integral membrane components, which are believed to form a hairpin loop in the plasma membrane with both NH2 and C termini oriented toward the cytoplasm (21, 24).
The molecular mechanism of caveolae assembly is largely unknown, but many studies have suggested that caveolin-1 is sufficient to drive organelle biogenesis and that caveolin-2 can modulate the actions of caveolin-1 during this process. Ectopic expression of caveolin-1 induces de novo formation of large and small noncoated vesicles that do not fuse significantly with the plasma membrane in cells that normally lack these organelles (23, 25–28). Evidence supporting the interdependence on both caveolins for the assembly of caveolae stems from studies showing that the interaction of caveolin-1 with caveolin-2 renders caveolin-2 detergent insoluble, targets Golgi localized caveolin-2 to the plasma membrane, and stabilizes caveolin-2 levels in cells. In Madin–Darby canine kidney (MDCK) cells, caveolin-1 targets to both apical and basolateral membranes, yet caveolae are only present on the basolateral surface where caveolin-2 is localized (29). More recently, by using immunogold microscopy of freeze fractured cells, it has been shown that overexpression of caveolin-2 with caveolin-1 promotes the enhanced appearance of “deep caveolae,” which are equivalent to plasmalemmal-attached caveolae (30). Interestingly, in mice genetically deficient in caveolin-1, plasmalemmal caveolae are absent in most cells examined, including endothelial cells, epithelial cells, embryonic fibroblasts, and adipocytes (31, 32). The absence of caveolae in these mice is associated with a marked reduction in caveolin-2 levels, suggesting that the interaction of caveolin-1 with caveolin-2 has a functional role in organelle biogenesis. Despite the evidence supporting an important role of caveolin-2 in caveolae formation in cultured MDCK (29) and in HEPG2 cells (30), caveolae are still present in ultra thin sections from lung capillary endothelium and in perigonadal adipose tissue of caveolin-2 null mice, suggesting that caveolin-2 is not necessary for caveolae formation (33). However, it is unclear whether the absolute number of caveolae in caveolin-2 null mice remains the same as in wild-type mice, because quantitative analysis of caveolae number has not been performed. Furthermore, interpretations of such knockout studies are complicated because of the possible compensatory mechanisms taking place during development.
To assess the functional role of caveolin-2 in the assembly of caveolae, we used cells deficient in both isoforms followed by reconstitution using adenoviral transduction with either of caveolin-1 or -2, or both. We show that caveolin-2 is necessary, in addition to caveolin-1, for the formation of deep plasma membrane attached caveolae in this reconstituted system. More importantly, we find that the ability of caveolin-2 to modulate caveolin-1-dependent caveolae assembly versus nonattached vesicles, relies on its phosphorylation state. Specifically, we map two serine phosphorylation sites in the N terminus of caveolin-2 and show that each site contributes to caveolin-1-dependent caveolae formation. Thus, our data provides molecular clues to explain the modulatory role of caveolin-2 in caveolae assembly.
Materials and Methods
More detailed description of each method is available in the Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Cell Lines. LNCaP cells (ATCC) were cultured in RPMI medium 1640 containing penicillin (100 units/ml), streptomycin (100 μg/ ml), and 10% (vol/vol) FBS (Life Technologies). MDCK cells were cultured in DMEM (Life Technologies) containing penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% (vol/vol) FBS.
Construction of Adenoviral Vectors and Transduction of Cells with Adenoviruses. Recombinant adenoviruses containing the cDNA encoding caveolin-1 (Adcav-1) or β-galactosidase (Adβ-gal) were generated as described (34). Adenoviral, myc-tagged caveolin-2 (Adcav-2) was obtained from Enrique Rodriguez-Boulan (Weill Medical College of Cornell University, New York), and was constructed as described (35).
Plasmid DNA Constructs and Transfection. Caveolin-2 and -1 cDNA constructs have been generated by PCR and expressed by transient or stable transfection of LNCaP cells.
Expression and Purification of GST-Fused Caveolin-2 Constructs. Caveolin-2 DNA constructs were subcloned into bacterial expression vector pGEX-4T-1, and purified by affinity chromatography using glutathione-agarose.
Immunofluorescence Labeling and Western Blotting. Cells were processed for immunofluorescence and Western blotting as described in Supporting Text.
Assessment of Biophysical Properties of Caveolins. Determination of such biophysical properties of caveolins as formation of SDS-resistant high molecular weight oligomers, their mass, insolubility in TX-100, and flotation in continuous sucrose gradient were performed as described in refs. 36, 37, 38 (details are available in Supporting Text).
In Vitro and in Vivo Phosphorylation Studies. In in vitro phosphorylation studies, caveolin-2 constructs were expressed in bacteria, purified, radiolabeled with [γ-32P]ATP in the presence of CK2, and analyzed by autoradiography or subjected to further analysis by matrix-assisted laser desorption ionization MS. CK2 labeled GST-caveolin-2 was purified by removal of GST via thrombin cleavage, the remaining labeled protein electrophoresed, isolated, subjected to trypsin digestion and the tryptic peptides chromatographed on reverse-phase HPLC followed by Cerenkov counting. In in vivo studies, cells were labeled with 32P orthophosphate, and processed for autoradiography as described in Supporting Text.
Production of Phosphorylated Serine-Specific Caveolin-2 Antibodies. Peptides containing phosphorylated serines 23 and 36 were synthesized and used for immunizing rabbits and for purifying of phospho-specific antibodies by Sulfolink Affinity Purification kit (Pierce) according to the manufacturer's procedure.
Electron Microscopy. Cells were prepared and processed for electron microscopy, and morphometric structures in a close proximity to plasma membrane were quantified similarly to that described (34).
Results
LNCaP Are Devoid of Caveolins and Require Both Caveolin-1 and -2 for Caveolae Assembly. To independently test the role of each caveolin in caveolae assembly, LNCaP cells were used. As seen in Fig. 1F, LNCaP cells were devoid of both caveolins as determined by Western blotting. As expected, LNCaP cells infected with control virus (Adβ-gal) lack caveolae in plasma membrane as shown by electron microscopy (Fig. 1A, quantitative analysis revealed 0 caveolae, 24 uncoated vesicles, 36 coated pits, and 47 coated vesicles per mm of basolateral plasma membrane). Adenoviral infection of LNCaP cells with myc-tagged caveolin-1 (Adcav-1, Fig. 1F, lane 2) did not result in the identification of any plasmalemmal-attached caveolae, even when myc-tagged caveolin-1 was grossly overexpressed to levels exceeding that of endogenous caveolin-1 in MDCK cells; however, it increased the number of uncoated vesicles (113 uncoated vesicles, 32 coated pits, and 36 coated vesicles per mm of membrane; depicted by arrowheads in Fig. 1 B and E Left). Also, infection of LNCaP cells with Adcav-2, which resulted in ample caveolin-2 expression (Fig. 1F, lane 3), did not cause the formation of caveolar structures nor appreciable increase in the number of uncoated vesicles (0 caveolae, 38 uncoated vesicles, 46 coated pits, and 38 coated vesicles per mm of basolateral membrane). However, coexpression of caveolins-1 and -2 (Fig. 1F, lane 4) provoked the formation of attached caveolae (arrows) and an increase in the number of uncoated vesicles on the basolateral surface (Fig. 1C, 53 caveolae, 182 uncoated vesicles, 46 coated pits, and 40 coated vesicles per mm of basolateral membrane) with no detectable caveolar structures on the apical surface. The presence of a neck structure with an electron dense diaphragm in LNCaP cells expressing both caveolins is exemplified by higher magnification of caveolae depicted by arrows (Fig. 1E Center), which are similar to those present in MDCK cells (Figs. 1 D and E Right, 311 caveolae, 161 uncoated vesicles, 63 coated pits and 40 coated vesicles per mm of basolateral membrane). Conversely the uncoated vesicles formed by caveolin-1 alone (Fig. 1 B and E Left depicted by arrowheads) do not have any connection with the plasma membrane. Furthermore, adenoviral infection of LNCaP with caveolin-1 alone, caveolin-2 alone or both together did not influence the biophysical properties of caveolin-1 including SDS-resistant oligomer formation, incorporation in buoyant membranes domains, TX-100 insolubility or trafficking to the plasma membrane. In addition, caveolin-1 was required for redistributing caveolin-2 into SDS-resistant oligomers and TX-100 insoluble fractions as well as redistributing caveolin-2 from the Golgi to the plasma membrane (Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 1.
Electron microphotographs and Western blots of control and caveolin-1 and/or caveolin-2 expressing LNCaP and MDCK cells. Two days after infection with adenoviruses encoding β-galactosidase (Adβ-gal; A), myc-tagged caveolin-1 (Adcav-1; B), or both myc-tagged caveolin-1 and -2 (C), cells were processed for electron microscopy or lysed with lysis buffer and subjected to Western blot analysis with caveolin antibodies (F). (D) Caveolae of MDCK cells for comparison. (E) Higher magnification of uncoated vesicles induced by Adcav-1 and caveolae transduced with Adcav-1 and -2 in LNCaP and MDCK cells for comparison. (F) Expression of caveolin-1 and -2 proteins determined by Western blotting in lysates of LNCaP cells infected with multiplicity of infection of viruses identical to that in cells subjected to electron microscopy as compared with MDCK cells, which express ample amounts of caveolin-2 (29); however, the mAb (Transduction Laboratories) that we used does not recognize canine caveolin-2 (lane 6). Lysates were blotted with caveolin-1 pAb (cav-1, Top), caveolin-2 mAb (cav-2, Middle), and β-actin mAb (actin, Bottom). Arrows denote caveolae (C, D, and E Center and Right). Arrowheads denote uncoated uncoated vesicles (B, C, and E Left). (Scale bars, 100 nm.)
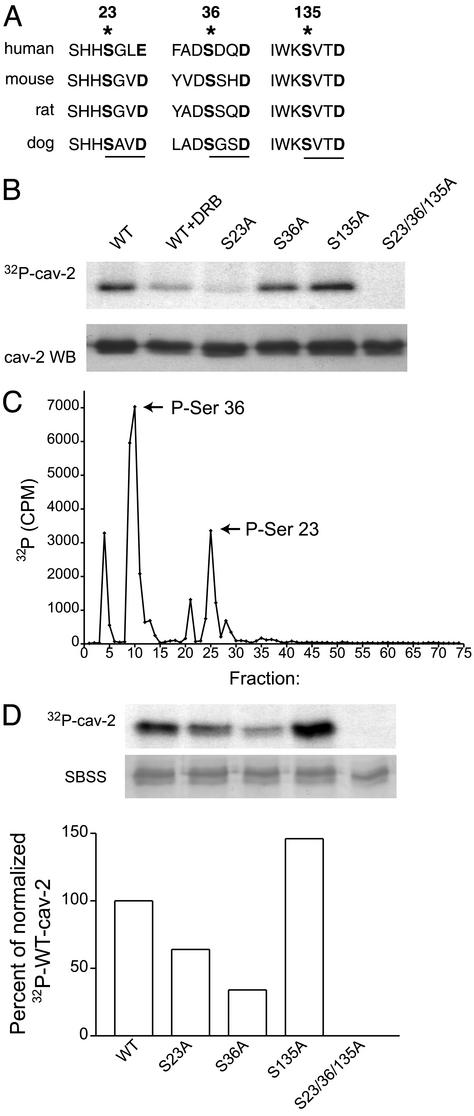
Caveolin-2 Is Constitutively Phosphorylated in Vivo: Evidence for the Involvement of Casein Kinase 2 (CK2). In search for a possible mechanism to explain the contribution of caveolin-2 to caveolae formation, we looked for unique features in caveolin-2 compared with caveolin-1. We determined that unlike caveolin-1, there was the overall negative charge on caveolin-2, especially prominent on the N terminus, which could be further increased assuming that it is a phospho-protein (29). Indeed, human caveolin-2 has several potential CK2 phosphorylation sites (amino acids 23, 36, and 135; Fig. 2A). Because CK2 substrates are involved in many fundamental cellular processes, we examined whether CK2 or CK2-like kinase can phosphorylate caveolin-2 in vivo. To this end, LNCaP cells were transfected with the cDNAs for wild-type caveolin-2, or the point mutants S23A, S36A, or S135A and the triple mutant S23/36/135A caveolin-2 and metabolically labeled cells with 32P orthophosphate. There was strong 32P incorporation into immunoprecipitated, wild-type caveolin-2 (Fig. 2B Upper, lane 1). The incorporation of 32P was inhibited (≈60%) by treatment with the specific inhibitor of CK2, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB; 30 μM); lane 2, suggesting the involvement of CK2 or a CK2-like kinase. Mutation of serine 23 to alanine (S23A) markedly inhibited 32P incorporation into caveolin-2 (lane 3), whereas mutation of serine 36 to alanine (S36A) only slightly decreased phosphate incorporation (lane 4), and mutation of serine 135 to alanine (S135A) had no effect on phosphate incorporation (lane 5). Finally, simultaneous mutation of all three serines to alanines (S23/36/135A) completely abolished phosphate incorporation (lane 6) into caveolin-2, suggesting that serines 23 and 36 were major sites of phosphorylation in vivo.
Fig. 2.
Phosphorylation of caveolin-2 in vivo and in vitro. (A) The putative CK 2 phosphorylation sites is conserved. The asterisks denote the potential phosphorylation sites and lines represent consensus sites for CK2. (B) The incorporation of 32P into immunoprecipitated wild-type caveolin-2 and various mutants is shown. LNCaP cells were transiently transfected with the cDNAs (Upper), followed by immunoblotting for caveolin-2 (Lower). (C) The distribution of radio-active peptides by reverse-phase HPLC is shown. The arrows depict fractions in which tryptic peptides containing serines 23 and 36 were detected by mass spectrometry. (D) 32P labeling of caveolin-2 and its mutated forms expressed as GST-fusion proteins were subjected to in vitro phosphorylation by CK2 (Top) and stained with SBSS (Middle). (Botom) Quantitative analysis of labeling data, normalized to 32P incorporation into wild-type caveolin-2.
CK2 Phosphorylates Serines 23 and 36 in Vitro. To examine whether CK2 can phosphorylate caveolin-2, GST fused caveolin-2 was incubated with recombinant CK2 in an in vitro kinase reaction and the phosphorylation sites mapped by HPLC/mass spectrometry. As seen in Fig. 2C, the well resolved radioactive peptides corresponding to fractions 4, 10, and 25 were then analyzed by matrix-assisted laser desorption ionization MS. The first radioactive fraction was composed of partially digested fragments, whereas fraction 10 contained a phosphopeptide mapped to serine 36 by radiosequence analysis and fraction 25 contained a phosphopeptide corresponding to serine 23.
To confirm the mass spectrometry data, GST fusions of wild type, S23A, S36A, S135A, and the triple mutant (S23/36/135A) caveolin-2 were generated and incubated in vitro with CK2. Mutations of serine 23 or 36 decreased 32P incorporation by 35% and 70%, respectively, compared with wild-type caveolin-2 (Fig. 2D), and the level of inhibition by each mutation strictly correlated with radioactivity measured by Cerenkov counting in fractions containing the tryptic peptides (see Fig. 2C). Consistent with the previous data, mutation of C-terminal serine 135 to alanine did not decrease the phosphorylation of caveolin-2. However, simultaneous mutation of the three serines to alanine resulted in a complete loss of phosphorylation of the caveolin-2 protein by CK2 in vitro, similar to the in vivo phosphorylation experiments. Collectively, these data show that CK2 can phosphorylate caveolin-2 on serines 23 and 36.
Phospho-Serine-Specific Antibodies Reveal That Caveolin-2 Is Phosphorylated in Vivo at Both Serines 23 and 36 in a CK2-Dependent Manner. The previous results are consistent with, but do not prove, that serines 23 and 36 are phosphorylated in vivo. To examine whether these residues are indeed phosphorylated in vivo, sequence-specific phospho-antibodies were generated. As seen in Fig. 3A, transfection of LNCaP cells with wild-type caveolin-2 results in phosphorylation on serine 23 by using the phospho-serine 23 (p-Ser 23) polyclonal antibody (Fig. 3A Top, lane 1), an effect absent in cells transfected with S23A caveolin-2 (Fig. 3A Top, lane 2). However, S23 phosphorylation is still present in cells transfected with the S36A caveolin-2 (Fig. 3A Top, lane 3). Conversely, transfection of LNCaP cells with wild-type caveolin-2 results in phosphorylation on serine 36 by using the phospho-serine 36 (p-Ser 36) polyclonal antibody (Fig. 3A Middle, lane 1), an effect absent in cells transfected with S36A caveolin-2 (Fig. 3A Middle, lane 3). However, S36 phosphorylation is still present in cells transfected with the S23A caveolin-2 (Fig. 3A Middle, lane 2). In cells transfected with S135A caveolin-2, phosphorylation on S23 and S36 is unperturbed (Fig. 3A Middle, lane 4), documenting the specificity of these antibodies. Moreover, the phosphorylation of caveolin-2 is found in other human cell lines expressing endogenous caveolin-2 including human umbilical vein endothelial cells and A431 cell line (not shown).
Fig. 3.
Phospho-serine-specific caveolin-2 antibodies reveal that caveolin-2 is phosphorylated in vivo at both serines 23 and 36 with the possible involvement of CK2. (A) The immunoblots of cell lysates from LNCaP cells expressing wild-type and mutant forms of caveolin-2 are shown. (B) Cells expressing caveolin-2 were lysed, immunoprecipitated with caveolin-2 mAb, control (lane 1), subjected to CK2 assay (lane 2), or treated with CIP at 37C (lane 3), and Western blotted with P-Ser 23 (Top), stripped, and reblotted with P-Ser 36 (Middle) pAbs, followed by reblotting with a monoclonal caveolin-2 antibody (Total cav-2; Bottom). (C) Cells expressing caveolin-2 were treated with 30 μM DRB at indicated times, and lysates containing equal amount of caveolin-2 were resolved and Western blotted as described in B.
To gain an appreciation for the relative stoichiometry of phosphorylation in vivo, we immunoprecipitated lysates of LNCaP cells transiently expressing caveolin-2 with caveolin-2 antibody. Lysates were equally divided and incubated with CK2 in an in vitro kinase assay, or after the in vitro kinase assay, treated with calf intestinal phosphatase (CIP). As seen in Fig. 3B, caveolin-2 is phosphorylated on serines 23 and 36 (Fig. 3B, lane 1) and the levels of phosphorylation on both residues can be increased by incubation with CK2 in vitro (Fig. 3B, lane 2). The densitometric analysis data of Western blot signal of control and subjected to in vitro phosphorylation by CK2 samples suggest that <30% of both serines are phosphorylated in vivo at a steady state. Finally, to examine the involvement of CK2 in phosphorylating these residues in vivo, LNCaP cells transiently expressing caveolin-2 were treated with DRB (30 μM) for the indicated times (Fig. 3C), and the levels of serine 23 and 36 phosphorylation were determined. DRB treatment for 12 h reduced the level of phosphorylation of both serine 23 and 36 by ≈50% relative to total caveolin-2 levels. Additionally, the inhibitor of phosphatidylinositol 3-kinase, LY 294002 that also inhibits CK2, reduced serine 23 and 36 phosphorylation but another phosphatidylinositol 3-kinase inhibitor, wortmannin, a generalized protein kinase C inhibitor, staurosporine, and a protein kinase A inhibitor, H-89, did not influence caveolin-2 phosphorylation using the phospho-specific antibodies (data not shown). These data suggest that CK2 or a CK-like kinase can constitutively phosphorylate serines 23 and 36 on caveolin-2, and that the turnover of phosphate on these residues is relatively slow.
Phosphorylation of Caveolin-2 Is Important for the Formation of Invaginated Caveolae. To examine the importance of caveolin-2 phosphorylation on caveolae assembly, we transiently expressed caveolin-2 and its phosphorylation mutants in LNCaP cells stably expressing caveolin-1 and performed quantitative morphometry. As seen in Table 1, LNCaP cells stably expressing caveolin-1 do not have any plasmalemmal attached caveolae, but do have uncoated (smooth) vesicles in close proximity to the plasma membrane. The number of smooth vesicles was 2-fold higher in caveolin-1 transfected LNCaP cells relative to parental cells (see previous results). Expression of caveolin-2 induced the formation of plasmalemmal attached caveolae and markedly increased the number of smooth vesicles. Mutation of serine 23 to alanine slightly (≈10%) decreased number of caveolae, and doubled the number of smooth vesicles found in proximity to the plasma membrane (Table 1), suggesting that phosphorylation of serine 23 may influence caveolae-type vesicle exocytosis/endocytosis. Moreover, mutation of serine 36 to alanine, greatly reduced number of plasmalemma attached caveolae by >70% but not the number of smooth vesicles within 50–100 nm of the plasma membrane. Finally, S23/36/135A caveolin-2, which cannot be phosphorylated because of simultaneous mutation of both serines 23 and 36, did not yield any detectable caveolae formation, but resulted in an ≈3-fold increase in the number of smooth vesicles within 50–100 nm of the plasma membrane surface compared with cells expressing wild-type caveolin-2. To exclude the likelihood that replacement of serine 36 by alanine could affect caveolae formation indirectly, perhaps via a nonspecific structural change that would influence the assembly of the protein coat of caveolae, we mutated serine 36 to aspartate (S36D) in an attempt to mimic the negative charge of the corresponding phosphate. Interestingly, transfection of S36D caveolin-2 increased the number of attached caveolae compared with cells expressing wild type caveolin-2, whereas the number of smooth vesicles was similar. Collectively, these data show that phosphorylations of caveolin-2 on serines 23 and 36 are crucial steps in the formation of plasmalemmal attached caveolae suggesting that the phospho-state of caveolin-2 can influence the relative number of smooth vesicles versus plasmalemma attached caveolae.
Table 1. Phosphorylation of caveolin-2 regulates the number of caveolae versus subplasmalemmal uncoated vesicles in LNCaP cells.
| cav-1 (clone 5) + various cav-2 cDNAs | Caveolae | Uncoated vesicles | Coated pits | Coated vesicles | Length of PM, mm |
|---|---|---|---|---|---|
| - | 0 | 49 | 45 | 26 | 0.245 |
| WT | 35 | 93 | 35 | 25 | 0.202 |
| S23A | 32 | 189 | 43 | 27 | 0.185 |
| S36A | 10 | 86 | 29 | 24 | 0.205 |
| S23/36/135A | 0 | 238 | 29 | 22 | 0.173 |
| S36D | 51 | 98 | 35 | 23 | 0.205 |
Wild-type and various mutant forms of caveolin-2 were expressed at equal levels (based on densitometric determination of Western blot signal) in stably expressing caveolin-1 LNCaP cells, fixed, postfixed, and processed for EM (Supporting Text). Numbers of plasma membrane structures, i.e., caveolae with a visible connection with the cell surface, as well as uncoated and coated vesicles and coated pits within 50 nm from the cell surface were assessed as described in Supporting Text.
To rule out the possibility that the concurrent mutation of three amino acids on caveolin-2 (S23/36/135A caveolin-2) could affect the general biophysical properties of the protein, we compared S23/36/136A caveolin-2 to the wild-type protein (Fig. 4) in several assays. S23/36/135A caveolin-2 behaved identically to wild-type protein in terms of targeting to the perinuclear Golgi region in the absence of caveolin-1 (Fig. 4A Left, depicted by arrowheads) and its ability to be redistributed to plasma membrane in cells stably expressing caveolin-1 (Fig. 4A Right, indicated by arrows), SDS-resistant oligomer formation (Fig. 4B), as well as its insolubility in TX-100 (Fig. 4C). In summary, phosphorylation of caveolin-2 is necessary for formation of plasma membrane attached caveolae in LNCaP cells, because simultaneous mutation of serines 23 and 36 prevents both phosphorylation of caveolin-2 and formation of these organelles in cells coexpressing caveolin-1.
Fig. 4.
Subcellular targeting and biophysical properties of S23/36/135A remain indistinguishable from wild-type caveolin-2. (A) Parental (Left) or stably expressing caveolin-1 clone 5 of LNCaP cells (Right) were fixed and labeled with caveolin-2 mAb. Arrowheads point to perinuclear caveolin-2 in LNCaP cells expressing caveolin-2 only. Arrows denote examples of plasma membrane caveolin-2 in LNCaP cells stably expressing caveolin-1. (Scale bar, 5 μm.) (B) LNCaP cells expressing either wild-type or S23/36/135A caveolin-2 were lysed in SDS/PAGE loading buffer and boiled (+) or left at room temperature for 30 min (–), and proteins were resolved on a 3–17% gradient SDS/PAGE and subsequently Western blotted with caveolin-2 and 1 Abs. Arrowheads point to oligomers, bands migrating above 200 kDa, and monomers, bands below 33 kDa, of caveolins. (C) Cells expressing wild-type or S23/36/135A caveolin-2 were lysed with MBS buffer (pH 6.5) containing 1% Triton X-100 and centrifuged, and pellets were resuspended with equal volumes of SDS/PAGE loading buffer and Triton X-100 soluble (S) and insoluble (I) proteins resolved on a SDS/13% PAGE, and subsequently Western blotted with caveolin-2 and -1 antibodies.
Discussion
Here we show that in a reconstituted system lacking caveolins, caveolin-1 and caveolin-2 are both necessary for the formation of plasmalemmal attached caveolae and smooth, uncoated vesicles. The modulatory effect of caveolin-2 on caveolin-1-dependent caveolae assembly involves the constitutive phosphorylation of caveolin-2 at serines 23 and 36 by CK2 or a CK2-like kinase. Mutation of these residues reduces the stimulatory effect of caveolin-2 on plasma membrane-attached caveolae, suggesting that serine phosphorylation of caveolin-2 may regulate the exocytosis/endocytosis of caveolin-1-dependent vesicles destined to be plasmalemmal caveolae.
Previously it was shown that canine caveolin-2 is constitutively phosphorylated in MDCK cells; however, the kinase(s) involved and the sites and function of phosphorylation were not examined (29). A recent report has identified phosphorylation of caveolin-2 at tyrosine 19 in a cell line stably overexpressing c-Src but not in control cells (39), based on it analogy to the src phosphorylation site, tyrosine 14, in caveolin-1. These authors speculate that tyrosine phosphorylation of caveolin-2 may provide an SH2 docking site for other adaptor molecules. In the present study, human caveolin-2 is phosphorylated on serine residues 23 and 36, sites that conform to the canonical consensus site for CK2 (*S/T-x-x-E/D). Indeed, serines 23 and 36 of caveolin-2 can be phosphorylated in vitro by CK2, and the overall incorporation of 32P in vivo can be attenuated by mutation of these residues or by treatment of cells with the specific CK2 inhibitor, DRB.
Further evidence supporting the endogenous phosphorylation of these residues is demonstrated by the successful generation of novel phosphorylation state-specific antibodies against phospho-serines 23 and 36 in human caveolin-2. Both antibodies recognize phosphorylated, but not unphosphorylated, serines (mutated to alanine or dephosphorylated with CIP) of caveolin-2. By using these antibodies, we were further able to confirm that both of these amino acids are phosphorylated in vivo and that CK2 is a candidate kinase. Treatment of cells with inhibitors of other protein kinases A, B, and C did not influence caveolin-2 serine phosphorylation (data not shown); however, we cannot rule out the possibility that another CK2 like kinase may also phosphorylate these sites in vivo.
Mutational analysis and quantitative morphometry of membrane-attached caveolae versus noncoated vesicles revealed that phosphorylation of serines 23 and 36 regulates caveolae assembly. For example, mutation of serine 36 to alanine (S36A caveolin-2) markedly reduced the number of plasmalemmaattached caveolae. Moreover, mutation of this serine to aspartate (S36D caveolin-2), to mimic the negative charge of phosphate, increased the number of attached caveolae to levels similar to wild-type caveolin-2. Phosphorylation of serine 23, though marginally influencing the formation of plasmalemma attached caveolae (i.e., S23A mutant,) enhanced the number of uncoated vesicles. The triple mutant S23/36/135A eliminated caveolae but markedly enhanced the appearance of noncoated vesicles. Importantly, the inability of S23/36/135A caveolin-2 to support the formation of plasmalemma attached caveolae and increase the population of noncoated vesicles is not caused by impaired biophysical properties or mislocalization of the mutant (see Fig. 4). However, we cannot rule out the possibility that serine 135 contributes to formation of a caveolae via some mechanism which is independent of phosphorylation (i.e., mutation of S135 to alanine alone did not influence 32P incorporation or detection of phospho-serines 23 and 36).
Interestingly, in our model cell system lacking caveolin-1 and -2, we demonstrate that both caveolin-1 and -2 are required for the formation of membrane-attached caveolae. Previous work has shown caveolin-1 overexpression increases the number of subplasmalemmal vesicles, and is some instances can generate few attached caveolae (23, 25–28, 40). Thus, caveolin-2 seems to serve an important accessory role by enhancing the formation of invaginated caveolae in the presence of caveolin-1 consistent with its role in caveolae formation in various cell types examined by us or others (29, 30). On the other hand, in the original description of caveolin-2 (–/–) mice, caveolae are still present in ultra thin sections from lung capillary endothelium and in perigonadal adipose tissue (33); however, quantitative profiles were not examined, nor were caveolae analyzed in multiple tissues. Future quantitative studies examining caveolae from caveolin-2 (–/–) mice and cells derived from these mice should help resolve these issues. Although there are several plausible explanations for this, including up-regulation of other compensatory systems during murine development, most are difficult to reconcile with experiments in cultured cells. Caveolin-1 and -2 are typically expressed in the same cells (most epithelia and endothelia) and caveolin-2 increases the number of “deep” caveolae in cells that have caveolin-1. Moreover, caveolin-1 is required for the trafficking of caveolin-2 from the Golgi to the plasma membrane and incorporation of caveolin-2 into high molecular weight oligomers in many cell types (27, 35). However, it is possible that caveolin-2 exerts cell- or tissue-specific functions in vivo as shown in caveolin-2 (–/–) mice and that cultured cells do not accurately reflect the functions of caveolin-2 in vivo.
The mechanisms of how caveolin-2 phosphorylation influences caveolae formation and function are presently not known. One possibility is that caveolae normally are formed by heterooligomers of caveolin-1 and 2, in which the phosphorylation of caveolin-2, in addition to the overall negative charge on its N terminus, could facilitate membrane targeting, vesicle fusion, invagination, and formation of deep caveolae. Perhaps the phosphorylation of caveolin-2 mediates the recruitment of other regulatory proteins to the complex that influence caveolae invagination and/or budding. Because the latter hypothesis seemed attractive, we metabolically labeled cells with [35S]methionine, and immunoprecipitated caveolin-1 from LNCaP cells expressing either caveolin-1 alone or in combination with caveolin-2. However in these preliminary experiments, we were unable to detect additional proteins stoichiometrically associated with a caveolin-1/2 complex. Similarly, Western blotting of caveolin-1/caveolin-2 immunoprecipitates with antibodies against known proteins that have been shown to associate with caveolin-1/caveolae and influence their budding such as dynamin (10, 41) were essentially negative (data not shown). Future studies dissecting the mechanistic relationships between caveolin isoforms, caveolin-2 phosphorylation vesicle trafficking, and caveolae assembly are clearly warranted, and may reveal a novel role for caveolin-2 in membrane remodeling.
Supplementary Material
Acknowledgments
We thank Dr. Michel Lisanti for the canine caveolin-1 cDNA, Dr. Enrique Rodriquez-Boulan for the Adcav-2 virus, and the University of Iowa Vector Core Laboratory for the Adcav-1 virus. This work was supported by National Institutes of Health Grants HL57665, HL61371, and HL64793 (to W.C.S.).
Abbreviations: MDCK, Madin–Darby canine kidney; CK2, casein kinase 2; DRB, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole.
References
- 1.Palade, G. E. (1953) J. Appl. Phys. 24, 1424. [Google Scholar]
- 2.Palade, G. E. (1961) Circulation 24, 368–384. [DOI] [PubMed] [Google Scholar]
- 3.Palade, G. E. (1968) J. Cell Biol. 37, 633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada, E. (1953) J. Biophys. Biochem. Cytol. 1, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, R. G. (1993) Proc. Natl. Acad. Sci. USA 90, 10909–10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parton, R. G. (1996) Curr. Opin. Cell Biol. 8, 542–548. [DOI] [PubMed] [Google Scholar]
- 7.Severs, N. J. (1988) J. Cell Sci. 90, 341–348. [DOI] [PubMed] [Google Scholar]
- 8.Fielding, P. E. & Fielding, C. J. (1995) Biochemistry 34, 14288–14292. [DOI] [PubMed] [Google Scholar]
- 9.Smart, E. J., Ying, Y., Donzell, W. C. & Anderson, R. G. (1996) J. Biol. Chem. 271, 29427–29435. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzer, J. E., Oh, P. & McIntosh, D. P. (1996) Science 274, 239–242. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, R. G., Kamen, B. A., Rothberg, K. G. & Lacey, S. W. (1992) Science 255, 410–411. [DOI] [PubMed] [Google Scholar]
- 12.Kurzchalia, T. V. & Parton, R. G. (1999) Curr. Opin. Cell Biol. 11, 424–431. [DOI] [PubMed] [Google Scholar]
- 13.Lisanti, M. P., Scherer, P. E., Vidugiriene, J., Tang, Z., Hermanowski-Vosatka, A., Tu, Y. H., Cook, R. F. & Sargiacomo, M. (1994) J. Cell Biol. 126, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto, C. T. (1998) Adv. Drug Delivery Rev. 29, 215–228. [DOI] [PubMed] [Google Scholar]
- 15.Shaul, P. W. & Anderson, R. G. (1998) Am. J. Physiol. 275, L843–L851. [DOI] [PubMed] [Google Scholar]
- 16.Glenney, J. R., Jr., & Soppet, D. (1992) Proc. Natl. Acad. Sci. USA 89, 10517–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurzchalia, T. V., Dupree, P., Parton, R. G., Kellner, R., Virta, H., Lehnert, M. & Simons, K. (1992) J. Cell Biol. 118, 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer, P. E., Okamoto, T., Chun, M., Nishimoto, I., Lodish, H. F. & Lisanti, M. P. (1996) Proc. Natl. Acad. Sci. USA 93, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang, Z., Scherer, P. E., Okamoto, T., Song, K., Chu, C., Kohtz, D. S., Nishimoto, I., Lodish, H. F. & Lisanti, M. P. (1996) J. Biol. Chem. 271, 2255–2261. [DOI] [PubMed] [Google Scholar]
- 20.Way, M. & Parton, R. G. (1995) FEBS Lett. 376, 108–112. [DOI] [PubMed] [Google Scholar]
- 21.Dupree, P., Parton, R. G., Raposo, G., Kurzchalia, T. V. & Simons, K. (1993) EMBO J. 12, 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R. & Anderson, R. G. (1992) Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- 23.Li, S., Song, K. S., Koh, S. S., Kikuchi, A. & Lisanti, M. P. (1996) J. Biol. Chem. 271, 28647–28654. [DOI] [PubMed] [Google Scholar]
- 24.Monier, S., Parton, R. G., Vogel, F., Behlke, J., Henske, A. & Kurzchalia, T. V. (1995) Mol. Biol. Cell 6, 911–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fra, A. M., Williamson, E., Simons, K. & Parton, R. G. (1995) Proc. Natl. Acad. Sci. USA 92, 8655–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipardi, C., Mora, R., Colomer, V., Paladino, S., Nitsch, L., Rodriguez-Boulan, E. & Zurzolo, C. (1998) J. Cell Biol. 140, 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parolini, I., Sargiacomo, M., Galbiati, F., Rizzo, G., Grignani, F., Engelman, J. A., Okamoto, T., Ikezu, T., Scherer, P. E., Mora, R., et al. (1999) J. Biol. Chem. 274, 25718–25725. [DOI] [PubMed] [Google Scholar]
- 28.Vogel, U., Sandvig, K. & van Deurs, B. (1998) J. Cell Sci. 111, 825–832. [DOI] [PubMed] [Google Scholar]
- 29.Scheiffele, P., Verkade, P., Fra, A. M., Virta, H., Simons, K. & Ikonen, E. (1998) J. Cell Biol. 140, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto, T., Kogo, H., Nomura, R. & Une, T. (2000) J. Cell Sci. 113, 3509–3517. [DOI] [PubMed] [Google Scholar]
- 31.Drab, M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., Menne, J., Lindschau, C., Mende, F., Luft, F. C., et al. (2001) Science 293, 2449–2452. [DOI] [PubMed] [Google Scholar]
- 32.Razani, B., Engelman, J. A., Wang, X. B., Schubert, W., Zhang, X. L., Marks, C. B., Macaluso, F., Russell, R. G., Li, M., Pestell, R. G., et al. (2001) J. Biol. Chem. 276, 38121–38138. [DOI] [PubMed] [Google Scholar]
- 33.Razani, B., Wang, X. B., Engelman, J. A., Battista, M., Lagaud, G., Zhang, X. L., Kneitz, B., Hou, H., Jr., Christ, G. J., Edelmann, W. & Lisanti, M. P. (2002) Mol. Cell. Biol. 22, 2329–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowa, G., Pypaert, M. & Sessa, W. C. (2001) Proc. Natl. Acad. Sci. USA 98, 14072–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mora, R., Bonilha, V. L., Marmorstein, A., Scherer, P. E., Brown, D., Lisanti, M. P. & Rodriguez-Boulan, E. (1999) J. Biol. Chem. 274, 25708–25717. [DOI] [PubMed] [Google Scholar]
- 36.Sargiacomo, M., Scherer, P. E., Tang, Z., Kubler, E., Song, K. S., Sanders, M. C. & Lisanti, M. P. (1995) Proc. Natl. Acad. Sci. USA 92, 9407–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danielsen, E. M. (1995) Biochemistry 34, 1596–1605. [DOI] [PubMed] [Google Scholar]
- 38.Lisanti, M. P., Tang, Z., Scherer, P. E. & Sargiacomo, M. (1995) Methods Enzymol. 250, 655–668. [DOI] [PubMed] [Google Scholar]
- 39.Lee, H., Park, D. S., Wang, X. B., Scherer, P. E., Schwartz, P. E. & Lisanti, M. P. (2002) J. Biol. Chem. 277, 34556–24567. [DOI] [PubMed] [Google Scholar]
- 40.Lee, S. W., Reimer, C. L., Oh, P., Campbell, D. B. & Schnitzer, J. E. (1998) Oncogene 16, 1391–1397. [DOI] [PubMed] [Google Scholar]
- 41.Oh, P., McIntosh, D. P. & Schnitzer, J. E. (1998) J. Cell Biol. 141, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.