Abstract
Certain C8-substituted and N7, C8-disubstituted guanine ribonucleosides comprise a class of small molecules with immunostimulatory activity. In a variety of animal models, these agents stimulate both humoral and cellular immune responses. The antiviral actions of these guanosine analogs have been attributed to their ability to induce type I IFNs. However, the molecular mechanisms by which the guanosine analogs potentiate immune responses are not known. Here, we report that several guanosine analogs activate Toll-like receptor 7 (TLR7). 7-Thia-8-oxoguanosine, 7-deazaguanosine, and related guanosine analogs activated mouse immune cells in a manner analogous to known TLR ligands, inducing cytokine production in mouse splenocytes (IL-6 and IL-12, type I and II IFNs), bone marrow-derived macrophages (IL-6 and IL-12), and in human peripheral blood leukocytes (type I IFNs, tumor necrosis factor α and IL-12). The guanosine congeners also up-regulated costimulatory molecules and MHC I/II in dendritic cells. Genetic complementation studies in human embryonic kidney 293 cells confirmed that the guanosine analogs activate cells exclusively via TLR7. The stimulation of TLR7 by the guanosine analogs in human cells appears to require endosomal maturation because inhibition of this process with chloroquine significantly reduced the downstream activation of NF-κB. However, TLR8 activation by R-848 and TLR2 activation by {S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-R-Cys-S-Ser-Lys4-OH, trihydrochloride)} were not inhibited by chloroquine, whereas TLR9 activation by CpG oligodeoxynucleotides was abolished. In summary, we present evidence that guanosine analogs activate immune cells via TLR7 by a pathway that requires endosomal maturation. Thus, the B cell-stimulating and antiviral activities of the guanosine analogs may be explained by their TLR7-activating capacity.
In a broad sense, immunity can be classified into an innate and an adaptive or acquired immune response. Each response is important in host defense against invading pathogens such as bacteria and viruses. The innate immune system is often the first line of defense against such pathogens and a conserved set of receptors called pattern-recognition receptors has evolved to recognize molecular patterns commonly associated with many microbial agents. These patterns are referred to as pathogen-associated molecular patterns (PAMPs). Thus, cells of the innate immune system, by recognizing these PAMPs, are able to distinguish self from nonself molecular structures and initiate the host defense consisting of the activation of signaling events that induce the expression of effector molecules, such as cytokines and costimulatory molecules. These effector molecules may subsequently induce an adaptive immune response. The pattern-recognition receptors in the mammalian innate immune system are called Toll-like receptors (TLRs) and represent a family of receptors, 10 distinct members of which have been identified so far. Mammalian TLRs are phylogenetically conserved type I transmembrane proteins with a Toll/IL-1 receptor homology region in their cytoplasmic domains (1). Microbial ligands have been identified for several of the mammalian TLRs. For example, TLR4 recognizes lipopolysaccharide (LPS) (2), TLR2 interacts with peptidoglycan, bacterial lipopeptides, and certain types of LPS (3), and TLR3 recognizes double-stranded RNA (4). TLR5 recognizes bacterial flagellin (5), whereas TLR9 was shown to recognize bacterial DNA (6). TLR7 is activated by small antiviral compounds such as various imidazoquinolines, imiquimod, and R-848 (7). R-848 also activates TLR8 in humans, and it was suggested that mouse TLR8 is not functional (8). However, no ligands for TLR7 or TLR8 from natural sources, either pathogens or host, have been reported so far. Certain TLRs (e.g., TLR1, TLR2, and TLR6) are recruited into macrophage phagosomes where they can heterodimerize, interact with their microbial ligands, and cause cell activation (9). This cooperation among different TLRs expands the ligand repertoire of the TLR family to discriminate among the large number of PAMPs found in the microbial environment (10). Recent studies have also proposed that host-derived factors can interact with TLRs to induce cell activation. In this respect, fibronectin (11) and HSP60 (12) have been implicated as ligands for TLR4. The importance of these latter interactions is not clear, but they may serve to alert the host to the presence of an invading microorganism through recognition of products released by tissue damage caused by microbes.
In the mid-1980s, our laboratory and others began studying a group of synthetic nucleosides that had the unique ability to activate the innate immune response. These compounds were mainly derivatives and analogs of guanine. Structure-activity studies within this class showed that structures in the thiazolo[4,5-d]pyrimidine (13), pyrazolo[3,4-d]pyrimidine (14), purine (15), 7-deazapurine (16), and 9-deazapurine ring systems (17) all were active. Examples in each group were found to be potent antiviral agents in mouse models because of their ability to rapidly induce the production of IFN (16, 18, 19). Among these guanosine analogs, 7-thia-8-oxoguanosine (TOG, Fig. 1) was studied in greatest detail and shown to exhibit broad spectrum antiviral activity and activate natural killer cells, macrophages, and B lymphocytes (20). Other guanosine analogs extensively studied were 7-deazaguanosine (21) and 7-allyl-8-oxoguanosine (loxoribine) (22). The exact mechanism of immune potentiation by these guanine derivatives has not been elucidated. As tremendous progress has been made over the past few years in the understanding of the signaling events surrounding activation of the innate immune system and the TLR family of receptors, we were prompted to investigate the possibility that these small molecules could be acting via signaling through one or more of the known TLRs. In this article, we present evidence that TOG and other guanosine analogs activate immune cells via TLR7 and the activation of TLR7 by guanosine analogs requires endosomal maturation.
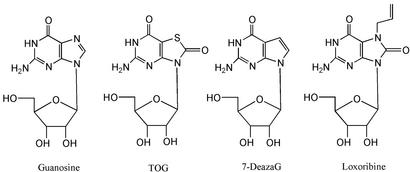
Fig. 1.
Guanosine and examples of immunostimulatory analogs. 7-deazaG, 7-deazaguanosine; loxoribine, 7-allyl-8-oxoguanosine.
Materials and Methods
Reagents. Guanosine, 2′-deoxy-G, 8-bromo-G, 7-methyl-G, 7-allyl-8-oxo-G (loxoribine), and chloroquine were purchased from Sigma. 8-OH-G, 9-p-chlorophenyl-8-aza-G, 9-phenyl-G, 9-hexyl-guanine, 7-deaza-G, and 7-deaza-9-benzyl-G were gifts from ICN. 6-Chloro-7-deazaguanine, 6-methoxy-7-deazaguanine, and 7-deaza-dG were prepared in our laboratories from 7-deazaguanine obtained from Berry and Associates (Ann Arbor, MI). TOG was synthesized as described (13). Antibodies to CD40, CD80, CD86, and MHC I and II were purchased from PharMingen. Imiquimod was obtained from Sequoia Research Products (Oxford), and R-848 {also called Resiquimod, 4-amino-2-ethoxymethyl-α, α-dimethyl-1H-imidazo[4,5-c]quinoline-1-ethanol} was from GLS Synthesis (Worcester, MA). Pam3Cys {S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-R-Cys-S-Ser-Lys-4-OH, trihydrochloride)} was obtained from EMC Microcollections (Tubingen, Germany), and p(I:C) was from Amersham Pharmacia. CpGODN for human cells, 5′-TCG TCG TTT GTC GTT TTG TCG TT-3′, and CpG-ODN for mouse cells, 5′-TCC ATG ACG TTC CTG ACG TT-3′, were purchased from Invitrogen. LPS from Salmonella minnesota Re595 was prepared and used as described (23).
Mouse Strains. C57BL/6 female mice, 6–8 weeks old, were purchased from The Jackson Laboratory.
Activation of Mouse Splenocytes, Bone Marrow-Derived Macrophages (BMDMs), and Bone Marrow-Derived Dendritic Cells (BMDDCs). BMDMs, BMDDCs, and splenocytes were prepared as described (24). Different doses of compounds were added to splenocytes or BMDMs, and the supernatants were collected after 24 h of stimulation. Induction of cytokines was measured by ELISA (IL-6, IL-12, or IFN-γ) using antibody from PharMingen. Type I IFN levels in supernatants from mouse splenocytes were measured by using an antiviral protection assay as described (25). BMDDCs were stimulated for 24 h with the indicated stimulus, and the induction of cell surface markers was analyzed by flow cytometry.
Flow Cytometry. Induction of cell surface markers by TLR ligands was analyzed with FACSCalibur (Becton Dickinson) by using the antibodies described above.
Activation of Human Peripheral Blood Leukocytes (PBLs) by Guanosine Analogs. Human blood samples from three donors were obtained from the San Diego Blood Bank. PBLs were isolated from the blood samples by Ficoll gradient using Ficoll-Plaque Plus (Amersham Pharmacia). Briefly, 7 ml of each sample was diluted with 7 ml of RPMI medium 1640, layered on 30 ml of Ficoll-Plaque Plus, and centrifuged at 2,000 rpm for 30 min with tabletop centrifuge (Beckman GS-6R). PBLs were washed twice with 50 ml of RPMI medium 1640, and the final pellet was resuspended in 10 ml of RPMI medium 1640. PBLs were stimulated for 36 h with the indicated stimulus, and the induction of cytokines was measured by ELISA according to the manufacturer's instructions [tumor necrosis factor α (TNF-α) and IFN-α from BioSource International (Camarillo, CA) and IL-12p40 from PharMingen].
RT-PCR. Total RNA from stimulated human PBLs from a single donor was isolated with a TRIzol kit (Invitrogen), and cDNA was generated with SuperScript II (Invitrogen). GAPDH was amplified at 68°C with the primers: sense, 5′-ACC ACA GTC CAT GCC ATC AC-3′, and antisense, 5′-TCC ACC ACC CTG TTG CTG TA-3′. IFN-β was amplified at 55°C with the primers: sense, 5′-ATG AAC TCC TTC TCC ACA AG-3′, and antisense, 5′-CTA CAT TTG CCG AAG AGC CC-3′.
Expression Vectors for Human TLRs. Human TLRs were cloned in pCMV vector (Sigma) with the flag tag at the N terminus and sequenced to confirm the correct ORF (26).
NF-κB Reporter Assay. Reporter assays for NF-κB were performed as described (26). Briefly, human embryonic kidney 293 (HEK293) cells were cultured in DMEM, supplemented with 10% FBS. The HEK293 cells were plated in six-well plates and transfected the next day with Lipofectamine 2000 (GIBCO/BRL) loaded with the following plasmids: human TLR expression vectors (TLR 1–10) or empty vector (0.1 μg), NF-κB reporter (endothelial leukocyte adhesion molecule 1 promoterluciferase plasmid; 0.1 μg), and β-galactosidase plasmid (0.1 μg) for normalization. The cells were lysed, and luciferase activities were determined by using reagents from Promega. Relative luciferase activities were calculated as folds of induction compared with vector control.
NF-κB Assay by Electrophoretic Mobility-Shift Assay (EMSA). EMSA was performed as described (23).
Inhibition by Chloroquine. For inhibition studies, chloroquine at 2–5 μM concentration was added to the cells 30 min before stimulation with TLR ligands.
Results
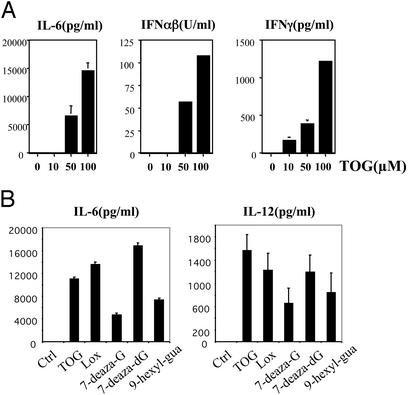
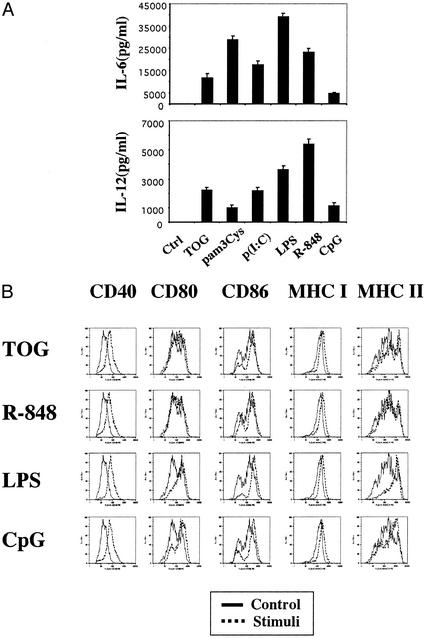
Guanosine Analogs Activate Mouse Immune Cells. TOG and several related guanosine analogs in mouse models were shown to provide protection against a spectrum of viruses that are sensitive to IFNs (20). In accordance with previous reports, TOG induced type I IFNs in mouse splenocytes in a dose-dependent manner (Fig. 2A). In addition, TOG induced IFN-γ, IL-6 and IL-12. Other guanosine analogs (loxoribine, 7-deaza-G, 7-deaza-dG, and 9-hexyl-guanine) also activated splenocytes in a similar manner, resulting in induction of IL-6 and IL-12 (Fig. 2B). TOG activated BMDMs in a manner comparable to other ligands of TLRs; TOG induced the production of IL-6 and IL-12 in mouse BMDMs (Fig. 3A). TOG also activated mouse BMDDCs, resulting in up-regulation of costimulatory molecules and MHC molecules (Fig. 3B). Up-regulation of CD40, CD86, MHC I, and MHC II by TOG was comparable to that by LPS or CpG. However, TOG or R-848 was less competent in CD80 induction compared with LPS or CpG (Fig. 3B). This finding is consistent with the report that the activation of individual TLRs results in different biological outcomes although the TLR signaling pathways share many aspects (27).
Fig. 2.
Guanosine analogs activate murine splenocytes. (A) TOG activates splenocytes. Splenocytes isolated from C57BL/6 mice were incubated with TOG at the indicated concentration for 24 h, and the induction of cytokines was measured by ELISA as described in Materials and Methods. (B) Other guanosine analogs activate splenocytes. Splenocytes were stimulated for 24 h with TOG, loxoribine (Lox), 7-deaza-G, 7-deaza-dG, and 9-hexyl-guanine (100 μM each), and the induction of IL-6 and IL-12(p40) was measured by ELISA. Results are representative of more than three separate experiments, and standard error was determined from duplicates.
Fig. 3.
TOG activates murine macrophages and dendritic cells. (A) TOG activates macrophages. BMDMs from C57BL/6 mice were stimulated for 24 h with TOG (100 μM), pam3Cys (5 μg/ml), p(I:C) (5 μg/ml), LPS (10 ng/ml), R-848 (1 μM), or CpG (5 μg/ml). The production of IL-6 and IL-12(p40) was measured by ELISA. (B) TOG activates dendritic cells. BMDDCs from C57BL/6 mice were stimulated for 24 h with TOG (100 μM), LPS (10 ng/ml), R-848 (1 μM), or CpG (5 μg/ml). The induction of cell surface markers was measured by flow cytometry.
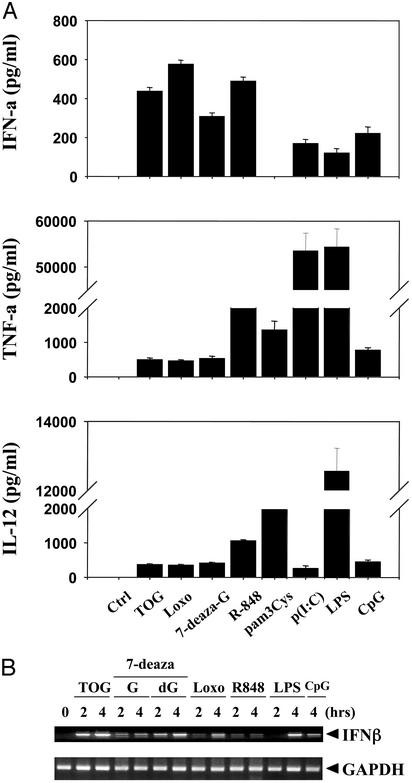
Guanosine Analogs Activate Human Immune Cells. To test whether guanosine analogs induce similar immune enhancement in humans, we stimulated human PBLs with guanosine analogs and other PAMPs. Activation of human PBLs by guanosine analogs resulted in induction of TNF-α, IL-12, and IFN-α (Fig. 4A). Compared with other TLR ligands, the guanosine analogs were strong inducers of IFN-α while much less potent in induction of TNF-α or IL-12 (Fig. 4A). Guanosine analogs also induced IFN-β in human PBLs (Fig. 4B). These data demonstrate that the guanosine analogs are capable of activating mouse and human immune cells in a manner similar to that of other PAMPs.
Fig. 4.
Guanosine analogs activate human PBLs. (A) Guanosine analogs induce TNF-α, IL-12, and IFN-α in human PBLs. Human PBLs were stimulated for 24 h with TOG, loxoribine (Loxo), 7-deaza-G (100 μM each), R-848 (0.5 μM), p(I:C) (5 μg/ml), LPS (10 ng/ml), or CpG (5 μg/ml). The induction of cytokines was measured by ELISA as described in Materials and Methods.(B) Guanosine analogs induce IFN-β in human PBLs. PBLs were stimulated for the indicated time periods with TOG (100 μM), 7-deaza-G (100 μM), 7-deaza-dG (100 μM), loxoribine (Loxo, 100 μM), R-848 (1 μM), LPS (10 ng/ml), or CpG (5 μg/ml), and the induction of IFN-β was measured by RT-PCR as described in Materials and Methods.
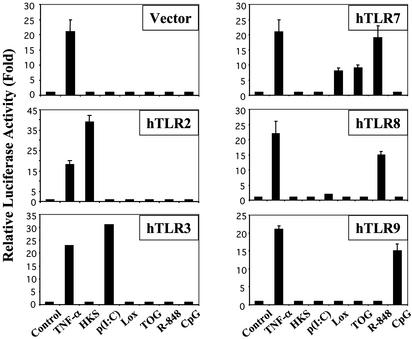
TOG Activates TLR7. Because the profile of TOG-induced activation of immune cells was very similar to that of other TLR ligands, we tested whether TOG activates cells via a TLR. To screen TLRs as possible receptor for guanosine analogs, individual human TLRs were expressed in HEK293 cells along with NF-κB–luciferase as a reporter. Luciferase activity was measured as readout for TOG-induced NF-κB activation via the transfected TLR. Among the tested TLRs, only TLR7 was able to transduce the TOG-induced signal, clearly demonstrating TLR7 as the receptor for TOG (Fig. 5). Positive controls demonstrated that pamCys3 activated TLR2, p(I:C) activated TLR3, R-848 activated TLR7/8, and CpG activated TLR9.
Fig. 5.
Guanosine analogs activate TLR7. HEK293 cells were transfected with either empty vector or the indicated human TLR. Twenty-four hours after transfection cells were stimulated for 6 h with TNF-α (10 ng/ml), heat-killed Staphylococcus aureus (HKS, 1 × 107/ml), p(I:C) (10 μg/ml), loxoribine (200 μM), TOG (200 μM), R-848 (20 μM), or CpG (5 μM). Relative luciferase activities were measured as described in Materials and Methods.
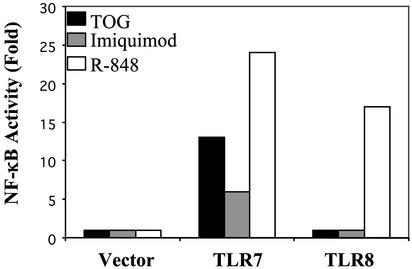
Guanosine Analogs Activate Human TLR7 But Not TLR8. Jurk et al. (8) recently demonstrated that R-848 can activate both TLR7 and TLR8 in human cells and suggested that TLR8 was not functional in the mouse, explaining why R-848 was not able to activate cells from TLR7-deficient mice. TOG activated TLR7 but not TLR8, whereas R-848 displayed both activities (Fig. 6). Surprisingly, imiquimod, which is closely related to R-848 in structure, activated only TLR7 but not TLR8 (Fig. 6). Besides TOG, 7-deaza-G, 7-deaza-deoxyG, and 7-ally-8-oxo-G (loxoribine) also activated TLR7 (Table 1). Although 9-hexyl-guanine activated mouse immune cells, it was not able to activate TLR7 in the reporter assay.
Fig. 6.
TOG and imiquimod activate TLR7 but not TLR8. HEK293 cells were transfected with empty vector, TLR7, or TLR8 and stimulated with TOG (200 μM), imiquimod (20 ng), or R-848 (20 μM) for 6 h. Relative luciferase activities were measured as described in Materials and Methods.
Table 1. Guanine analogs activate TLR7 but not TLR8.
| Guanine derivatives | Activation of TLR7 | Activation of TLR8 |
|---|---|---|
| Guanosine (G) | No | No |
| Deoxy-Guanosine (dG) | No | No |
| 8-OH-dG | No | No |
| 8-Aza-chlorophenyl-G | No | No |
| 9-Phenyl-G | No | No |
| 7-Deaza-9-benzyl-G | No | No |
| 6-Chloro-7-deaza-G | No | No |
| 6-Methoxy-7-deaza-G | No | No |
| 7-Deaza-dG | Yes | No |
| 7-Deaza-G | Yes | No |
| 7-Thia-8-oxo-G (TOG) | Yes | No |
| 8-Bromo-G | No | No |
| 7-Methyl-G | No | No |
| 7-Allyl-8-oxo-G (Loxoribine) | Yes | No |
| Imiquimod | Yes | No |
| R-848 | Yes | Yes |
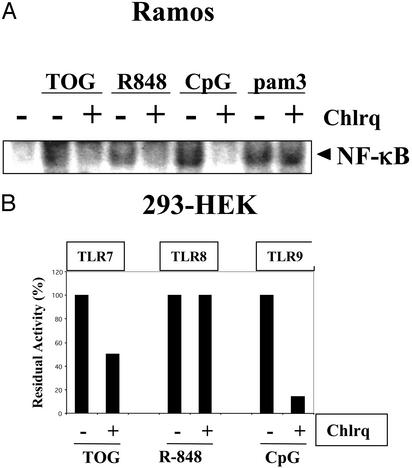
Activation of TLR7 Depends on Endosomal Maturation. Activation of immune cells by bacterial DNA or CpG has been shown to require endosomal maturation because compounds such as chloroquine, which inhibit endosomal acidification, completely abolish TLR9-mediated cell activation. The requirement for endosomal acidification in TLR signaling is unique for TLR9 in immune cells (28). Indeed, TLR9 in immune cells is expressed in endosomes rather than on the plasma membrane, which further supports this notion (29). We tested whether activation of TLR7 by guanosine analogs requires endosomal acidification. TLR7 activation by guanosine analogs or R-848 was sensitive to chloroquine inhibition in a human B cell line (Ramos) (Fig. 7A). Because R-848 can activate both TLR7 and TLR8 in human cells, we tested whether TLR7, TLR8, or both are sensitive to chloroquine in HEK293 cells. Inhibition of TLR7 activation by chloroquine was ≈50%, whereas that of TLR9 was >90%, suggesting that the activation mechanism of TLR7 is not identical to that of TLR9 (Fig. 7B). However, TLR8 activation by R-848 or TLR2 by pam3Cys was not affected significantly by chloroquine (Fig. 7).
Fig. 7.
Activation of TLR7 requires endosomal maturation. (A) Activation of Ramos cells by TOG or R-848 is inhibited by chloroquine. Ramos cells were pretreated with or without chloroquine (5 μM) for 30 min and stimulated with TOG (100 μM), R-848 (1 μM), CpG (5 μg), or pam3Cys (pam3, 5 μg) for 2 h, and activation of NF-κB was measured by electrophoretic mobility-shift assay. (B) Activation of TLR7 but not TLR8 is inhibited by chloroquine. HEK293 cells were transfected with TLR7, TLR8, or TLR9 and stimulated with TOG (200 μM), R-848 (20 μM), or CpG (5 μM) for 6 h. Relative luciferase activities were measured as described in Materials and Methods.
Discussion
A number of derivatives of guanosine and analogs thereof have been studied over the years for their ability to stimulate the innate immune system in mouse and human models. The structural requirements for guanosine analogs to be immunostimulatory are such that the pattern of purine ring substitution at the 7- and 8-positions (and to a lesser extent the 9-position) is absolutely critical for immune stimulation. Thus, when a 7-nitrogen is present in the ring, such as in the case of purine or 9-deazapurine nucleosides, the 8-position must be oxidized (Fig. 1). The consequences of immune stimulation by guanosine analogs include both humoral and cellular immune responses. Most importantly, these guanosine analogs provide strong protection against a broad spectrum of viruses that are sensitive to type I IFNs. However, the molecular details of the mechanism leading to immune potentiation have remained unclear.
Our recent studies, reported here, shed light on these events. As reported, several guanosine analogs activated both mouse and human immune systems and were particularly efficient in inducing type I IFNs (Figs. 2 and 4). The profile of immune activation by guanosine analogs resembled very much that by LPS or other PAMPs that immune systems evolved to recognize as danger signals. These findings led us to hypothesize that guanosine analogs might activate immune cells via a TLR. TLRs are the receptors for the PAMPs and are phylogenetically conserved from flies to humans, indicating the importance of these receptors in immune functions. So far 10 members of TLR have been identified in mammals, and ligands for some TLRs have been identified. By a genetic complementation assay, we confirmed that guanosine analogs activate TLR7 (Fig. 5). Interestingly, the only known ligands for TLR7 are the small antiviral compounds, imiquimod and R-848 (7), but no natural endogenous ligand for TLR7 has been reported. We previously reported that imiqimod activates NF-κB and IRF-1 and induces cytokines such as IFN-α in human peripheral blood monocytes (30). Imiquimod and R-848 activate TLR7 in a MyD88-dependent manner, and MyD88 is an essential adapter molecule in the signal transduction pathways initiated by TLRs. Later it was found that R-848 can also activate TLR8 in human cells, but it was suggested that TLR8 in the mouse is nonfunctional (8). We demonstrated here that, unlike R-848, guanosine analogs signal exclusively through TLR7 and that imiquimod, a close structural relative of R-848, also activates only TLR7 (Fig. 6).
The mode of TLR9 activation by CpG motif is unique among TLRs in that endosomal acidification or maturation is required for its activation (28). We report here that activation of TLR7 by guanosine analogs also goes through endosomal processing because an inhibitor of endosomal maturation such as chloroquine inhibited TLR7 activation by guanosine analogs (Fig. 7). However, the activation mechanism of TLR7 is not entirely identical to that of TLR9 because TLR9 activation is abolished completely by chloroquine whereas TLR7 activation is inhibited only partially, but significantly (Fig. 7). The total dependence of TLR9 activation was explained by the localization of TLR9 on the inner face of endosomes rather than in the cytoplasm (29). Based on this information, we speculate that TLR7 might be expressed in more than one cellular compartment. Early studies have indicated the presence of two distinctive binding activities of guanosine analogs in murine B cells, and they exhibited dissociation constants that were proportional to their distinctive activities (31). The availability of specific antibodies to TLR7 will be needed to prove this point.
Phagocytic white blood cells produce reactive oxidants that play critical roles in host defenses by destroying invading microbial pathogens. These oxidants comprise a group of electrophilic reactants that include hypochlorous and hypobromous acids, reactive nitrogen species such as peroxynitrite, nitrogen dioxide, and nitrosyl chloride, and hydrogen peroxide and other reactive oxygen species. Macromolecules of the invading pathogens and host tissues become the targets of these reactive species, resulting in oxidative damage to lipids, proteins, and nucleic acids. Oxidative damage to nucleic acid components generally involves alterations at the 8-position of susceptible purine bases and the 5-position of pyrimidines. Guanine and cytosine, of all of the nucleobases, are the most susceptible bases to undergo oxidative damage because of their intrinsic higher electron density at the available ring carbon atoms. Indeed, oxidized guanine derivatives have been observed at sites of inflammation and infection, including 8-oxo-, 8-bromo-, 8-chloro- and 8-nitroguanosine (32). Based on this information, we hypothesized that certain naturally oxidized nucleosides might activate innate immunity in a manner similar to that of TOG and other guanosine analogs. We tested three of these compounds (8-chloro-G, 8-nitro-G, and 5-chloro-C) and found that both 8-nitro-G and 5-chloro-C, but not 8-chloro-G, activated NF-κB in both Ramos cells and human PBLs. However, we were unable to show that these three compounds activate TLR7 (data not shown). Although it is not clear by what mechanisms the oxidized nucleosides activate NF-κB, it is tempting to speculate that they are weak activators of TLR7 but that the transfection assay system was not sensitive enough to detect this activity, possibly because, in part, of their instability. Finally, the proposal that certain oxidized guanosines produced during inflammation or infection activate the innate immune system via TLR is analogous to the recent finding that β-defensins, small antimicrobial peptides produced in response to microbial infection of mucosal tissue and skin, activate TLR4 (33).
Taken together, our data provide evidence that certain guanosine analogs and derivatives activate immune cells via TLR7 and that endosomal maturation is required for this activation to occur.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grant AI40682 (to E.R.), a grant from the Stein Institute for Research on Aging (to J.L.), and a Research Agreement from Salmedix, Inc. (to H.B.C.).
Abbreviations: TOG, 7-thia-8-oxoguanosine; TNF-α, tumor necrosis factor α; TLR, Toll-like receptor; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular pattern; pam3Cys, {S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-R-Cys-S-Ser-Lys4-OH, trihydrochloride)}; BMDM, bone marrow-derived macrophage; BMDDC, bone marrow-derived dendritic cell; PBL, peripheral blood leukocyte; HEK, human embryonic kidney.
References
- 1.Means, T. K., Golenbock, D. T. & Fenton, M. J. (2000) Life Sci. 68, 241–258. [DOI] [PubMed] [Google Scholar]
- 2.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Huffel, C. V., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S. & Hemmi, H. (2003) Immunol. Lett. 85, 85–95. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., Eng, J. K., Akira, S., Underhill, D. M. & Aderem, A. (2001) Nature 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi, H., Takeuchi, O., Kawai, T., Kaisho, T., Sato, S., Sanjo, H., Matsumoto, M., Hoshino, K., Wagner, H., Takeda, K. & Akira, S. (2000) Nature 408, 740–745. [DOI] [PubMed] [Google Scholar]
- 7.Hemmi, H., Kaisho, T., Takeuchi, O., Sato, S., Sanjo, H., Hoshino, K., Horiuchi, T., Tomizawa, H., Takeda, K. & Akira, S. (2002) Nat. Immunol. 3, 196–200. [DOI] [PubMed] [Google Scholar]
- 8.Jurk, M., Heil, F., Vollmer, J., Schetter, C., Krieg, A. M., Wagner, H., Lipford, G. & Bauer, S. (2002) Nat. Immunol. 3, 499. [DOI] [PubMed] [Google Scholar]
- 9.Ozinsky, A., Smith, K. D., Hume, D. & Underhill, D. M. (2000) J. Endotoxin Res. 6, 393–396. [PubMed] [Google Scholar]
- 10.Ozinsky, A., Underhill, D. M., Fontenot, J. D., Hajjar, A. M., Smith, K. D., Wilson, C. B., Schroeder, L. & Aderem, A. (2000) Proc. Natl. Acad. Sci. USA 97, 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamura, Y., Watari, M., Jerud, E. S., Young, D. W., Ishizaka, S. T., Rose, J., Chow, J. C. & Strauss, J. F., 3rd (2001) J. Biol. Chem. 276, 10229–10233. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. (2000) J. Immunol. 164, 558–561. [DOI] [PubMed] [Google Scholar]
- 13.Nagahara, K., Anderson, J. D., Kini, G. D., Dalley, N. K., Larson, S. B., Smee, D. F., Jin, A., Sharma, B. S., Jolley, W. B., Robins, R. K., et al. (1990) J. Med. Chem. 33, 407–415. [DOI] [PubMed] [Google Scholar]
- 14.Bontems, R. J., Anderson, J. D., Smee, D. F., Jin, A., Alaghamandan, H. A., Sharma, B. S., Jolley, W. B., Robins, R. K. & Cottam, H. B. (1990) J. Med. Chem. 33, 2174–2178. [DOI] [PubMed] [Google Scholar]
- 15.Michael, M. A., Cottam, H. B., Smee, D. F., Robins, R. K. & Kini, G. D. (1993) J. Med. Chem. 36, 3431–3436. [DOI] [PubMed] [Google Scholar]
- 16.Smee, D. F., Alaghamandan, H. A., Gilbert, J., Burger, R. A., Jin, A., Sharma, B. S., Ramasamy, K., Revankar, G. R., Cottam, H. B., Jolley, W. B., et al. (1991) Antimicrob. Agents Chemother. 35, 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girgis, N. S., Michael, M. A., Smee, D. F., Alaghamandan, H. A., Robins, R. K. & Cottam, H. B. (1990) J. Med. Chem. 33, 2750–2755. [DOI] [PubMed] [Google Scholar]
- 18.Smee, D. F., Alaghamandan, H. A., Jin, A., Sharma, B. S. & Jolley, W. B. (1990) Antiviral Res. 13, 91–102. [DOI] [PubMed] [Google Scholar]
- 19.Smee, D. F., Huffman, J. H., Gessaman, A. C., Huggins, J. W. & Sidwell, R. W. (1991) Antiviral Res. 15, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smee, D. F., Alaghamandan, H. A., Cottam, H. B., Sharma, B. S., Jolley, W. B. & Robins, R. K. (1989) Antimicrob. Agents Chemother. 33, 1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smee, D. F., Alaghamandan, H. A., Ramasamy, K. & Revankar, G. R. (1995) Antiviral Res. 26, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitz, A. B., Goodman, M. G., Pope, B. L., Argentieri, D. C., Bell, S. C., Burr, L. E., Chourmouzis, E., Come, J., Goodman, J. H., Klaubert, D. H., et al. (1994) J. Med. Chem. 37, 3561–3578. [DOI] [PubMed] [Google Scholar]
- 23.Lee, J., Mira-Arbibe, L. & Ulevitch, R. J. (2000) J. Leukocyte Biol. 68, 909–915. [PubMed] [Google Scholar]
- 24.Chu, W., Gong, X., Li, Z., Takabayashi, K., Ouyang, H., Chen, Y., Lois, A., Chen, D. J., Li, G. C., Karin, M. & Raz, E. (2000) Cell 103, 909–918. [DOI] [PubMed] [Google Scholar]
- 25.Cheung, T. H., Lo, K. W., Yu, M. M., Yim, S. F., Poon, C. S., Chung, T. K. & Wong, Y. F. (2001) Cancer Lett. 172, 93–98. [DOI] [PubMed] [Google Scholar]
- 26.Chuang, T. H., Lee, J., Kline, L., Mathison, J. C. & Ulevitch, R. J. (2002) J. Leukocyte Biol. 71, 538–544. [PubMed] [Google Scholar]
- 27.Jones, B. W., Means, T. K., Heldwein, K. A., Keen, M. A., Hill, P. J., Belisle, J. T. & Fenton, M. J. (2001) J. Leukocyte Biol. 69, 1036–1044. [PubMed] [Google Scholar]
- 28.Krieg, A. M. (2002) Annu. Rev. Immunol. 20, 709–760. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad-Nejad, P., Hacker, H., Rutz, M., Bauer, S., Vabulas, R. M. & Wagner, H. (2002) Eur. J. Immunol. 32, 1958–1968. [DOI] [PubMed] [Google Scholar]
- 30.Megyeri, K., Au, W. C., Rosztoczy, I., Raj, N. B., Miller, R. L., Tomai, M. A. & Pitha, P. M. (1995) Mol. Cell. Biol. 15, 2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman, M. G. & Goodman, J. H. (1994) J. Immunol. 153, 4081–4087. [PubMed] [Google Scholar]
- 32.Henderson, J. P., Byun, J., Williams, M. V., Mueller, D. M., McCormick, M. L. & Heinecke, J. W. (2001) J. Biol. Chem. 276, 7867–7875. [DOI] [PubMed] [Google Scholar]
- 33.Biragyn, A., Ruffini, P. A., Leifer, C. A., Klyushnenkova, E., Shakhov, A., Chertov, O., Shirakawa, A. K., Farber, J. M., Segal, D. M., Oppenheim, J. J. & Kwak, L. W. (2002) Science 298, 1025–1029. [DOI] [PubMed] [Google Scholar]