Abstract
Retinoblastoma (Rb)-deficient embryos show severe defects in neurogenesis, erythropoiesis, and lens development and die at embryonic day 14.5. Our recent results demonstrated a drastic disorganization of the labyrinth layer in the placenta of Rb-deficient embryos, accompanied by reduced placental transport function. When these Rb-/- embryos were supplied with a wild-type placenta by using either tetraploid aggregation or genetic approaches, animals survived until birth. Here we analyze the role of extraembryonic Rb in regulating proliferation, apoptosis, and differentiation in the rescued animals at different developmental stages. Many of the neurological and erythroid abnormalities thought to be responsible for the embryonic lethality of Rb-/- animals, including the ectopic apoptosis in the CNS, were virtually absent in rescued Rb-/- pups. However, rescued animals died at birth with severe skeletal muscle defects. Like in Rb knockout embryos, rescued animals showed a marked increase in DNA replication and cell division in the CNS. In sharp contrast, the typical widespread neuronal apoptosis was absent in Rb-deficient embryos reconstituted with a normal placenta. In lens fiber cells, however, the inappropriate proliferation and apoptosis that is normally observed in Rb-/- embryos continued unabated in rescued animals. These results demonstrate that Rb function in extraembryonic lineages plays an important role in the survival of neuronal cells and in the differentiation of the erythroid lineage, providing mechanistic insight into the cell autonomous and nonautonomous functions of Rb during development.
Twenty years ago the retinoblastoma (Rb) gene was discovered as the first tumor suppressor in humans. Although loss-of-function mutations in Rb were initially observed in inherited retinoblastoma (1), inactivation of Rb has since been implicated in a wide variety of human tumors, including familial osteosarcomas, as well as sporadic bladder, lung, prostate, and breast carcinomas (2). Indeed, alterations of the Rb signaling pathway, by activation of positive acting components such as G1 cyclins and cyclin-dependent kinases (cdk), by inactivation of negative acting components such as cdk inhibitors (i.e., p16INK4a) and p53, or by mutations in Rb itself, have been detected in virtually all human cancers (3). Interestingly, mice heterozygous for Rb are not predisposed to retinoblastoma but instead are highly susceptible to pituitary and thyroid carcinomas (4–7).
Deletion of Rb in mice results in widespread ectopic cell cycle entry and increased apoptosis in the CNS, peripheral nervous system (PNS), lens, and liver (4–6, 8). In addition to the pivotal role in balancing proliferation and apoptosis, Rb is important in determining cell fate. In vitro studies point to a function for Rb in regulating terminal differentiation of many cell types, including adipocytes, osteoblasts, erythrocytes, and skeletal muscle cells (9–13). Ablation of Rb in mice leads to differentiation defects in embryonic neurogenesis and erythropoiesis (4–6). In each case, Rb-deficient cell lineages appear competent in initiating differentiation programs, as indicated by their ability to express early differentiation markers, but fail to achieve a fully differentiated state. These studies have fueled current tumor suppressor models supporting the notion that cancer is a developmental disease heavily impacted by gene expression programs that affect cell fate and differentiation processes (14).
Rb-/- embryos die between embryonic days (E) 13.5 and 15.5, and this is thought to result from a defect in erythropoiesis, leading to anemia (4–6). Interestingly, we have recently demonstrated that Rb-/- mice have dramatic defects in the labyrinth layer of the placenta, characterized by marked hyperplasia of trophoblast cells and severe dysplasia of the labyrinth architecture, associated with a decrease in placental transport function. By supplying a normal placenta, either via tetraploid aggregation or by genetic approaches, Rb-/- embryos were able to survive to full term, suggesting that an abnormal placenta is the primary cause for the embryonic lethality of Rb-/- animals (15). These Rb-deficient animals supplied with a normal placenta provide a powerful tool to evaluate the mechanism for the cell autonomous versus non-cell autonomous roles of Rb in fetal development.
The goal of the current study was to directly assess the role of extraembryonic Rb in controlling the proliferation, apoptosis, and cell fate of cells in tissues of rescued Rb-/- fetuses and newborn pups. Remarkably, many of the previously characterized phenotypes of Rb mutant animals in the CNS and hematopoietic organs were almost completely absent in rescued animals. We show that Rb has a non-cell autonomous function in apoptosis of periventricular hindbrain neurons and a cell autonomous function in neuronal proliferation. In contrast, rescued animals showed excessive proliferation and apoptosis in lens fiber cells, accompanied with the same disorganized lens architecture as seen in Rb-/- embryos. Taken together these results define a key function of Rb in extraembryonic lineages that is essential for fetal neurogenesis and erythropoiesis.
Experimental Procedures
Mouse Strains and Genotyping. Rb+/- mice (5), Mox2+/cre transgenic mice (16), and RbloxP/loxP conditional knockout mice (17) were maintained on a mixed (C57BL/6 × 129/Sv or FVB/N × 129/Sv) background. Genotypes of mice were determined by PCR as described (5, 16, 17).
Tetraploid Aggregation. Tetraploid aggregation experiments were performed as described (15).
Histological Analysis, Immunofluorescence, and Immunohistochemistry. Embryos and neonates were fixed in 10% formalin. Fixed samples were embedded in paraffin and were cut as 5-μm serial sections, which were stained with hematoxylin/eosin for general histopathological analysis. For analysis of skeleton, newborn mice were deskinned, eviscerated, and fixed in 95% ethanol overnight. Cartilage and bones were stained with alcian blue and alizarin red, respectively, following previously described procedures (18).
For immunofluorescence, BrdUrd (100 μg per g of body weight) was injected i.p. into timed pregnant females 1.5 h before euthanasia and tissue collection. BrdUrd-positive cells were detected by using a fluorescence-based staining procedure. Phosphorylated histone 3 (PH3)-positive cells were detected by using the ABC detection system (Santa Cruz Biotechnology). Apoptosis was assayed by using a terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)/peroxidase assay kit (Intergen, Purchase, NY). Multiple nonconsecutive sections at an interval of at least 40 μm were counted for both immunostainings and the TUNEL assay. For the BrdUrd assay, nuclei were stained by 4,6-diamidino-2-phenylindole (DAPI). Approximately 200–700 fiber cells were counted for each lens section and 2,000–3,000 nuclei from the intermediate zone of the hindbrain for each CNS section. Data are reported as mean percentages or average numbers of positive cells over at least three nonconsecutive sections, with error bars representing SD of different sections for each sample.
Results
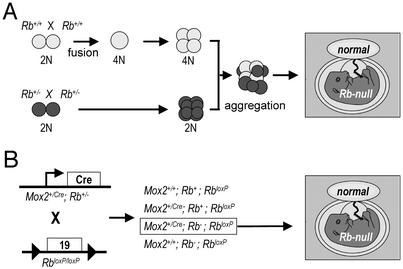
Recent studies identified a novel phenotype in Rb knockout mice where overproliferation of labyrinth trophoblasts results in a severe disruption of placental architecture and function (15). The fact that Rb-/- embryos supplied with a normal placenta can be carried to term provided us with a unique circumstance under which to directly assess the role of Rb in the differentiation of tissues that fully develop during the latter stages of gestation. We therefore reconstituted Rb-/- embryos with wild-type trophoblast cells by two separate but complementary methods and analyzed the offspring at different developmental stages. In the first method, we aggregated wild-type tetraploid embryos with diploid embryos derived from Rb heterozygote intercrosses (Fig. 1A). Under these circumstances, wild-type tetraploid cells contribute exclusively to the placenta, whereas diploid cells contribute primarily to the embryo proper, with some contribution to the labyrinth of the placenta (19). As reported previously, this strategy led us to recover several live and apparently normal Rb-/- embryos, at developmental stages where no Rb-/- embryos derived from natural matings can be recovered (15).
Fig. 1.
Schematic drawing of the tetraploid aggregation (A) and conditional knockout (B) experiments.
In addition to the tetraploid aggregation strategy described above, we used a second approach, using RbloxP/loxP conditional knockout mice (17) and Mox2+/cre transgenic mice (16), to supply Rb-deficient embryos with wild-type placentas (Fig. 1B). The Mox2 promoter drives expression of Cre within the embryo proper but not in the tissues of extraembryonic origin, which form the bulk of the placenta (15, 16). By crossing Mox2+/cre mice harboring one Rb-null allele (Mox2+/creRb+/-) with homozygous RbloxP/loxP conditional knockout mice, we were able to recover Rb-deficient embryos possessing normal Rb-/loxP placentas at E13.5, E15.5, E18.5, and birth, but they died immediately or soon after birth.
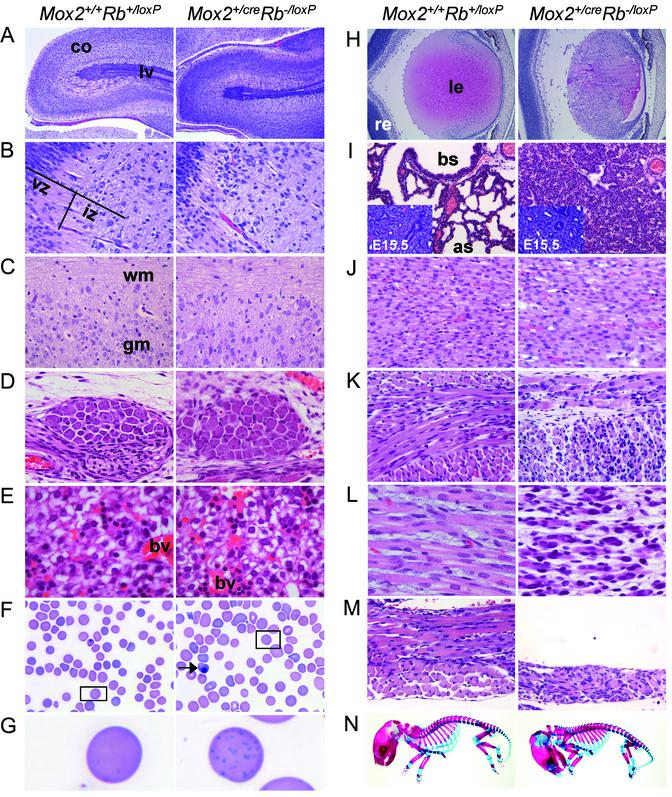
Suppression of the CNS Defects in Rb-/- Embryos. Interestingly, Rb-deficient embryos reconstituted with a normal placenta via conditional knockout or tetraploid methods lack many of the characteristic Rb-/- phenotypes (Fig. 2; and see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org). Histological analysis of CNS organs, including brain, spinal cord, and retinas, revealed no significant microscopic differences between the rescued and control animals (Figs. 2 A–C and H and 5 A–C and G).
Fig. 2.
Neurogenic and hematopoietic defects are strongly suppressed in Rb-deficient animals supplied with a normal placenta by the conditional knockout approach. Shown is the histology of multiple organs from Mox2+/+Rb+/loxP and Mox2+/creRb-/loxP newborn mice. (A) Cerebrum, sagittal section. (B) Hindbrain, adjacent to the fourth ventricle, sagittal section. (C) Spinal cord, sagittal section. (D) Dorsal root ganglia (DRG), sagittal section. (E) Liver. (F) Blood smear. (G) Higher magnification of erythrocyte from inset in F.(H) Eye. (I) Lung (Inset, E15.5 lung). (J) Cardiac muscle. (K) Intercostal skeletal muscle. (L) Limb skeletal muscle. (M) Diaphragm. (N) Skeleton. co, cerebral cortex; lv, lateral ventricle; vz, ventricular zone; iz, intermediate zone; wm, white matter; gm, gray matter; bv, blood vessel; le, lens; re, retina; bs, bronchiolar space; as, alveolar space. (Original magnification: A, ×2; B–E and J–M, ×40; F, ×100; G, ×1,000; H and I, ×10; N, ×0.8.)
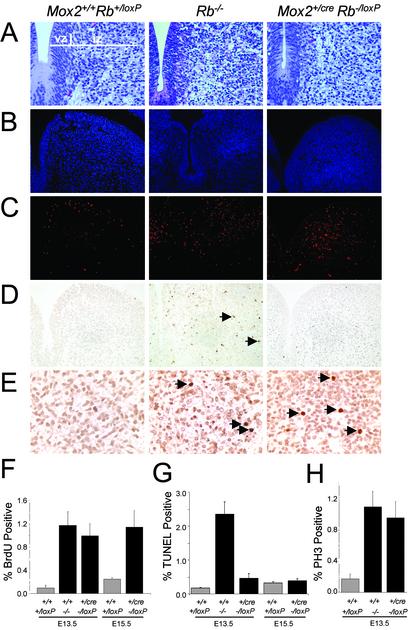
Many in vitro and in vivo experiments have demonstrated an important role for Rb in the control of cell cycle progression, cellular proliferation, and apoptosis. Inactivation of Rb in mice leads to excessive unscheduled DNA replication and ectopic apoptosis in the developing CNS (4–6). We therefore assessed the proliferative status of the CNS in E13.5 and E15.5 embryos derived from Mox2+/creRb-/+ and RbloxP/loxP crosses, as well as E13.5 embryos derived from tetraploid aggregation experiments, by BrdUrd incorporation and TUNEL assays. As expected, the normally postmitotic hindbrain neurons surrounding the fourth ventricle of Rb-/- E13.5 embryos showed high levels of BrdUrd incorporation, indicative of inappropriate DNA replication in these cells. Equivalent levels of inappropriate DNA replication were found in hindbrain neurons from Mox2+/creRb-/loxP and tetraploid-rescued (TR) Rb-/- embryos as compared with Rb-/- E13.5 embryos (Fig. 3 C and F; and see Fig. 6 C and E, which is published as supporting information on the PNAS web site). Moreover, immunohistochemical staining for the mitotic marker PH3 also revealed significant increases in mitotic neurons of Mox2+/creRb-/loxP E13.5 embryos, suggesting that most BrdUrd-positive neuronal cells complete S phase and proceed through mitosis (Fig. 3 E and H). In striking contrast to Rb-/- fetuses, there was no evidence of ectopic apoptosis in neurons of Mox2+/creRb-/loxP and TR Rb-/- embryos (Figs. 3 D and G and 6 D and F). These findings identify a key role for Rb in extraembryonic lineages in the survival of fetal neuronal cells.
Fig. 3.
A wild-type placenta suppresses aberrant apoptosis but not the ectopic proliferation in the CNS when using the conditional knockout approach. Histology (A), DAPI staining (B), BrdUrd immunofluorescence staining (C), TUNEL assays (D), and phosphorylated histone 3 (PH3) immunohistochemical staining (E) of the hindbrain (adjacent to the fourth ventricle) from genetically rescued E13.5 and E15.5 embryos. (F) Quantification of C. (G) Quantification of D.(H) Quantification of E. Similar sections from an E13.5 Rb mutant embryo were used as controls. Arrows indicate positive-staining cells. vz, ventricular zone; iz, intermediate zone. (Original magnification: A–D, ×40; E, ×63.)
We also analyzed DRG of Rb-/- animals supplied with normal placentae. Histological evaluation of DRG in E13.5 Rb-/- embryos, and to a lesser extent in E13.5 Rb-/- rescued embryos, revealed an increase in mitotic figures and karyorrhexis, characterized by nuclear fragmentation, compared with littermate normal controls (data not shown). However, DRG from E15.5 TR Rb-/- embryos or newborn Mox2+/cre Rb-/lox pups have mostly normal ganglion cells with occasional nuclear fragmentation, indicating an amelioration in unscheduled proliferation and apoptosis at later stages of development (Figs. 2D and 5D).
Suppression of Erythropoietic Defects in Rb-/- Embryos. During normal development, the major site of hematopoiesis shifts from the yolk sac to the liver by approximately E12.5. Rb-/- animals display severe defects in erythrocyte maturation, characterized by increased numbers of nucleated RBCs in both the liver and peripheral circulation (4–6). By E13.5, Rb-/- embryos are visually paler than littermate controls and eventually die by E14.5. Remarkably, the hematopoietic phenotype in the liver was almost completely absent in Rb-/- embryos provided with a normal placenta (Figs. 2E and 5E). Although hepatic hematopoiesis was within normal limits and embryos had normal coloration, there was a small but significant increase in the number of nucleated RBCs relative to the controls, indicating a slight delay in erythroid differentiation (Figs. 2 F and G and 5F; ref. 15). This delay was also reflected by the presence of more basophilic stippling of erythrocytes in blood smears prepared from Mox2+/creRb-/loxP pups compared with that of littermate Mox2+/+Rb+/loxP normal controls (Fig. 2G).
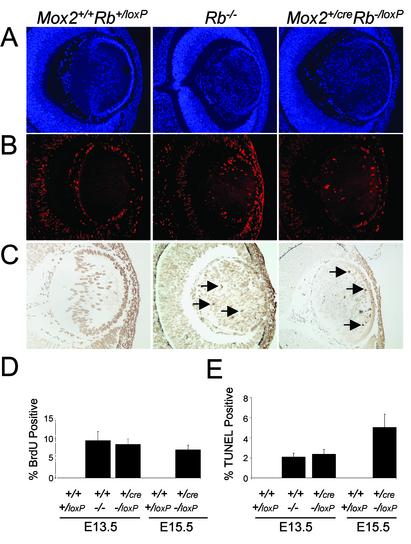
Abnormal Lens Development Persists in Mox2+/creRb-/loxP and TR Rb-/- Mice. Previous studies also documented a role for Rb in lens development, because lens fiber cells in Rb-/- embryos enter S phase and undergo apoptosis inappropriately (8). Interestingly, neither the proliferation nor the apoptotic defects in lens fiber cells caused by the disruption of Rb were rescued by supplying Rb-deficient embryos with a wild-type placenta (Fig. 4; and see Fig. 7, which is published as supporting information on the PNAS web site). Lens fiber cells in E13.5 Rb-/-, Mox2+/creRb-/loxP, and TR Rb-/- embryos exhibited equal levels of unscheduled DNA synthesis and apoptosis, and had the same disorganized histological appearance. Similarly, E15.5 lenses from Mox2+/creRb-/loxP embryos displayed high levels of inappropriate proliferation and apoptosis relative to control lenses (Fig. 4 D and E). Lenses of Mox2+/creRb-/loxP newborn pups and TR Rb-/- E15.5 fetuses were highly disorganized, hypernuclear, and severely necrotic (Figs. 2H and 5G). Consequently, one hallmark phenotype emanating from loss of Rb function, the cataract, remained in these genetically and tetraploid rescued embryos and newborn pups. The retina of rescued Rb-deficient animals, however, appeared normal and lacked visible apoptotic cells (Figs. 2H and 5G). The phenotypic difference between the retina and lens in these Rb-deficient mice likely reflects the different origins of these tissues; whereas cells of the retina are an extension of the CNS derived from neural ectoderm, lens fiber cells are derived from a surface ectoderm precursor (20). These results imply that Rb acts in the developing lens to control proliferation and apoptosis in a cell autonomous manner.
Fig. 4.
A wild-type placenta does not rescue aberrant apoptosis and ectopic proliferation in the lens when using the conditional knockout approach. DAPI staining (A), BrdUrd immunofluorescence staining (B), and TUNEL assays (C)of the lens from genetically rescued E13.5 and E15.5 embryos. (D) Quantification of B.(E) Quantification of C. Similar sections from an E13.5 Rb mutant embryo were used as controls. Arrows indicate positive-staining cells. (Original magnification: A–C, ×10.)
Severe Skeletal Muscle Dysplasia in Mox2+/creRb-/loxP and TR Rb-/- Animals. Histological analysis of fetal tissues revealed no apparent defects in the brain, heart, liver, lung, kidney, or spleen of Mox2+/creRb-/loxP and TR Rb-/- animals (Figs. 2 and 5; data not shown). However, these rescued embryos and newborn pups were severely deficient in skeletal muscle, as illustrated by hypoplastic and dysplastic myofibers within the intercostal muscles, diaphragm, limbs, and, to a lesser extent, tongue (Figs. 2 J–M and 5 I–L; data not shown). Although the lungs of Mox2+/creRb-/loxP and TR Rb-/- embryos at E15.5 were indistinguishable from control embryos, the lungs of Mox2+/creRb-/loxP animals after birth showed marked fetal atelectasis, a phenotype characterized by the collapse of alveolar air spaces (Figs. 2I and 5H). This pulmonary phenotype, together with the severe diaphragmatic muscle fiber defect, suggests that the sudden postpartum death of these Rb-deficient pups was due to their inability to respire. Moreover, Mox2+/creRb-/loxP pups had a characteristic “hunchback” appearance and lacked the normal S-shaped curvature of the vertebral column (Fig. 2N). No additional skeletal abnormalities or defects in ossification were detected in the rescued Rb-deficient pups. The absence of a cervical curvature and the forward tilt of the head in Mox2+/creRb-/loxP animals are most likely an indirect consequences of an inadequacy in skeletal musculature that is required to support the proper alignment of the skeleton during development.
Discussion
Previous studies have established a central role for Rb in the control of cellular proliferation, apoptosis, and differentiation of various cell lineages (21–23). Homozygous loss of Rb in mice results in ectopic proliferation and marked apoptosis, leading to severe defects in neurogenesis, liver hematopoiesis, and embryonic death by E14.5 (4–6). More recently, our work has demonstrated a profound placental defect in Rb-/- embryos (15). Interestingly, disruption of the E2F heterodimeric partner DP1 in mice leads to faulty placentation and early lethality, suggesting an important role for the Rb/E2F pathway in placental development (24). We now find that by supplying wild-type extraembryonic cells that form the bulk of the placenta, Rb-deficient fetuses develop to birth without many of the severe phenotypes typically associated with inactivation of Rb in the entire embryo. Thus, these results and others recently presented (15) identify an essential function of Rb in the placenta that profoundly impacts on apoptosis and differentiation of fetal tissues and ultimately dictates fetal development and viability.
Disruption of Rb function in mice leads to a marked induction of unscheduled DNA replication and ectopic apoptosis that is most pronounced in postmitotic neurons of the ventricular regions of the hindbrain, DRG, and spinal cord. Provision of wild-type extraembryonic lineages suppressed the severe defects in neurogenesis that are associated with loss of Rb during development. Direct analysis of the CNS in rescued Rb-null embryos revealed that although unscheduled proliferation continues, the ectopic apoptosis normally seen in postmitotic neurons of Rb-/- embryos is completely abrogated. Consequently, the architecture and differentiation of these tissues are relatively normal. Our findings are consistent with a recent detailed analysis of Rb+/+ and Rb-/- chimeric mice demonstrating that Rb has a cell autonomous role in the control of S-phase entry and a non-cell autonomous role in the suppression of apoptosis and maintenance of terminal differentiation in the developing CNS (25). Lipinski et al. (25) speculated that in Rb chimeras, the wild-type cells could alter the fate of neighboring Rb-/- neuronal cells by providing them with a specific survival or antiapoptotic signal(s) or by creating an improved growth environment that supports the continued survival of Rb-deficient cells. Our findings suggest that disruption of the extraembryonic function of Rb can account for much of the ectopic apoptosis observed in Rb-/- embryos. We propose that loss of Rb function in extraembryonic lineages leads to overproliferation of labyrinth trophoblast cells and a disruption of placental development and function that consequently contributes to the induction of apoptosis in the CNS of Rb-/- embryos. Although the exact mechanism is not yet clear, apoptosis in the CNS could be influenced directly by factors transported by or produced in the placenta. Alternatively, apoptosis could be an indirect effect of placental malfunction. Erythropoietic defects in the Rb-/- embryos, for example, may create a hypoxic state that promotes apoptosis in neurons of the CNS. Consistent with our proposal, conditional ablation of Rb from neuronal cells of the CNS does not induce apoptosis in these cells (26, 27). Whereas disruption of Rb in extraembryonic lineages results in apoptosis in the CNS that is clearly cell nonautonomous, inactivation of Rb in the lens and peripheral nervous system by Cre-mediated recombination (ref. 27 and this work) or in choroid plexus epithelial cells by the simian virus 40 T-antigen results in apoptosis that appears to be cell autonomous (28, 29). However, several other explanations may exist for these latter observations, including the effects imposed by the restricted nature of the lens architecture and additional functions of T-antigen that could impact on the apoptosis observed in the brains of these mice.
Erythropoiesis is also nearly normal in rescued Rb-deficient embryos, suggesting that a non-cell autonomous function of Rb in extraembryonic lineages can account for the appropriate differentiation of diverse tissues in the embryo. Although our data suggest that the placental defect is the most likely primary cause for the anemia of Rb-/- embryos, inactivation of Rb from other extraembryonic derived cells may also contribute to this phenotype. For example, mutant yolk sac endoderm, which is derived from the extraembryonic endoderm, could in principle adversely affect yolk sac erythropoiesis and explain the severe anemia in Rb knockout embryos. The facts that the yolk sac is the primary site of erythropoiesis between E8.5 and E12.5 and that the erythropoietic defects in Rb knockout embryos only become evident just before their death at E14.5 suggest that yolk sac erythropoiesis is normal and is unlikely to be responsible for their anemia and lethality. Although the exact nature of the placenta-derived signals responsible for the developmental defects and lethality of Rb-/- embryos is not yet known, we suspect that oxygen and/or nutrient deprivation could lead to the increase in immature RBC production. Alternatively, because the placenta produces erythropoietic differentiation factors, including erythropoietin (Epo) and placental prolactin-e (Plp-e), it is possible that the placenta may more directly affect erythropoiesis.
The early embryonic lethality of Rb-/- embryos has hampered studies on the potential role for Rb during late fetal development. Therefore, extending the lifespan of Rb-/- animals by supplying them with a normal placenta provides an opportunity to investigate Rb function at later stages of development. Interestingly, Rb-/- animals supplied with a normal placenta have a severe deficiency in skeletal muscle and die during late embryonic development or shortly after birth. Our observations demonstrate that the severe defects in skeletal muscle differentiation that occur later in development can be accounted for by the loss of Rb alone. Furthermore, in studies where skeletal structure has been examined (refs. 13 and 30, and this work), rescued Rb-/- animals lacked a normal cervical curvature and typically had craniums that were bent forward, which we now propose to be a secondary effect of a severe deficiency in skeletal muscle mass. These observations provide in vivo evidence for a role of Rb in skeletal muscle differentiation and indicate that this function of Rb, as in lens development, is likely cell autonomous.
Interestingly, loss of the cell cycle regulators E2F1, E2F3, or Id2 can also rescue, at least in part, the early lethality of Rb-/- embryos and some of the Rb-/--associated consequences (30–32). These observations, together with our current findings pointing to a key role of Rb in placental development, raise the possibility that E2F1, E2F3, and Id2 may function as downstream effectors of Rb in the control of proliferation and differentiation of extraembryonic lineages that form the placenta. If this were the case, the inactivation of these cell cycle regulators from Rb-/- placentae might be sufficient to restore placental function and thus rescue some of the phenotypic consequences stemming from Rb inactivation, including the early lethality of Rb-/- embryos. Although suppression of the erythropoietic defects and apoptosis in the CNS of Rb-/- embryos by loss of E2F1 or E2F3 may be mechanistically related to restoration of the placenta, our data suggest that suppression of inappropriate S-phase entry in double mutant embryos likely reflects cell-autonomous functions of Rb and E2Fs. The current findings will prompt a more careful evaluation of the cell autonomous or non-cell autonomous nature of the interactions between Rb, E2F, and Id2 during development.
In summary, embryonic manipulation and conditional knockout strategies have led us to identify and define a key function of Rb in extraembryonic lineages that is required for fetal development and viability, allowing a dissection of the cell-autonomous and non-cell-autonomous functions of Rb in the fetus. Identification of cell-autonomous and non-cell-autonomous functions of Rb represents a first step toward the elucidation of the molecular mechanism by which Rb acts during development. In the future, the identification and isolation of the precise placenta-derived cells defective in Rb-/- embryos will be a key second step in the understanding of Rb function.
Supplementary Material
Acknowledgments
We thank A. Berns, P. Soriano, and T. Jacks for Rb+/loxP, Mox2+/cre, and Rb+/- mice, respectively. This work was supported by grants from the National Cancer Institute (to G.L. and T.J.R.), the National Institutes of Health (to M.W. and G.L.), and the National Center for Research Resources (to T.J.R.). L.W. and H.I.S. were supported by National Institutes of Health awards, A.d.B. was supported by an Up on the Roof Human Cancer Genetics postdoctoral fellowship, and G.L. is a V Foundation and Pew Scholar.
Abbreviations: DRG, dorsal root ganglia; En, embryonic day n; TR, tetraploid-rescued; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Friend, S. H., Bernards, R., Rogelj, S., Weinberg, R. A., Rapaport, J. M., Albert, D. M. & Dryja, T. P. (1986) Nature 323, 643–646. [DOI] [PubMed] [Google Scholar]
- 2.Goodrich, D. W. & Lee, W. H. (1993) Biochim. Biophys. Acta 1155, 43–61. [DOI] [PubMed] [Google Scholar]
- 3.Nevins, J. R. (2001) Hum. Mol. Genet. 10, 699–703. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, A. R., Maandag, E. R., van Roon, M., van der Lugt, N. M., van der Valk, M., Hooper, M. L., Berns, A. & te Riele, H. (1992) Nature 359, 328–330. [DOI] [PubMed] [Google Scholar]
- 5.Jacks, T., Fazeli, A., Schmitt, E. M., Bronson, R. T., Goodell, M. A. & Weinberg, R. A. (1992) Nature 359, 295–300. [DOI] [PubMed] [Google Scholar]
- 6.Lee, E. Y., Chang, C. Y., Hu, N., Wang, Y. C., Lai, C. C., Herrup, K., Lee, W. H. & Bradley, A. (1992) Nature 359, 288–294. [DOI] [PubMed] [Google Scholar]
- 7.Williams, B. O. (1994) Cold Spring Harbor Symp. Quant. Biol. 59, 449–457. [DOI] [PubMed] [Google Scholar]
- 8.Morgenbesser, S. D., Williams, B. O., Jacks, T. & DePinho, R. A. (1994) Nature 371, 72–74. [DOI] [PubMed] [Google Scholar]
- 9.Chen, P. L., Riley, D. J., Chen, Y. & Lee, W. H. (1996) Genes Dev. 10, 2794–2804. [DOI] [PubMed] [Google Scholar]
- 10.Novitch, B. G., Mulligan, G. J., Jacks, T. & Lassar, A. B. (1996) J. Cell Biol. 135, 441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novitch, B. G., Spicer, D. B., Kim, P. S., Cheung, W. L. & Lassar, A. B. (1999) Curr. Biol. 9, 449–459. [DOI] [PubMed] [Google Scholar]
- 12.Thomas, D. M., Carty, S. A., Piscopo, D. M., Lee, J. S., Wang, W. F., Forrester, W. C. & Hinds, P. W. (2001) Mol. Cell 8, 303–316. [DOI] [PubMed] [Google Scholar]
- 13.Zacksenhaus, E., Jiang, Z., Chung, D., Marth, J. D., Phillips, R. A. & Gallie, B. L. (1996) Genes Dev. 10, 3051–3064. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan, D. & Weinberg, R. A. (2000) Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 15.Wu, L., de Bruin, A., Saavedra, H. I., Starovic, M., Trimboli, A., Yang, Y., Opavska, J., Wilson, P., Thompson, J. C., Ostrowski, M. C., et al. (2003) Nature 421, 942–947. [DOI] [PubMed] [Google Scholar]
- 16.Tallquist, M. D. & Soriano, P. (2000) Genesis J. Genet. Dev. 26, 113–115. [DOI] [PubMed] [Google Scholar]
- 17.Marino, S., Vooijs, M., van Der Gulden, H., Jonkers, J. & Berns, A. (2000) Genes Dev. 14, 994–1004. [PMC free article] [PubMed] [Google Scholar]
- 18.Lufkin, T., Mark, M., Hart, C. P., Dolle, P., LeMeur, M. & Chambon, P. (1992) Nature 359, 835–841. [DOI] [PubMed] [Google Scholar]
- 19.James, R. M., Klerkx, A. H., Keighren, M., Flockhart, J. H. & West, J. D. (1995) Dev. Biol. 167, 213–226. [DOI] [PubMed] [Google Scholar]
- 20.Chow, R. L. & Lang, R. A. (2001) Annu. Rev. Cell Dev. Biol. 17, 255–296. [DOI] [PubMed] [Google Scholar]
- 21.Lipinski, M. M. & Jacks, T. (1999) Oncogene 18, 7873–7882. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen, E. S., Buckmaster, C., Chen, T. T., Feramisco, J. R. & Wang, J. Y. (1998) Genes Dev. 12, 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen, K. E., Booth, D., Naderi, S., Sever-Chroneos, Z., Fribourg, A. F., Hunton, I. C., Feramisco, J. R., Wang, J. Y. J. & Knudsen, E. S. (2000) Mol. Cell. Biol. 20, 7751–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn, M. J., Bronson, R. T., Harlow, E., Dyson, N. & Yamasaki, L. (2003) Development (Cambridge, U.K.) 130, 1295–1305. [DOI] [PubMed] [Google Scholar]
- 25.Lipinski, M. M., Macleod, K. F., Williams, B. O., Mullaney, T. L., Crowley, D. & Jacks, T. (2001) EMBO J. 20, 3402–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson, K. L., Vanderluit, J. L., Hebert, J. M., McIntosch, W. C., Tibbo, E., MacLaurin, J. G., Park, D. S., Wallace, V. A., Vooijs, M., McConnell, S. K. & Slack, R. S. (2002) EMBO J. 21, 3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacPherson, D., Sage, J., Crowley, D., Trumpp, A., Bronson, R. T. & Jacks, T. (2003) Mol. Cell. Biol. 23, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Symonds, H., Krall, L., Remington, L., Saenz-Robles, M., Lowe, S., Jacks, T. & Van Dyke, T. (1994) Cell 78, 703–711. [DOI] [PubMed] [Google Scholar]
- 29.Xiao, A., Wu, H., Pandolfi, P. P., Louis, D. N. & Van Dyke, T. (2002) Cancer Cell 1, 157–168. [DOI] [PubMed] [Google Scholar]
- 30.Lasorella, A., Noseda, M., Beyna, M., Yokota, Y. & Iavarone, A. (2000) Nature 407, 592–598. [DOI] [PubMed] [Google Scholar]
- 31.Tsai, K. Y., Hu, Y., Macleod, K. F., Crowley, D., Yamasaki, L. & Jacks, T. (1998) Mol. Cell 2, 293–304. [DOI] [PubMed] [Google Scholar]
- 32.Ziebold, U., Reza, T., Caron, A. & Lees, J. A. (2001) Genes Dev. 15, 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.