Abstract
The antifolate methotrexate is one of the most successful drugs in cancer chemotherapy. Although its efficacy is widely attributed to a decrease in nucleotide biosynthesis (1), methotrexate is known to increase homocysteine (2), a compound associated with an elevated risk of heart disease, Alzheimer's disease (3), and neural tube defects (4). A potential mechanism for the detrimental effects of homocysteine is cellular hypomethylation from an increase in S-adenosylhomocysteine (5), an inhibitor of methyltransferases including isoprenylcysteine carboxyl methyltransferase (Icmt). Among the substrates of Icmt is the monomeric G protein Ras, a critical component of many signaling pathways that regulate cell growth and differentiation. Because carboxyl methylation of Ras is important for proper plasma membrane localization and function (6), we investigated the role of Icmt in the antiproliferative effect of methotrexate. After methotrexate treatment of DKOB8 cells, Ras methylation is decreased by almost 90%. This hypomethylation is accompanied by a mislocalization of Ras to the cytosol and a 4-fold decrease in the activation of p44 mitogen-activated protein kinase and Akt. Additionally, cells lacking Icmt are highly resistant to methotrexate. Whereas cells expressing wild-type levels of Icmt are inhibited by methotrexate, stable expression of myristoylated H-Ras, which does not require carboxyl methylation for membrane attachment (7), confers resistance to methotrexate. These results suggest that inhibition of Icmt is a critical component of the antiproliferative effect of methotrexate, expanding our understanding of this widely used drug and identifying Icmt as a target for drug discovery.
Methotrexate has been used to treat cancer for over 50 yr. Developed in the early 1950s to treat children with acute lymphocytic leukemia, methotrexate is still a key component of the standard chemotherapy regimen for treating leukemia and several other cancers. Methotrexate also has several applications outside the field of oncology, including the treatment of psoriasis and rheumatoid arthritis (1, 8). Methotrexate is an antifolate; as such, it interferes with the cellular metabolism of folate. In particular, methotrexate inhibits the activity of dihydrofolate reductase, along with three enzymes involved in purine and thymidine biosynthesis (1). The efficacy of methotrexate in treating cancer is widely attributed to a decrease in the production of nucleotides (1, 3).
In addition to effects on nucleotide biosynthesis, methotrexate treatment has been linked to a decrease in cellular methylation (9, 10). Methylation reactions use S-adenosyl methionine (AdoMet) as a methyl group donor to produce a methylated product and S-adenosyl homocysteine (AdoHcy). AdoHcy is broken down by AdoHcy hydrolase into adenosine and homocysteine. Homocysteine is then rapidly remethylated to form methionine in a reaction that uses 5-methyltetrahydrofolate as a methyl group donor (11, 12). In folate-deficient cells, including cells treated with antifolates, depletion of 5-methyltetrahydrofolate (13) blocks the remethylation of homocysteine. The resulting accumulation of homocysteine drives AdoHcy hydrolase to catalyze the energetically favorable reverse reaction and synthesize AdoHcy (14, 15, 5), a potent product inhibitor of cellular methyltransferases (12). Such a decrease in methylation has been overlooked as a mechanism for the antiproliferative effect of antifolates.
One major class of methylated proteins is the CaaX-type prenyl proteins, including the Ras family of GTP-binding proteins. Ras is a central component in many signal transduction pathways, including pathways important for cell growth and differentiation, and proper membrane association is important for Ras function (16–18). Ras and other CaaX proteins are classified by a C-terminal tetrapeptide motif that signals a series of posttranslational modifications, beginning with the addition of an isoprenoid lipid. After the covalent attachment of the isoprenoid lipid to the cysteine of the CaaX motif, the “aaX” tripeptide is removed in a reaction catalyzed by a prenyl protein-specific protease, Rce1 (19, 20). Finally, in a reaction that consumes AdoMet, the now C-terminal prenylcysteine is methylated by isoprenylcysteine carboxyl methyltransferase (Icmt; ref. 21).
Although isoprenoid modification of Ras proteins is the primary determinant of their ability to associate with, and function at, cellular membranes, the subsequent steps of proteolysis and methylation are clearly important. Disruption of either the Rce1 or Icmt genes in mice results in embryonic lethality (22, 23). Ras is largely mislocalized to the cytosol in Icmt–/– mouse embryonic stem cells, suggesting that this processing step is critical for trafficking and/or stable membrane association (6).
Because antifolates are known to increase intracellular levels of AdoHcy (10), we speculated that a resulting decrease in carboxyl methylation of Ras might be a mechanism of action for methotrexate. In this study, we show that AdoHcy inhibits Icmt and that, in methotrexate-treated cells, this inhibition leads to a decrease in the methylation of Ras. After methotrexate treatment, Ras is mislocalized to the cytosol, and its signaling functions are impaired. In addition, we demonstrate that cells lacking Icmt are significantly resistant to the growth inhibitory effects of methotrexate. These studies suggest that methotrexate has an additional mechanism of action involving a decrease in Icmt activity that leads to an inhibition in Ras signaling.
Materials and Methods
Determination of Icmt Activity. The cDNA encoding human ICMT (21) was cloned from a human fetal brain library. After sequence verification, the cDNA was subcloned into the baculovirus expression vector pDEST8. Recombinant baculovirus was prepared following established protocols, and membranes containing recombinant human ICMT were isolated as described (20). ICMT activity was determined by using farnesylated, Rce1-treated K-Ras (20, 6) and [3H-methyl]AdoMet [7.5 Ci/mmol; (1 Ci = 37 GBq)] as substrates. The final concentration of K-Ras in the reaction mixtures was 2.5 μM, and the final AdoMet concentration was 6.5 μM. To initiate methyltransferase reactions, Sf9 membranes containing recombinant ICMT (100 ng of protein) were added, and the reaction mixtures were incubated at 37° for 10 min. Reactions were terminated and [3H-methyl]K-Ras was quantified by filter binding as described (20).
Analysis of Base-Labile Methylation in Treated Cells. DKOB8 cells (a gift from Gideon Bollag, Onyx Pharmaceuticals) were grown to 70% confluence and labeled with L-[3H-methyl]methionine (Perkin–Elmer) as described (24). Concurrent with labeling, cells were treated with 10 μM ponasterone A and 10 μM methotrexate (Sigma) where indicated. After 24 h, cells were harvested, resuspended in lysis buffer (10 mM Tris·HCl, pH 7.7/1 mM MgCl2/0.5 mM PMSF/10 μg/ml leupeptin/10 μg/ml aprotinin/1 μM DTT/0.2% cholate), and lysed in a dounce homogenizer. NaCl was added to a final concentration of 150 mM, and lysates were centrifuged at 5,000 × g for 10 min. Lysates were fractionated on 15% SDS/polyacrylamide gels and processed for incorporation of [3H] into base-labile methyl groups as described (24). Total Ras levels in the DKOB8 cells were assayed by immunoblot analysis using anti-Ras antibody 873 (20).
Localization of GFP-Tagged Proteins. Mouse embryonic fibroblasts (MEFs) were grown as described (23). Icmt+/+ MEFs (line L2), grown to 60% confluence, were either left untreated or treated with 1 μM methotrexate for 24 h before transfecting with pEGFP H-, K-, or N-Ras (25), or pYesGFP (26; a gift from Luc Berthiaume of the University of Alberta) as described. Cells were imaged 18 to 24 h after transfection by using an LSM-410 laser scanning confocal microscope with a ×40 or ×63 oil-immersion objective (Carl Zeiss).
Analysis of Ras Signaling Pathways. To establish Icmt–/–/ICMT cells (designated line E8-84B), the human ICMT cDNA was cloned into pcDNA6 and Icmt–/– MEFs (line E8) transfected with 1 μg of FspI-linearized vector. Clonal cell lines were isolated after selection for 14 days with blasticidin (12 μg/ml). ICMT activity in the Icmt–/–/ICMT cells was ≈50% of that of Icmt+/+ MEFs. For analysis of Ras signaling, Icmt+/+, Icmt–/–, and Icmt–/–/ICMT MEFs were grown to 70% confluence and either left untreated or treated for 24 h with 1 μM methotrexate, after which the media were replaced with drug-free media containing 1% FBS. After 48 h in 1% FBS, half of the samples were treated with 10 ng/ml epidermal growth factor (EGF; Calbiochem) for 10 min, whereupon cells were rinsed in PBS and harvested. Samples containing equal quantities of protein were resolved on 13% SDS/polyacrylamide gels and proteins transferred to nitrocellulose for immunoblot analysis. Blots were probed with anti phospho-p42/44 mitogen-activated protein kinase (MAPK) antibody (Cell Signaling Technology, Beverly, MA), anti phospho-Akt antibody (Cell Signaling Technology), or anti β-tubulin antibody (Santa Cruz Biotechnology) as indicated.
Analysis of Cell Growth. To examine the effect of drug treatment on cell growth, MEFs were seeded at 1,700 cells per cm2 as above and grown for 24 h before addition of drug (1 μM methotrexate or 5 nM vinblastine). After 24 h of drug treatment, the media on control and methotrexate-treated cells was replaced with drug-free media, and cell culture was continued for 3 days. Cells were harvested by trypsin treatment and quantitated by using a Coulter cell counter (Beckman Coulter); triplicate samples were processed and analyzed for each condition. Because Icmt+/+ cells grow approximately twice as fast as Icmt–/– cells (data not shown), cell growth was normalized to the growth of matched untreated cells over the same time period. In one set of experiments (see Fig. 4B), the media were supplemented with thymidine (1 μM; Sigma) daily as noted.
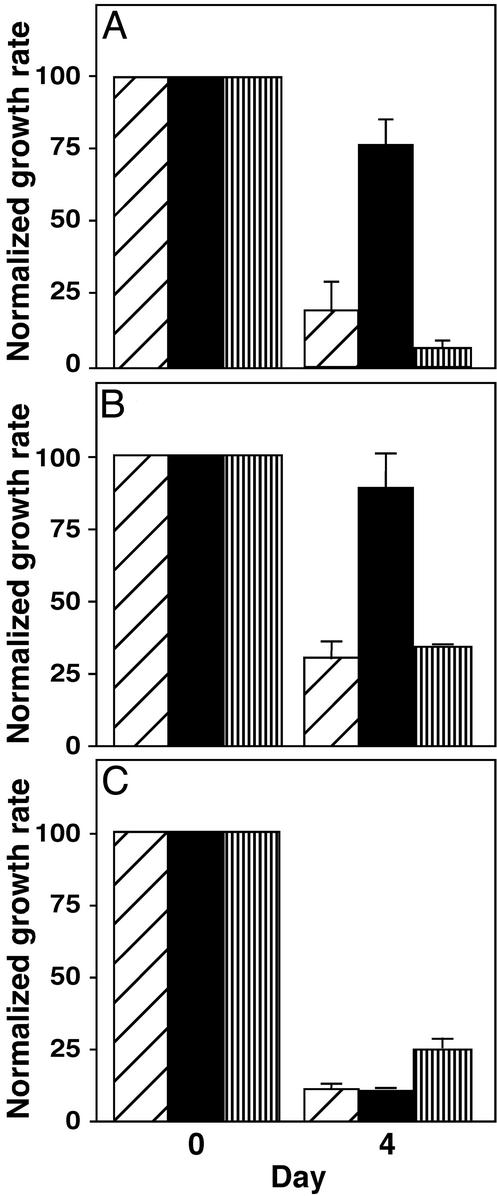
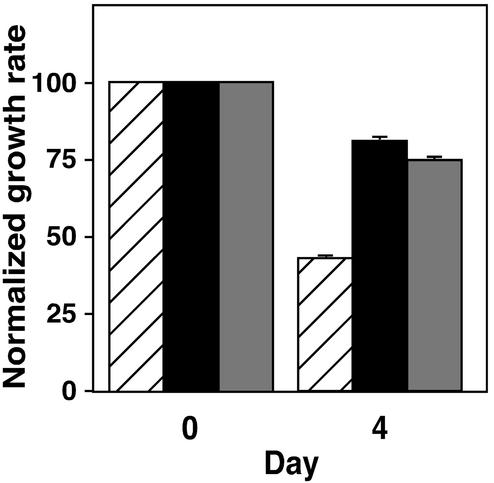
Fig. 4.
Effect of methotrexate on growth of Icmt+/+ and Icmt–/– mouse embryonic fibroblasts. (A) Inhibition of Icmt+/+, Icmt–/–, and Icmt–/–/ICMT mouse embryonic fibroblasts after methotrexate treatment. Icmt+/+ cells (diagonally striped bars), Icmt–/– cells (black bars), and Icmt–/– cells stably transfected with a gene expressing human ICMT (Icmt–/–/ICMT cells; vertically striped bars) were treated with 1 μM methotrexate for 24 h. Four days after the addition of methotrexate, cells were harvested and counted as described in Materials and Methods. The data represent the mean and standard deviation of triplicate analyses of each condition. Because Icmt+/+ cells grow approximately twice as fast as Icmt–/– cells (data not shown), cell growth was normalized to the growth of matched untreated cells. (B) Effect of thymidine on the sensitivity of Icmt+/+, Icmt–/–, and Icmt–/–/ICMT cells to methotrexate treatment. Icmt+/+ cells (diagonally striped bars), Icmt–/– cells (black bars), and Icmt–/–/ICMT cells (vertically striped bars) were treated and harvested as in A, except that media were supplemented daily with thymidine (1 μM). Cell growth was normalized to the growth of matched untreated cells. (C) Effect of vinblastine on the growth of Icmt+/+, Icmt–/–, and Icmt–/–/ICMT cells. Icmt+/+ cells (diagonally striped bars), Icmt–/– cells (black bars), and Icmt–/–/ICMT cells (vertically striped bars) were treated and harvested as in A except that the drug used was vinblastine (5 nM) rather than methotrexate, and the vinblastine level was maintained throughout the course of the experiment. Cell growth was normalized to the growth of matched untreated cells.
Retroviral Transfection of Mouse Embryonic Fibroblasts. Retroviral constructs were generated by cloning activated H-Ras (Q61L) with or without an 11-aa N-terminal myristoylation signal (a gift from Dr. Adrienne Cox, University of North Carolina at Chapel Hill; ref. 7) into the pLPCX retroviral vector (CLONTECH). These constructs, along with empty vector, were transfected into EcoPak, an ecotropic packaging cell line (CLONTECH). Virus was harvested 48 h after transfection and used to infect Icmt+/+ MEFs at ≈30% confluence. Cells were incubated with virus for 24 h, then allowed to recover for an additional 24 h. Cells were then selected for expression of the retroviral construct in 2 μg/ml puromycin, a concentration that killed all uninfected cells.
Miscellaneous Procedures. AdoHcy levels in mouse embryonic fibroblasts were determined as described (27). Visualization of protein levels on immunoblots was performed by using Renaissance chemiluminescence reagents (Perkin–Elmer) as per the manufacturer's instructions.
Results and Discussion
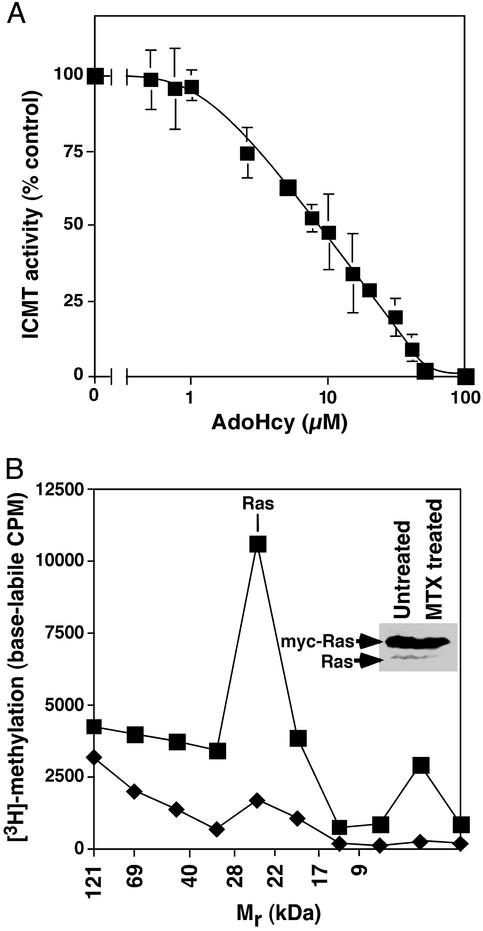
AdoHcy Inhibits Mammalian Icmt. Antifolates increase homocysteine and AdoHcy in vivo (10, 28). Consistent with these reports, treatment of mouse embryonic fibroblasts or DKOB8 human colon cancer cells with 1 μM methotrexate results in a marked increase in intracellular AdoHcy (data not shown). AdoHcy is known to inhibit many methyltransferases (12, 29), but because Icmt has a unique AdoMet binding motif (21), we felt it important to determine the sensitivity of Icmt to AdoHcy. Inhibition of recombinant human ICMT was detectable at an AdoHcy concentration of 2.5 μM, with 50% inhibition occurring at 7.5 μM (Fig. 1 A).
Fig. 1.
Inhibition of ICMT-catalyzed methylation. (A) Inhibition of ICMT by AdoHcy. ICMT activity on farnesylated, RCE1-treated K-Ras was determined in the absence of AdoHcy and in the presence of the indicated concentrations of the competitor AdoHcy as described in Materials and Methods. Product formation is reported as a percentage of that determined in the absence of the AdoHcy competitor. (B) Inhibition of ICMT-catalyzed methylation in methotrexate-treated cells. DKOB8 cells were labeled with [3H]methionine for 24 h in the presence (♦) or absence (▪)of10 μM methotrexate. K-Ras-myc expression was induced by the addition of 10 μM ponasterone A. Total cell lysates were processed on SDS/polyacrylamide gels, and dried gel slices were treated with 1 M NaOH to liberate base-labile carboxyl methyl groups as described in Materials and Methods.(Inset) Results of an immunoblot analysis of total Ras in the untreated and methotrexate-treated cells.
Ras Carboxyl Methylation Is Decreased After Methotrexate Treatment. A predicted consequence of the elevation of AdoHcy resulting from antifolate treatment is an inhibition of Icmt and a resultant decrease in the methylation of Icmt substrates such as Ras. To test this prediction, we used [3H-methyl]methionine to label DKOB8 cells, a human colon cancer cell line engineered to express high levels of myc-tagged, mutationally activated K-Ras in response to ecdysone (24, 30). This cell line was chosen because the highly expressed, inducible K-Ras was necessary to give a good signal in this assay. Concurrent with [3H-methyl]methionine labeling, K-Ras expression was induced and cells were treated with 10 μM methotrexate. Compared with untreated controls, methotrexate treatment resulted in an overall 70% decrease in base-labile [3H]carboxyl methylation and an 88% decrease in the Mr region of Ras (Fig. 1B). Immunoblot analysis of total Ras in these samples revealed a modest decrease in the accumulation of both myc-tagged K-Ras and endogenous Ras in methotrexate-treated cells (Fig. 1B Inset) that is likely due to a decrease in the half-life of unmethylated Ras (31).
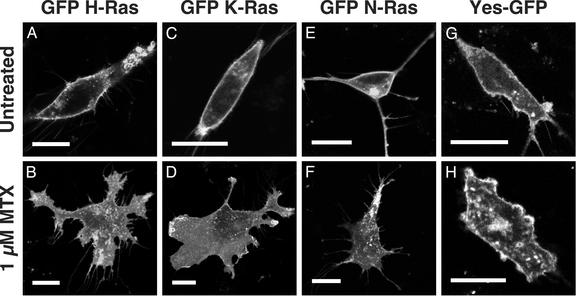
Ras Proteins Are Mislocalized in Methotrexate-Treated Cells. Ras proteins are substantially mislocalized in mouse cells that lack Icmt (6), suggesting that methotrexate treatment of cells should lead to a similar mislocalization of Ras. When the localization of GFP-tagged versions of H-, K- and N-Ras was examined in cells treated with methotrexate, all three forms of Ras were substantially mislocalized to the cytosol and/or endomembrane system (Fig. 2 A–F). As a control, methotrexate-treated cells were transfected with an N-terminal myristoylated, palmitoylated GFP (YesGFP; ref. 26). Although these cells showed a similar morphological change after exposure to methotrexate, they were still able to correctly localize YesGFP to the plasma membrane (Fig. 2 G and H).
Fig. 2.
Altered localization of Ras in methotrexate-treated mouse embryonic fibroblasts. Icmt+/+ mouse embryonic fibroblasts were left untreated (A, C, E, and G) or treated with 1 μM methotrexate (MTX; B, D, F, and H) for 24 h, whereupon the cells were transfected with expression constructs encoding GFP-tagged forms of H-Ras (A and B), K-Ras (C and D), N-Ras (E and F), or pYesGFP (G and H). Live cells were imaged on a confocal microscope 18 to 24 h after transfection. (A–H) Scale bars = 25 μm.
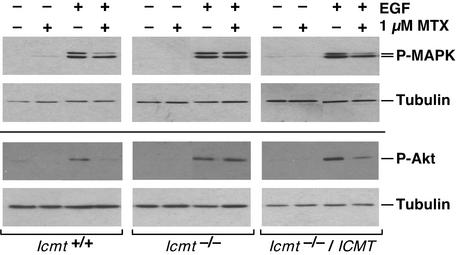
Signaling Through Ras Pathways Is Decreased in Methotrexate-Treated Cells. Ras is a key component of signaling pathways, such as those triggered by the binding of EGF to its receptor, that lead to the phosphorylation and activation of MAPK and Akt (32). After EGF activation of serum-starved mouse embryonic fibroblasts, phosphorylation of p44 MAPK and Akt was decreased by 75% in methotrexate-treated Icmt+/+ cells (Fig. 3 Left). In contrast, methotrexate treatment had no effect on the phosphorylation and activation of these proteins in Icmt–/– cells (Fig. 3 Center). Because a decrease in Ras carboxyl methylation has been correlated with a decrease in MAPK activity (18), we were surprised to detect EGF-dependent phosphorylation of p42/44 MAPK, as well as Akt, in Icmt–/– cells. The most likely explanation for this finding is that, because these signaling pathways are so critical for cell growth and survival, during the process of immortalization the Icmt–/– cells adapted an alternate mechanism to respond to EGF. However, because reintroduction of human ICMT into these cells restored the methotrexate sensitivity of p44 MAPK and Akt activation (Fig. 3 Right), the difference in response to methotrexate was clearly due to the difference in Icmt activity and not other mutations carried by these cell lines. These results provide compelling evidence that Icmt is a major target of methotrexate action. Curiously, the decrease in MAPK activation is seen in p44 MAPK but not in p42 MAPK (Fig. 3 Left), suggesting that p42 MAPK can be activated by an independent pathway that does not recognize p44 MAPK.
Fig. 3.
Effect of methotrexate on Ras signaling in Icmt+/+ and Icmt–/– mouse embryonic fibroblasts. Icmt+/+, Icmt–/–, and Icmt–/– cells stably transfected with a gene expressing human ICMT (Icmt–/–/ICMT cells) were either left untreated or treated with 1 μM methotrexate (MTX) for 24 h, then maintained for an additional 48 h without drug in reduced-serum media as described in Materials and Methods. Where indicated, cells were treated with EGF for the final 10 min before harvesting. Cell lysates containing equal amounts of protein were resolved on a 13% SDS/polyacrylamide gel and probed with antiphospho-p42/44 MAPK, antiphospho-Akt, or anti-β-tubulin antibody as indicated.
Icmt-/- Cells Are Resistant to Methotrexate. We reasoned that, if Icmt is indeed a key target of methotrexate, then Icmt–/– cells should be resistant to the antiproliferative effects of methotrexate. To test this, we treated Icmt+/+ and Icmt–/– mouse embryonic fibroblasts with methotrexate under a regimen that mimicked clinical use of the drug (i.e., a 24-h exposure followed by withdrawal of the drug). Cell growth was monitored 4 days after drug treatment. This treatment protocol revealed that Icmt–/– cells are remarkably resistant to the effects of methotrexate (Fig. 4A). Whereas treatment with 1 μM methotrexate caused an 80% inhibition in the growth of Icmt+/+ cells, under those same conditions the growth of Icmt–/– cells was inhibited by less than 25%. Because addition of thymidine to the media afforded only a slight recovery (Fig. 4B), methotrexate inhibition of thymidine biosynthesis seems to have minimal impact on these cells. Importantly, expression of human ICMT in Icmt–/– cells entirely restored their sensitivity to methotrexate (Fig. 4 A and B). Furthermore, all three cell lines were equally sensitive to the effects of a pharmacologically distinct chemotherapy drug, vinblastine (Fig. 4C). The sharp difference in methotrexate sensitivity between Icmt+/+ and Icmt–/– cells suggests that, in these cells, a major component of the activity of methotrexate is mediated through Icmt.
Myristoylated Ras Confers Resistance to Methotrexate. The addition of a myristoylation signal to the N terminus of Ras directs membrane association in the absence of C-terminal farnesylation (7). Because the CaaX sequence has been removed, these proteins are not substrates for Icmt. To assay the specific involvement of Ras proteins in cellular responses to methotrexate, we stably expressed myristoylated H-Ras in Icmt+/+ MEFs. Cell growth was then monitored after exposure to 1 μM methotrexate. In the presence of additional thymidine, methotrexate slowed the growth of vector transfected Icmt+/+ cells by greater than 50%. In contrast, the Icmt+/+ MEFs expressing myristoylated Ras had significantly less growth inhibition, with a level of methotrexate resistance similar to that seen in Icmt–/– cells (Fig. 5).
Fig. 5.
Effect of methotrexate on growth of Icmt+/+ mouse embryonic fibroblasts expressing myristoylated H-Ras. Vector-transfected Icmt+/+ cells (diagonally striped bars), Icmt–/– cells (black bars), and Icmt+/+ cells stably expressing activated myristoylated H-Ras (ref. 7; gray bars) were treated with 1 μM methotrexate for 24 h. Media were supplemented daily with thymidine (1 μM). Four days after the addition of methotrexate, cells were harvested and counted as described in Materials and Methods. The data shown represent the mean and SD of triplicate determinations from a single experiment that is representative of three such experiments. Cell growth was normalized to the growth of matched untreated cells.
A central component of many signal transduction pathways, Ras has long been recognized as a proto-oncoprotein (33). As a result, Ras has been a favorite target for drug discovery in the search for new and more effective anticancer drugs (34). Farnesyltransferase inhibitors, an emerging class of anticancer drugs, were designed specifically to decrease the membrane association of Ras; several farnesyltransferase inhibitors are showing promise in clinical trials (35). It now seems that methotrexate, one of the most successful chemotherapy drugs ever developed, may have been targeting Ras well before anyone thought to design anti-Ras drugs.
Inhibition of Icmt, the link between antifolates and Ras, is an unexpected mechanism of action for methotrexate. The finding that myristoylated Ras confers resistance to methotrexate suggests that a significant component of methotrexate's effects is mediated through Ras pathways. However, Ras isoforms represent only a fraction of the substrates of Icmt, and alteration of the activities or stability of other CaaX proteins may contribute to this mode of action of methotrexate. In this regard, it is interesting to note that, although farnesyltransferase inhibitors were initially developed as anti-Ras drugs, there is accumulating evidence that they affect cell growth by blocking modification of other CaaX proteins as well (35). Additionally, the involvement of the CaaX protein Rac in superoxide release may connect Icmt with the efficacy of methotrexate in the treatment of rheumatoid arthritis (9, 36). Finally, although our findings do not refute the evidence that inhibition of nucleotide biosynthesis is an important component of methotrexate activity, they do indicate that Icmt is a critical additional target of this chemotherapeutic agent. It is likely that the relative importance of any single mechanism of action varies from tumor to tumor, perhaps even cell to cell. This combination of effects may explain why, even though other compounds have been developed to specifically target thymidine or purine biosynthesis, antifolates remain among the most broadly successful classes of therapeutics.
Acknowledgments
We thank Ying Chen and Patrick Kelly for technical assistance and James Otto for recombinant human RCE1 and ICMT. This work was supported in part by a Howard Hughes Medical Institute Predoctoral Fellowship (to A.M.W.-V.), National Institutes of Health Grants GM46372 (to P.J.C.) and HL41633 and AG15451 (to S.G.Y.), the University of California Tobacco-Related Disease Research Program (to M.O.B. and S.G.Y.), and the Swedish Cancer Foundation (to M.O.B.). B.A.K. is an American Cancer Society Clinical Research Professor.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Icmt, isoprenylcysteine carboxyl methyltransferase; AdoMet, S-adenosyl methionine; AdoHcy, S-adenosyl homocysteine; MEF, mouse embryonic fibroblast; EGF, epidermal growth factor; MAPK, MAP kinase.
References
- 1.Takimoto, C. H. & Allegra, C. J. (1995) Oncology (Huntingt) 9, 649–56, 659; discussion 660, 662, 665.8924375 [Google Scholar]
- 2.Refsum, H., Helland, S. & Ueland, P. M. (1989) Clin. Pharmacol. Ther. 46, 510–520. [DOI] [PubMed] [Google Scholar]
- 3.Molloy, A. M. & Scott, J. M. (2001) Public Health Nutr. 4, 601–609. [DOI] [PubMed] [Google Scholar]
- 4.Rosenquist, T. H., Ratashak, S. A. & Selhub, J. (1996) Proc. Natl. Acad. Sci. USA 93, 15227–15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi, P., Melnyk, S., Pogribna, M., Pogribny, I. P., Hine, R. J. & James, S. J. (2000) J. Biol. Chem. 275, 29318–29323. [DOI] [PubMed] [Google Scholar]
- 6.Bergo, M. O., Leung, G. K., Ambroziak, P., Otto, J. C., Casey, P. J. & Young, S. G. (2000) J. Biol. Chem. 275, 17605–17610. [DOI] [PubMed] [Google Scholar]
- 7.Buss, J. E., Solski, P. A., Schaeffer, J. P., MacDonald, M. J. & Der, C. J. (1989) Science 243, 1600–1603. [DOI] [PubMed] [Google Scholar]
- 8.Kamen, B. A., Cole, P. D. & Bertino, J. R. (2000) in Cancer Medicine, eds. Bast, R. C., Jr., Kufe, D. W., Pollock, R. E., Weichselbaum, R. R., Holland, J. F. & Frei, E., III (B. C. Decker, Hamilton, ON, Canada), 5th Ed., pp. 612–624.
- 9.Nesher, G., Moore, T. L. & Dorner, R. W. (1991) Ann. Rheum. Dis. 50, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishi, T., Tanaka, Y. & Ueda, K. (2000) Cancer 89, 925–931. [DOI] [PubMed] [Google Scholar]
- 11.Wagner, C. (1995) in Folate in Health and Disease, ed. Bailey, L. B. (Dekker, New York), pp. 23–42.
- 12.Chiang, P. K., Gordon, R. K., Tal, J., Zeng, G. C., Doctor, B. P., Pardhasaradhi, K. & McCann, P. P. (1996) Faseb J 10, 471–480. [PubMed] [Google Scholar]
- 13.Allegra, C. J., Fine, R. L., Drake, J. C. & Chabner, B. A. (1986) J. Biol. Chem. 261, 6478–6485. [PubMed] [Google Scholar]
- 14.Selhub, J. (1999) Annu. Rev. Nutr. 19, 217–246. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein, J. D. (1998) Eur. J. Pediatr. 157, Suppl. 2, S40–S44. [DOI] [PubMed] [Google Scholar]
- 16.Kato, K., Cox, A. D., Hisaka, M. M., Graham, S. M., Buss, J. E. & Der, C. J. (1992) Proc. Natl. Acad. Sci. USA 89, 6403–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haklai, R., Weisz, M. G., Elad, G., Paz, A., Marciano, D., Egozi, Y., Ben-Baruch, G. & Kloog, Y. (1998) Biochemistry 37, 1306–1314. [DOI] [PubMed] [Google Scholar]
- 18.Wang, H., Yoshizumi, M., Lai, K., Tsai, J. C., Perrella, M. A., Haber, E. & Lee, M. E. (1997) J. Biol. Chem. 272, 25380–25385. [DOI] [PubMed] [Google Scholar]
- 19.Fu, H. W. & Casey, P. J. (1999) Recent Prog. Horm. Res. 54, 315–342. [PubMed] [Google Scholar]
- 20.Otto, J. C., Kim, E., Young, S. G. & Casey, P. J. (1999) J. Biol. Chem. 274, 8379–8382. [DOI] [PubMed] [Google Scholar]
- 21.Dai, Q., Choy, E., Chiu, V., Romano, J., Slivka, S. R., Steitz, S. A., Michaelis, S. & Philips, M. R. (1998) J. Biol. Chem. 273, 15030–15034. [DOI] [PubMed] [Google Scholar]
- 22.Kim, E., Ambroziak, P., Otto, J. C., Taylor, B., Ashby, M., Shannon, K., Casey, P. J. & Young, S. G. (1999) J. Biol. Chem. 274, 8383–8390. [DOI] [PubMed] [Google Scholar]
- 23.Bergo, M. O., Leung, G. K., Ambroziak, P., Otto, J. C., Casey, P. J., Gomes, A. Q., Seabra, M. C. & Young, S. G. (2001) J. Biol. Chem. 276, 5841–5845. [DOI] [PubMed] [Google Scholar]
- 24.Choi, Y. J., Niedbala, M., Lynch, M., Symons, M., Bollag, G. & North, A. K. (2001) Methods Enzymol. 332, 103–114. [DOI] [PubMed] [Google Scholar]
- 25.Chen, Z., Otto, J. C., Bergo, M. O., Young, S. G. & Casey, P. J. (2000) J. Biol. Chem. 275, 41251–41257. [DOI] [PubMed] [Google Scholar]
- 26.McCabe, J. B. & Berthiaume, L. G. (1999) Mol. Biol. Cell 10, 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melnyk, S., Pogribna, M., Pogribny, I. P., Yi, P. & James, S. J. (2000) Clin. Chem. 46, 265–272. [PubMed] [Google Scholar]
- 28.Hilton, M. A., Hoffman, J. L. & Sparks, M. K. (1983) Cancer Res. 43, 5210–5216. [PubMed] [Google Scholar]
- 29.Hoffman, D. R., Cornatzer, W. E. & Duerre, J. A. (1979) Can. J. Biochem. 57, 56–65. [DOI] [PubMed] [Google Scholar]
- 30.Shirasawa, S., Furuse, M., Yokoyama, N. & Sasazuki, T. (1993) Science 260, 85–88. [DOI] [PubMed] [Google Scholar]
- 31.Backlund, P. S. (1997) J. Biol. Chem. 272, 33175–33180. [DOI] [PubMed] [Google Scholar]
- 32.Shields, J. M., Pruitt, K., McFall, A., Shaub, A. & Der, C. J. (2000) Trends Cell. Biol. 10, 147–154. [DOI] [PubMed] [Google Scholar]
- 33.Bos, J. L. (1989) Cancer Res. 49, 4682–4689. [PubMed] [Google Scholar]
- 34.Adjei, A. A. (2001) J. Natl. Cancer Inst. 93, 1062–1074. [DOI] [PubMed] [Google Scholar]
- 35.Karp, J. E., Kaufmann, S. H., Adjei, A. A., Lancet, J. E., Wright, J. J. & End, D. W. (2001) Curr. Opin. Oncol. 13, 470–476. [DOI] [PubMed] [Google Scholar]
- 36.Kreck, M. L., Freeman, J. L., Abo, A. & Lambeth, J. D. (1996) Biochemistry 35, 15683–15692. [DOI] [PubMed] [Google Scholar]