Abstract
Teeth were lost in birds 70–80 million years ago. Current thinking holds that it is the avian cranial neural crest-derived mesenchyme that has lost odontogenic capacity, whereas the oral epithelium retains the signaling properties required to induce odontogenesis. To investigate the odontogenic capacity of ectomesenchyme, we have used neural tube transplantations from mice to chick embryos to replace the chick neural crest cell populations with mouse neural crest cells. The mouse/chick chimeras obtained show evidence of tooth formation showing that avian oral epithelium is able to induce a nonavian developmental program in mouse neural crest-derived mesenchymal cells.
Tooth development, in common with the formation of many other vertebrate organs, involves reciprocal series of epithelial–mesenchymal interactions (1). These inductive interactions are mediated by diffusible factors between oral epithelium and neural crest-derived mesenchyme (2, 3). In the mouse embryo, the initiation period of tooth development extends from embryonic day (E) 8, when crest cells first emerge from the cranial neural folds, to E11, when local thickenings of the oral epithelium are formed (4–6). The epithelium then invaginates into the underlying mesenchyme to form the tooth bud (E12.5–13).
Classical tissue recombination experiments and more recent molecular analysis have identified the oral epithelium as providing the instructive information for the initiation of mouse tooth development. The E9–11 presumptive dental epithelium can elicit tooth formation even in neural crest-derived mesenchyme that does not normally form teeth, but is not able to induce tooth formation in a nonneural crest-derived mesenchyme, such as the limb mesenchyme (4, 7). By E12, the odontogenic potential shifts to the mesenchyme, which can instruct any kind of epithelium to form tooth-specific structures (7–9). These experiments point to the importance of epithelial signals in the initiation of mouse tooth formation.
Birds lost their dentition almost 70–80 million years ago, but a number of genes that initiate odontogenesis continue to be expressed in their jaws (10, 11). During avian embryonic development, rudimentary structures consisting of local epithelial thickenings are formed transiently in the mandibular arch (12, 13), and these thickenings closely resemble the murine dental thickenings. Although these epithelial ingrowths share similarities in organization and morphology with mouse tooth primordia, the molecular mechanisms regulating their outgrowth appear to be distinct because their development is arrested at this stage, which may be due to the origin of the neural crest cells and/or regional differences in the oral epithelium. Several in vitro studies have suggested that a number of genes involved in tooth formation, and which remain silent in modern birds, can be reactivated upon appropriate signaling (8, 14). Isolated chick epithelium cultured in combination with mouse tooth mesenchyme produced dental structures, suggesting that it is the cranial neural crest-derived mesenchyme of modern Aves that has lost odontogenic capacity, whereas the oral epithelium retains the signaling properties to induce odontogenesis in competent mesenchyme (8, 14, 15). Unfortunately, such recombination approaches suffer from being performed in vitro, and moreover, it is difficult to eliminate completely contamination of mouse mesenchyme with residual epithelium. To determine whether teeth can form in the developing jaws of avian embryos in ovo, we grafted mouse cranial neural crest in place of chick.
Materials and Methods
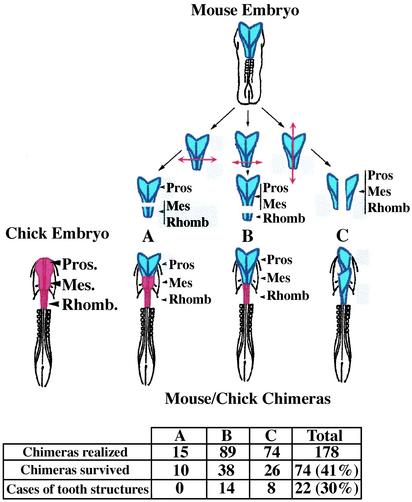
Preparation of Mouse/Chick Chimeras. JA657 chick embryos at 1 day of incubation (seven somites) and Swiss mice embryos at E8 (four to six somites) were used for the microsurgery. Reciprocal exchanges of precisely defined regions of the neural tube were performed between chick and mouse embryos as previously described (16). The cephalic region to be grafted was first delimited in the mouse donor and the chick host, then removed from the host and replaced by the donor graft (Fig. 1 Upper). Chimera embryos were incubated in ovo for different periods (1–18 days).
Fig. 1.
Mouse neural tube transplantation into chick host embryos. (Upper) Experimental procedure of mouse neural tube transplantation into chick host embryos. Graft level in mouse donor embryos (E8, four to six somites) shown in blue. The implantation level in six somite-stage chick host embryos is shown in red. Depending on the series (A–C), different zones were grafted. In series A, the prosencephalon (Pros) of the chick embryo was replaced by the equivalent encephalic region of the mouse embryo (homotopic and homochronic graft). In series B, the mouse prosencephalon–mesencephalon (Pros–Mes) was implanted into a chick host embryo (homotopic and homochronic graft). In series C, the chick prosencephalon–mesencephalon–rhombencephalon (Pros–Mes–Rhomb, the whole red region) was replaced by two half parts of the mouse encephalon disposed one behind the other (homocoronic graft). (Lower) Number of cases studied. One hundred seventy-eight chick embryos were grafted at the cephalic level and 74 (41%) survived between 1 and 18 days after surgery. For the three series, the grafts involved 15 chimeras in series A, with 10 survivals; 89 in series B, with 38 survivals; and 74 in series C, with 26 survivals. The chimeras were removed at various developmental stages (between 1 and 18 days of incubation), fixed, and then prepared for different analyses.
Visualization of Neural Crest Cell Migration. Chimeras killed 1–2 days after surgery were prepared for whole-mount in situ hybridization with mouse probes to check mouse neural crest cell migration into the facial territories of the chick host embryos. Heads of older chimeras were prepared for histological examination and in situ hybridization on sections. To ascertain the presence of donor tissue in hosts, several sections of each chimera were stained with bis-benzimide (Hoechst staining), as described (16). The distribution of mouse neural crest cells in the head of chimeras was visualized by a DNA repartition that differs in the mouse and chick nuclei. (Mouse cells had a brighter appearance after Hoechst staining.)
In Situ Hybridization and Immunohistochemistry on Tissue Sections. For in situ hybridization, digoxigenin-labeled mouse and chick specific riboprobes (mMK, mMsx1, mPax9, mBarx1, cPitx2, cFGF8, cBMP4, cShh) were used. Whole-mount in situ hybridization and in situ hybridization on cryosections were performed according to Wilkinson (17).
For immunohistochemistry, affinity-purified antibodies against several proteins (dentin sialoprotein, nestin) were used. Immunoperoxidase (ABC Kit, Vector Laboratories) staining was performed as previously described (18, 19). Positive peroxidase staining produces red color on light microscopy.
Results and Discussion
Several molecules are well known to be involved in the initiation of murine tooth formation. Pitx2 is a homeobox gene that is initially expressed throughout the mouse oral epithelium and progressively becomes restricted to the dental epithelium (20). Bone morphogenetic protein-4 (BMP4), fibroblast growth factor-8 (FGF8), and sonic hedgehog (Shh) are involved in the determination of tooth-forming sites in mice and the stepwise determination of ectomesenchyme into dental papilla (21–25). Expression of BMP4 and FGF8 has been documented in chick oral epithelium in broad domains during early embryonic development (10). Although Shh is expressed in areas of facial epithelium during chick development, it is not expressed in early (st21) dental epithelium (11, 26). The restricted expression of these molecules to dental placodes in oral epithelium during mouse development is thus an indication of the induction of odontogenesis.
To investigate the odontogenic capacity of murine ectomesenchymal cells and also to examine whether avian oral epithelium can undergo full tooth development, we performed a series of interspecific homotopic neural-tube transplantations. The whole dorsoventral aspect of the rostral murine neural tube, before its closure (when it still contains all of the premigratory cranial neural crest cells), was transplanted into an avian host from which the equivalent tissues had been surgically removed (Fig. 1 Upper). Mouse neural crest cells had already invaded the maxillary and mandibular processes of the chick host by 1 to 2 days after surgery (Fig. 2A). These cells contribute to the formation of tooth-like germ structures at different time points after grafting in the chick hosts (Fig. 2 B–D and Fig. 1 Lower). Transplants of prosencephalon-mesencephalon (series B) and prosencephalon-mesenchephalon-rhombencephalon (series C) both contributed to the formation of tooth structures in the mouse/chick chimeras, whereas no tooth structures were obtained from transplants of prosencephalon (series A).
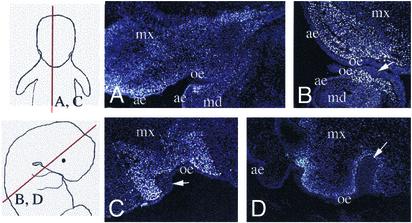
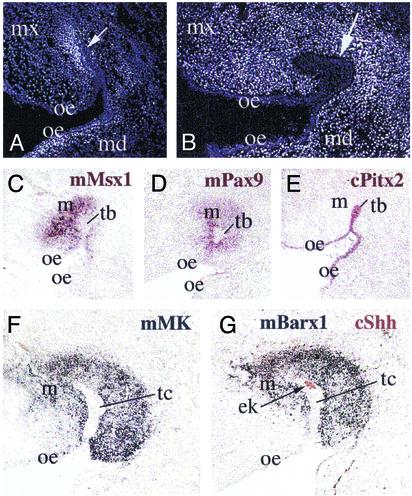
Fig. 2.
Visualization of mouse ectomesenchymal cells surrounding the ingrowths of the oral chick epithelium in chimeras and formation of a mineralized dental-like structure in a mouse/chick chimera. Mouse cells detected after Hoechst staining, in transverse sections of the head region of mouse/chick chimeras, 2 (A),3(B),5(C), and 9 (D) days after grafting. (A–D) Migrations and localization of mouse neural crest cells (brighter cells) in maxillary and mandibular processes. Note the invaginations of the chick oral epithelium (arrows in B, C, and D), and the acquisition of a tooth-bud configuration (D). ae, aboral epithelium; md, mandibular process; mx, maxillary process; oe, oral epithelium.
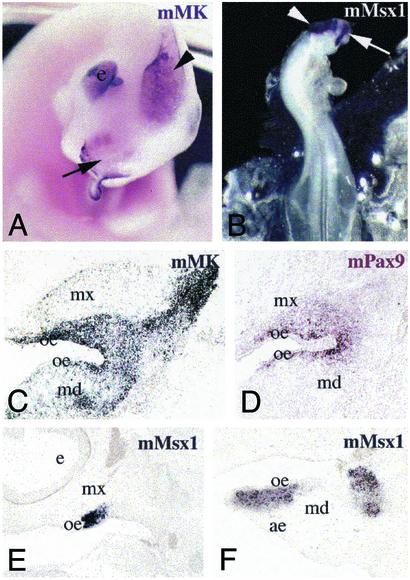
We undertook a molecular analysis to examine expression of both early and late markers of tooth development in chimeras. Midkine (MK) is a heparin-binding growth/differentiation factor that is expressed in all neural crest cells during the early stages of mouse embryonic development (18). Subsequently, MK expression becomes down-regulated in the vast majority of tissues and organs, but its expression persists in the developing murine teeth until E18 (late bell stage) (19). In chimeric embryos, MK was found to be expressed in a large population of mouse neural crest cells migrating into the maxillary and mandibular processes of the chick host (Fig. 3 A and C). Expression of the mouse homeodomain-containing transcription factors Msx1 and Pax9 was far more restricted, occurring specifically in those cell populations surrounding or contacting the chick oral epithelium (Fig. 3 D–F). These findings suggest that neural crest cells expressing Pax9 and Msx1 with MK possess odontogenic potential, because the expression of Pax9 and Msx1 is limited to dental mesenchyme from the earliest stages of murine odontogenesis (21, 22, 25). We conclude that the reciprocal signaling interactions between neural crest-derived mesenchyme from mouse and localized regions of the chick oral epithelium are responsible for the subsequent elaboration of the specific form and structure of the teeth described above. The loss of teeth in Aves is thus probably due to the lack of appropriate neural crest-derived signaling molecules involved in epithelial–mesenchymal interactions.
Fig. 3.
Visualization of mouse neural crest cell populations in the forming maxillary and mandibular processes of mouse/chick chimeras, 1 to 5 days after grafting. Whole-mount digoxigenin in situ hybridization (A and B) and in situ hybridization on sections (C–F). (A) Expression of mouse MK (mMK) in the grafted area (arrowhead), eye, and facial region (arrow) of a mouse/chick chimera 2 days after grafting. (B) Detection of mouse Msx1 (mMsx1) transcripts in the grafted area (arrowhead) and the facial process (arrow) in a chimera 1 day after grafting. (C) Widespread mMK expression in migrating cells of a chimera 3 days after grafting. (D) Restricted mouse Pax9 (mPax9) expression in mesenchymal cells surrounding the chick oral epithelium of 3 days after grafting chimera. (E and F) Mouse Msx1 expression limited to mesenchymal cell populations in contact with the oral epithelium in two different chimeras (3 and 5 days after grafting). ae, aboral epithelium; e, eye; md, mandibular process; mx, maxillary process; oe, oral epithelium.
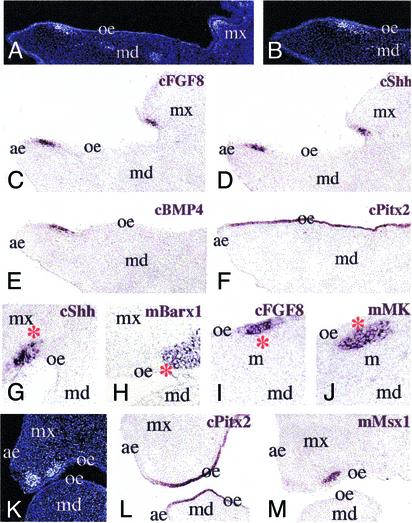
It had been suggested that loss of BMP4 in chick “vestigial” tooth rudiments is responsible for their failure to progress into teeth (13). To test if mouse cranial neural crest contains specific signals that can ectopically induce localized BMP4 and Shh expression in the chick oral epithelium, we performed in situ hybridization in chimeras, 2 to 4 days after surgery. Although Pitx2 was expressed throughout the oral epithelium (Fig. 4 F and L), ectopic chick FGF8, Shh, and BMP4 expression was restricted to those areas that form the epithelial placodes described above (Fig. 4 C–E, G, and I). These localized regions of epithelial expression correspond to the sites overlying mouse neural crest cells (Fig. 4 A, B, and K) that express the mouse Msx1, Barx1, and MK genes (Fig. 4 H, J, and M). These results indicate that, in vivo, neural crest cells may play a role in the activation of BMP4 and Shh expression in tooth-forming sites of the murine oral epithelium.
Fig. 4.
Formation of dental placodes in the oral epithelium of mouse/chick chimeras. Hoechst staining (A, B, and K) and digoxigenin in situ hybridization on sections of 2 (A–F) to 4 (G–M) days after grafting chimeras, by using chick epithelial (C–G, I, and L) and mouse mesenchymal (H, J, and M) markers. (A and B) Mouse cells appear brighter after Hoechst staining in the maxillary and mandibular processes. (C–E) Expression of chick FGF8 (cFGF8; C), Shh (cShh; D), and BMP4 (cBMP4; E) in restricted areas of the oral epithelium. (F) Expression of chick Pitx2 (cPitx2) in all cells of the oral epithelium. Compare C, D, E, and F with A and B, showing the arrival of mouse neural crest cells at the sites of cFGF8, cShh, and cBMP4 expression. (G) Restricted cShh expression in oral epithelium. The asterisk shows the mesenchymal area in which the mouse Barx1 gene (mBarx1) was detected in an adjacent section. (H) Expression of the mBarx1 gene in mesenchymal cells that are in contact with cShh-expressing epithelial cells (asterisk). (I and J) Similarly, the chick epithelium expressing cFGF8 (I, asterisk in J) is in contact with the mesenchyme in which the mMK gene was expressed (J, asterisk in I). (L) Expression of cPitx2 to the oral epithelium of maxillary and mandibular processes. Note the absence of the hybridization signal in the aboral epithelium. (M) Mouse Msx1 (mMsx1) expression in a population of mesenchymal cells in contact with the oral epithelium of the maxilla. ae, aboral epithelium; md, mandibular process; mx, maxillary process; oe, oral epithelium.
Seven to nine days after grafting, the chick oral epithelium had invaginated into the underlying ectomesenchyme and acquired a bud configuration (Fig. 5 A and B). As during the bud stage of mouse tooth development, mouse ectomesenchymal cells surrounding the chick epithelial invaginations expressed the tooth-specific genes Msx1, Pax9, and Barx1 (Fig. 5 C, D, and G). Although each of these genes is expressed in cells other than dental mesenchyme, dental mesenchyme cells are the only cells in the embryo that express all three genes. MK expression, which was initially widespread in the migrating neural crest cells, became restricted around the invaginated chimera epithelium (Fig. 5F). The oral origin of the epithelium was evident from the detection of the chick Pitx2 gene (Fig. 5E). The differential growth and subsequent folding of the dental epithelium is thought to be directed by a transient signaling center known as the enamel knot (2, 3, 26). Shh expression has been reported in the enamel knot of developing mouse teeth (27). Similarly, ectopic chick Shh expression was seen in a restricted population of epithelial cells in chimera tooth germs (Fig. 5G), indicating the presence of a tooth-shape regulator in these structures.
Fig. 5.
Formation of dental-like epithelial bud structures in mouse/chick chimeras. The chick oral epithelium invaginates into the underlying mouse neural crest-derived mesenchyme and acquires bud configurations, 7–9 days after grafting. Hoechst staining (A and B) and in situ hybridization on sections by using tooth-specific molecular markers (C–G). Examination of the chick origin and dental potential of the epithelial ingrowths by in situ hybridization by using either fluorescein-labeled (G, brown color) or digoxigenin-labeled (C–G, violet and blue colors) species-specific probes. (A and B) Visualization of mouse neural crest cells surrounding chick epithelial bud/cap structures (arrows) in the maxillary processes. (C, D, F, and G) Detection of mouse Msx1 (mMsx1; C), Pax9 (mPax9; D), MK (mMK; F), and Barx1 (mBarx1; G) transcripts in mesenchymal cells surrounding the epithelial bud structures. Restricted expression of the chick Shh gene (cShh) to cells of the epithelial bud structure (G; brown color, arrow) that represents the equivalent population of cells forming the “enamel knot” in developing mouse teeth. (E) Expression of the chick Pitx2 gene (cPitx2) in the bud epithelium. ek, enamel knot; oe, epithelium; m, mesenchyme; md, mandibular process; mx, maxillary process; tb, tooth bud; tc, tooth cap-like invagination.
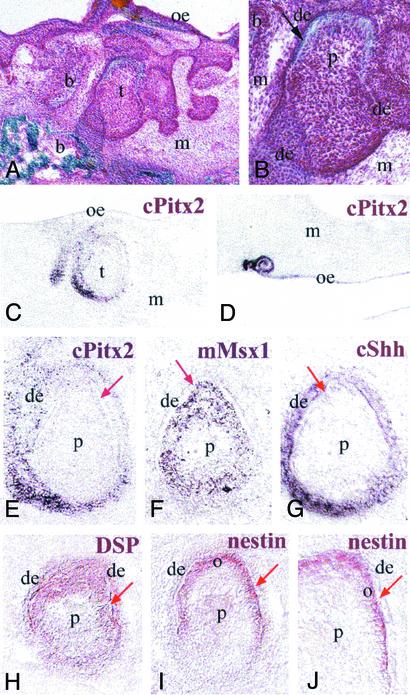
Fourteen days after grafting, multiple ingrowths of the oral epithelium were evident, and mineralized structures resembling tooth germs were observed beneath the oral epithelium (Fig. 6 A and B). These tooth-like structures were clearly distinguishable from the surrounding mineralized bone. The deposition of the mineral matrix in the tooth-like structures was typical of the dentin matrix deposition observed in developing teeth of murine embryos, which starts at the tip of the cusps, proceeds apically and follows odontoblast differentiation. No dentin tubules were evident because these teeth represent an early stage of mineral deposition. Significantly, the epithelial-derived enamel matrix was absent from these structures. The lack of enamel formation may be due to the early termination of the interaction between the epithelium and mesenchyme. This possibility can be supported by the fact that the dentin matrix is not as mature as in teeth when enamel deposition is occurring in a deeper layer of dentin. Chick Pitx2 was expressed in the epithelium of the chimera tooth germs (Fig. 6 C and E) and in several unusual structures formed at the anterior part of the oral epithelium (Fig. 6D). Chick Pitx2 was not expressed in the epithelial cells contacting the mineralized matrix, which is reminiscent of Pitx2 down-regulation in ameloblasts observed in mouse teeth. The mouse Msx1 (Fig. 6F) and the chick Shh genes (Fig. 6G) were expressed, respectively, in dental mesenchyme and epithelium of the chimera teeth. Dentin sialoprotein, a noncollagenous extra-cellular matrix protein produced by odontoblasts (28) and osteoblasts (29), was detected in ectomesenchymal cells and the mineralized matrix (Fig. 6H). The intermediate filament protein nestin, a marker of neurons, muscles (30) and differentiating and mature odontoblasts (31), was also detected in cells producing dentin matrix in the chimera tooth germs (Fig. 6 I and J). The presence of both these markers together in the same cell layer provides a strong indication that these cells are odontoblasts.
Fig. 6.
Formation of tooth-like structures in mouse/chick chimeras, 14 days after grafting. Histology (A and B), digoxigenin in situ hybridization (C–J), and immunohistochemistry (H–J) by using tooth-specific markers. Arrows indicate the sites of mineral matrix deposition. (A) Histological section of the mineralized structure resembling a tooth germ in a mouse/chick chimera. The mineralized matrix has a blue color after Masson's trichrome staining. Spongy bone has formed around the dental-like structure. (B) Higher magnification of Fig. 2 A, showing mineral matrix deposition (arrow), formation of a single cusp (conical or incisal shape), and the absence of enamel matrix and polarized epithelial cells. (C) Exclusive chick Pitx2 (cPitx2) expression in the epithelium of the tooth germ. Note that the gene is absent in oral epithelium. (D) An unusual structure expressing cPitx2 in the anterior part of the oral epithelium. (E–G) Higher magnification of the tooth germ, showing cPitx2 (E) and chick Shh (cShh; G) expression in the epithelium, and mouse Msx1 (mMsx; F) expression in the mesenchyme. Note that the cPitx2 gene is down-regulated in epithelial cells contacting the mineralized matrix (E, arrow). (H) Dentin sialoprotein (DSP) distribution (red color) in cells of the dental mesenchyme and in mineralized matrix of the chimera tooth. (I) Nestin expression (red color) in odontoblast-like cells responsible for mineral matrix production in the chimera teeth. (J) Higher magnification of I. b, bone; de, dental epithelium; m, jaw mesenchyme; o, odontoblast-like cells; oe, oral epithelium; p, dental papilla; t, tooth-like germ.
These results show that, although within a species cranial neural crest cells do not appear to be prepatterned with respect to their skeletal fates, they do contain the information to interpret generic epithelial signals and to behave in a species-specific way.
Acknowledgments
We thank W. Butler (University of Texas, Houston) for the dentin sialoprotein antibody; U. Lendahl (Karolinska Institute, Stockholm) for the nestin antibody; S. Artavanis-Tsakonas, N. Le Douarin, P. Lemaire, M. Maroto, and O. Pourquié for valuable comments; K. Dale for comments and improving the manuscript; and M. Zampieri for help with Masson's trichrome staining. This work was partly realized at the Institut de la Biologie du Développement de Marseille, in the laboratory of C. Goridis. The research was supported by grants from the Association pour la Recherche sur le Cancer, Association Française contre les Myopathies, Centre National de la Recherche Scientifique, the Wellcome Trust, Medical Research Council, and the University of Nantes.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: En, embryonic day n; BMP, bone morphogenetic protein; FGF, fibroblast growth factor; Shh, sonic hedgehog; MK, midkine.
References
- 1.Kollar, E. J. (1983) in Epithelial-Mesenchymal Interactions in Development, eds. Sawyer, R. H. & Fallon, J. F. (Praeger, New York), pp. 27–74.
- 2.Peters, H. & Balling, R. (1999) Trends Genet. 15, 59–65. [DOI] [PubMed] [Google Scholar]
- 3.Thesleff, I. & Sharpe, P. (1997) Mech. Dev. 67, 111–123. [DOI] [PubMed] [Google Scholar]
- 4.Lumsden, A. G. S. (1988) Development (Cambridge, U.K.) 103, Suppl., 155–169. [DOI] [PubMed] [Google Scholar]
- 5.Lumsden, A. G. S. & Buchanan, J. A. G. (1986) Arch. Oral Biol. 31, 301–311. [DOI] [PubMed] [Google Scholar]
- 6.Nichols, D. H. (1981) J. Embryol. Exp. Morphol. 64, 105–120. [PubMed] [Google Scholar]
- 7.Mina, M. & Kollar, E. J. (1987) Arch. Oral Biol. 32, 123–127. [DOI] [PubMed] [Google Scholar]
- 8.Kollar, E. J. & Fisher, C. (1980) Science 207, 993–995. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, C. A., Tucker, A. S. & Sharpe, P. T. (2000) Development (Cambridge, U.K.) 127, 403–412. [DOI] [PubMed] [Google Scholar]
- 10.Francis-West, P., Ladher, R., Barlow, A. & Graveson, A. (1998) Mech. Dev. 75, 3–28. [DOI] [PubMed] [Google Scholar]
- 11.Schneider, R. A., Hu, D. & Helms, J. A. (1999) Cell Tissue Res. 296, 103–109. [DOI] [PubMed] [Google Scholar]
- 12.Romanoff, A. L. (1960) The Avian Embryo (Macmillan, New York), pp. 459–460.
- 13.Chen, Y.-P. Zhang, Y., Jiang, T.-X., Barlow, A. J., St. Amand, T. R., Hu, Y., Heaney, S., Francis-West, P., Chuong, C.-M. & Maas, R. (2000) Proc. Natl. Acad. Sci. USA 97, 10044–10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, Y.-H., Upholt, W. B., Sharpe, P. T., Kollar, E. J. & Mina, M. (1998) Dev. Dyn. 213, 386–397. [DOI] [PubMed] [Google Scholar]
- 15.Kollar, E. J. & Mina, M. (1991) J. Craniofac. Genet. Dev. Biol. 11, 223–228. [PubMed] [Google Scholar]
- 16.Fontaine-Pérus, J., Halgand, P., Chéraud, Y., Rouaud, T., Velasco, M. E., Cifuentes Diaz, C. & Rieger, F. (1997) Development (Cambridge, U.K.) 124, 3025–3036. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson, D. G. (1992) in In Situ Hybridization: A Practical Approach, ed. Wilkinson, D. G. (Oxford Univ. Press, Oxford), pp. 75–83.
- 18.Mitsiadis, T. A., Salmivirta, M., Muramatsu, T., Muramatsu, H., Rauvala, H., Lehtonen, E., Jalkanen, M. & Thesleff, I. (1995) Development (Cambridge, U.K.) 121, 37–51. [DOI] [PubMed] [Google Scholar]
- 19.Mitsiadis, T. A., Muramatsu, T., Muramatsu, H. & Thesleff, I. (1995) J. Cell Biol. 129, 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucchielli, M.-L., Mitsiadis, T., Raffo, S., Brunet, J.-F., Proust, J.-P. & Goridis C. (1997) Dev. Biol. 189, 275–284. [DOI] [PubMed] [Google Scholar]
- 21.Neubüser, A., Peters, H., Balling, R. & Martin, G. R. (1997) Cell 90, 247–255. [DOI] [PubMed] [Google Scholar]
- 22.Tucker, A. S., Khamis, A. A. & Sharpe, P T. (1998) Dev. Dyn. 212, 533–539. [DOI] [PubMed] [Google Scholar]
- 23.Tucker, A. S., Yamada, G., Grigoriou, M., Pachnis, V. & Sharpe, P. T. (1999) Development (Cambridge, U.K.) 126, 51–61. [DOI] [PubMed] [Google Scholar]
- 24.Hardcastle, Z., Mo, R., Hui, C. C. & Sharpe, P. T. (1998) Development (Cambridge, U.K.) 125, 2803–2811. [DOI] [PubMed] [Google Scholar]
- 25.Vainio, S., Karavanova, I., Jowett, A. & Thesleff, I. (1993) Cell 75, 45–58. [PubMed] [Google Scholar]
- 26.Hu, D. & Helms, J. A. (1999) Development (Cambridge, U.K.) 126, 4873–4884. [DOI] [PubMed] [Google Scholar]
- 27.Vaahtokari, A., Aberg, T., Jernvall, J., Keränen, S. & Thesleff, I. (1996) Mech. Dev. 54, 39–43. [DOI] [PubMed] [Google Scholar]
- 28.Butler, W. T., Bhown, M., Brunn, J. C., D'Souza, R. N., Farach-Carson, M. C., Happonen, R. P., Schrohenloher, R. E., Seyer, J. M., Somerman, M. J., Foster, R. A., et al. (1992) Matrix 12, 343–351. [DOI] [PubMed] [Google Scholar]
- 29.Qin, C., Brunn, J. C., Cadena, E., Ridall, A., Tsujigiwa, H., Nagatsuka, H., Nagai, N. & Butler, W. T. (2002) J. Dent. Res. 81, 392–394. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman, L., Parr, B., Lendahl, U., Cunningham, M., McKay, R., Gavin, B., Mann, J., Vassileva, G. & McMahon, A. (1994) Neuron 12, 11–24. [DOI] [PubMed] [Google Scholar]
- 31.About, I., Laurent-Maquin, D., Lendahl, U. & Mitsiadis, T. A. (2000) Am. J. Pathol. 157, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]