Abstract
Anthrax protective antigen (PA) is a 735-aa polypeptide that facilitates the exit of anthrax lethal factor (LF) from the endosome to the cytosol where the toxin acts. We recently found, however, that a fusion protein of the detoxified N-terminal domain of lethal factor (LFn) with a foreign peptide could induce CD8 T cell immune responses in the absence of PA. Because CD8 T cells recognize peptides derived from proteins degraded in the cytosol, this result suggests that lethal factor may be capable of entering the cytosol independently of PA. To investigate this further, the intracellular trafficking of an LFn-enhanced green fluorescent protein fusion protein (LFn-GFP) in the presence or absence of PA was examined by using confocal microscopy. LFn-GFP is able to enter the cytosol without PA. Moreover, it efficiently colocalizes with the proteosome 20s subunit, which degrades proteins into peptides for presentation to CD8 T cells by the MHC class I pathway. We further demonstrate that in the presence of an immune adjuvant LFn fusion protein without PA is able to effectively elicit anti-HIV cytotoxic T lymphocyte in inbred mice. These results indicate that LFn may be used without PA in a protein vaccine as a carrier to deliver antigens into the cytosol for efficient induction of T lymphocyte responses. Furthermore, these results enable us to propose a modified molecular mechanism of anthrax lethal toxin.
Bacillus anthracis is the causative agent of anthrax in animals and humans. It produces two bipartite protein exotoxins, lethal toxin (LeTx) and edema toxin. LeTx is composed of protective antigen (PA) and lethal factor (LF). The molecular mechanism of anthrax toxin action is currently hypothesized as follows: PA is a 735-aa polypeptide that binds to the surface of mammalian cells via anthrax toxin receptors (1). Once bound, PA is activated by proteolytic cleavage by cellular proteases to a 63-kDa molecule capable of forming a ring-shaped heptamer in the plasma membrane of the targeted cell (2, 3). The PA heptamer then binds to LF, both of which are subsequently internalized by endocytosis. LF inactivates mitogen-activated protein kinase (4), thus inhibiting its signaling pathways with lethal effects on host animals. LF is a 796-aa polypeptide, and the functional toxin domain is located between amino acids 383 and 796 (5). The N-terminal LF (LFn) without this catalytic domain completely lacks any toxic effect when mixed with PA and added to cultured macrophages or when injected into animals. It does, however, still effectively bind to PA. The crystal structure of LF has been recently reported (6).
The combination of PA plus LFn fused to a foreign peptide can be used as a “molecular syringe” to introduce foreign proteins into the cytosol for antigen presentation to CD8 T cells. Previous studies have demonstrated successful cytotoxic T lymphocyte (CTL) induction in inbred mice after administration of PA plus LFn carrying an 8- to 9-aa peptide epitope. For example, immunization of BALB/c mice with PA plus LFn-LLO, a fusion protein of LFn with a CTL epitope from the listeriolysin protein of Listeria monocytogenes, stimulated a significant CTL response, which protected animals challenged with L. monocytogenes. Immunized mice showed a significant reduction of colony-forming units in the spleen and liver compared with untreated mice. Similarly, immunization with PA plus LFn fused to a lymphocytic choriomeningitis virus (LCMV) nucleoprotein epitope protects against lethal LCMV infection (7). Another study demonstrated CTL induction in C57BL/6 mice by PA plus an LFn fusion protein carrying a CTL epitope from ovalbumin (LFn-OVA; ref. 8). We reported the production of stable and soluble recombinant fusion proteins between LFn and a variety of HIV antigens that were up to 500 aa in length. We demonstrated that immunization with these HIV candidate vaccines is safe in mice and rabbits and can elicit CD8 T cell responses in mice. The proteins also stimulated human peripheral blood mononuclear cells in an in vitro activation protocol (9).
However, we recently observed that LFn fusion proteins in the absence of PA were also capable of sensitizing CTL target cells in an MHC-I-restricted manner (10). We demonstrate that this cytosolic delivery of LFn fusion proteins relies on functional TAP (transport-associated proteins) for intracellular antigen processing that transports the peptides into the endoplasmic reticulum (ER) lumen for binding to MHC class I. We further showed that this PA-independent LFn delivery could be abrogated in the presence of cytochalasin B, which is a phagocytosis inhibitor, thus suggesting an endocytosis-mediated internalization process. This unique antigen presentation pathway is also sensitive to the treatment of the target cells by brefeldin A that inhibits exocytosis of proteins from the ER and Golgi complexes and prevents newly assembled peptide-MHC complexes from reaching the cell surface. Finally, we tested whether the PA-independent LFn delivery could be blocked by the treatment of the antigen-presenting cells by chloroquine, which raises the pH in the endosomal and lysosomal compartments and inhibits protein hydrolysis by cathepsins. It has been reported that the presentation of some exogenous antigens on MHC class I molecule involves proteolysis in the endocytic compartment and the peptides subsequently gain access to the cytosol where it enters the MHC class I pathway. Our results, however, showed that the chloroquine treatment had no effect on the PA-independent LFn fusion protein-mediated antigen presentation, suggesting that phagosomes with leaky properties were not requisite for the entry of the LFn fusion proteins into the cytosol. In contrast, if targets were pulsed with the optimal 8-mer epitopes at the surface in all these experiments, the treatment of cytochalasin B, brefeldin A, and chloroquine, or the use of TAP-deficient target cells, did not affect lysis of target cells. Taken together, we conclude that, even in the absence of PA, LFn enables exogenous proteins to enter the classical MHC-I pathway in cytosol.
In this study, we demonstrate that enhanced GFP fused with LFn can enter into cells in the absence of PA. We further show that this PA-independent LFn delivery of exogenous GFP seems to colocalize with proteosomes, which degrade proteins into peptides capable of binding to MHC class I molecules. Finally, we present evidence that this interesting property of the LFn molecule can be used as an effective method to elicit cell-mediated immune responses in animals without resorting to live bacterial or viral vectors and DNA vaccination.
Materials and Methods
Construction and Purification of LFn-GFP, GFP, and PA. LFn-GFP fusion protein was constructed by insertion of the GFP ORF from pEGFP-C1 (CLONTECH) into the LFn expression vector pET15b as described. The fusion protein is soluble in bacterial cell extracts, has an N-terminal His-6 tag, and can be purified in one-step chromatography by metal chelation as described (9). The purified fusion protein has a molecular mass of ≈55 kDa, and its solution has a bright green color (data not shown). To have an appropriate control for the experiments, GFP alone was constructed into the same bacterial expression vector so that the only difference in the expression, purification, and use of GFP and LFn-GFP is the lack of LFn sequences in the former. PA was produced and purified as described (11).
The DNA fragments encoding HIV-1 env gp120, nef p27, and gag p24 were amplified by PCR, cloned into pET15bLFn, and sequenced to verify the in-frame fusion between the LFn and the HIV coding sequence. The longest HIV-1 antigen among these fusion proteins is LFn-NG, which is created by adding Nef p27 between LFn and Gag p24. The molecular mass of LFn-NG is ≈83 kDa, containing 51 kDa of combined HIV-1 antigens. As reported (9), the purified proteins of LFn-p24, LFn-NG, and LFn-ENV reacted to various HIV-1 patient sera and monoclonal and polyclonal antibodies against HIV-1 antigens.
Staining and Confocal Imaging of LFn-GFP-Loaded Cells. HeLa cells (American Type Culture Collection), grown on collagen-treated chamber slides (BD Science) to reach ≈80% confluence, were incubated with 40 μg/ml purified GFP or LFn-GFP at 37°C for 1 or 2 h. Some incubations were performed in the presence of 100 μg/ml Texas red-conjugated transferrin (Molecular Probes) as a marker for the endocytic pathway or with 10 μg/ml PA. For the transferrin experiments, cells were washed four times with cold DMEM and then fixed for 15 min in 4% paraformaldehyde in cold PBS. For antibody labeling, slides were then incubated on ice for 15 min in 50 mM NH4Cl in PBS and then in PBS containing 0.1% saponin for 20 min on ice. After further washing in PBS, slides were incubated at room temperature for 1 hr in a moisture chamber with PBS containing 4% donkey serum and the following primary antibodies: mouse anti-early endosome antigen 1 (EEA-1) (BD Laboratory) to stain early endosomes, mouse anti-Lamp1 and anti-Lamp2 (Developmental Studies Hybridoma Banks, University of Iowa, Iowa City) to stain late endosomes and lysosome, mouse Ab-1(Oncogene) to stain the Golgi apparatus, mouse anti-mitochondrial antibody from Calbiochem, rabbit anti-calreticulin (StressGen Biotechnologies, Victoria, Canada), or anti-major E. R. glycoproteins (MERG) (kind gift from D. Meyer, University of California, Los Angeles) for endoplasmic reticulum staining and rabbit anti-20s proteosome α subunit and anti-20s proteosome β subunit antibodies (Calbiochem). After further washing with PBS, slides were incubated at room temperature for 45 min in PBS containing 4% donkey serum and Alexa Fluor 568- or Alexa Fluor 594-conjugated donkey anti-mouse or rabbit IgG (Molecular Probes). After extensive washing with PBS, slides were mounted with ProLong anti-fade medium (Molecular Probes) and analyzed on a Bio-Rad Radiance 2000 laser scanning confocal microscopy system enhanced with a Ti Sapphire Tsunami laser from Spectra-Physics. All images represent a single section through the focal plane.
CTL Induction in Mice by Vaccination with the LFn Fusion Proteins. Groups of 8- to 10-wk-old BALB/c mice (four in each group) were used in these studies. One i.p. immunization was given with 15 μg of the purified LFn fusion proteins with or without 5 μg of PA and with or without 150 μg of Alum. Chromium (51Cr) release CTL tests were conducted at 1 wk after immunization by using protocols as described (4). In brief, splenic mononuclear cells from the immunized mice were used as the source of CTL. The precursor CTLs in the splenic cultures were induced to mature in vitro by culturing with gamma-irradiated and peptide-pulsed BALB/c splenocytes from unimmunized animals. The peptide used for testing LFn-p24 and LFn-NG was the peptide p20 (GHQAAMQMLKETINEEAAEW, ref. 12), and for LFn-V3 and LFn-ENV the peptide was p18 (RIQRGPGRAFVTIGK, ref.13). After 6 days of culturing in a 37°C and 5% CO2 incubator, mature CTLs (effectors) were tested for their ability to lyse either 51Cr-labeled and peptide-pulsed P815 cells (positive targets) or 51Cr-labeled non-peptide pulsed cells (negative targets). A standard 4-hr cytotoxicity assay format was used with 10,000 targets per well and a titration of effector-to-target ratios of 100:1, 50:1, 25:1, and 12.5:1. The percentage lysis was calculated by using the formula: {[(experimental release) — (spontaneous release)]/[(maximum release) — (spontaneous release)]} × 100.
Results
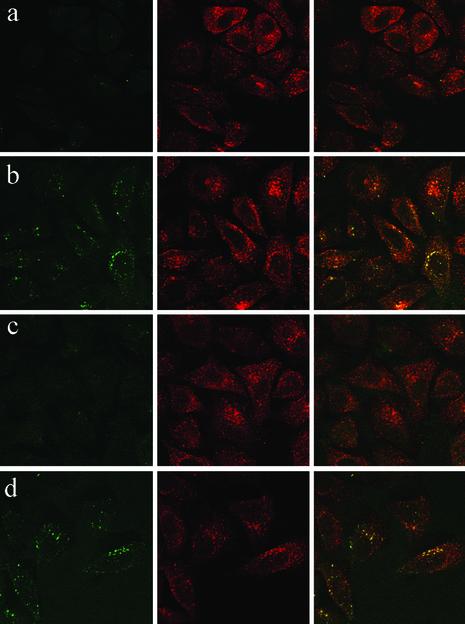
LFn-GFP Enters HeLa Cells Whereas GFP Cannot. To determine whether LFn fusion proteins can enter cells in the absence of PA, HeLa cells were incubated with purified LFn-GFP or GFP in the presence of Texas red-conjugated transferrin for 1 or 2 hr and were examined by confocal microscopy (Fig. 1). Although the control GFP protein was not taken up into cells, internalization of LFn-GFP was clearly detected within 1 hr and persisted in comparable amounts for 2 hr after incubation. The LFn-GFP staining pattern was cytosolic and granular, suggesting that LFn-GFP might be associated with a vesicular compartment within cells. However, LFn-GFP and transferrin signals did not seem to colocalize significantly.
Fig. 1.
HeLa cells internalize LFn-GFP in the absence of PA, but do not internalize GFP. HeLa cells were incubated with Texas red-conjugated transferrin and GFP (a and c) or LFn-GFP (b and d) for 1 hr (a and b) or 2 hr (c and d) and imaged by confocal microscopy. (Right) Overlay of red and green staining. (Center) Uptake of transferrin in red. (Left) Internalized LFn-GFP or GFP in green. Similar results were found for COS cells (data not shown).
Colocalization of Internalized LFn-GFP with the Proteosome 20s Subunit. The internalization of transferrin has been well characterized to occur via the endocytic pathway (14). Because LFn-GFP and transferrin staining did not coincide, to determine the trafficking pathway for LFN-facilitated uptake, we stained cells incubated with either protein for 1 and 2 hr with markers associated with a variety of subcellular organelles, including the early endosome, late endosome, and lysosome (Lamp-1 and Lamp-2), Golgi apparatus, endoplasmic reticulum, and mitochondria. (Fig. 2 and data not shown). Transferrin was mostly detected within the early endosome after 1 hr and was present in both early endosomes and late endosomes after 2 hr (data not shown). None of these markers coincided well with the pattern of LFn-GFP staining. Although there may have been some colocalization of LFn-GFP with the early endosomal marker EEA-1, this was incomplete. Computer generated 3D z plots did not clearly show that EEA-1 and LFn-GFP were in identical compartments (data not shown).
Fig. 2.
Internalized LFn-GFP does not completely localize with the endocytic or secretory pathways. HeLa cells incubated for 1 hr with LFn-GFP in the absence of PA were stained with markers for early endosomes (EEA-1; a), late endosomes (Lamp-1; b), lysosomes (Lamp-2; c), and the Golgi apparatus (Ab-1; d) and visualized by confocal microscopy. (Right) Overlay of red and green staining. (Center) Red organelle antibody staining. (Left) Green fluorescence of LFn-GFP.
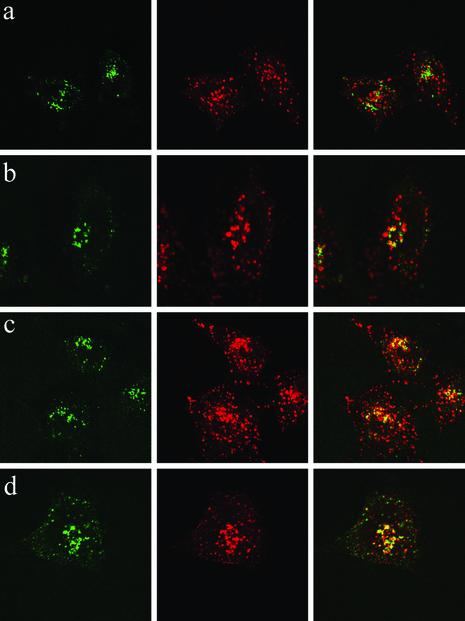
Incubation of cells with LFn fusion proteins in the absence of PA clearly stimulates CD8 T cell responses (10). Because the proteosome is responsible for processing of antigen into the MHC class I pathway for presentation to CD8 T cells, we next stained LFn-GFP-loaded cells with antibodies to the proteosome 20s subunit (Fig. 3). The staining for the proteosome colocalizes well with LFn-GFP fluorescence 1 or 2 hr after loading. Although the colocalization is not complete, it is much better than for any of the organelle markers. The same results were found with two different antibodies to the proteosome 20s subunit, respectively (data not shown).
Fig. 3.
Colocalization of LFn-GFP with the proteosome 20s subunit. HeLa cells were stained with the mixture of an antibody to the α-subunit and the other to the β-subunit of the 20s proteosome 2 hr after incubation with LFn-GFP. Similar results were also found after 1 hr incubation (see Fig. 1) and with a single antibody to the α-subunit or β-subunit of the proteosome, respectively (data not shown). (Top) Green fluorescence of LFn-GFP. (Middle) Red fluorescence of the proteosome antibody. (Bottom) Overlay of both channels.
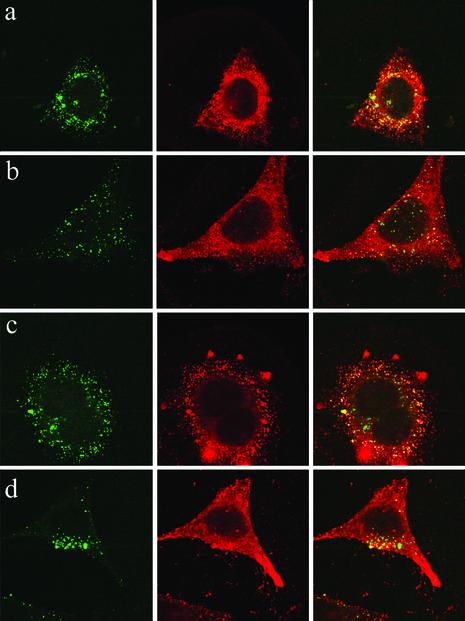
PA Does Not Enhance Cellular Intake of LFn-GFP. We further examined whether the presence of PA would enhance the intake of LFn-GFP under these experimental conditions. As shown in Fig. 4, adding PA did not increase the uptake of LFn-GFP inside cells. Moreover, the presence of PA seems to have reduced the colocalization of LFn-GFP with the proteosome 20s subunit. These results suggest that the presentation of LFn-GFP to the class I pathway may actually be enhanced in the absence of PA.
Fig. 4.
Internalization and colocalization of LFn-GFP with the proteosome 20s subunit is more efficient in the absence of PA. HeLa cells were stained for the proteosome 20s α-subunit 1 hr (a and b)or2hr(c and d) after incubation with LFn-GFP in the absence (a and c) or presence (b and d) of PA. (Right) Overlay. (Center) Red fluorescent staining for the proteosome. (Left) Green fluorescence of LFn-GFP.
LFn-p24, LFn-NG, and LFn-ENV Are Capable of Stimulating Anti-HIV CTL in Inbred Mice in the Absence of PA. Unlike LFn-V3 (9), LFn-p24 in the presence of PA did not show any significant CTL induction in mice after three immunizations (data not shown); neither did LFn-NG in similar experiments. Although LFn-ENV contains the same CTL epitope as the LFn-V3, it failed to induce the CTL response in mice that LFn-V3 easily did.
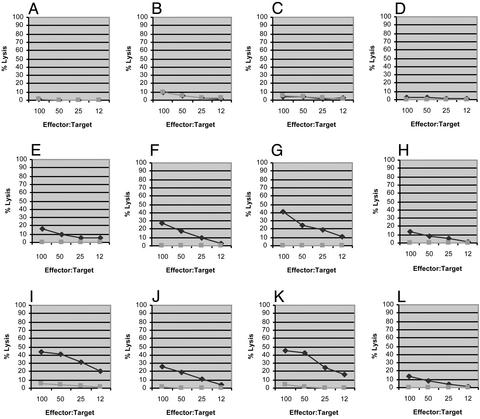
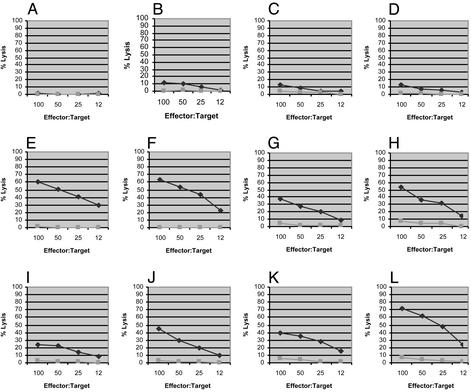
We then tested whether adding an immunoadjuvant could improve the in vivo immunogenicity of these LFn fusion proteins. As shown in Fig. 5 (Middle, animals E–H), we found that LFn-p24 plus PA in the presence of 150 μg of Alum (aluminum oxyhydroxide, or Alhydrogel) could induce significant anti-p20 CTL in mice after only one immunization. Under the same conditions, the same immunogen without the adjuvant remained inactive (Fig. 5, animals A–D). However, in the presence of Alum, the animals that received the same amount of LFn-24 without PA also showed comparable CTL induction (Fig. 5, animals I–L). This result was somewhat unexpected because Alum has not been known as an adjuvant to enhance CTL induction. In fact, this adjuvant has been reported to have a negative effect on CTL induction in mice by recombinant protein-based Hepatitis B vaccine (15, 16). As a negative control, groups of animals that received Alum alone (150 μg) or Alum with 15 μg recombinant HIV-1 p24 (Trinity Biotech, Bray, Ireland/Bartels) did not show any significant antigen-specific CTL activities (data not shown).
Fig. 5.
CTL induction in mice by LFn-p24 with Alum in the presence and absence of PA. Chromium release CTL was conducted 1 wk after a single i.p. immunization with 15 μg of LFn-p24 plus 5 μg of PA (animals A–D), 15 μg of LFn-p24 plus 5 μg of PA formulated with 150 μg of Alum (animals E–H), and 15 μg of LFn-p24 with 150 μg of Alum, respectively. The diamonds represent lysis of target cells pulsed with the p20 peptide (antigen-specific cell killing), whereas the squares represent lysis of target cells that were not sensitized by the peptide (nonspecific cell lysis).
We repeated this experiment using LFn-NG to replace LFn-p24. As shown in Fig. 6 (animal H), in the presence of Alum, one of the four animals that received LFn-NG plus PA showed a low level of anti-p20 CTL activity after one immunization. In the same experiment, three of the four animals that received LFn-NG without PA but with Alum had significantly higher CTL activities (Fig. 6, animals I, J, and L). As aforementioned, in the absence of the adjuvant, none of the animals that received LFn-NG plus PA showed detectable CTL activity (Fig. 6, animals A–D).
Fig. 6.
CTL induction in mice by LFn-NG with Alum in the presence and absence of PA. CTL assay was conducted 1 wk after a single i.p. immunization with 15 μg of LFn-NG plus 5 μg of PA (animals A–D), 15 μg of LFn-NG plus 5 μgofPA formulated with 150 μg of Alum (animals E–H), and 15 μg of LFn-NG with 150 μg of Alum, respectively. The diamonds represent lysis of target cells pulsed with the p20 peptide and the squares represent nonspecific cell lysis.
We then tested LFn-ENV for its ability to induce anti-p18 CTL activity in mice and obtained a similar result. As shown in Fig. 7 (animals E–H), all of the animals that received LFn-ENV/PA plus Alum showed high levels of CTL activity after one immunization; so did all of the animals that received LFn-ENV plus Alum, but without PA (Fig. 7, animals I–L). As shown in Fig. 7, three of the four animals that were immunized with LFn-ENV/PA in the absence of Alum showed only low levels of CTL response to the immunization (animals B, C, and D).
Fig. 7.
CTL induction in mice by LFn-ENV with Alum in the presence and absence of PA. CTL assay was conducted 1 wk after a single i.p. immunization with 15 μg of LFn-ENV plus 5 μg of PA (animals A–D), 15 μg of LFn-ENV plus 5 μg of PA formulated with 150 μg of Alum (animals E–H), and 15 μg of LFn-ENV with 150 μg of Alum, respectively. The diamonds represent lysis of target cells pulsed with the p18 peptide and the squares represent nonspecific cell lysis.
In summary, these large LFn fusion proteins, including LFn-p24, LFn-NG, and LFn-ENV, are capable of inducing substantial CTL activities in mice by a single immunization. Whereas adding an immunoadjuvant such as Alum turns out to be essential, the presence of PA is not. In the case of LFn-NG, the presence of PA even seemed to have a negative effect (compare Fig. 6 E–H and I–L). These results are consistent with the previous report that, by using cloned human CTLs in vitro, these LFn fusion proteins were able to sensitize the CTL target cells in the absence of PA (10).
Discussion
Previous studies showed that LF and PA must act together as a toxin unit. For example, genetic deletion of either the PA or LF gene in B. anthracis attenuated bacterial pathogenesis to an extent comparable to the deletion of the whole LeTx (17). Although LF by itself inactivates mitogen-activated protein kinase kinase (MAPKK) in solution, which is believed to be the mechanism for the profound pathogenic effect of LeTx within cells, such enzymatic activities in live cells are totally PA-dependent. Furthermore, i.v. injection of LF in the absence of PA into experimental rats seems harmless compared with the lethal effect of injecting LF in the presence of PA (18). Experiments that looked at the induction of cell death in mouse macrophages as a pathogenic indicator of LeTx action reached the same conclusion (19). It is, therefore, concluded that the pathogenic effect of LF is PA-dependent.
These observations also lead to the hypothesis that PA enables LF to enter host cells to exert its pathogenic enzymatic activities. Based on the polymeric nature of PA molecules and the fact that a cellular receptor has been identified for PA, it is hypothesized that PA forms a pore-like structure on the surface of the cell membrane, through which LF enters into the cytosol (2, 3). The experimental results presented in this study and the previous publication (10) shed more light into the molecular mechanism by which LF functions as part of a bipartite anthrax LeTx and as an individual protein. Our data suggest that LF may be able to enter into cells in the absence of PA. The new hypothesis is, therefore, that the PA dependence of LeTx is not because LF cannot enter the cytosol without PA, but because LF needs PA binding to function as a toxin. PA may target LF to the endosomal pathway and allow it to enter the cytosol in such as way that it escapes from proteosomal colocalization, as we observed with LFn-GFP, and subsequent degradation. An alternate possibility is that sequences in LF that are not present in the N-terminal fragment LFn may be involved in targeting LF to mitogen-activated protein kinase kinase (MAPKK) or to protecting it from proteosomal degradation. Experiments to examine this finding are beyond the scope of the present study.
PA-independent LF entry may have a potential application to vaccine research and development. Growing evidence shows that there may be a number of pathways through which an exogenous protein antigen can elicit MHC class I-restricted CTLs. These pathways include the classic MHC class I antigen presentation pathway, the phagocytosis-mediated pathway, the endosomal “recycling” model, and the outside “loading” pathway. In the first two pathways, the MHC-I/peptide complexes are formed in the cytosol. The classical MHC-I pathway requires protein expression within the cell, secretion into the cytosol (BfA-sensitive), and intracellular antigen processing (TAP-dependent). In the phagocytosis pathway, protein antigens in the form of particles are processed in endosomes rather than in the proteosome (TAP-independent and BfA-sensitive). The “recycling” and “loading” models share the same characteristic. The exogenous antigens can be degraded either inside endosomes and then are bound to “recycled” MHC-I molecules there. Alternatively, they can be degraded outside cells and are then “loaded” as peptides onto empty MHC-I pockets on the surface of cells. These latter pathways are not as efficient as protein processing of proteins produced within cells, and the loading pathway is effective only for small epitopic peptides. In vaccine research, much effort has been devoted to making soluble protein antigens to use these different pathways. For example, using antigens coded in “naked” DNA as vaccines enables the antigens to enter into the classic MHC-I pathway as endogenous proteins do, thus eliciting cell-mediated immune responses. Using live recombinant viruses or bacteria as vaccine delivery vehicles achieves the same purpose, allowing the antigens to be synthesized endogenously so that they can enter the classic MHC-I pathway. Alternatively, antigens in the form of virus particles or associated with cell debris are designed to use the “phagocytosis” pathway to elicit CTL, whereas synthetic peptide vaccines are believed to load on MHC-I molecules.
In a previous publication (10), we showed that the entry of LFn fusion proteins into the classic MHC-I pathway can be blocked by using TAP-deficient target cell lines or a number of chemical inhibitors that interrupt the class I antigen-processing pathway. In this study, we show that the LFn fusion protein can indeed enter inside cells and appear in locations occupied by the cellular proteosome. The efficient colocalization of LFn-GFP with the proteosome in the absence of PA suggests that fusion proteins of LFn without PA may efficiently be used to induce CD8 T cell responses in vivo. This finding is supported by the fact that LFn fusion proteins without PA induce stronger CTL responses in vivo than fusion proteins administered with PA.
Regardless of the molecular mechanism of LeTx's function as a lethal toxin, what we have learned thus far about its entry into the cytosol has allowed us to develop a vaccine delivery vehicle without resorting to live viral vectors or naked DNA. Previous studies demonstrated that, in the presence of PA, which is hypothesized to serve as a “molecular syringe,” LFn can deliver a variety of short CTL epitopes up to 33 aa long (7, 8, 9). Our recent study showed that, in the presence of an immune adjuvant, larger LFn fusion proteins that carry various viral antigens (up to 550 aa long) could induce CTL responses in experimental animals with or without the molecular syringe. We hypothesize, therefore, that there may be two different mechanisms that govern the entry of different LFn fusion proteins, which subsequently determine the effectiveness of the antigen presentation by MHC-I molecules. In the case of LFn fusion proteins that carry only short pieces of CTL epitopes, the entry is perhaps through the molecule syringe made of PA. Although such a PA-dependent delivery seems to be limited by the size of the fusion proteins, it is sufficiently effective in that it requires a very low amount of antigen and works in the absence of any immune adjuvant (7, 8, 9). On the other hand, when LFn fusion proteins carry longer pieces of “foreign” antigens, they may not be able to use the molecular syringe as effectively as those shorter molecules and may have to enter the cytosol through a different mechanism. The PA-independent delivery thus requires a relatively higher amount of the proteins and the presence of an immune adjuvant.
The results in this study put LF into the small class of proteins capable of entering into cells through mechanisms that are yet to be understood. Other proteins in this class include the HIV-1 Tat protein and bacterial heat-shock proteins (20, 21). Further studies are warranted to understand their entry mechanism and their subsequent trafficking within cells.
Acknowledgments
We thank Chanc E. VanWinkle for editorial assistance. This work is supported by National Institutes of Health Grant AI47539 (to Y.L.).
Abbreviations: LeTx, lethal toxin; PA, protective antigen; LF, lethal factor; LFn, N-terminal LF; CTL, cytotoxic T lymphocyte; TAP, transport-associated protein; Alum, aluminum oxyhydroxide, or Alhydrogel; EEA-1, early endosome antigen 1.
References
- 1.Bradley, K., Mogridge, J., Mourez, M., Collier, J. & Young, J. (2001) Nature 414, 225–229. [DOI] [PubMed] [Google Scholar]
- 2.Milne, J., Hanna, P., Wall, J. & Collier, J. (1994) J. Biol. Chem. 269, 20607–20612. [PubMed] [Google Scholar]
- 3.Koehler, T. & Collier, J. (1991) Mol. Microbiol. 5, 1501–1506. [DOI] [PubMed] [Google Scholar]
- 4.Duesbery, N., Webb, C., Leppla, S., Gordon, V., Klimpel, K., Copeland, T., Ahn, A., Oskarsson, M., Fukasawa, K., Paull, K. & Vander Woude, G. (1998) Science 280, 734–737. [DOI] [PubMed] [Google Scholar]
- 5.Milne, J., Blanke, S., Hanna, P. & Collier, J. (1995) Mol. Microbiol. 15, 661–666. [DOI] [PubMed] [Google Scholar]
- 6.Pannifer, A., Wong, T., Schwarzenbacher, R., Renatus, M., Petosa, C., Blenkowska, J., Lacy, D., Collier, J., Park, S., Leppla, S., et al. (2001) Nature 414, 229–233. [DOI] [PubMed] [Google Scholar]
- 7.Ballard, J., Collier, J. & Starnbach, M. (1996) Proc. Natl. Acad. Sci. USA 93, 12531–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard J., Doling, A., Beauregard, K., Collier, J. & Starnbach, M. (1998) Infect. Immun. 66, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, Y., Friedman, R., Kushner, N., Doling, A., Thomas, L., Touzjian, N., Starnbach, M. & Lieberman, J. (2000) Proc. Natl. Acad. Sci. USA 97, 8027–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, H., Agrawal, D., Kushner, N., Touzjian, N., Essex, M. & Lu, Y. (2002) J. Infect. Dis. 185, 244–251. [DOI] [PubMed] [Google Scholar]
- 11.Ivins, B. & Welkos, S. (1986) Infect. Immun. 54, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel, F. R., Hegde, S., Lieberman, J. & Paterson, Y. (1995) J. Immunol. 155, 4775–4782. [PubMed] [Google Scholar]
- 13.Takeshita, T., Takaharshi, H., Kozlowski, S., Ahlers, J. D., Pendleton, C. D., Moore, R. L., Nakagawa, Y., Yokomuro, K., Fox, B. S., Margulies, D. H. & Berzofsky, J. A. (1995) J. Immunol. 154, 1973–1986. [PubMed] [Google Scholar]
- 14.Jackle, S., Runquist, E., Miranda-Brady, S. & Havel, R. (1991) J. Biol. Chem. 266, 1396–1402. [PubMed] [Google Scholar]
- 15.Schirmbeck, R., Melber, K., Kuhrober, A., Janowicz, Z. A. & Reimann, J. (1994) J. Immunol. 152, 1110–1119. [PubMed] [Google Scholar]
- 16.Schirmbeck, R., Melber, K., Mertens, T. & Reimann, J. (1994) J. Virol. 68, 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezard, C., Berche, P. & Mock, M. (1991) Infect. Immun. 59, 3472–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephen, J. (1986) in Pharmacology of Bacterial Toxins, eds. Dorner, F. & Drews, J. (Pergamon, Oxford), pp. 381–395.
- 19.Hanna, P. C., Acosta, D. & Collier, J. (1993) Proc. Natl. Acad. Sci. USA 90, 10198–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarze, S., Ho, A., Vocero-Akbani, A. & Dowdy, S. (1999) Science 285, 1569–1572. [DOI] [PubMed] [Google Scholar]
- 21.Rojas, M., Donahue, J., Tan, Z. & Lin, Y.-Z. (1998) Nat. Biotechnol. 16, 370–375. [DOI] [PubMed] [Google Scholar]